Abstract
Although there are considerable reports of magnetic field effects (MFE) on organisms, very little is known so far about the MFE-related signal transduction pathways. Here we establish a manipulative near-zero magnetic field (NZMF) to investigate the potential signal transduction pathways involved in MFE. We show that exposure of migratory white-backed planthopper, Sogatella furcifera, to the NZMF results in delayed egg and nymphal development, increased frequency of brachypterous females, and reduced longevity of macropterous female adults. To understand the changes in gene expression underlying these phenotypes, we examined the temporal patterns of gene expression of (i) CRY1 and CRY2 as putative magnetosensors, (ii) JHAMT, FAMeT and JHEH in the juvenile hormone pathway, (iii) CYP307A1 in the ecdysone pathway, and (iv) reproduction-related Vitellogenin (Vg). The significantly altered gene expression of CRY1 and CRY2 under the NZMF suggest their developmental stage-specific patterns and potential upstream location in magnetic response. Gene expression patterns of JHAMT, JHEH and CYP307A1 were consistent with the NZMF-triggered delay in nymphal development, higher proportion of brachypterous female adults, and the shortened longevity of macropterous female adults, which show feasible links between hormone signal transduction and phenotypic MFE. By conducting manipulative NZMF experiments, our study suggests an important role of the geomagnetic field (GMF) in modulating development and physiology of insects, provides new insights into the complexity of MFE-magnetosensitivity interactions, and represents an initial but crucial step forward in understanding the molecular basis of cryptochromes and hormone signal transduction involved in MFE.
Introduction
Magnetoreception or magnetosensitivity, the ability of living organisms to respond to magnetic environments such as the geomagnetic field (GMF) and various artificial magnetic fields, has been well documented, especially in the animal kingdom [1, 2]. Much work has been done in relation to animal magnetic orientation in birds, fish, turtles, lobsters and even insects [3, 4]. At present, there are two non-mutually exclusive models of magentoreception, the light-independent magnetite-based system and the light-dependent radical pair mechanism (RPM). Both models have received experimental and theoretical support [3]. In particular, the putative RPM occurring in the flavoprotein photoreceptor cryptochromes (e.g. RPM with FAD–Trp, or FAD-Z) has received much more attention [5–8]. Cryptochromes can be grouped into three classes including animal cryptochromes (Type I, Type II and Type I+II), plant cryptochromes, and CRY-DASH cryptochromes [9]. Despite this seeming diversity, cryptochromes are fundamentally similar in both structure and photochemistry enabling them to have the potential to detect not only light, but also redox state and geomagnetic field [10]. The similar characteristics in different types of cryptochromes may also be the reason why animals that don’t migrate retain the capacity for magnetosensitivity, e.g. the honeybee Apis mellifera [11], the fruit fly Drosophila melanogaster [6]. Moreover, using a transgenic approach, human CRY2 was proved to even function as a magnetosensor in the magnetoreception system of Drosophila [12]. Thus, cryptochrome is likely to be a conserved and common magnetosensor of animals in a light-dependent manner, that is not limited to use in orientation.
Marley et al. (2014) advanced our understanding of the neuronal signal transduction process involved in the immediate electrophysiological magnetosensitivity by demonstrating a cryptochrome-dependent magnetic field effect on seizure response in Drosophila melanogaster larvae. And the data is consistent with a magnetosensitive, photochemical radical pair reaction in cryptochrome that alters levels of neuronal excitation [13]. It is commonly known that hormone signal transduction conventionally coexists and works together with neuronal signal transduction. The juvenile hormone (JH) and the principal molting hormone (MH) (i.e. ecdysone), 20-hydroxy-ecdysone (20E) are highly versatile insect hormones that coordinate development, growth and reproduction [14, 15], and signaling by these two hormones is regulated by neuropeptides and environmental signals [14–19]. By analogy, we hypothesized that the phenotypic MFE on development and physiology as we observed in small brown planthopper, Laodelphax striatellus and brown planthopper, Nilaparvata lugens subjected to a near-zero magnetic field (NZMF) [2], may be associated with cryptochrome-related hormone signal transduction which would alter the secretion of JH and ecdysone. Actually, NZMF has also been reported to affect circadian rhythm [20, 21]. And it is generally accepted that cryptochromes are not only the putative primary magnetosensors, but also key components in regulating circadian rhythms, which are ultimately involved in controlling the downstream expression of specific hormones in both vertebrates [22] and invertebrates [23, 24].
To investigate the hypothesis, we used the white-backed planthopper, Sogatella furcifera, which has two cryptochromes, CRY1 and CRY2. This insect is a major migratory insect pest of rice crops, causing serious loss by sap-sucking and transmitting the southern rice black-streaked dwarf virus [25]. Adult females of S. furcifera exhibit wing dimorphism and occur in two forms, macropterous with functional wings and brachypterous with reduced wings. Males are usually monomorphic macropterous [26]. Previously, we have found the phenotypic MFE on development and physiology of the two other species of rice planthoppers when subjected to a NZMF [2]. This is also the first reason we chose the NZMF as the experimental magnetic environment in this experiment, which ensure the feasibility of the NZMF in inducing MFE on rice planthoppers. Besides, there are two more practical advantages. First, GMF decay is a real phenomenon. A recent snapshot of the GMF based on data from the ESA’s Swarm satellite indicates a GMF decay 10 times faster than expected [27]. Moreover, the current trend of changing GMF looks persistent [28]. NZMF is a good approximation to simulate GMF decay. Ongoing decay in GMF provide a unique opportunity to study its potential bioeffects on organisms as well as role of field intensity in magnetoreception and life evolution [3, 29, 30]. Second, the strength of the galactic magnetic field does not exceed 0.1 nT, and interplanetary navigation will expose life to magnetic environments near 1 nT that are well below the typical value of approx. 0.05mT at the earth’s surface [30]. Spaceflight missions are proved to impact circadian clocks and disrupt sleep which may be due to the absence of electromagnetic field in space [31, 32]. Therefore, NZMF is also regarded as a good simulator for the magnetic environment in space, and it would help a lot in exploring the mechanisms of the adverse effects in a MFE context.
To date, no mechanisms have been identified whether hormone signal transduction is a part of a GMF- or artificial SMF-induced phenotypic response. To explore the potential hormone signal transduction and its link to cryptochrome in a molecular context, we exposed S. furcifera to a manipulative NZMF and quantified the temporal patterns of gene expression of the putative magnetosensors, CRY1 and CRY2, JHAMT, FAMeT and JHEH in JH pathway, CYP307A1 in ecdysone pathway, and Vitellogenin (Vg) which is downstream the JH hormone pathway [33] and crucial for fecundity [34].
Materials and Methods
Ethics statement
No specific permission was required for the collection location of Sogatella furcifera, a rice planthopper, and no endangered or protected species were involved, all experiments were done in controlled laboratory conditions.
Insect rearing
The white-backed planthopper, Sogatella furcifera, was collected from the paddy fields of Jiangsu Academy of Agricultural Science at Nanjing, Jiangsu Province of China. The planthopper stocks were reared on rice seedlings (cv. TN1; 15–30 days after planting, DAP) grown in the same environment chambers (HPG280H, Ningbo JIANGNAN Ltd., Ningbo, China) at 70–80% RH, 27±1°C/26±1°C day/night temperatures, and 14:10h (L:D) photoperiod for twelve generations to obtain uniform colonies. The brachypterous wing forms were only found in female adults, as previously reported [26]. Considering that macropterous females are of considerable significance as pests due to their migratory ability and potential magnetic sensitivity for orientation. we used macropterous females to measure fecundity, longevity and the expression patterns of selected genes.
Setup of magnetic field and insect exposure treatments
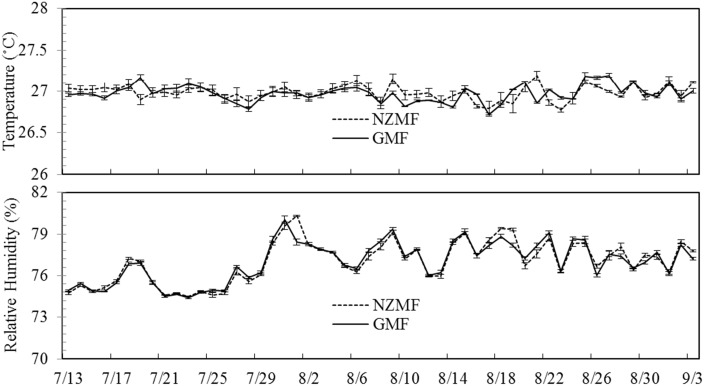
A static near-zero static magnetic field (NZMF) was generated by four pairs of Helmholtz coils mounted in a laboratory with an average intensity of ~500nT measured using a fluxgate magnetometer (CTM-5W01B, National Institute of Metrology, China, sensitivity: ±1 nT) in the center cubical space (300 × 300 × 300 mm3) as described in our previous work [2]. An identical set of equipment was used as control to simulate the real geomagnetic field, and both sets of equipment were interchanged during the experiment to minimize variation due to equipment. As described in Wan et al. (2014), the magnetic field equipment for both treatments were each housed in customized cylindrical chambers to maintain constant lighting and environmental factors. The experimental S. furcifera treatment groups were both reared on 15–30DAP rice seedlings (cv. TN1) in glass tubes (diameter: height = 3.0cm: 15cm), and exposed to either a NZMF or simulated GMF. The rice seedlings were exchanged every 2 days and randomly arranged within the NZMF or GMF treatments to minimize the coils' fringe field effects. Each glass tube was covered with a piece of 40-mesh nylon gauze to prevent the escape of planthoppers. Kimura B culture solution was added along the tube wall daily to provide sufficient water and nutrients for the seedlings. The magnetic flux density of the NZMF and GMF was measured daily. The NZMF and GMF treatments were located at the same position within the effective magnetic field area to ensure that illumination intensity, temperature, humidity, vibrations and disturbances potentially induced by experimenters were as uniform as possible. The experiment was conducted under a 14:10 h (light: dark) photoperiod and monitored continuously using an automatic temperature analysis system (U23-001, HOBO Pro V2 Temp/RH Data Logger, MicroDAQ.com, Ltd., Contoocook, NH, USA) with an accuracy of ±0.02°C from 0 to 50°C. No significant differences in temperature (paired t test, P≥0.11, n = 53) or RH (paired t test, P≥0.13, n = 53) between NZMF and the GMF during the period from egg to adult respectively were found (Fig 1).
Fig 1. The dynamics of temperature and relative humidity (RH) in near-zero magnetic field (NZMF) vs. geomagnetic field (GMF) treatments from 13 July to 3 September 2014.
Significant differences in temperature or RH between NZMF and GMF treatments during the egg, nymphal, adult and total developmental period of the white-backed planthopper, Sogatella furcifera were respectively tested for using paired t-tests with an alpha value of P<0.05.
Developmental period of eggs and nymphs
Thirty female and male pairs of newly emerged macropterous S. furcifera were randomly selected from the insect stocks, and separately reared and mated in pairs on rice seedlings within glass tubes for 2 days in a greenhouse. Each mated female was then transferred into either NZMF or GMF treatments in the same cage to oviposit on fresh rice seedlings for one day, and then all adults were removed. Cages were monitored at the same time each day for newly hatched 1st-instar nymphs to quantify the egg period (days). Next, newly-hatched 1st-instar nymphs of S. furcifera were randomly collected from the NZMF and the GMF treatments, individually transferred to rice seedlings in new numbered glass tubes (one insect per tube), and re-exposed to the NZMF and GMF treatments from which they were collected to complete development. These individuals were checked for molting at the same time daily to monitor development from the 1st-5th instar. The remaining newly-hatched 1st-instar nymphs were reared for the gene expression experiments, and nymphs at 0h, 24h and 60h after molting into the 5th instar from each treatment group meeting the requirements of the molecular experiments were individually transferred into 1.5ml clear microtubes (Axygen MCT-150-C) at and stored in a -80°C freezer (Thermo Scientific Forma 702, USA) (90 individuals for each sampling time).
Wing dimorphism, fecundity and longevity of female adults
Once the S. furcifera adults emerged, individuals from each treatment group were identified to sex and wing form. Fifteen pairs of macropterous females and males were randomly selected to mate and oviposit in numbered glass tubes with rice seedlings (Male NZMF x female NZMF and male GMF x Female GMF). Since each S. furcifera female adult mates multiple times with male adults, a new male adult was added to the cage if the original died before the female. Fecundity was measured by dissecting rice stems every day under a stereomicroscope (MOTIC SMZ-168) until death of the given female individual and counting number of eggs laid per female. Another 40 newly-emerged macropterous females from each treatment group were transferred to large beakers with rice seedlings, maintained continuously under their corresponding magnetic field treatments, and checked for mortality daily until death. The remaining macropterous female adults on the 1st, 4th, 8th day after emergence from each treatment group meeting the requirements of the molecular experiments were individually transferred into 1.5ml clear microtubes (Axygen MCT-150-C) at and stored in a -80°C freezer (Thermo Scientific Forma 702, USA) for the gene expression experiments (90 individuals for each sampling time). Fresh rice seedlings were provided every 3rd day.
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of cryptochromes, genes in juvenile and ecdysone pathways, and Vitellogenin
The duration of the 5th instar, longevity and frequency of wing polymorphism in the S. furcifera female adults were significantly affected by the NZMF (see Results). For the qRT-PCR analysis of the cryptochromes (CRY1 and CRY2), JHAMT, FAMeT, JHEH and CYP307A1, RNA was extracted with Trizol (Invitrogen) from newly-molted to 60 hour-old (i.e., 0, 24 and 60 hour-old) 5th instar nymphs, and from newly emerged to 8-day-old (i.e., 1, 4 and 8 day-old) macropterous virgin female adults from both the NZMF and GMF treatment groups. For the qRT-PCR analysis of Vg, total RNA was extracted from newly-emerged to 8 day-old (i.e., 1, 4 and 8 days) macropterous virgin female adults from both treatment groups. A pooled sample of 30 heads for each sampling time was randomly mixed as one replicate, and three replicates were analyzed for each sampling interval and magnetic field treatment. Concentration and quality of total RNA was determined using a NanoDrop spectrophotometer (Thermo Scientific) and first-strand complementary cDNA was synthesized using PrimeScript RT reagent kit (TaKaRa). QRT-PCR was performed using SYBR Premix Ex Taq (Tli RNaseH Plus) (TaKaRa) in combination with a 7500 Real-Time PCR Detection System. Reactions were performed in a 20μl final volume reaction, using primers in a final concentration of 200nM. Two μl of a 1/2 dilution of the cDNA template of 5th instar nymph and 2μl of a 1/10 dilution of the cDNA template from female adults were used to make the Ct values fall within the suitable range of 15 to 35 based on preliminary runs. No template was added to negative control reactions. ARF and RPL9 were used for housekeeping reference genes for 5th instar nymphs, whereas 18S and RPL9 were used for female adults. The gene-specific qRT-PCR primers are listed in Table 1.
Table 1. Primers used in the qRT-PCR experiments to measure gene expression levels of CRY1, CRY2, JHAMT, FAMeT, JHEH, CYP307A1 in 5th instar nymphs, and CRY1, CRY2, JHAMT, CYP307A1, Vg in macropterous virgin female adults of Sogatella furcifera.
| Gene | Description | Primer forward (Sequence 5' to 3') | Primer reverse (Sequence 5' to 3') | GeneBank Accession |
|---|---|---|---|---|
| 18S | Housekeeping genes | TGTCTGCTTAATTGCGATAACGAAC | CCTCAAACTTCCATCGGCTTG | JF773150.1 |
| RPL9 | Housekeeping genes | TGTGTGACCACCGAGAACAACTCA | ACGATGAGCTCGTCCTTCTGCTTT | From Guo-Qing Li |
| ARF | Housekeeping genes | CACAATATCACCGACTTTGGGATTC | CAGATCAGACCGTCCGTACTCTC | From Guo-Qing Li |
| CRY1 | Cryptochrome1 | CTGTTCTTCCAGCGGCAAC | TGCTCTCACTGCGTCTGTCC | From Guo-Qing Li |
| CRY2 | Cryptochrome2 | CCTTCTGCTATCACGTTTGCT | CACGCCAAATTATTTCAAGTTCGTC | From Guo-Qing Li |
| JHAMT | JH acid methyltransferase | TGAATTGACTGCCATTACGGTT | CAGTTGTGTTGTTCCCGCTCA | From Guo-Qing Li |
| FAMeT | Farsoic acid methyltransferase | TGAGTATAAGCCTTCAGTACCTAGC | GTTTCACAAGGCATTCCTCTCG | From Guo-Qing Li |
| JHEH | JH epoxide hydrolase | GCACTATAACATCTTCAATGCGACT | AACTCATTTGGGAATCTTGCACA | From Guo-Qing Li |
| Vg | Vitellogenin | CTGATCTGGCTTTCATAGCTCT | GCTGCCAACATGGATCAGAAC | From Guo-Qing Li |
| CYP307A1 | Cytochrome P450, family 307, subfamily A | GAGCCCAAAGACTTCACCGAT | CCAGCTCATAAAGAATGTGATGCC | KC701459.1 |
Data analysis
All data were analyzed using the SPSS 20.0 (SPSS Inc., Chicago, IL, USA). Significant differences in temperature or RH between the NZMF and GMF treatments during the egg, nymphal, adult and total developmental periods were tested with paired t-test at α = 0.05. The developmental periods of the 1st-5th instar nymphs and total nymph periods were separately analyzed using two-way analyses of variance (ANOVAs), with the magnetic field as main factor (NZMF vs. GMF) and sex (female vs. male) as sub-factor for the macropterous S. furcifera, and with the magnetic field as main factor (NZMF vs. GMF) and wing form (macropterous female vs. brachypterous female) as sub-factor for the female S. furcifera. Moreover, two-way ANOVAs were also used to analyze the effects of the magnetic field (NZMF vs. GMF), sampling time, and their interactions on gene expression levels of CRY1, CRY2, JHAMT, FAMeT, JHEH and CYP307A1 in the 5th instar nymphs, and on the gene expression levels of CRY1, CRY2, JHAMT, CYP307A1 and Vg in the macropterous virgin female adults. If significant effects of magnetic field, sex/wing form/sampling time or their interactions on the growth, development and reproduction of S. furcifera were found, follow-up pairwise student's t tests were used to compare the means between NZMF and GMF, female and male, or macropterous female and brachypterous female at α = 0.05. Given that vitellin (Vn) of the female is the main source of nutrients for eggs they laid [33], we analyzed the effects of the magnetic field (NZMF vs. GMF) on egg period by one-way ANOVAs, not considering the effect of wing form. One-way ANOVAs were also used to analyze the effects of the magnetic field (NZMF vs. GMF) on longevity and fecundity of macropterous female adults. The Chi-square test was performed to analyze the frequency of brachypterous and macropterous female adults under the NZMF vs. GMF.
Results
Effects of the NZMF on the developmental period of eggs laid by macropterous female adults
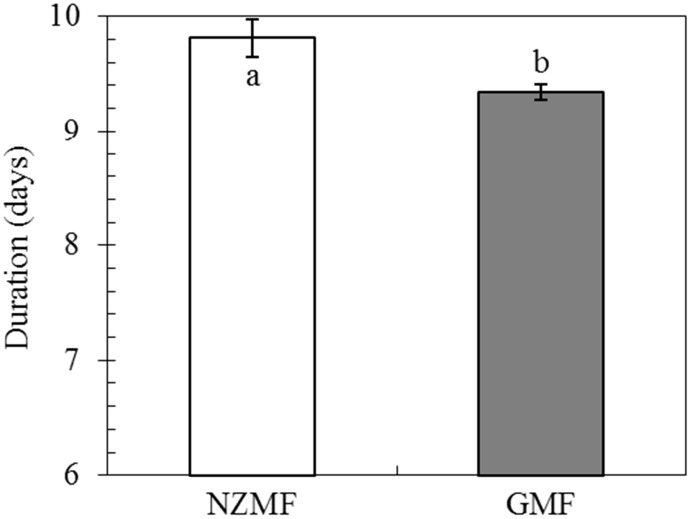
Compared with the GMF, NZMF exposure significantly lengthened the developmental period of eggs laid by macropterous female adults by 5.08% on average (F = 45.33, P<0.001; Fig 2).
Fig 2. Developmental period of eggs laid by macropterous female adults of Sogatella furcifera, under near-zero magnetic field (NZMF) vs. geomagnetic field (GMF).
N = 279 and 300 for eggs under the NZMF and GMF, respectively. The columns represent averages with vertical bars indicating SE. Different lowercase letters indicate significant differences between NZMF and GMF treatments for eggs laid by macropterous female adults by one-way ANOVA at P<0.05.
Effects of NZMF on nymphal development
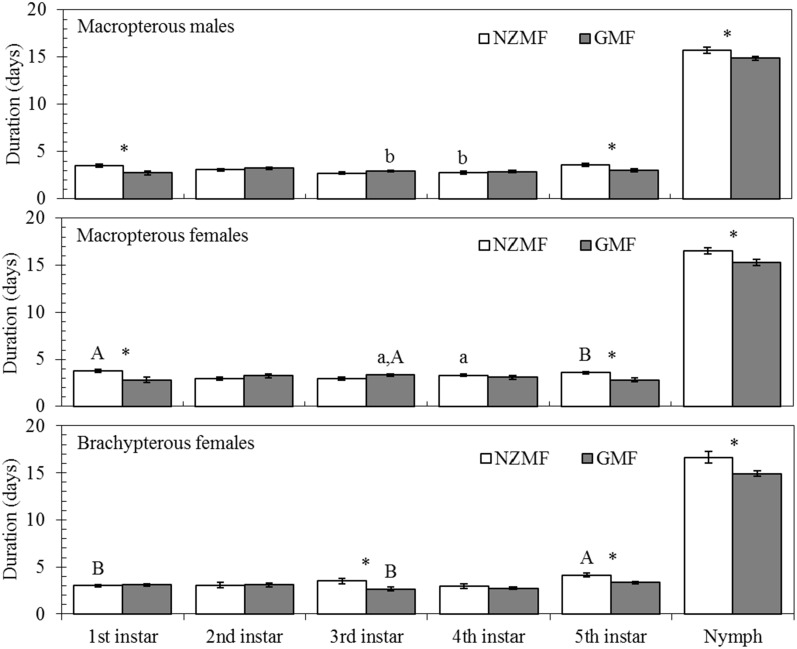
Across all female and male nymphs, NZMF significantly prolonged the duration of the 1st (F = 17.85, P<0.001), 5th instar (F = 16.98, P<0.001) and total nymphal period (F = 9.77, P = 0.002), and no significant effects of the interaction between magnetic field and sex were found (F≤1.01, P≥0.32) (Table 2). For the macropterous and brachypterous females, NZMF significantly prolonged the duration of the 1st (F = 4.32, P = 0.04), 5th instar (F = 22.15, P<0.001) and total nymphal period (F = 9.73, P = 0.003) (Table 2). Significant effects of the interaction between magnetic field and wing form were also observed in the 1st (F = 6.00, P = 0.02) and 3rd instar (F = 8.15, P = 0.006) (Table 2). In addition, the durations of the 1st, 5th instar and total nymphal stage were significantly prolonged by 27.54%, 19.41% and 5.78% on average for the macropterous male (P<0.05; Fig 3), and 33.22%, 26.50% and 7.89% on average for macropterous females (P<0.05; Fig 3), respectively. The duration of the 3rd, 5th and total nymphal stage were significantly prolonged by 31.09%, 24.02% and 11.46% on average for the brachypterous females (P<0.05; Fig 3). Moreover, significant differences were also found between macropterous males and females in the 3rd instar for the GMF and in the 4th instar for the NZMF treatments (P<0.05; Fig 3). Significant differences were also found between macropterous and brachypterous females for NZMF in the 1st and 5th instars, and for GMF in the 3rd instar (P<0.05; Fig 3).
Table 2. Two-way analyses of variance (ANOVAs) with magnetic field (MF) as main factor and sex/wing form as sub-factor on the duration of the 1st–5th instars and total nymphal periods (F/P).
| Nymph duration | Male & female | Female | ||||
|---|---|---|---|---|---|---|
| MF a | Sex | MF a × Sex | MF a | Wing form b | MF × Wing form b | |
| 1st instar | 17.85 / <0.001 *** | 0.65 / 0.42 | 0.20 / 0.66 | 4.32 / 0.04 * | 1.62 / 0.21 | 6.00 / 0.02 * |
| 2nd instar | 2.00 / 0.16 | 0.16 / 0.69 | 0.21 / 0.65 | 1.11 / 0.30 | 0.07 / 0.80 | 0.11 / 0.74 |
| 3rd instar | 3.30 / 0.07 | 3.34 / 0.07 | 0.22 / 0.64 | 1.36 / 0.25 | 0.17 / 0.69 | 8.15 / 0.006 ** |
| 4th instar | 0.14 / 0.71 | 5.08 / 0.03 * | 1.01 / 0.32 | 0.69 / 0.41 | 2.54 / 0.12 | 0.12 / 0.73 |
| 5th instar | 16.98 / <0.001 *** | 0.64 / 0.43 | 0.23 / 0.64 | 22.15 / <0.001 *** | 9.5 / 0.003 ** | 0.05 / 0.84 |
| Nymph | 9.77 / 0.002 ** | 3.58 / 0.06 | 0.27 / 0.60 | 9.73 / 0.003 ** | 0.04 / 0.89 | 0.15 / 0.70 |
a MF—the near-zero magnetic field (NZMF) vs. the geomagnetic field (GMF).
b Wing form—macropterous vs. brachypterous female.
* P<0.05.
** P<0.01.
*** P<0.001.
Fig 3. Nymphal periods of macropterous males, macropterous females and brachypterous females of Sogatella furcifera under near-zero magnetic field (NZMF) vs. geomagnetic field (GMF).
N = 30 for each treatment. The columns represent averages with vertical bars indicating SE. Only significant differences are marked with letters. Different lowercase letters indicate significant differences between macropterous females and males. Different uppercase letters indicate significant differences between macropterous and brachypterous female under the NZMF or GMF by one-way ANOVA at P<0.05; * Significant differences between NZMF and GMF for males or females were measured by one-way ANOVA at P<0.05.
Effects of the NZMF on wing dimorphism of female adults
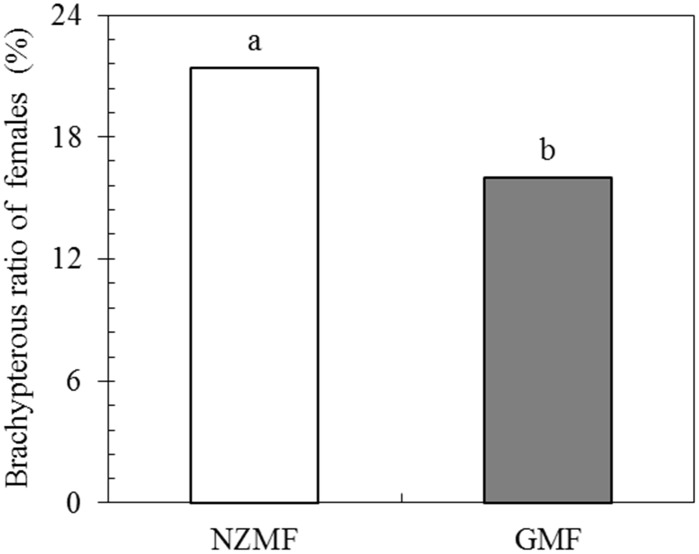
There was a significant difference in the frequency of brachypterous females produced among insects exposed to NZMF for one generation (X 2 = 4.524, two-tailed P = 0.033 by the Chi-square test; Fig 4). Compared with GMF, NZMF significantly increased the frequency of brachypterous female adults by 5.42% (P < 0.05; Fig 4).
Fig 4. The percentage of brachypterous female adults of Sogatella furcifera produced under near-zero magnetic field (NZMF) vs. geomagnetic field (GMF).
Under NZMF, 452 and 123 individual newly emerged female adults were macropterous and brachypterous, respectively, whereas under GMF, 342 and 65 females were macropterous and brachypterous, respectively. Insects in both magnetic field treatments came from the same number of parents and were reared for one generation under either NZMF or GMF. Different lowercase letters showed significant differences between NZMF and GMF by the Chi-square test at P<0.05.
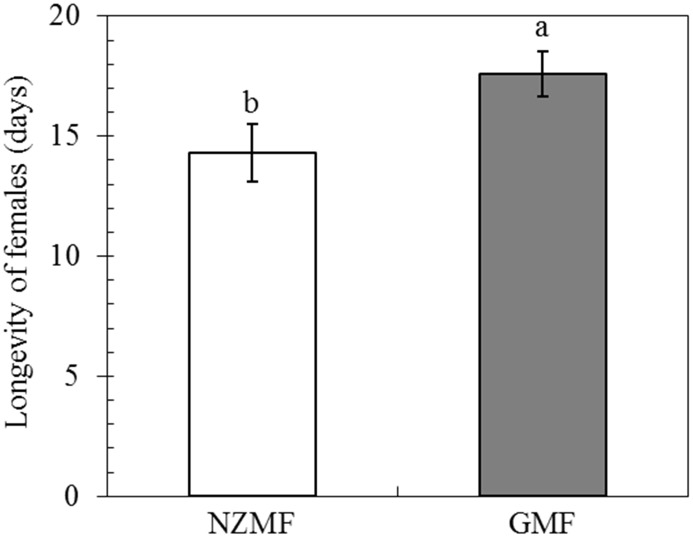
Effects of the NZMF on longevity of macropterous virgin female adults
Compared with the GMF, the NZMF significantly reduced the longevity of macropterous virgin female adults by 18.67%(F = 5.28, P = 0.025; Fig 5).
Fig 5. Longevity of macropterous virgin female adults of Sogatella furcifera, under near-zero magnetic field (NZMF) vs. geomagnetic field (GMF).
N = 30 and 45 for macropterous virgin female adults under the NZMF and GMF, respectively. The columns represent averages with vertical bars indicating SE. Significant differences between NZMF and GMF were measured by one-way ANOVA at P<0.05.
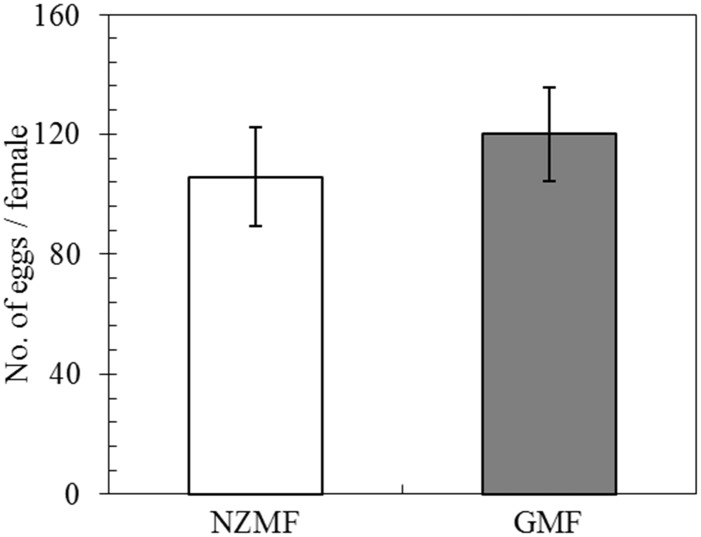
Effects of the NZMF on fecundity of macropterous female adults
NZMF had no significant effects on the fecundity of macropterous female adults mated with macropterous male adults compared to the GMF (F = 0.92, P = 0.35; Fig 6).
Fig 6. The fecundity of macropterous female adults of Sogatella furcifera under near-zero magnetic field (NZMF) vs. geomagnetic field (GMF).
N = 15 for both macropterous female adults under NZMF and GMF. The columns represent averages with vertical bars indicating SE. No significant differences between NZMF and GMF were found by one-way ANOVA at P<0.05.
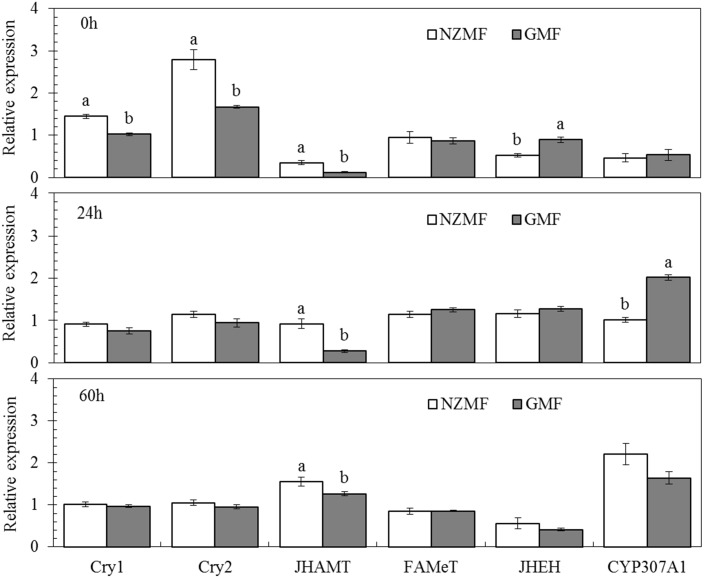
Temporal expression levels of cryptochromes and genes in ecdysone and juvenile hormone (JH) pathways in 5th instar nymphs
The expression of the cryptochrome genes (CRY1 and CRY2), CYP307A1, JHAMT, FAMeT and JHEH in 5th instar insects at 0h, 24h and 60h after molting varied significantly over time (F≥11.13, P≤0.002; Table 3). Magnetic field significantly affected the expression of JHAMT and both cryptochromes (CRY1 and CRY2) (F≥25.03, P≤0.001; Table 3). The temporal patterns of expression varied for the different genes under different magnetic field treatments as evidenced by significant interactions between magnetic field and sampling time for CRY1, CRY2, CYP307A1, JHAMT and JHEH (F≥5.64, P≤0.019; Table 3). Interestingly, the cryptochromes (CRY1 and CRY2) were significantly up-regulated by NZMF vs. GMF at 0h after molting into the 5th instar (P<0.05; Fig 7). Compared with the GMF, NZMF significantly increased JHAMT expression levels at the 0h, 24h and 60h after molting into the 5th instar (P<0.05). Simultaneously, NZMF reduced the JHEH expression level at the 0h after molting into the 5th instar (P<0.05; Fig 7). CYP307A1 exhibited significantly lower expression at 24h (P<0.05), but higher expression levels at the 60h after molting into the 5th instar nymphs under NZMF vs. GMF (P>0.05; Fig 7). No significant differences were found in the FAMeT expression levels between the NZMF and the GMF at any time during the 5th instar (P>0.05; Fig 7).
Table 3. Two-way ANOVAs with magnetic field (MF) as main factor and sampling time as sub-factor on the gene expression levels of cryptochromes (CRY1 and CRY2), JHAMT, FAMeT, JHEH and CYP307A1 for 5th instar nymphs (F/P).
| Genes | MF a | Sampling time b | MF a × Sampling time b |
|---|---|---|---|
| CRY1 | 26.49 / <0.001 *** | 33.21 / <0.001 *** | 7.74 / 0.007 ** |
| CRY2 | 25.03 / <0.001 *** | 73.28 / <0.001 *** | 11.86 / 0.001 ** |
| JHAMT | 71.37 / <0.001 *** | 145.38 / <0.001 *** | 13.64 / 0.001 ** |
| FAMeT | 0.03 / 0.87 | 11.13 / 0.002 ** | 0.77 / 0.485 |
| JHEH | 3.39 / 0.09 | 47.70 / <0.001 *** | 5.64 / 0.019 * |
| CYP307A1 | 2.11 / 0.17 | 54.06 / <0.001 *** | 15.70 / <0.001 *** |
a MF—the near-zero magnetic field (NZMF) vs. the geomagnetic field (GMF).
b Sampling time—0h, 24h and 60h after molting into the 5th instar under NZMF and GMF.
* P<0.05.
** P<0.01.
*** P<0.001.
Fig 7. The temporal patterns of gene expression of cryptochromes (CRY1 and CRY2), JHAMT, FAMeT, JHEH and CYP307A1 at 0h, 24h and 60h after molting into the 5th instar of Sogatella furcifera, under near-zero magnetic field (NZMF) vs. geomagnetic field (GMF).
Thirty individual 5th instar nymphs were randomly mixed as one sample for each sampling time with three repeats. The columns represent averages with vertical bars indicating SE. Only significant differences are marked with letters. Different lowercase letters indicate significant differences between NZMF and GMF for the same sampling time by the Student's t-test at P<0.05.
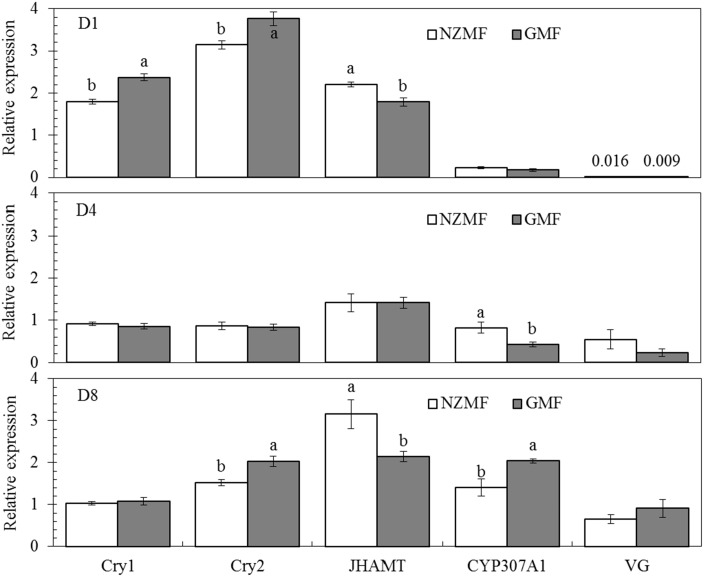
Temporal expression levels of Vg, cryptochromes and genes in ecdysone and juvenile hormone (JH) pathways in macropterous virgin female adults
The two-way ANOVAs indicated that NZMF significantly affected the expression of CRY1, CRY2 and JHAMT in macropterous virgin female adults (F≥9.90, P≤0.008; Table 4). Significant effects of sampling time (F≥11.13, P≤0.002; Table 4) were found for all five of the genes assayed. Moreover, significant magnetic field × sampling time interactions for the cryptochromes (CRY1 and CRY2) and CYP307A1 (F≥5.21, P≤0.02; Table 4) indicated that the expression patterns of these genes differed over time. Compared with the GMF, NZMF significantly reduced the expression levels of CRY1 on the 1st day, CRY2 on the 1st, 8th day and CYP307A1 on the 8th day after emergence of macropterous virgin female adults (P<0.05; Fig 8), while the expression levels of JHAMT on the 1st, 8th day, and CYP307A1 on the 4th day after emergence of macropterous virgin female adults were significantly up-regulated by the NZMF (P<0.05; Fig 8).
Table 4. Two-way ANOVAs with magnetic field (MF) as main factor, sampling time as sub-factor on the gene expression of cryptochromes (CRY1 and CRY2), JHAMT, CYP307A1 and Vg for the macropterous virgin female adults (F/P).
| Genes | MF a | Sampling time b | MF a × Sampling time b |
|---|---|---|---|
| CRY1 | 12.14 / 0.005 ** | 194.71 / <0.001 *** | 13.16 / 0.001 ** |
| CRY2 | 16.87 / 0.001 ** | 293.52 / <0.001 *** | 5.21 / 0.02 * |
| JHAMT | 9.90 / 0.008 ** | 22.06 / <0.001 *** | 3.83 / 0.052 |
| CYP307A1 | 0.55 / 0.47 | 115.56 / <0.001 *** | 12.90 / 0.001** |
| Vg | 0.03 / 0.86 | 14.72 / 0.001 ** | 2.03 / 0.174 |
a MF—the near-zero magnetic field (NZMF) vs. the geomagnetic field (GMF).
b Sampling time—the 0, 4 and 8 days after emergence of female adult and the GMF.
* P<0.05.
** P<0.01.
*** P<0.001.
Fig 8. Temporal gene expression patterns of cryptochromes (CRY1 and CRY2), JHAMT, CYP307A1 and Vg on the 1st (D1), 4th (D4), 8th (D8) day after emergence of macropterous female adult Sogatella furcifera under near-zero magnetic field (NZMF) vs. the geomagnetic field (GMF).
Thirty individual macropterous female adults were randomly mixed as one sample for each sampling time with three repeats. The columns represent averages with vertical bars indicating SE. Only significant differences are marked with letters. Different lowercase letters indicate significant differences between NZMF and GMF for the same sampling time by the Student's t-test at P<0.05.
Discussion
We have demonstrated a series of significant MFE induced by exposure to NZMF on the development and physiology of S. furcifera ranging from variation in gene transcription to the expression of alternative phenotypes. Combined with the results of our previous work using the same approach [2], this study broadens our understanding of NZMF triggered MFE on S. furcifera development to include variation depending on species, sex and alternative wing-form phenotype.
It is well-known that the main ecdysone, 20E and JH coordinately orchestrate larva to larva (or nymph to nymph) and metamorphosis development [14–18]. Positive correlations have been revealed between JHAMT expression levels and JH titer as well as between CYP307A1 expression levels and 20E titer. It was also reported that one major route of insect JH degradation is epoxide hydration by JH epoxide hydrolase (JHEH). Given these relationships, our findings in gene expression levels of JHAMT, JHEH and CYP307A1 in 5th instar nymphs are consistent with an up-regulation of JH and a down-regulation of 20E. Therefore, change patterns of these two hormones in S. furcifera should be related to the significantly prolonged nymphal development we observed in this and the previous study [2]. Besides the roles of JH in juvenile development, a role for JH in regulating insect dormancy was recently shown in experiments in which surgical removal of the CA led to reduced fecundity or sterility and extended longevity in grasshoppers, butterflies, hemipterans, and fruit flies, and it was suggested that JH is a positive regulator of fecundity, but a negative regulator of life span [35]. It was also reported that JH is involved in the regulation of wing dimorphism and the development of brachypterous wings in N. lugens [36]. Our findings in the related S. furcifera demonstrated changes in gene expression levels of JHAMT, JHEH in 5th instar nymphs and JHAMT in macropterous virgin female adults that are consistent with an up-regulation of JH, which should be responsible for the significantly higher brachypterous forms and significantly shorter life span of macropterous virgin female adults under the NZMF compared to the GMF. Although hormone levels in the egg stage were not determined in our study, the prolonged hatching period we observed was likely to be triggered by hormone signal transduction as well [37]. All insects provision their eggs with Vn as a major yolk protein, and Vg acts as the precursor of Vn. Vg molecules in the fat body are important in facilitating the transport of nutrients to the ovaries [33]. Zhai et al. (2013) have shown that the number of brown planthopper offspring decreased after its nymphs (third to fifth instars) were continuously treated with dsVg [34], which indicated a positive correlation between gene expression level of Vg and fecundity. This positive correlation may be the reason why significant differences in fecundity were not found between GMF and NZMF treated macropterous adult females (Fig 6). However, in comparison with the temporal gene expression pattern of Vg under the GMF in our study, it seemed that the transcription of Vg commenced earlier when exposed to the NZMF. Given that the hormones involved in Vg gene regulation include juvenile hormone, ecdysone and several neuropeptides [32], our observation of alternating changes between JHAMT and CYP307A1 may have been responsible for the gene expression pattern of Vg in female adults, though with a time lag.
In a recent transcriptome profiling analysis of human neuroblastoma cells, MAPK1 and CRY2 showed significant up- and down-regulation, respectively, during the first 6 h of a NZMF exposure [38]. We also found significant changes in the expression of both CRY1 and CRY2 in 5th instar and adult females of S. furcifera. With the exception of the down-regulation of CRY2 on day 8 in macropterous females, significant changes in both life history stages were only early in the developmental period, implying that cryptochromes may be located upstream in magnetic response as putative magnetosensors [9]. Cryptochrome proteins precisely act as components of the central circadian clock of metazoans [39] that times a range of important behavioral and physiological processes in animals including sleep-wake cycles [40], locomotion patterns [41], feeding [42], mating [43], and cell division [44]. Interestingly, NZMF has been reported to affect circadian rhythm [20, 21]. Circadian clock is also found to be altered in space where is absence of electromagnetic field [31, 32]. The work of Yoshii et al. (2009) further reveals that the magnetosensitivity of Drosophila’s circadian clock need light activation of cryptochrome and depend on the applied field strength [45]. In insects, levels of JH and ecdysone are controlled by the neuroendocrine system [46–50]. Many experiments have shown relationships between the circadian clock and neuroendocrine hormones that may indirectly regulate secretion of JH and ecdysone (e.g. 20E) [14, 18, 50–53]. Most likely, the pacemaker neurons that produce the principal circadian transmitter called pigment-dispersing factor (PDF) may connect the circadian clock to hormone signal transduction. This is supported by the fact that axons projecting from the PDF-producing neurons terminate in close proximity to the dendritic arbors of the prothoracicotropic hormone-producing neurons [17], and PDF mutants show altered prothoracicotropic hormone transcript levels [18] which is reported negatively correlated with JH while positively correlated with ecdysone [15, 18]. In addition, mammalian-type cryptochromes in insects such as the monarch butterfly seem to work as a transcriptional repressor in the feedback loop of the circadian clock [54]. Therefore, the MFE involved in hormone signal transduction here may due to potential roles for magnetic fields in the regulation of circadian clock [5, 45].
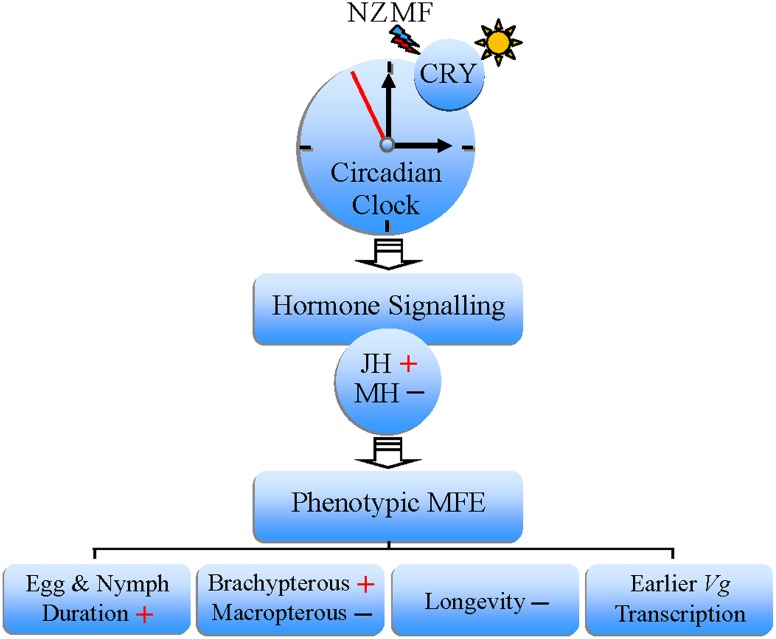
It is commonly known that continuous treatment with abnormal or unexpected signals would result in stress reactions. Observations with mole-rats suggested that magnetosensitivity is no different to the other senses, and is involved in multi-sensory integration with other inputs [55, 56]. Presumably, the MFE in our study may also be stress responses of sensory systems [56], induced by continuous treatment with the manipulative NZMF. Based on some previous research, our current results, and the published evidence for the putative role of cryptochromes as magnetosensors [6, 9, 12, 13, 45, 57, 58], a conceptual model describing potential links among MFE on the development and physiology of S. furcifera, hormone signal transduction and magnetosensitivity is provided in Fig 9. Here we proposed that one pathway could be specifically originated from the putative light-dependent magnetosensor, cryptochrome. The effects of the NZMF on the development and physiology of S. furcifera may be owing to the dual function of cryptochromes, that there may be interactions between their putative function as magnetosensors and as crucial components of the circadian clock [10]. Furthermore, given the above discussion of the potential roles for magnetic fields in the regulation of circadian clock, our results indicating temporal changes induced by NZMF exposure in the expression patterns of cryptochromes and genes in juvenile hormone (JH) and ecdysteroid pathway provide a feasible causal link among magnetosensitivity, hormone signal transduction and the phenotypic MFE.
Fig 9. A conceptual model of hormone signal transduction-involved magnetic field effects (MFE), triggered by the near-zero magnetic field (NZMF) on the development and physiology of Sogatella furcifera.
Abbreviation, CRY: Cryptochrome; JH: Juvenile hormone; MH: Molting hormone (Ecdysone); Vg: vitellogenin; +: up-regulated or increased;–: down-regulated or decreased.
Except for the functional links as light-dependent CRY-mediate pathway in Fig 9, it is also possible that the MFE on cryptochromes, hormone signal transduction and circadian clock of S. furcifera are isolated and non-functional since the precise biophysical origin of magnetosensitivity or MFE remains unclear. Although the phenotypic MFE of prolonged egg and nymph duration, earlier transcription of Vg, decreased longevity of macropterous female adults and increased brachypterous ratio of female adults in S. furcifera occurred when exposed to the NZMF, there still have much possibilities to be further studied in the future. For example, whether changes in development and physiology under NZMF is also related to altered circadian rhythms of cell division [44], cell proliferation [59], apoptosis [60] and efficiency of food digestion [23], the level of locomotor activity [5] or nutrient efficiency [61]. In summary, our findings of MFE triggered by the NZMF on transcriptional levels of cryptochromes and other genes in growth and development-related hormone pathways suggest the presence of a hormone signal transduction mechanism in S. furcifera. Importantly, such a pathway is conventionally suggested to coexist with neural signal transduction [13], together making up the signal transduction systems of MFE. To our knowledge, this study provides the first molecular evidence that insect hormone signal transduction can contribute to MFE. The use of manipulative NZMF in this study also provides valuable references for exploring potential bioeffects triggered by decrease of the GMF intensity [27, 30] and bioeffects on astronauts in space where is absence of magnetic field [30–32].
Acknowledgments
We thank Prof. Guo-Qing Li of the Department of Entomology, Nanjing Agricultural University (NJAU) for his help in supplying gene information. We also thank Kai-Yun Fu of the Department of Entomology, NJAU for advice on the gene expression experiment.
Data Availability
All relevant data are within the paper.
Funding Statement
FJC was funded by the National Basic Research Program of China "973" grant no. 2010CB126200, the National Nature Science Foundations of China grants no. 31470454 and 31272051, the Fok Ying Tung Education Foundation grant no. 122033 and the Qing Lan Project. WDP was funded by the National Nature Science Foundations of China grant no. 31170362 and 31070755. GJW was funded by the Research Grant from the Innovation Project for Graduate Student of Jiangsu Province grant no. KYLX_0576. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gould JL. Magnetoreception. Curr Biol. 2010;20(10):R431–5. 10.1016/j.cub.2010.03.045 [DOI] [PubMed] [Google Scholar]
- 2. Wan GJ, Jiang SL, Zhao ZC, Xu JJ, Tao XR, Sword GA, et al. Bio-effects of near-zero magnetic fields on the growth, development and reproduction of small brown planthopper, Laodelphax striatellus and brown planthopper, Nilaparvata lugens . J Insect Physiol. 2014;68:7–15. 10.1016/j.jinsphys.2014.06.016 [DOI] [PubMed] [Google Scholar]
- 3. Lohmann KJ. Q&A: Animal behaviour: magnetic-field perception. Nature. 2010;464(7292):1140–2. 10.1038/4641140a [DOI] [PubMed] [Google Scholar]
- 4. Guerra PA, Gegear RJ, Reppert SM. A magnetic compass aids monarch butterfly migration. Nat Commun. 2014;5:4164 10.1038/ncomms5164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fedele G, Edwards MD, Bhutani S, Hares JM, Murbach M, Green EW, et al. Genetic analysis of circadian responses to low frequency electromagnetic fields in Drosophila melanogaster . PLoS Genet. 2014;10(12):e1004804 10.1371/journal.pgen.1004804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gegear RJ, Foley LE, Casselman A, Reppert SM. Animal cryptochromes mediate magnetoreception by an unconventional photochemical mechanism. Nature. 2010;463(7282):804–7. 10.1038/nature08719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee AA, Lau JCS, Hogben HJ, Biskup T, Kattnig DR, Hore PJ. Alternative radical pairs for cryptochrome-based magnetoreception. J R Soc Interface. 2014;11(95). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahmad M, Cashmore AR. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993;366(6451):162–6. [DOI] [PubMed] [Google Scholar]
- 9. Dodson CA, Hore PJ, Wallace MI. A radical sense of direction: signalling and mechanism in cryptochrome magnetoreception. Trends Biochem Sci. 2013;38(9):435–46. 10.1016/j.tibs.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 10. Chaves I, Pokorny R, Byrdin M, Hoang N, Ritz T, Brettel K, et al. The cryptochromes: blue light photoreceptors in plants and animals. Annu Rev Plant Biol. 2011;62:335–64. 10.1146/annurev-arplant-042110-103759 [DOI] [PubMed] [Google Scholar]
- 11. Walker MM, Bitterman ME. Honeybees can be trained to respond to very small changes in geomagnetic-field intensity. J Exp Biol. 1989;145:489–94. [Google Scholar]
- 12. Foley LE, Gegear RJ, Reppert SM. Human cryptochrome exhibits light-dependent magnetosensitivity. Nature Commun. 2011;2:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marley R, Giachello CN, Scrutton NS, Baines RA, Jones AR. Cryptochrome-dependent magnetic field effect on seizure response in Drosophila larvae. Sci Rep. 2014;4:5799 10.1038/srep05799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dubrovsky EB. Hormonal cross talk in insect development. Trends Endocrinol Metab. 2005;16(1):6–11. [DOI] [PubMed] [Google Scholar]
- 15. Gilbert LI, Rybczynski R, Warren JT. Control and biochemical nature of the ecdysteroidogenic pathway. Annu Rev Entomol. 2002;47:883–916. [DOI] [PubMed] [Google Scholar]
- 16. Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 2013;58:181–204. 10.1146/annurev-ento-120811-153700 [DOI] [PubMed] [Google Scholar]
- 17. McBrayer Z, Ono H, Shimell M, Parvy JP, Beckstead RB, Warren JT, et al. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila . Dev Cell. 2007;13(6):857–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamanaka N, Rewitz KF, O'Connor MB. Ecdysone control of developmental transitions: lessons from Drosophila research. Annu Rev Entomol. 2013;58:497–516. 10.1146/annurev-ento-120811-153608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Loof A. Ecdysteroids, juvenile hormone and insect neuropeptides: Recent successes and remaining major challenges. Gen Comp Endocrinol. 2008;155(1):3–13. [DOI] [PubMed] [Google Scholar]
- 20. Bliss VL, Heppner FH. Circadian activity rhythm influenced by near zero magnetic field. Nature. 1976;261(5559):411–2. [DOI] [PubMed] [Google Scholar]
- 21. Zamoshchina TA, Krivova NA, Khodanovich M, Trukhanov KA, Tukhvatulin RT, Zaeva OB, et al. Influence of simulated hypomagnetic environment in a far space flight on the rhythmic structure of rat's behavior. Aviakosm Ekolog Med. 2012;46(1):17–23. [PubMed] [Google Scholar]
- 22. Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–54. 10.1126/science.1195027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bajgar A, Jindra M, Dolezel D. Autonomous regulation of the insect gut by circadian genes acting downstream of juvenile hormone signaling. Proc Natl Acad Sci U S A. 2013;110(11):4416–21. 10.1073/pnas.1217060110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meelkop E, Temmerman L, Schoofs L, Janssen T. Signalling through pigment dispersing hormone-like peptides in invertebrates. Prog Neurobiol. 2011;93(1):125–47. 10.1016/j.pneurobio.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 25. Zhou GH, Xu DL, Xu DG, Zhang MX. Southern rice black-streaked dwarf virus: a white-backed planthopper-transmitted fijivirus threatening rice production in Asia. Front Microbiol. 2013;4:270 10.3389/fmicb.2013.00270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsumura M. Genetic analysis of a threshold trait: density-dependent wing dimorphism in Sogatella furcifera (Horváth) (Hemiptera: Delphacidae), the whitebacked planthopper. Heredity. 1996;76(3):229–37. [Google Scholar]
- 27. Sneed A. Earth's impending magnetic flip. Sci Am. 2014;311(4):29 [DOI] [PubMed] [Google Scholar]
- 28. De Santis A. How persistent is the present trend of the geomagnetic field to decay and, possibly, to reverse? Phys Earth Planet In. 2007;162(3–4):217–26. [Google Scholar]
- 29. Lohmann KJ, Lohmann CMF. Detection of magnetic field intensity by sea turtles. Nature. 1996;380(6569):59–61. [Google Scholar]
- 30. Maffei ME. Magnetic field effects on plant growth, development, and evolution. Front Plant Sci. 2014;5:445 10.3389/fpls.2014.00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mallis MM, DeRoshia CW. Circadian Rhythms, Sleep, and Performance in Space. Aviat Space Environ Med. 2005;76(6):B94–B107. [PubMed] [Google Scholar]
- 32. Monk TH, Kennedy KS, Rose LR, Linenger JM. Decreased Human Circadian Pacemaker Influence After 100 Days in Space: A Case Study. Psychosom Med. 2001;63(6):881–5. [DOI] [PubMed] [Google Scholar]
- 33. Tufail M, Nagaba Y, Elgendy AM, Takeda M. Regulation of vitellogenin genes in insects. Entomol Sci. 2014;17(3):269–82. [Google Scholar]
- 34. Zhai YF, Zhang JQ, Sun ZX, Dong XL, He Y, Kang K, et al. Proteomic and transcriptomic analyses of fecundity in the brown planthopper Nilaparvata lugens (Stål). J Proteome Res. 2013;12(11):5199–212. 10.1021/pr400561c [DOI] [PubMed] [Google Scholar]
- 35. Flatt T, Amdam GV, Kirkwood TB, Omholt SW. Life-history evolution and the polyphenic regulation of somatic maintenance and survival. Q Rev Biol. 2013;88(3):185–218. [DOI] [PubMed] [Google Scholar]
- 36. Iwanaga K, Tojo S. Effects of juvenile hormone and rearing density on wing dimorphism and oöcyte development in the brown planthopper, Nilaparvata lugens . J Insect Physiol. 1986;32(6):585–90. [Google Scholar]
- 37. Van Ekert E, Powell CA, Shatters RG Jr., Borovsky D. Control of larval and egg development in Aedes aegypti with RNA interference against juvenile hormone acid methyl transferase. J Insect Physiol. 2014;70:143–50. 10.1016/j.jinsphys.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 38. Mo WC, Liu Y, Bartlett PF, He RQ. Transcriptome profile of human neuroblastoma cells in the hypomagnetic field. Sci China Life Sci. 2014;57(4):448–61. 10.1007/s11427-014-4644-z [DOI] [PubMed] [Google Scholar]
- 39. Yuan Q, Metterville D, Briscoe AD, Reppert SM. Insect cryptochromes: gene duplication and loss define diverse ways to construct insect circadian clocks. Mol Biol Evol. 2007;24(4):948–55. [DOI] [PubMed] [Google Scholar]
- 40. Czeisler CA, Allan JS, Strogatz SH, Ronda JM, Sanchez R, Rios CD, et al. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233(4764):667–71. [DOI] [PubMed] [Google Scholar]
- 41. Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382(6594):810–3. [DOI] [PubMed] [Google Scholar]
- 42. Nishio T, Shiosaka S, Nakagawa H, Sakumoto T, Satoh K. Circadian feeding rhythm after hypothalamic knife-cut isolating suprachiasmatic nucleus. Physiol Behav. 1979;23(4):763–9. [DOI] [PubMed] [Google Scholar]
- 43. Sakai T, Ishida N. Circadian rhythms of female mating activity governed by clock genes in Drosophila . Proc Natl Acad Sci U S A. 2001;98(16):9221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matsuo T. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302(5643):255–9. [DOI] [PubMed] [Google Scholar]
- 45. Yoshii T, Ahmad M, Helfrich-Forster C. Cryptochrome mediates light-dependent magnetosensitivity of Drosophila's circadian clock. PLoS Biol. 2009;7(4):813–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Henrich VC, Rybczynski R, Gilbert LI. Peptide hormones, steroid hormones, and puffs: Mechanisms and models in insect development In: Gerald L, editor. Vitamins & Hormones. Volume 55: Academic Press; 1998. pp. 73–125. [DOI] [PubMed] [Google Scholar]
- 47. Yamanaka N, Romero NM, Martin FA, Rewitz KF, Sun M, O'Connor MB, et al. Neuroendocrine control of Drosophila larval light preference. Science. 2013;341(6150):1113–6. 10.1126/science.1241210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hoffmann KH, Lorenz MW. Allatostatins and allatotropins: Is the regulation of corpora allata activity their primary function? Eur J Entomol. 1999;96:255–66. [Google Scholar]
- 49. Stay B. A review of the role of neurosecretion in the control of juvenile hormone synthesis: a tribute to Berta Scharrer. Insect Biochem Mol Biol. 2000;30(8–9):653–62. [DOI] [PubMed] [Google Scholar]
- 50. Kawakami A, Kataoka H, Oka T, Mizoguchi A, Kimura-Kawakami M, Adachi T, et al. Molecular cloning of the Bombyx mori prothoracicotropic hormone. Science. 1990;247(4948):1333–5. [DOI] [PubMed] [Google Scholar]
- 51. Reppert SM. A colorful model of the circadian clock. Cell. 2006;124(2):233–6. [DOI] [PubMed] [Google Scholar]
- 52. Sandrelli F, Costa R, Kyriacou CP, Rosato E. Comparative analysis of circadian clock genes in insects. Insect Mol Biol. 2008;17(5):447–63. 10.1111/j.1365-2583.2008.00832.x [DOI] [PubMed] [Google Scholar]
- 53. Sehadova H, Markova EP, Sehnal FS, Takeda M. Distribution of circadian clock-related proteins in the cephalic nervous system of the silkworm, Bombyx mori . J Biol Rhythms. 2004;19(6):466–82. [DOI] [PubMed] [Google Scholar]
- 54. Tomioka K, Matsumoto A. A comparative view of insect circadian clock systems. Cell Mol Life Sci. 2010;67(9):1397–406. 10.1007/s00018-009-0232-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Burger T, Lucova M, Moritz RE, Oelschlager HH, Druga R, Burda H, et al. Changing and shielded magnetic fields suppress c-Fos expression in the navigation circuit: input from the magnetosensory system contributes to the internal representation of space in a subterranean rodent. J R Soc Interface. 2010;7(50):1275–92. 10.1098/rsif.2009.0551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Close J. Are stress responses to geomagnetic storms mediated by the cryptochrome compass system? Proc Biol Sci. 2012;279(1736):2081–90. 10.1098/rspb.2012.0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Maeda K, Robinson AJ, Henbest KB, Hogben HJ, Biskup T, Ahmad M, et al. Magnetically sensitive light-induced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor. Proc Natl Acad Sci U S A. 2012;109(13):4774–9. 10.1073/pnas.1118959109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wiltschko R, Wiltschko W. Sensing magnetic directions in birds: radical pair processes involving cryptochrome. Biosensors. 2014;4(3):221–42. 10.3390/bios4030221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mo WC, Zhang ZJ, Liu Y, Bartlett PF, He RQ. Magnetic shielding accelerates the proliferation of human neuroblastoma cell by promoting G1-phase progression. PloS One. 2013;8(1):e54775 10.1371/journal.pone.0054775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bree RT, Stenson-Cox C, Grealy M, Byrnes L, Gorman AM, Samali A. Cellular longevity: role of apoptosis and replicative senescence. Biogerontology. 2002;3(4):195–206. [DOI] [PubMed] [Google Scholar]
- 61. Berg BN, Simms HS. Nutrition and longevity in the rat. II. Longevity and onset of disease with different levels of food intake. J Nutr. 1960;71:255–63. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.