Abstract
Commensal microbiota-specific Th17 cells are enriched in the intestines, which can convert into Tfh in Peyer’s patches, and are crucial for production of intestinal IgA against microbiota, however, the role of Th17 and Tfh cytokines in regulating the mucosal IgA response to enteric microbiota is still not completely known. In this study, we found that intestinal IgA was impaired in mice deficient in IL-17 or IL-21 signaling. IL-21, but not IL-17, is able to augment B cell differentiation to IgA+ cells as mediated by TGFβ1, and accelerate IgA class switch recombination (CSR). IL-21 and retinoic acid (RA) induce IgA+ B cell development and IgA production, and drives autocrine TGFβ1 production to initiate IgA CSR. Repletion of T cell-deficient TCRβxδ−/− mice with Th17 cells specific for commensal bacterial antigen, increased levels of IgA+ B cells and IgA production in the intestine, which was blocked by neutralizing IL-21. Thus, IL-21 functions to strongly augment IgA production under intestinal environment. Furthermore, IL-21 promotes intestinal B cell homing through α4β7 expression, alone or with TGFβ and RA. Together, IL-21 from microbiota-specific Th17 and/or Tfh cells contributes to robust intestinal IgA levels by enhancing IgA+ CSR, IgA production, and B cell trafficking into the intestine.
Introduction
The human intestinal tract is home to over 100 trillion microorganisms, the majority of which reside peacefully without insult or challenge to the host. The mucosal surfaces are the most frequent access point for the microbiota, which is lined by a single layer of epithelial cells. Breach of the epithelial layer by pathogens results in enteric infections and disease, while chronic infiltration by the commensal microbiota leads to continued exposure and activation of the intestinal immune system1. Over time, chronic and dysregulated immune responses against the commensal microbiota results in increased inflammation and the onset of inflammatory bowel disease2. Among the multiple regulatory mechanisms regulating host response to microbiota, IgA, which is enriched in mucosal secretions, plays crucial roles in the maintenance of intestinal homeostasis against microbiota. IgA functions to neutralize and aid in clearance of extracellular pathogens by preventing adherence to epithelial surfaces and limiting access to the intestines and the immune system3. The high level of IgA production is driven by microbial colonization of the intestine, as germ-free mice have low levels of IgA and IgA+ B cells, whereas colonization with commensal bacteria restores IgA production4, and the majority of intestinal plasmablasts produce antibodies that are specific for intestinal antigens5. Notably, monocolonization of germ-free mice with segmented filamentous bacteria (SFB) selectively increases IgA production and secretion6, and intestinal IgA-deficiency in wild-type mice leads to SFB overgrowth7. A recent report revealed that colonization by segmented filamentous bacteria induced both IgA+ B cells and Th17 cells in multiple locations in the intestine8. With the observations that SFB colonization can control both Th17 cells and IgA production, therein suggests a link between intestinal T cell function and IgA production.
As with all subtypes of CD4+ T cells, Th17 and T follicular helper (Tfh) cells exhibit influence over B cell responses. Transfer of Th17 cells into T cell-deficient TCRα−/− mice results in increased serum IgG titers across all measured subtypes (IgG1, IgG2a, IgG2b, and IgG3), with strongest increases in IgG1 and IgG2b9. Furthermore, transfer of Th17 cells induces the generation of germinal centers in the spleen and draining lymph nodes, structures that are mostly lacking in the absence of T cells. These effects are dependent on both IL-17 and IL-21, as transfer of Th17 cells into IL-17ra−/− or IL-21r−/− mice do not increase the number of germinal centers present. Direct addition of IL-17 to B cells in vitro triggers production of IgG2a and IgG3, whereas IL-21 induces production of IgG1, IgG2a, IgG2b and IgG39, indicating that sources of IL-21 and IL-17 are competent B cell helpers in generating systemic IgG responses. The effects of IL-17 and IL-21 on IgG induction is further demonstrated in the role of IL-17 during systemic lupus erythematosus (SLE), characterized by autoreactive B cells and pathogenic autoantigen antibody production. Patients with SLE have increased serum levels of IL-17, IL-21, and BAFF, which promote survival and antibody production from autoantigen B cells10–13. We recently demonstrated that intestinal Th17 cells promote secretory IgA response through IL-17 stimulation of intestinal epithelial expression of polymeric Ig receptor14. A recent report further demonstrates that Th17 cells convert into Tfh cells in Peyer’s patches and induce intestinal IgA15. It has been shown that IL-21 can modulate B cell differentiation by enhancing IL-4-driven IgG production16 and TGFβ-driven IgA production17. However, whether Th17 and Tfh cell cytokines directly influence mucosal IgA production has not been fully investigated. In this report, we demonstrate that IL-21, produced by both Th17 and Tfh cells, can augment IgA responses mediated by TGFβ1 and retinoic acid in the intestine, and intestinal sources of IL-21 directly induce IgA production.
Materials and Methods
Mice
TCRβxδ−/− mice were obtained from Jackson Laboratory. CBir1 flagellin-specific TCR transgenic (CBir1-Tg) mice were maintained in the Animal Facilities at University of Texas Medical Branch. All experiments were reviewed and approved by the Institutional Animal Care and Use Committees of the UTMB and the MD Anderson Cancer Center. All the mice strains were bred in the UTMB animal facility, and housed together from 3 weeks of age. IL-21−/− and IL-21r−/− mice18 were maintained at the Animal Facilities at MD Anderson Cancer Center. All mice are bred onto the C57BL/6 background, and contain SFB as verified via PCR. 8–12 week-old mice were used for all experiments.
Antibody and reagents
The following antibodies were used for flow cytometry: PE-IL-17A (TC11-18H10.1), FITC-B220 (RA3-6B2), PE-α4β7 (DATK32), FITC-CD4 (RM4-5), PE/Cy7-CD38 (90), APC-CD138 (281-2), and PE/Cy7-PD-1 (29F.1A12) were purchased from Biolegend; APC-RORγt (B2D), APC-GL-7, and eFluor450-Streptavidin were from eBioscience; Brilliant Violet 421-CD95 (Jo2) and biotinylated CXCR5 (2G8) were from BD Biosciences; recombinant mouse IL-21R human FC chimera was from R&D Systems; AlexaFluor 647 antihuman IgG FCγ (polyclonal) was from Jackson ImmunoResearch; PE-IgA (polyclonal) was from Southern Biotechnology. Intracellular permeabilization was performed with Foxp3 perm/fix kit from eBioscience. Live cells were gated using Live/Dead Fixable Dead Cell stain kit from Life Technologies. Mouse recombinant IL-6, and human recombinant IL-17A, TGFβ1 were purchased from R&D Systems. Recombinant IL-21 was purchased from eBioscience. Antibody against IL-21r (4A9) was from BioXCell. Antibodies against IgA and IgD were purchased from Kirkegaard & Perry Labs and Southern Biotechnology. Anti-µ was purchased from Jackson ImmunoResearch Laboratories. All-trans-retinoic acid, TGFβ receptor I inhibitor SB505124, and collagenase IV were purchased from Sigma-Aldrich. Anti-biotin microbeads from Miltenyi were used to sort naïve IgD+ B cells.
Polarization of Th17 cells
CD4+ T cells were isolated from spleens of CBir1 Tg mice using anti-mouse CD4-magnetic beads (GK1.5, BD Biosciences) as previously described19. To polarize Th17 cells, CBir1-Tg CD4+ T cells were cultured with 10ng/ml TGFβ1, 20ng/ml IL-6, 10µg/ml anti-IFNγ (XMG1.2, BioXCell), and 10µg/ml anti-IL-4 (11B11, BioXCell) with irradiated splenic APC20.
Fecal pellet preparation
Fecal pellets were homogenized in PBS containing 0.04mg/ml soybean trypsin inhibitor, 20mM EDTA, and 2mM PMSF and centrifuged to remove bacteria and insoluble debris as described previously21.
ELISA
96-well plates (Nunc) were coated with 1µg/ml anti-IgA (Kirkegaard & Perry Labs) overnight at 4°, blocked in PBS with 1% BSA, and incubated with sera or fecal pellets for 2 hours. After washing, adherent antibodies were detected by biotinylated anti-IgA or anti-IgG, followed by HRP-labeled streptavidin. Plates were developed using 3,3’,5,5’-TMB and analyzed at 450nm.
Intestinal lamina propria isolation
As described previously19, intestines were removed and the Peyer’s patches excised. Intestines were sliced and incubated with 50mM EDTA to remove epithelial cells. Intestines were then digested by collagenase IV. Liberated cells were resuspended in 40% Percoll and overlaid onto 70% Percoll. The interface containing lymphocytes was collected. Lymphocytes were directly stained (for B cells) or restimulated with PMA (50ng/ml), ionomycin (750ng/ml), and monensin for 2 hours (for T cells) before staining for flow cytometry.
TGFβ bioassay
As described previously22, MFB-F11 cells are embryonic fibroblasts from Tgfb1−/− mice that are stably transfected with a reporter plasmid consisting of TGFβ responsive SMAD-binding elements coupled to a secreted alkaline phosphatase reporter gene. Secreted alkaline phosphatase activity shown as chemiluminescence units was measured using Great EscApe SEAP Chemiluminescence kit 2.0 (Clontech), following the manufacturer’s instructions and represents biologically active TGFβ activity.
Quantitative Real-Time/Reverse transcription PCR
RNA was extracted with TRIzol (Invitrogen) and followed by cDNA synthesis with Revertaid reverse transcriptase (Fermentas). Quantitative PCR was performed using TaqMan Gene Expression Assays. Predesigned primers and probes for Aicda and Gapdh were ordered from Applied Biosystems, and data were normalized to Gapdh mRNA expression. To detect germ-line α transcripts the primers IαF (5′- CCA GGC ATG GTT GAG ATA GAG ATA G-3′) and CαR (5′-GAG CTG GTG GGA GTG TCA GTG-3′) were used23. Circular transcripts were detected by a nested PCR using the outer primers Iµ4 (5′-ACC CTG GAT GAC TTC AGT GT-3′) and Iαup4 (5′-CAT CTG GAC TCC TCT GCT CA-3′) followed by the inner primers IαF (5′-CCA GGC ATG GTT GAG ATA GAG ATA G-3′) and CµR (5′-AAT GGT GCT GGG CAG GAA GT-3′)23. Post-switch α transcripts were detected with the primers IµF (5′-GAG CTG GTG GGA GTG TCA GTG-3′)17 and CαR. Aliquots of PCR products were visualized by electrophoresis on 1.5% agarose gels.
Immunofluorescence
Deparaffinized and rehydrated Peyer’s patch sections were incubated for an hour with biotinylated-GL-7 (eBioscience), and FITC-B220 (clone RA3-6B2, Biolegend), followed by incubation with AlexaFluor 594-streptavidin (Jackson ImmunoResearch). Images were taken and analyzed with an Olympus BX51 imaging system.
Statistical analysis
For comparisons between samples, levels of significance were determined by Student’s t test in Prism 5.0 (Graphpad). Where appropriate, mean ± SEM is represented on graphs.
Results
IL-21 but not IL-17 promotes intestinal B cell production of IgA
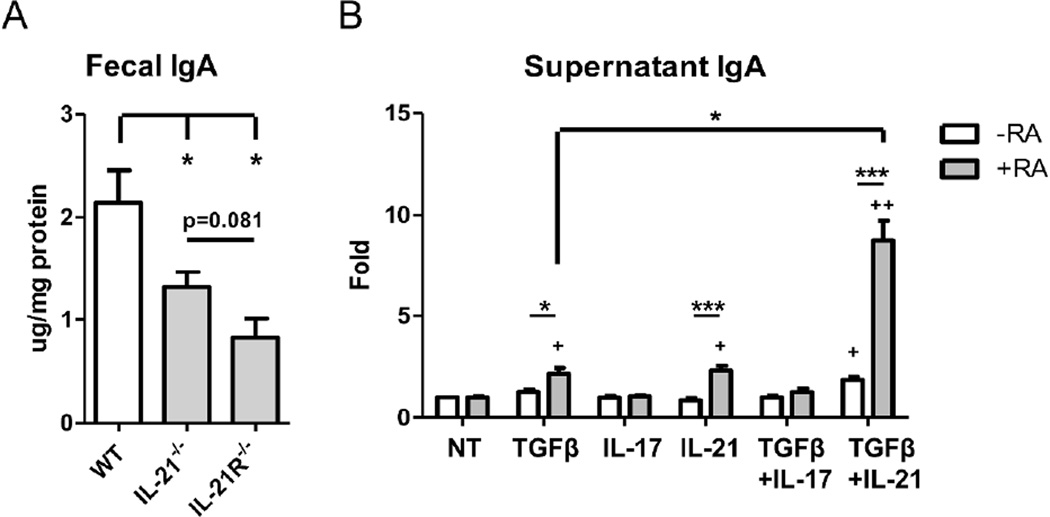
CD4+ T cells play an important role in the induction of IgA in the intestine, as T cell-deficient TCRβxδ−/− mice have significantly lower amounts of fecal IgA19. Our previous work has detailed an important role for Th17 cells in the regulation of the intestinal IgA response through induction of pIgR expression on intestinal epithelial cells as mediated by IL-1714. Th17 cells produce high levels of IL-17, IL-21, and IL-2224, 25. B cells do not express the IL-22r26, 27, so we investigated the roles of IL-17 and IL-21 in the intestinal IgA response. Analysis of fecal IgA levels between wild-type and IL-17r−/− mice demonstrate decreased levels of intestinal IgA (data not shown) as previously published14. We further determined the role of IL-21 in the intestinal IgA response by analyzing IgA levels in mice deficient in IL-21 signaling. Quantification of fecal IgA from IL-21−/−, IL-21r−/− and wild-type mice revealed a significant decrease in IgA levels in the absence of IL-21 signaling (Figure 1A), demonstrating that IL-21 regulates intestinal IgA production.
Figure 1. IL-21 regulates IgA production in cooperation with TGFβ1 and/or retinoic acid.
(A) Fecal pellets were collected from age-matched wild-type, IL-21−/−, and IL-21R−/− B6 mice. IgA levels were quantified through ELISA and normalized to total protein. N = 2 mice per group, data is reflective of 2 independent experiments. *p<0.05 compared to wild-type mice. (B) Naïve IgD+ B cells were activated with anti-µ (5µg/ml) and anti-CD40 (5µg/ml) in the presence of TGFβ1 (5ng/ml), IL-17 (50ng/ml), IL-21 (20ng/ml), or retinoic acid (1µM), or a combination of TGFβ1, IL-17, IL-21, and/or retinoic acid. On day 5, IgA was measured from the supernatant by ELISA. Data is reflective of 6 independent experiments. *p<0.05, ***p<0.001 compared between samples. +p<0.05, ++p<0.01 compared to untreated cells.
Given that the separate absence of both IL-17 and IL-21 resulted in deficiencies in the intestinal IgA response, we then investigated whether IL-17 and IL-21 could directly stimulate B cells to produce IgA. We isolated splenic naïve IgD+ B cells and cultured them with anti-µ and CD40L for B cell activation to mimic T cell contact, together with TGFβ1 in the presence or absence of IL-17 or IL-21. IgA in the supernatant was measured by ELISA after 5 days of culture. As seen in Figure 1B, addition of IL-17 did not induce IgA production in vitro, alone or in combination with TGFβ1. Addition of IL-21 alone had little effect on IgA production, but moderately increased TGFβ1-induced IgA. Retinoic acid (RA), which is enriched in the intestine, has been shown to enhance B cell IgA production as mediated by other cytokines, 10 including TGFβ128 and IL-629. To this end, we observed that addition of RA greatly enhanced IgA production in conjunction with TGFβ and IL-21. Surprisingly, IL-21 also cooperated with RA to increase IgA production in the absence of exogenous TGFβ1. Taken together, these data indicated that IL-21 is an effective Th17 cell cytokine for mediating IgA production by synergizing with retinoic acid.
IL-21 augments TGFβ-mediated IgA CSR
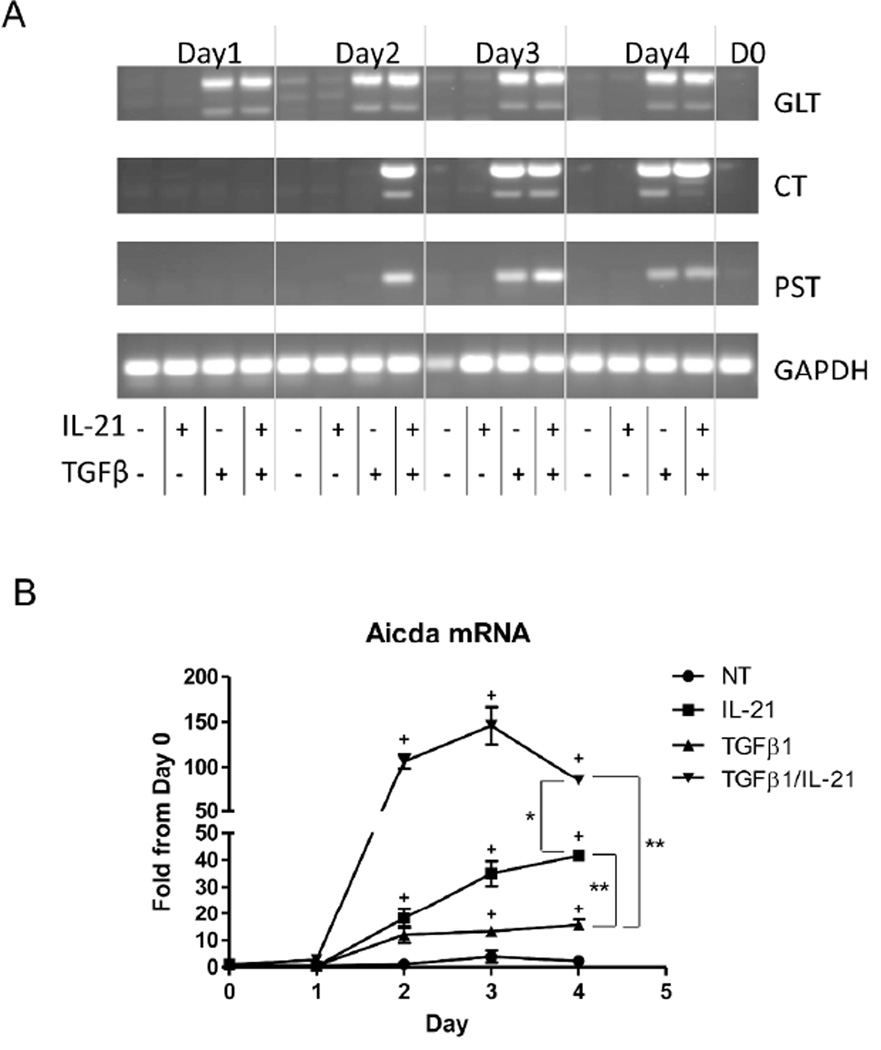
It has been shown that IL-21 is able to modulate B cell differentiation by enhancing IL-4- driven IgG production16 and TGFβ-driven IgA production17. We then investigated the mechanisms by which IL-21 regulates B cell IgA production. In order for B cells to fully differentiate, activated B cells undergo class switch recombination (CSR), by which the Ig heavy chain locus becomes rearranged and portions of the locus become looped-out and excised from the genome. The physical recombination of the Ig locus is facilitated by the enzyme activation-induced cytokine deaminase (Aicda)30. B cells undergo IgA CSR after exposure to TGFβ1, and the loci for IgG, IgE, and IgM are removed from the genome. These B cells are permanently differentiated and can only produce IgA. CSR is a rapid process, however, the looped-out chromosomes stay within the cell for 24–48 hours30, and continue to be transcribed until they are degraded. As a result of the continued transcription, detection of these mRNAs as remaining circular transcripts (CT) via RT-PCR are indicative of cells that are actively undergoing CSR to IgA-producing cells23, 30. To determine if IL-21 can induce CSR, splenic B cells were cultured with anti-µ and CD40L. TGFβ1 and/or IL-21 were added to the cultures, and RNA was collected daily to detect when CSR began. As shown in Figure 2A, TGFβ1 immediately induced germline production of IgA (GLT), with CSR to IgA commencing on day 3 (CT). Transcription of IgA after CSR (PST) was also detectable on day 3, in accordance with CSR induction. As expected9, 31, treatment of B cells with IL-21 alone did not induce germ-line IgA, nor CSR to IgA. However, IL-21 augmented and accelerated TGFβ-mediated IgA CSR as treatment with both TGFβ1 and IL-21 continued to induce IgA production and CSR. Furthermore, CSR and post-class switched IgA were detected one day earlier when IL-21 was added with TGFβ1. Analysis of Aicda mRNA, required to initiate CSR to any Ig subtype, revealed that TGFβ1 and IL-21 each induced Aicda, with TGFβ1 inducing IgA CSR and IL-21 inducing IgG CSR (Figure 2B). Interestingly, treatment with both TGFβ1 and IL-21 greatly enhanced Aicda transcription from day 2 on, correlating with the appearance of circular transcripts and post-class switched IgA transcripts seen on day 2. Collectively, our data reveals that although IL-21 itself does not facilitate IgA CSR, it potently augments and accelerates TGFβ-mediated IgA CSR. In contrast, IL-17 alone did not induce Aicda, nor did it enhance TGFβ1-induced Aicda (data not shown).
Figure 2. IL-21 enhances TGFβ-mediated class switching towards IgA.
Naïve IgD+ B cells were activated with anti-µ (5µg/ml) and anti-CD40 (5µg/ml) in the presence of TGFβ1 (5ng/ml) or IL-21 (20ng/ml), or a combination of TGFβ1 and IL-21. (A) RNA was collected from cultured B cells on various days during culture and 2µg RNA was used to analyze molecular markers for IgA CSR by RT-PCR. GLT = α germ-line transcripts. CT = circular transcripts. PST = α post-switch transcripts. Image is representative of three independent experiments. (B) Aicda mRNA was analyzed from cells on various days during culture by RT-PCR. Aicda expression values were normalized to Gapdh expression. Data is reflective of three independent experiments. *p<0.05, **p<0.01 compared between Day 4 samples. +p<0.05 compared to untreated samples.
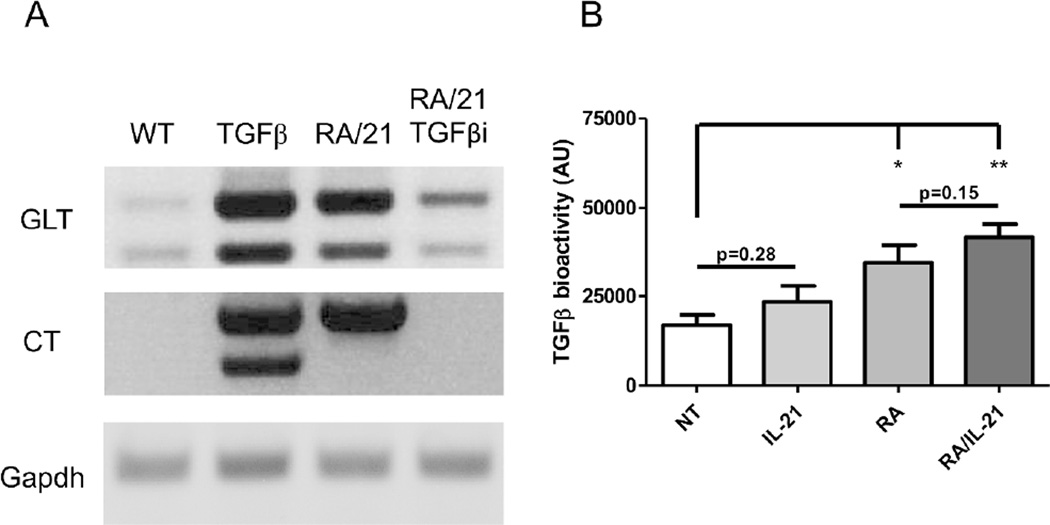
As RA and IL-21 also increased IgA production in the absence of exogenous TGFβ1, we next investigated how IL-21 and RA regulate IgA production. Analysis of the mRNA transcripts revealed that IL-21 and RA together were capable of inducing an overall IgA response, particularly at the germ-line transcriptional level (Figure 3A). Interestingly, we were able to detect circular transcripts in B cells treated with IL-21 and RA, without exogenous TGFβ1. Treatment of B cells with IL-21, RA, and a TGFβ receptor I inhibitor (SB505124) negated the IgA class switch recombination process, revealing that IL-21 and RA stimulation of IgA-secreting B cell development is still reliant upon TGFβ, which could be mediated by enhancing B cell response to TGFβ or promoting B cell production of TGFβ.
Figure 3. IL-21 and retinoic acid induce class switch recombination to IgA-secreting cells by inducing TGFβ production from B cells.
(A) Naïve IgD+ B cells were activated with anti-µ (5µg/ml) and anti-CD40 (5µg/ml) in the presence of TGFβ1 (5ng/ml) or IL-21 (20ng/ml) or retinoic acid (1µM) and a TGFβ receptor I inhibitor (1µM). RNA was collected from cultured B cells after 3 days and 2µg RNA was used to analyze molecular markers for IgA CSR by RTPCR. GLT = α germ-line transcripts. CT = circular transcripts. Image is representative of three independent experiments. (B) Naïve IgD+ B cells were activated with anti-µ and anti-CD40 in the presence of IL-21 and/or retinoic acid. On day 5, supernatant was collected from cultured B cells as conditioned medium. To detect bioactive TGFβ, the TGFβ-reporter cell line MFB-F11 was cultured with B cell-conditioned medium for 24 hours. Secreted alkaline phosphatase was measured as a reflection of TGFβ bioactivity. Data is reflective of 4 independent experiments. *p<0.05, **p<0.01 compared to conditioned medium from untreated B cells.
We sought to determine if IL-21 and RA signals were able to modulate the B cell response to TGFβ. While the naïve B cells continued to produce IgA in the absence of exogenous TGFβ, there remained some TGFβ in the culture as a result of medium supplemented with FBS. However, IL-21 and RA (separately or in tandem) did not increase B cell expression of tgfbr1 or tgfbr2 (data not shown), and thus, they did not influence B cell sensitivity to TGFβ1. Our previous work has demonstrated that that RA induces dendritic cell TGFβ1 production, resulting in increased regulatory T cell differentiation in the intestine22. We then investigated whether IL-21 and RA could induce B cell production of TGFβ and therefore drive IgA CSR. Consistently, we revealed that RA has a similar effect in B cells, in that RA is able to induce TGFβ production from B cells. Although IL-21 has little effect on TGFβ induction, it appears to promote RA-induced TGFβ production (Figure 3B). Appropriately, the induction of TGFβ by RA, and synergism with IL-21 is sufficient to drive IgA production in the absence of exogenous TGFβ1.
Transfer of Th17 cells promote IgA+ B cell development in the intestinal lamina propria but not in the spleen of TCRβxδ−/− mice
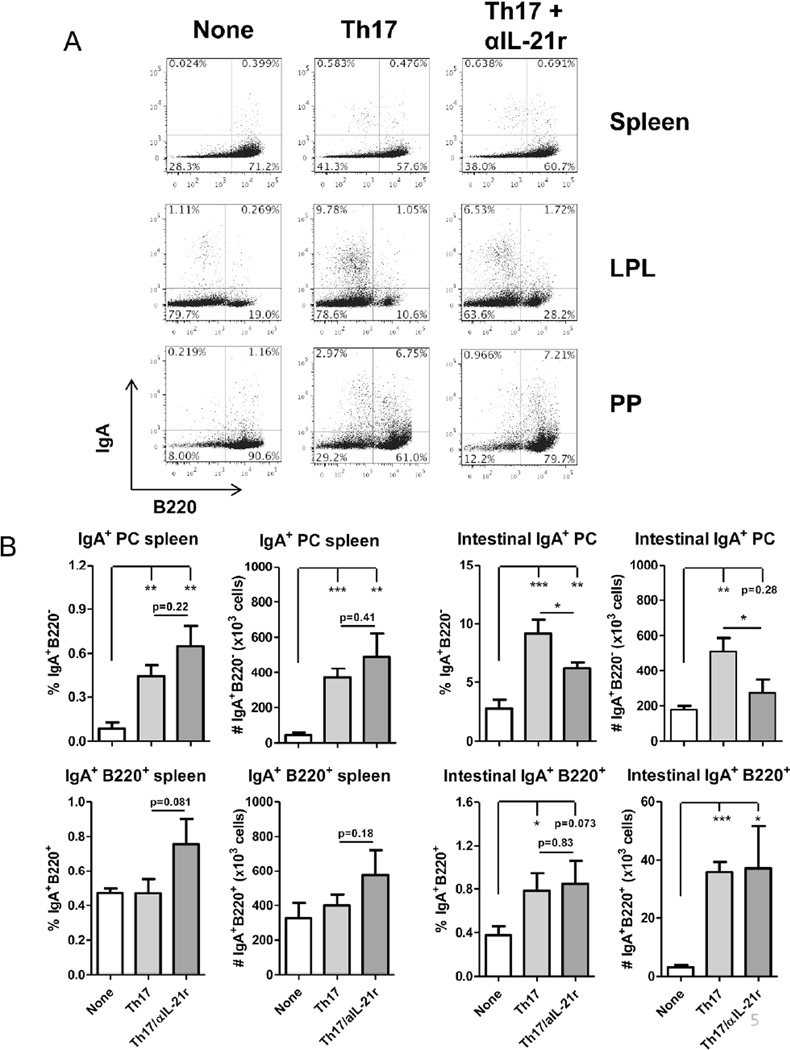
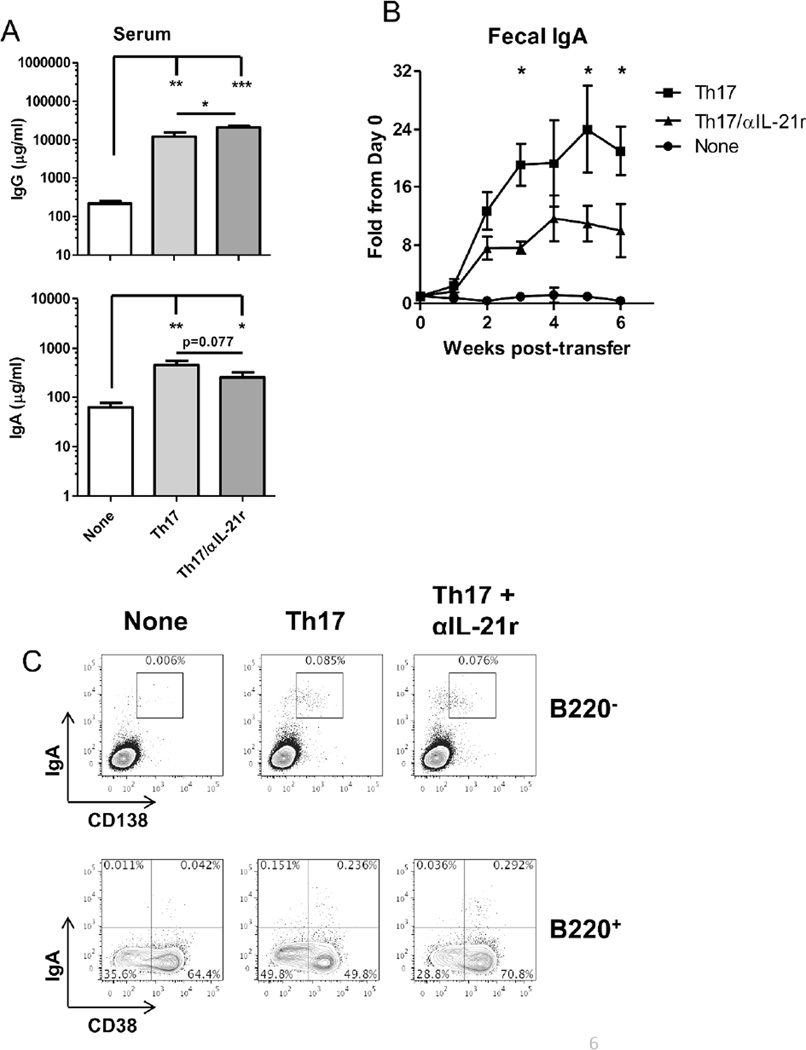
Thus far, our results in vitro have demonstrated that IL-21 promotes B cell development towards IgA production. As Th17 cells produce IL-21, in addition to IL-17, we then transferred Th17 cells into TCRβxδ−/− mice, which contain B cells but lack T cells, to determine if they can affect intestinal IgA+ B cell development and B cell IgA production in vivo, and if IL-21 was required for their action. We generated Th17 cells from microbiota antigen CBir1-specific TCR transgenic mice20 in vitro and transferred them into TCRβxδ−/− mice, and treated them with a neutralizing antibody against the IL-21 receptor (IL-21r). Recipient mice were sacrificed 44 days after transfer, and frequencies of IgA+ B cells were measured in the spleen and intestines. Antibody levels were also measured from feces and serum and compared to naïve TCRβxδ−/− mice. Transfer of Th17 cells did not significantly increase populations of IgA+ B220+ cells in the spleen; however, both frequencies and the total population of IgA+ B220- plasma cells were increased in the spleen (Figure 4A and 4B). Blockade of IL-21 signaling did not affect levels of both IgA+ B220+ and B220- cells. Analysis of intestinal B cells revealed greater numbers of IgA+ B cells in the lamina propria of intestines (Figure 4A and 4B) after Th17 cell transfer. Within the intestinal lamina propria, levels of both IgA+ B220+ and B220− plasma cells were significantly increased. Notably, blockade of IL-21 primarily decreased populations of IgA+ plasma cells, but had no discernible effect on IgA+ B220+ cells. This remained consistent with B cell populations within the Peyer’s patches, as Th17 cell transfer increased IgA production from B220+ and B220− cells, however blockade of IL-21 reduced IgA+ plasma cell populations without affecting B220+ cells. Consistently, transfer of Th17 cells increased serum IgA when compared to naïve TCRβxδ−/− mice (Figure 5A), which was slightly attenuated by blockade of IL-21r. Levels of IgG in the serum also increased significantly after Th17 cell transfer. Notably, blockade of IL-21 increased serum IgG, likely indicating a role for IL-21 in the switching from IgG to IgA production (Figure 5A). Th17 cells were capable of inducing strong intestinal IgA responses as measured from 2 weeks after Th17 cell transfer (Figure 5B), which is consistent with previous reports14, 15. Blockade of IL-21r decreased the IgA levels in the feces, consistent with the decrease in IgA-secreting plasma cells. Given that we used CBir1 Tg Th17 cells that respond to the immunodominant bacterial flagellin CBir119, the transferred cells would become activated and exhibit effector function after migrating to the intestine and encountering the intestinal antigens. That may in part explain why we observe that the expansion in B220+ cells primarily occurs in the intestine. The resulting plasma cells appear to re-enter circulation in the spleen. Furthermore, IgA+ cells were found in the bone marrow of Th17 cell recipients, whereas the bone marrow of naïve TCRβxδ−/− mice did not contain IgA-producing cells. IgA+B220- CD138+ plasma cells migrated into or were formed in the bone marrow after Th17 cell transfer. We also found a population of IgA+B220+CD38+ cells in the bone marrow, which could be partially comprised of memory B cells. Blockade of IL-21 did not have an effect on IgA+ B cell populations in the bone marrow (Figure 5C). While IL-21 was important for plasma cell generation in the periphery and the intestines, IL-21 did not contribute to plasma cell populations in the bone marrow, possibly indicating that bone marrow plasma cells were generated in the periphery and entered the bone marrow, or the neutralizing antibodies were limited in penetrating the bone marrow32. Consistently, the increased levels of IgA results in both increased secretion into the intestinal lumen, as well as increased circulation in the serum, to which IL-21 contributes.
Figure 4. Transfer of CBir1 Th17 cells into TCR®x™−/− mice increases IgA+ B cells in the intestine.
106 CBir1 Th17 cells were i.v. transferred into TCR®x™−/− mice. (A) On day 44 post-Th17 cell transfer, expression of surface IgA on splenic, intestinal lamina propria, or Peyer’s patch B cells of CBir1 Th17 cell recipients, Th17 cell recipients receiving neutralizing IL-21r (Th17+αIL-21r), or control TCRβxδ−/− mice was determined by flow cytometry. FACS plots are representative of 4 mice per group. (B) IgA+ plasma cells and mature B cells in the spleens and intestines. Bar charts reflect mean +/− SEM. Data reflects 3 independent experiments. *p<0.05, **p<0.01, ***p<0.001 between compared samples. LPL = intestinal lamina propria lymphocytes; PP = Peyer’s patch; PC = plasma cell
Figure 5. Transfer of CBir1 Th17 cells into TCR®x™−/− mice increases both systemic and intestinal IgA production.
(A) On day 44 post-Th17 cell transfer, IgG and IgA in the serum was measured by ELISA. *p<0.05, **p<0.01, ***p<0.001 (B) Fecal pellets were collected from Th17 cell recipients during the course of the experiment. IgA levels were quantified through ELISA and normalized to total protein. Changes in expression over time are expressed as a fold change from individuals pre-transfer. N = 4 mice per group, from 3 independent experiments. *p<0.05 as compared to anti-IL-21r group. (C) Intracellular IgA production was measured from total bone marrow cells of CBir1 Th17 cell recipients, Th17 cell recipients receiving neutralizing IL-21r (Th17+αIL-21r), or control TCRβxδ−/− mice by flow cytometry. FACS plots are representative of 4 mice per group.
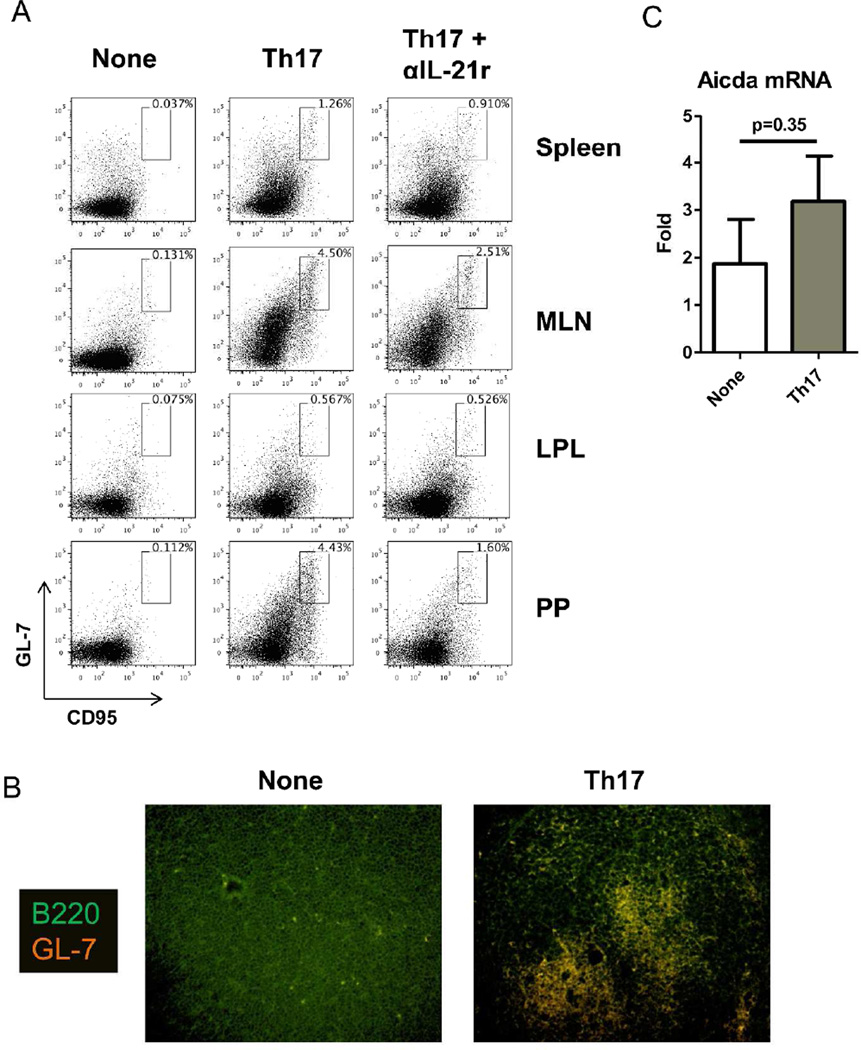
Germinal center reactions are critical for T-dependent antibody responses. It has been shown that IL-17 and IL-21 non-redundantly contribute towards germinal center formation within the lymphoid tissues9. Within naïve TCRβxδ−/− mice, we found no evidence of germinal B cells based on co-expression of GL7 and CD95. GC B cells were increased after transfer of Th17 cells (Figure 6A). We found the highest incidences of germinal centers within the mesenteric lymph node and Peyer’s patches. With the associated increase in GC B cells (Figure 6A), we observed that structured germinal centers had developed in the Peyer’s patches of Th17 cell recipients (Figure 6B). Consistent with previously-described roles for IL-219, 31, we found that neutralizing IL-21r decreased the frequencies of GC B cells in both the MLN and Peyer’s patches, however frequencies remained higher than those found in naive TCRβxδ−/− mice (Figure 6A). B cells in the intestinal lamina propria also reflected a small number of GC B cells, however, we were unable to detect active IgA class switching in the intestinal tissue. Further, Aicda expression was not significantly changed after Th17 cell transfer (Figure 6C). Collectively, these data indicated that the intestinal lamina propria was not the primary site for IgA induction.
Figure 6. Transfer of CBir1 Th17 cells into TCR®x™−/− mice induces germinal center formation in the intestinal lymphoid tissues.
106 CBir1 Th17 cells were i.v. transferred into TCR®x™−/− mice. (A) On day 44 post-Th17 cell transfer, expression of germinal center markers GL-7 and CD95 on splenic, mesenteric lymph node, intestinal lamina propria, or Peyer’s patch B220+ cells of CBir1 Th17 cell recipients, Th17 cell recipients receiving neutralizing IL-21r (Th17+αIL-21r), or control TCRβxδ−/− mice was determined by flow cytometry. FACS plots are representative of 4 mice per group. (B) IF image of Peyer’s patch from control TCRβxδ−/− mice or CBir1 Th17 cell recipients with 20× objective. Germinal center formation was analyzed by staining for GL-7-AlexaFluor 594 and B220-FITC. (C) RNA was collected from ileal tissue on day 44 post-Th17 cell transfer from CBir1 Th17 cell recipients or control TCRβxδ−/− mice and Aicda mRNA was analyzed by RT-PCR. Data is reflective of 4 mice per group, from 3 independent experiments.
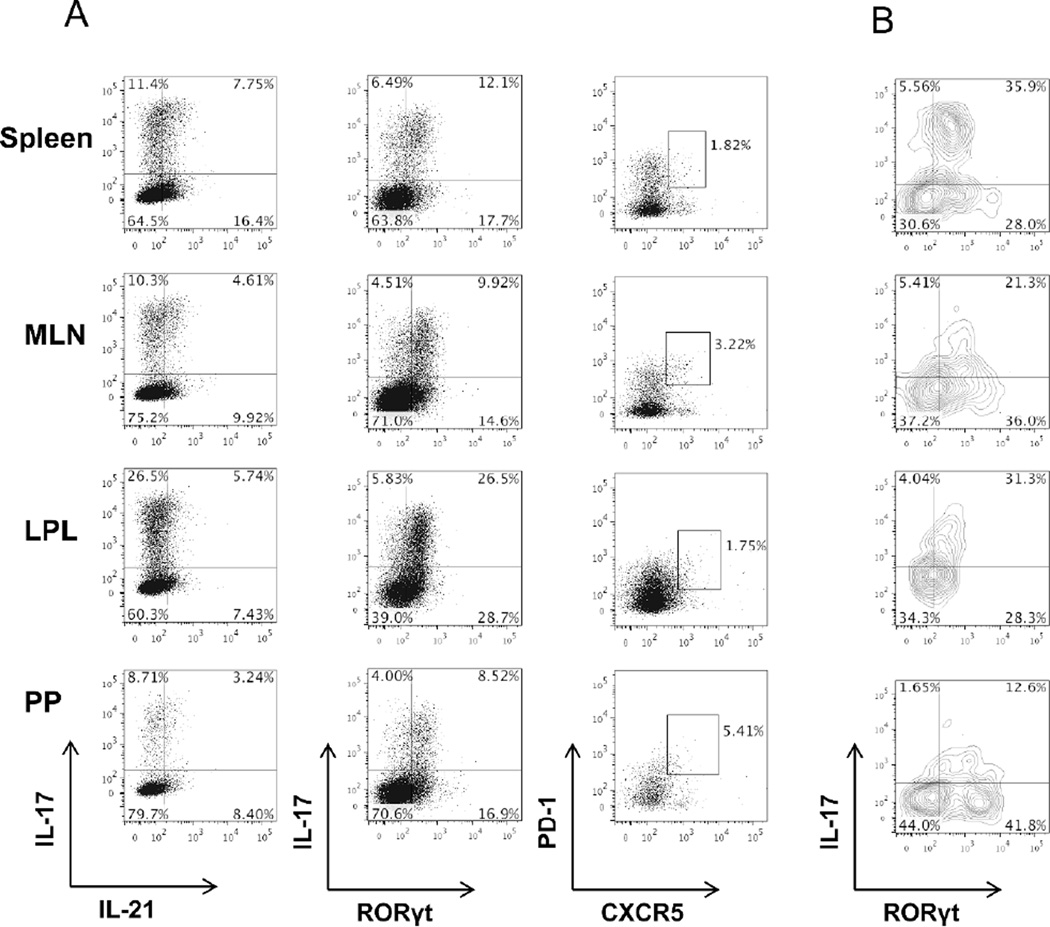
Follicular T cells are also a substantial source of IL-21 and are abundantly present in the lymphoid tissues. It has been recently discovered that Th17 cells can undergo conversion into Tfh in the Peyer’s patches, where they produce IL-21 and drive IgA responses15. We therefore investigated whether our transferred Th17 cells were converting into Tfh in the intestines. We found that the transferred Th17 cells retained IL-17 and RORγt expression throughout the spleen and intestines, including the Peyer’s patches. Notably, the intestinal lamina propria contained the highest populations of Th17 cells (Figure 7A). Many of the transferred cells also expressed IL-21, either independently or in tandem with IL-17. Few of the transferred cells co-expressed PD-1 and CXCR5, the characteristic surface markers of Tfh cells; however, those cells also retained IL-17 and RORγt expression (Figure 7B). Consistent with the previous report15, the Peyer’s patches had the highest frequencies of transferred cells expressing Tfh markers; however these cells did not completely lose IL-17 production. It is notable that the Peyer’s patches had the overall lowest frequency of IL-17+ cells.
Figure 7. Transferred CBir1 Th17 cells into TCR®x™−/− mice retain IL-17 and RORγt expression.
106 CBir1 Th17 cells were i.v. transferred into TCR®x™−/− mice. (A) On day 44 post-Th17 cell transfer, analysis of IL-17, IL-21, RORγt, PD-1, and CXCR5 expression on spleen, mesenteric lymph node, intestinal lamina propria, or Peyer’s patch CD4 T cells of CBir1 Th17 cell recipients was determined by flow cytometry. (B) Analysis of IL-17 and RORγt expression from PD-1+CXCR5+ CD4 T cells of CBir1 Th17 cell recipients was determined by flow cytometry. FACS plots are representative of 4 mice per group.
IL-21 induces mucosal homing on B cells
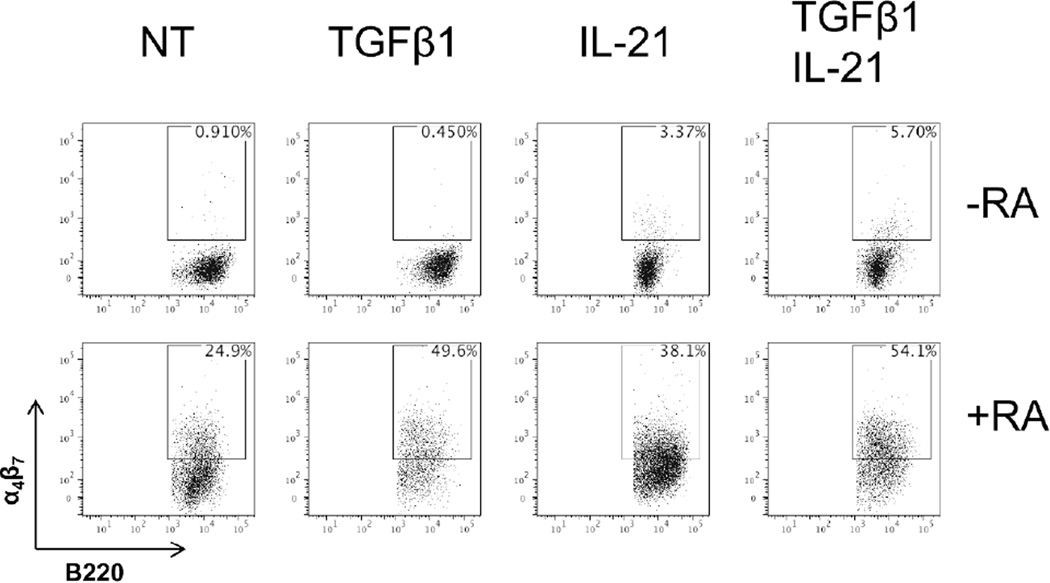
It has been shown that RA is able to induce the expression of homing molecules on lymphocytes to migrate to the intestinal tract22, 33. We then questioned if IL-21 could synergize with RA to influence B cell mucosal homing through induction of α4β7, thus concentrating to Th17 cell induction of intestinal IgA production. Ligation of α4β7 on lymphocytes with mucosal addressin cell adhesion molecule (MAdCAM-1) on endothelial vessels results in lymphocyte migration into the intestinal tract. We cultured splenic B cells with anti-µ and CD40L in the presence or absence of TGFβ1, and IL-21, alone or in combination, with or without RA to determine if Th17 cell cytokines could induce B cell expression of α4β7. Five days after culture, cells were analyzed for α4β7 expression via FACS. As expected, RA was able to induce α4β7 expression, which was enhanced with TGFβ1 (Figure 8). Interestingly, IL-21 alone was able to induce α4β7 expression, which was enhanced with both TGFβ1 and RA. While the synergy between IL-21 and RA was not quite to the degree as TGFβ1 and RA, the combination of all three molecules resulted in optimal α4β7 expression, concomitant with IgA production as seen in Figure 1B. Notably, IL-17 had no visible effect on α4β7 induction, both alone and in conjunction with RA (data not shown). These data indicates that IL-21 contributes to mucosal homing in addition to IgA production in conjunction with TGFβ1 and RA.
Figure 8. IL-21 induces mucosal homing markers alone and in synergism with TGFβ1 and/or retinoic acid.
Naïve IgD+ B cells were activated with anti-µ (5µg/ml) and anti-CD40 (5µg/ml), and cultured with TGFβ1 (5ng/ml), IL-21 (20ng/ml), alone or in combination, with or without retinoic acid (1µM). On day 5, α4β7 was measured by flow cytometry. FACS plots are representative of 3 independent experiments. NT = no treatment.
Discussion
The gastrointestinal tract exists in a mutualistic relationship with the commensal flora within. Multiple mechanisms have evolved to regulate and conserve homeostasis between the immune system and microbiota. The intestines act as a natural reservoir for IgA-producing B cells, where they are critical for maintaining intestinal homeostasis by containing the vast population of commensal bacteria. Our data demonstrated that Th17 cells and their cytokine IL-21 in the intestine strongly promote intestinal IgA production by promoting differentiation of B cells to IgA-secreting cells. Furthermore, IL-21 can influence B cells to migrate into the intestine, where they can continue to differentiate into IgA-secreting cells.
Treg, Th17, and Tfh cells have all been shown to promote the IgA response in the intestine14, 15, 19. Both Th17 and Tfh cells produce IL-17A and IL-2124, 34, cytokines that are important for establishing germinal center architecture and thus the humoral response. Further, IL-21 induces B cells to undergo CSR and produce large amounts of IgG9. TGFβ can override IL-21-mediated CSR to IgG in favor of class switching to IgA in mucosal tissues31. IL-21 has also been described to have a number of pro-B cell effects, which may contribute to increasing IgA production, particularly during T-dependent antibody responses. Both retinoic acid and IL-21 promote overall B cell proliferation35–37, as well as selected proliferation of TGFβ-mediated IgA+ cells28, 31. In our current study, our results indicate that IL-21 is a potent inducer of CSR, as indicated by Aicda transcription, and when combined with TGFβ1, results in highly specific and accelerated induction of CSR to promote IgA-secreting B cells. It remains notable that retinoic acid is required to achieve optimal IgA induction from IL-21-treated B cells in vitro. Furthermore, it is likely that IL-21 from Th17 cells synergizes with physiologic RA and TGFβ to drive intestinal IgA production in vivo. This may indeed help to explain the mechanism by which the intestinal environment is so favorable for IgA+ B cells. As described previously, the intestinal environment is rich in molecular cues, including TGFβ and RA, to promote IgA production and differentiation. As such, our data further reveals that in the intestinal environment with high levels of TGFβ and RA, Th17 and Tfh cell production of IL-21 is capable of inducing strong IgA+ B cell differentiation and IgA production from B cells.
It is interesting to note that IL-21 and RA are capable of inducing IgA production in the absence of exogenous TGFβ1 (Figures 1B). Our previous work has detailed an important role for RA in the maintenance of the intestinal regulatory T cell population22. Intestinal dendritic cells are able to produce TGFβ after exposure to RA, which in turn induces Foxp3 expression in CD4+ T cells and promotes regulatory T cell differentiation. As shown in Figure 3B, RA also stimulates B cells production of TGFβ. As B cells have already been described to have regulatory roles through production of IgA and IL-1038, our finding broadens their regulatory roles, specifically that B cells are capable of directing their own differentiation into IgA-secreting cells. Although IL-21 alone does not induce TGFβ production from B cells, it appears to promote RA-induced TGFβ production, which is consistent with the role of IL-21 in promoting IgG responses rather than IgA. The observation that IL-21 and RA can cooperate in the regulation of B cell homing to intestines through α4β7 suggests that Th17 and Tfh cells can indirectly regulate the development of IgA+ B cells outside of the intestinal tract. Environments such as the bone marrow are rich in RA and IL-2122, 39, 40, and may influence naïve B cells from the bone marrow to migrate en masse to the intestines, where the environment is ripe for the induction of IgA+ B cells. Interestingly, IL-17 and IL-21 differentially regulate intestinal IgA response in a cooperative manner.
The primary observation that IL-21 can promote IgA+ B cells appears to occur primarily in the intestinal tract. After transferring Th17 cells into naïve TCRβxδ−/− mice, we observed a strong increase in IgA+ cells in the periphery and intestines; however, blockade of IL-21 only decreased IgA+ cells in the intestines. Accordingly, blockade of IL-21 led to a decrease in fecal IgA, while it did not significantly decrease serum IgA. This indicates that: 1) IL-21 augments the favorable environment for promoting IgA responses in the intestine; and 2) IL-21 may provide an important switch for B cells to produce IgA rather than IgG. Previous reports have indicated that Th17 cells produce IL-21 and support IgA production only after conversion into Tfh cells15 in the Peyer’s patches. However, we found that our Th17 cells retained IL-17 and RORγt expression 7 weeks after transfer. Transferred cells also expressed IL-21, while lacking PD-1 and CXCR5 co-expression, suggesting limited conversion to Tfh cells. While the Th17-to-Tfh conversion described by Hirota et al15 was observed after 3 months, we found few PD-1/CXCR5 co-expressing cells in the Peyer’s patches after 7 weeks. It remains possible that Th17 reprogramming is an extended process as the Peyer’s patches contained fewer IL-17+ cells than those in the lamina propria or MLN; however, we observed an increase in fecal IgA within 2 weeks after Th17 cell transfer, indicating that Th17 cells are competent in supporting IgA production.
While TGFβ1 is required for CSR to IgA, a number of other factors can facilitate and modify IgA production or IgA+ cell differentiation. Previous studies have indicated that TGFβ can also induce IgG2b production; however, IL-21 appears to inhibit IgG2b production and skews toward IgA class switching17. While our results concur on the final differentiation pattern of IgA+ B cells, we present a more rapid and efficient differentiation pattern. The increased robustness of our IL-21 treatment may be a result of the differing activation strategies; Seo et al17 utilized T-independent activation via LPS, whereas our experiments used anti-CD40 and anti-µ antibodies in order to focus on a T-dependent mechanism. As such, our results remain consistent with observations made in human mucosal tissues, such that IL-21 can greatly increase AID and IgA production in conjunction with TGFβ treatment31. Previous evidence has demonstrated that IgA class switching primarily occurs in the Peyer’s patches, but can occur in the intestinal lamina propria3. These studies revealed that both T-dependent and T-independent responses could occur in the intestinal lamina propria of normal mice and humans41, 42. While we observed expression of Aicda in the intestines of naïve TCRβxδ−/− mice, expression was not enhanced by the presence of Th17 cells, nor was genetic evidence of IgA class switching detected, despite the high numbers of IgA+ plasma cells in the lamina propria.
In summary, our current study demonstrates that IL-21, which is produced by Th17 and Tfh cells that are particularly enriched in the intestinal lamina propria, promote IgA+ B cell differentiation and IgA production in the intestinal environment that contains high levels of TGFβ and RA. IL-21 directly induces B cell IgA CSR to promote IgA-secreting B cells and B cell IgA production, whereas IL-17 stimulates pIgR expression in epithelial cells, thereby enhancing IgA translocation into the intestinal lumen. Thus, IL-21 and IL-17 coordinately regulate intestinal IgA response to microbiota.
Acknowledgements
We thank Mats Bemark and Peter Bergqvist of University of Gothenburg, Sweden for critical advice in detection of IgA CSR, GLT, and PST.
Grant Support: This work was supported by NIH grants DK079918 and DK098370, and John Sealy Memorial Endowment Fund. ATC is a recipient of the J.W. McLaughlin Postdoctoral Fellowship, UTMB.
Abbreviations used
- IL
interleukin
- Tg
transgenic
- IgA
immunoglobulin A
Footnotes
No authors have conflicting financial interests
References
- 1.Kuchroo VK, Ohashi PS, Sartor RB, Vinuesa CG. Dysregulation of immune homeostasis in autoimmune diseases. Nat. Med. 2012;18:42–47. doi: 10.1038/nm.2621. [DOI] [PubMed] [Google Scholar]
- 2.Feng T, Elson CO. Adaptive immunity in the host-microbiota dialog. Mucosal Immunol. 2011;4:15–21. doi: 10.1038/mi.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lycke NY, Bemark M. The role of Peyer's patches in synchronizing gut IgA responses. Front. Immunol. 2012;3:329. doi: 10.3389/fimmu.2012.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benckert J, et al. The majority of intestinal IgA+ and IgG+ plasmablasts in the human gut are antigen-specific. J. Clin. Invest. 2011;121:1946–1955. doi: 10.1172/JCI44447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talham GL, Jiang HQ, Bos NA, Cebra JJ. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect. Immun. 1999;67:1992–2000. doi: 10.1128/iai.67.4.1992-2000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki K, et al. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc. Natl. Acad. Sci. U. S. A. 2004;101:1981–1986. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lecuyer E, et al. Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity. 2014;40:608–620. doi: 10.1016/j.immuni.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Mitsdoerffer M, et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crispin JC, Tsokos GC. Interleukin-17-producing T cells in lupus. Curr. Opin. Rheumatol. 2010;22:499–503. doi: 10.1097/BOR.0b013e32833c62b0. [DOI] [PubMed] [Google Scholar]
- 11.Crispin JC, Kyttaris VC, Terhorst C, Tsokos GC. T cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6:317–325. doi: 10.1038/nrrheum.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bubier JA, et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolff S, et al. Increase in IL-21 producing T-cells in patients with systemic lupus erythematosus. Arthritis Res. Ther. 2011;13:R157. doi: 10.1186/ar3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao AT, Yao S, Gong B, Elson CO, Cong Y. Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J. Immunol. 2012;189:4666–4673. doi: 10.4049/jimmunol.1200955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirota K, et al. Plasticity of Th17 cells in Peyer's patches is responsible for the induction of T cell-dependent IgA responses. Nat. Immunol. 2013;14:372–379. doi: 10.1038/ni.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avery DT, Bryant VL, Ma CS, de Waal Malefyt R, Tangye SG. IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4. J. Immunol. 2008;181:1767–1779. doi: 10.4049/jimmunol.181.3.1767. [DOI] [PubMed] [Google Scholar]
- 17.Seo GY, Youn J, Kim PH. IL-21 ensures TGF-beta 1-induced IgA isotype expression in mouse Peyer's patches. J. Leukoc. Biol. 2009;85:744–750. doi: 10.1189/jlb.0708450. [DOI] [PubMed] [Google Scholar]
- 18.Nurieva RI, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc. Natl. Acad. Sci. U. S. A. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng T, Qin H, Wang L, Benveniste EN, Elson CO, Cong Y. Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production. J. Immunol. 2011;186:6313–6318. doi: 10.4049/jimmunol.1001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cong Y, et al. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J. Exp. Med. 1998;187:855–864. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng T, Cong Y, Qin H, Benveniste EN, Elson CO. Generation of mucosal dendritic cells from bone marrow reveals a critical role of retinoic acid. J. Immunol. 2010;185:5915–5925. doi: 10.4049/jimmunol.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergqvist P, Gardby E, Stensson A, Bemark M, Lycke NY. Gut IgA class switch recombination in the absence of CD40 does not occur in the lamina propria and is independent of germinal centers. J. Immunol. 2006;177:7772–7783. doi: 10.4049/jimmunol.177.11.7772. [DOI] [PubMed] [Google Scholar]
- 24.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolls JK, Khader SA. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 2010;21:443–448. doi: 10.1016/j.cytogfr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lecart S, et al. IL-22, in contrast to IL-10, does not induce Ig production, due to absence of a functional IL-22 receptor on activated human B cells. Int. Immunol. 2002;14:1351–1356. doi: 10.1093/intimm/dxf096. [DOI] [PubMed] [Google Scholar]
- 27.Gelebart P, Zak Z, Dien-Bard J, Anand M, Lai R. Interleukin 22 signaling promotes cell growth in mantle cell lymphoma. Transl. Oncol. 2011;4:9–19. doi: 10.1593/tlo.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe K, et al. Requirement for Runx proteins in IgA class switching acting downstream of TGF-beta 1 and retinoic acid signaling. J. Immunol. 2010;184:2785–2792. doi: 10.4049/jimmunol.0901823. [DOI] [PubMed] [Google Scholar]
- 29.Mora JR, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita K, Harigai M, Fagarasan S, Muramatsu M, Honjo T. A hallmark of active class switch recombination: transcripts directed by I promoters on looped-out circular DNAs. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12620–12623. doi: 10.1073/pnas.221454398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dullaers M, et al. A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity. 2009;30:120–129. doi: 10.1016/j.immuni.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br. J. Pharmacol. 2009;157:220–233. doi: 10.1111/j.1476-5381.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammerschmidt SI, et al. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J. Clin. Invest. 2011;121:3051–3061. doi: 10.1172/JCI44262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuchen S, et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J. Immunol. 2007;179:5886–5896. doi: 10.4049/jimmunol.179.9.5886. [DOI] [PubMed] [Google Scholar]
- 36.Zotos D, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ertesvag A, Naderi S, Blomhoff HK. Regulation of B cell proliferation and differentiation by retinoic acid. Semin. Immunol. 2009;21:36–41. doi: 10.1016/j.smim.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Kalampokis I, Yoshizaki A, Tedder TF. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res. Ther. 2013;15(Suppl 1):S1. doi: 10.1186/ar3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodge LS, et al. IL-21 in the bone marrow microenvironment contributes to IgM secretion and proliferation of malignant cells in Waldenstrom macroglobulinemia. Blood. 2012;120:3774–3782. doi: 10.1182/blood-2012-03-419440. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Bayona B, Ramos-Amaya A, Bernal J, Campos-Caro A, Brieva JA. Cutting edge: IL-21 derived from human follicular helper T cells acts as a survival factor for secondary lymphoid organ, but not for bone marrow, plasma cells. J. Immunol. 2012;188:1578–1581. doi: 10.4049/jimmunol.1102786. [DOI] [PubMed] [Google Scholar]
- 41.Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413:639–643. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- 42.Bergqvist P, Stensson A, Lycke NY, Bemark M. T cell-independent IgA class switch recombination is restricted to the GALT and occurs prior to manifest germinal center formation. J. Immunol. 2010;184:3545–3553. doi: 10.4049/jimmunol.0901895. [DOI] [PubMed] [Google Scholar]