Abstract
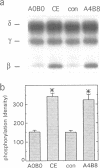
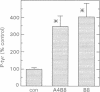
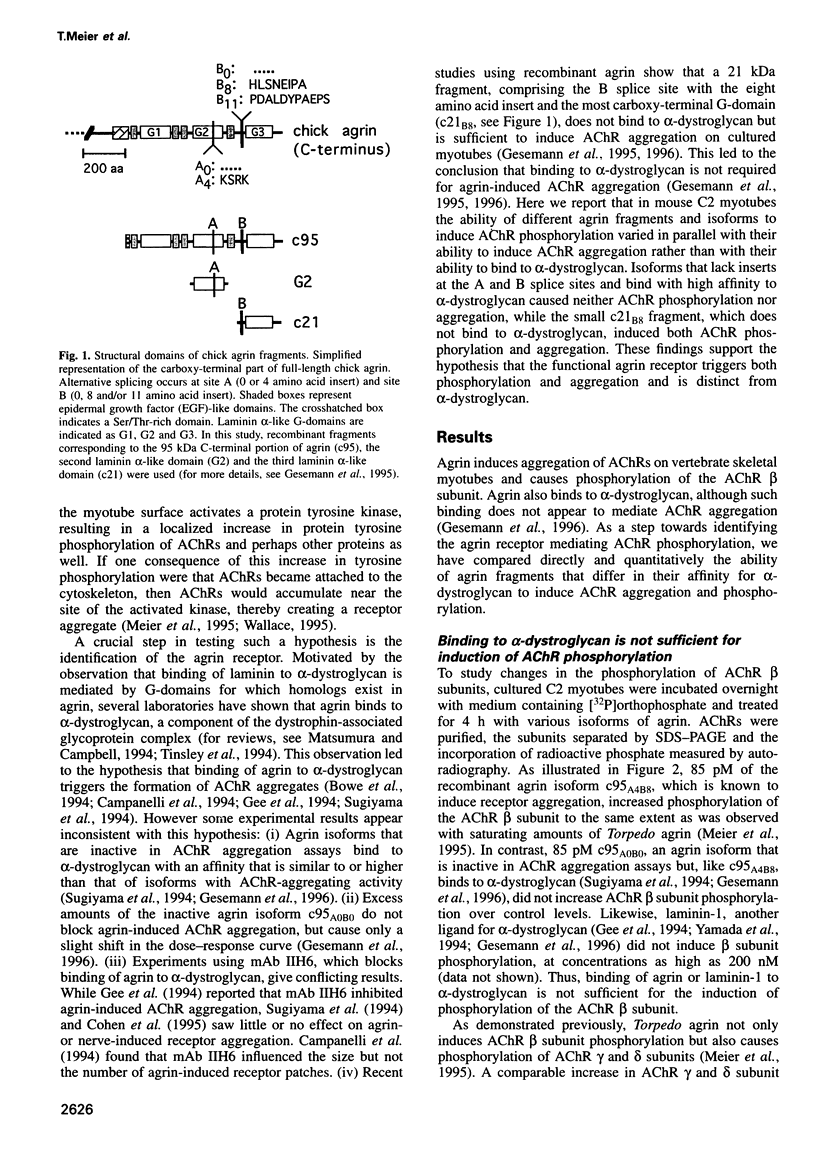
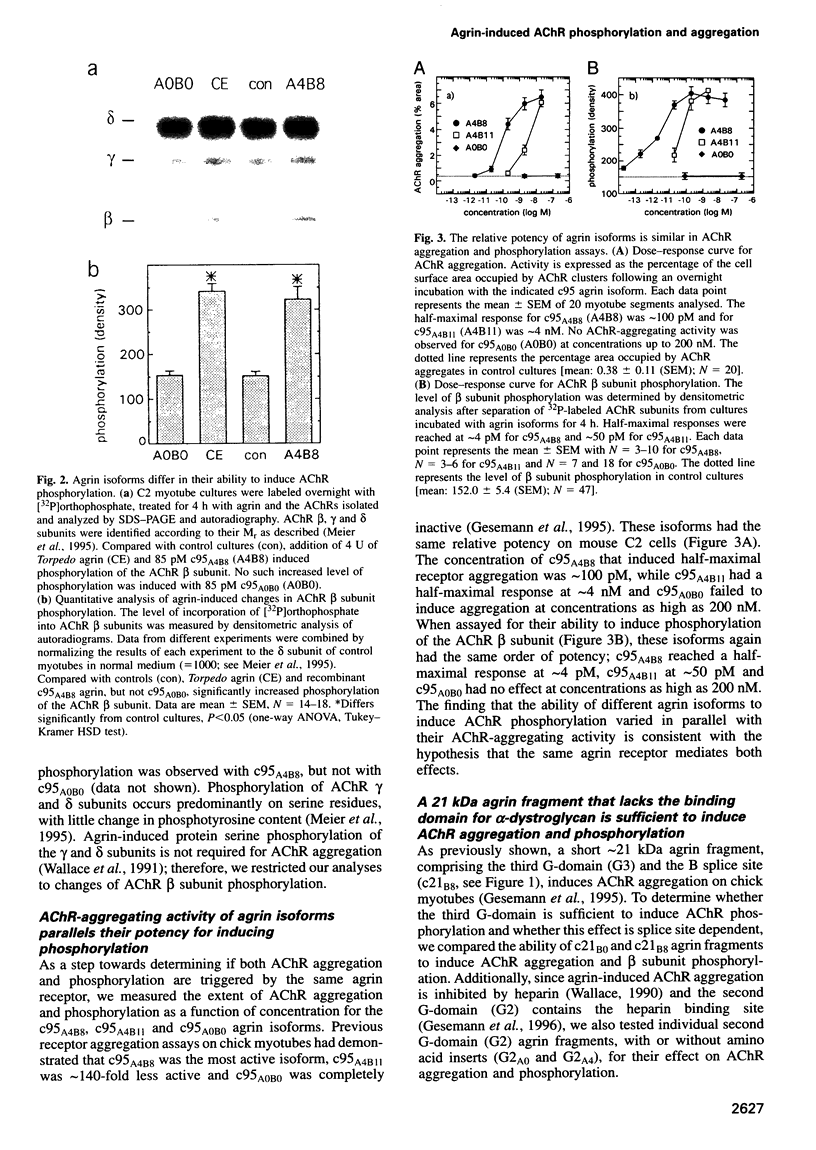
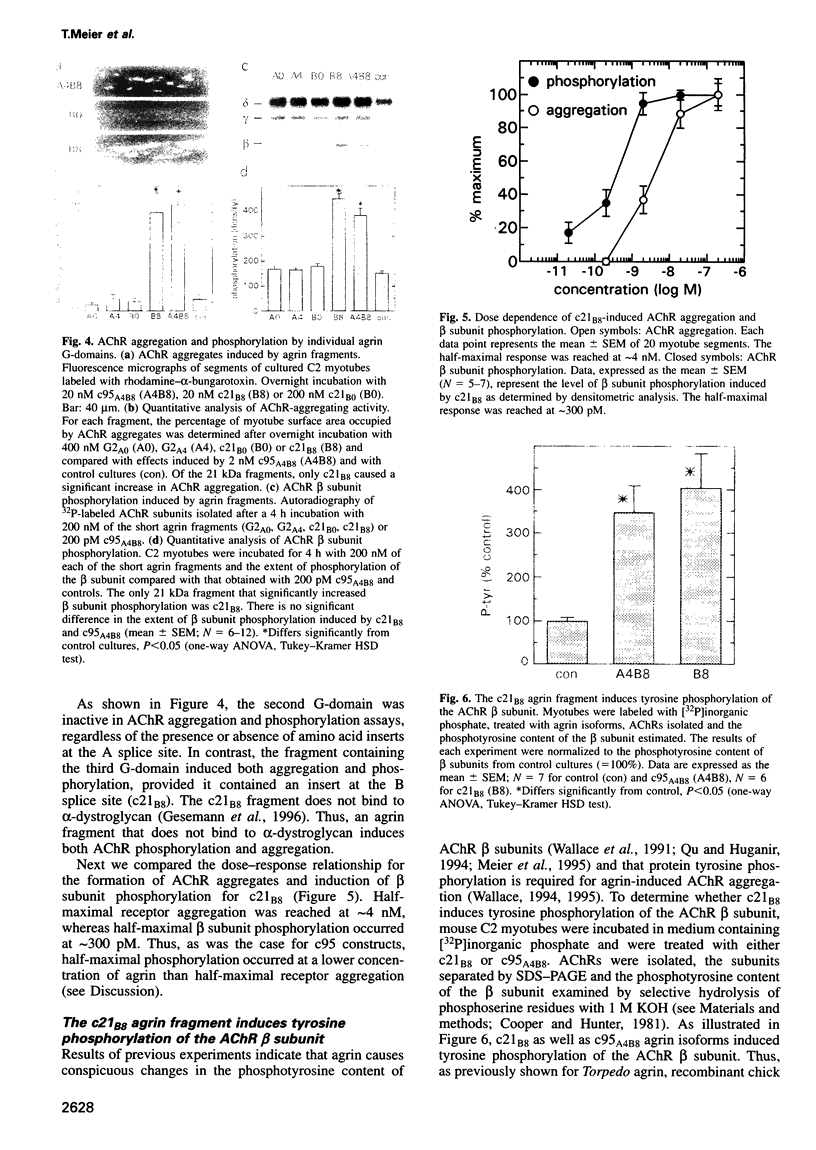
Agrin induces both phosphorylation and aggregation of nicotinic acetylcholine receptors (AChRs) when added to myotubes in culture, apparently by binding to a specific receptor on the myotube surface. One such agrin receptor is alpha-dystroglycan, although binding to alpha-dystroglycan appears not to mediate AChR aggregation. To determine whether agrin-induced AChR phosphorylation is mediated by alpha-dystroglycan or by a different agrin receptor, fragments of recombinant agrin that differ in affinity for alpha-dystroglycan were examined for their ability to induce AChR phosphorylation and aggregation in mouse C2 myotubes. The carboxy-terminal 95 kDa agrin fragment agrin-c95(A0B0), which binds to alpha-dystroglycan with high affinity, failed to induce AChR phosphorylation and aggregation. In contrast, agrin-c95(A4B8) which binds less strongly to alpha-dystroglycan, induced both phosphorylation and aggregation, as did a small 21 kDa fragment of agrin, agrin-c21(B8), that completely lacks the binding domain for alpha-dystroglycan. We conclude that agrin-induced AChR phosphorylation and aggregation are triggered by an agrin receptor that is distinct from alpha-dystroglycan.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel E. D., Merlie J. P. Assembly of the postsynaptic apparatus. Curr Opin Neurobiol. 1995 Feb;5(1):62–67. doi: 10.1016/0959-4388(95)80088-3. [DOI] [PubMed] [Google Scholar]
- Apel E. D., Roberds S. L., Campbell K. P., Merlie J. P. Rapsyn may function as a link between the acetylcholine receptor and the agrin-binding dystrophin-associated glycoprotein complex. Neuron. 1995 Jul;15(1):115–126. doi: 10.1016/0896-6273(95)90069-1. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Chiu C. P., Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983 Apr;32(4):1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Bowe M. A., Deyst K. A., Leszyk J. D., Fallon J. R. Identification and purification of an agrin receptor from Torpedo postsynaptic membranes: a heteromeric complex related to the dystroglycans. Neuron. 1994 May;12(5):1173–1180. doi: 10.1016/0896-6273(94)90324-7. [DOI] [PubMed] [Google Scholar]
- Campanelli J. T., Roberds S. L., Campbell K. P., Scheller R. H. A role for dystrophin-associated glycoproteins and utrophin in agrin-induced AChR clustering. Cell. 1994 Jun 3;77(5):663–674. doi: 10.1016/0092-8674(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Cohen M. W., Jacobson C., Godfrey E. W., Campbell K. P., Carbonetto S. Distribution of alpha-dystroglycan during embryonic nerve-muscle synaptogenesis. J Cell Biol. 1995 May;129(4):1093–1101. doi: 10.1083/jcb.129.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer A. J., Gesemann M., Schumacher B., Ruegg M. A. An amino-terminal extension is required for the secretion of chick agrin and its binding to extracellular matrix. J Cell Biol. 1995 Dec;131(6 Pt 1):1547–1560. doi: 10.1083/jcb.131.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferns M. J., Campanelli J. T., Hoch W., Scheller R. H., Hall Z. The ability of agrin to cluster AChRs depends on alternative splicing and on cell surface proteoglycans. Neuron. 1993 Sep;11(3):491–502. doi: 10.1016/0896-6273(93)90153-i. [DOI] [PubMed] [Google Scholar]
- Ferns M. J., Hall Z. W. How many agrins does it take to make a synapse? Cell. 1992 Jul 10;70(1):1–3. doi: 10.1016/0092-8674(92)90525-h. [DOI] [PubMed] [Google Scholar]
- Ferns M., Hoch W., Campanelli J. T., Rupp F., Hall Z. W., Scheller R. H. RNA splicing regulates agrin-mediated acetylcholine receptor clustering activity on cultured myotubes. Neuron. 1992 Jun;8(6):1079–1086. doi: 10.1016/0896-6273(92)90129-2. [DOI] [PubMed] [Google Scholar]
- Froehner S. C. Regulation of ion channel distribution at synapses. Annu Rev Neurosci. 1993;16:347–368. doi: 10.1146/annurev.ne.16.030193.002023. [DOI] [PubMed] [Google Scholar]
- Gautam M., Noakes P. G., Mudd J., Nichol M., Chu G. C., Sanes J. R., Merlie J. P. Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature. 1995 Sep 21;377(6546):232–236. doi: 10.1038/377232a0. [DOI] [PubMed] [Google Scholar]
- Gee S. H., Montanaro F., Lindenbaum M. H., Carbonetto S. Dystroglycan-alpha, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell. 1994 Jun 3;77(5):675–686. doi: 10.1016/0092-8674(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Gesemann M., Denzer A. J., Ruegg M. A. Acetylcholine receptor-aggregating activity of agrin isoforms and mapping of the active site. J Cell Biol. 1995 Feb;128(4):625–636. doi: 10.1083/jcb.128.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Z. W., Sanes J. R. Synaptic structure and development: the neuromuscular junction. Cell. 1993 Jan;72 (Suppl):99–121. doi: 10.1016/s0092-8674(05)80031-5. [DOI] [PubMed] [Google Scholar]
- Hoch W., Ferns M., Campanelli J. T., Hall Z. W., Scheller R. H. Developmental regulation of highly active alternatively spliced forms of agrin. Neuron. 1993 Sep;11(3):479–490. doi: 10.1016/0896-6273(93)90152-h. [DOI] [PubMed] [Google Scholar]
- Ma E., Morgan R., Godfrey E. W. Agrin mRNA variants are differentially regulated in developing chick embryo spinal cord and sensory ganglia. J Neurobiol. 1995 Apr;26(4):585–597. doi: 10.1002/neu.480260411. [DOI] [PubMed] [Google Scholar]
- Ma E., Morgan R., Godfrey E. W. Distribution of agrin mRNAs in the chick embryo nervous system. J Neurosci. 1994 May;14(5 Pt 2):2943–2952. doi: 10.1523/JNEUROSCI.14-05-02943.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K., Campbell K. P. Dystrophin-glycoprotein complex: its role in the molecular pathogenesis of muscular dystrophies. Muscle Nerve. 1994 Jan;17(1):2–15. doi: 10.1002/mus.880170103. [DOI] [PubMed] [Google Scholar]
- McMahan U. J., Horton S. E., Werle M. J., Honig L. S., Kröger S., Ruegg M. A., Escher G. Agrin isoforms and their role in synaptogenesis. Curr Opin Cell Biol. 1992 Oct;4(5):869–874. doi: 10.1016/0955-0674(92)90113-q. [DOI] [PubMed] [Google Scholar]
- McMahan U. J. The agrin hypothesis. Cold Spring Harb Symp Quant Biol. 1990;55:407–418. doi: 10.1101/sqb.1990.055.01.041. [DOI] [PubMed] [Google Scholar]
- McMahan U. J., Wallace B. G. Molecules in basal lamina that direct the formation of synaptic specializations at neuromuscular junctions. Dev Neurosci. 1989;11(4-5):227–247. doi: 10.1159/000111903. [DOI] [PubMed] [Google Scholar]
- Meier T., Perez G. M., Wallace B. G. Immobilization of nicotinic acetylcholine receptors in mouse C2 myotubes by agrin-induced protein tyrosine phosphorylation. J Cell Biol. 1995 Oct;131(2):441–451. doi: 10.1083/jcb.131.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitkin R. M., Smith M. A., Magill C., Fallon J. R., Yao Y. M., Wallace B. G., McMahan U. J. Identification of agrin, a synaptic organizing protein from Torpedo electric organ. J Cell Biol. 1987 Dec;105(6 Pt 1):2471–2478. doi: 10.1083/jcb.105.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips W. D., Noakes P. G., Roberds S. L., Campbell K. P., Merlie J. P. Clustering and immobilization of acetylcholine receptors by the 43-kD protein: a possible role for dystrophin-related protein. J Cell Biol. 1993 Nov;123(3):729–740. doi: 10.1083/jcb.123.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z., Huganir R. L. Comparison of innervation and agrin-induced tyrosine phosphorylation of the nicotinic acetylcholine receptor. J Neurosci. 1994 Nov;14(11 Pt 2):6834–6841. doi: 10.1523/JNEUROSCI.14-11-06834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reist N. E., Werle M. J., McMahan U. J. Agrin released by motor neurons induces the aggregation of acetylcholine receptors at neuromuscular junctions. Neuron. 1992 May;8(5):865–868. doi: 10.1016/0896-6273(92)90200-w. [DOI] [PubMed] [Google Scholar]
- Ruegg M. A., Tsim K. W., Horton S. E., Kröger S., Escher G., Gensch E. M., McMahan U. J. The agrin gene codes for a family of basal lamina proteins that differ in function and distribution. Neuron. 1992 Apr;8(4):691–699. doi: 10.1016/0896-6273(92)90090-z. [DOI] [PubMed] [Google Scholar]
- Rupp F., Ozçelik T., Linial M., Peterson K., Francke U., Scheller R. Structure and chromosomal localization of the mammalian agrin gene. J Neurosci. 1992 Sep;12(9):3535–3544. doi: 10.1523/JNEUROSCI.12-09-03535.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp F., Payan D. G., Magill-Solc C., Cowan D. M., Scheller R. H. Structure and expression of a rat agrin. Neuron. 1991 May;6(5):811–823. doi: 10.1016/0896-6273(91)90177-2. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Kleinman H. K., Huber H., Deutzmann R., Yamada Y. Laminin, a multidomain protein. The A chain has a unique globular domain and homology with the basement membrane proteoglycan and the laminin B chains. J Biol Chem. 1988 Nov 15;263(32):16536–16544. [PubMed] [Google Scholar]
- Smith M. A., O'Dowd D. K. Cell-specific regulation of agrin RNA splicing in the chick ciliary ganglion. Neuron. 1994 Apr;12(4):795–804. doi: 10.1016/0896-6273(94)90332-8. [DOI] [PubMed] [Google Scholar]
- Sugiyama J., Bowen D. C., Hall Z. W. Dystroglycan binds nerve and muscle agrin. Neuron. 1994 Jul;13(1):103–115. doi: 10.1016/0896-6273(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Tinsley J. M., Blake D. J., Zuellig R. A., Davies K. E. Increasing complexity of the dystrophin-associated protein complex. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8307–8313. doi: 10.1073/pnas.91.18.8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsen G., Halfter W., Kröger S., Cole G. J. Agrin is a heparan sulfate proteoglycan. J Biol Chem. 1995 Feb 17;270(7):3392–3399. doi: 10.1074/jbc.270.7.3392. [DOI] [PubMed] [Google Scholar]
- Tsim K. W., Ruegg M. A., Escher G., Kröger S., McMahan U. J. cDNA that encodes active agrin. Neuron. 1992 Apr;8(4):677–689. doi: 10.1016/0896-6273(92)90089-v. [DOI] [PubMed] [Google Scholar]
- Wallace B. G. Inhibition of agrin-induced acetylcholine-receptor aggregation by heparin, heparan sulfate, and other polyanions. J Neurosci. 1990 Nov;10(11):3576–3582. doi: 10.1523/JNEUROSCI.10-11-03576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. G. Mechanism of agrin-induced acetylcholine receptor aggregation. J Neurobiol. 1992 Jul;23(5):592–604. doi: 10.1002/neu.480230512. [DOI] [PubMed] [Google Scholar]
- Wallace B. G., Qu Z., Huganir R. L. Agrin induces phosphorylation of the nicotinic acetylcholine receptor. Neuron. 1991 Jun;6(6):869–878. doi: 10.1016/0896-6273(91)90227-q. [DOI] [PubMed] [Google Scholar]
- Wallace B. G. Regulation of the interaction of nicotinic acetylcholine receptors with the cytoskeleton by agrin-activated protein tyrosine kinase. J Cell Biol. 1995 Mar;128(6):1121–1129. doi: 10.1083/jcb.128.6.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. G. Staurosporine inhibits agrin-induced acetylcholine receptor phosphorylation and aggregation. J Cell Biol. 1994 May;125(3):661–668. doi: 10.1083/jcb.125.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yamada H., Shimizu T., Tanaka T., Campbell K. P., Matsumura K. Dystroglycan is a binding protein of laminin and merosin in peripheral nerve. FEBS Lett. 1994 Sep 19;352(1):49–53. doi: 10.1016/0014-5793(94)00917-1. [DOI] [PubMed] [Google Scholar]