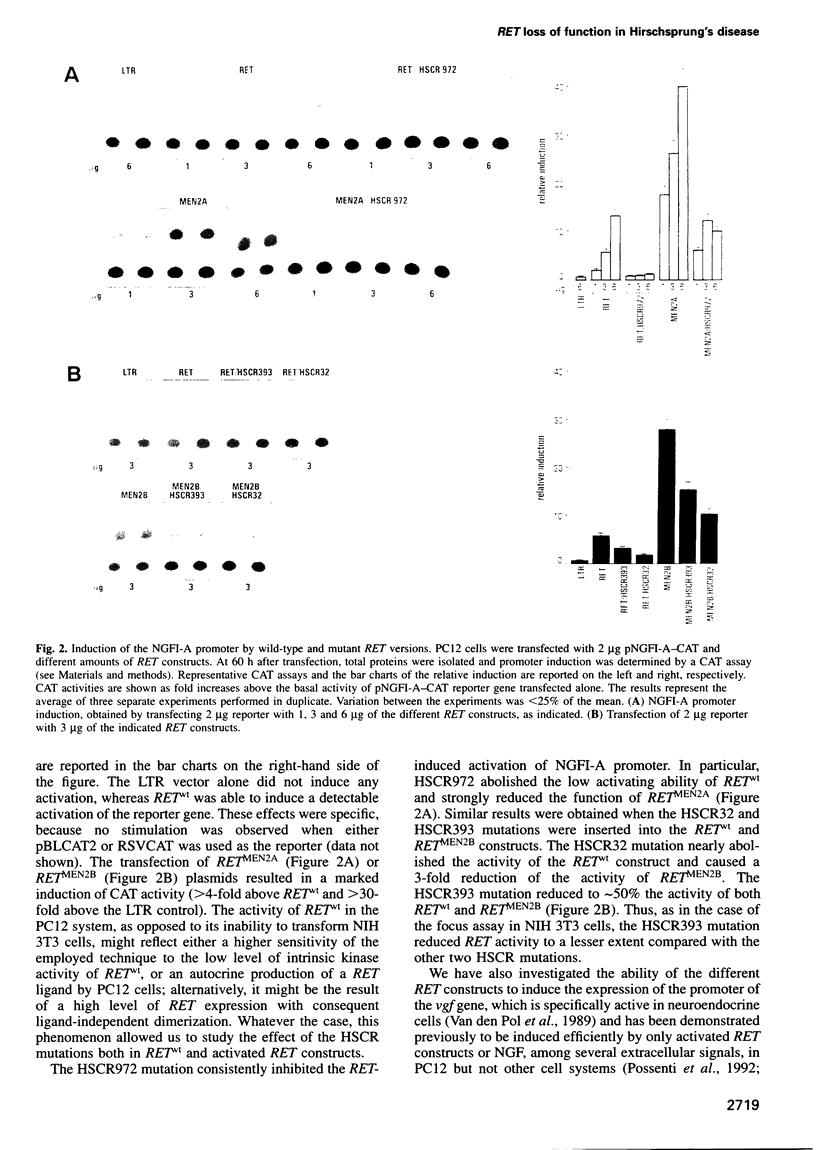
Abstract
The RET proto-oncogene encodes a receptor with tyrosine kinase activity (RET) that is involved in several neoplastic and non-neoplastic diseases. Oncogenic activation of RET, achieved by different mechanisms, is detected in a sizeable fraction of human thyroid tumors, as well as in multiple endocrine neoplasia types 2A and 2B (MEN2A and MEN2B) and familial medullary thyroid carcinoma tumoral syndromes. Germline mutations of RET have also been associated with a non-neoplastic disease, the congenital colonic aganglionosis, i.e. Hirschsprung's disease (HSCR). To analyse the impact of HSCR mutations on RET function, we have introduced into wild-type RET and activated RET(MEN2A) and RET(MEN2B) alleles three missense mutations associated with HSCR. Here we show that the three mutations caused a loss of function of RET when assayed in two model cell systems, NIH 3T3 and PC12 cells. The effect of different HSCR mutations was due to different molecular mechanisms. The HSCR972 (Arg972-->Gly) mutation, mapping in the intracytoplasmic region of RET, impaired its tyrosine kinase activity, while two extracellular mutations, HSCR32 (Ser32-->Leu) and HSCR393 (Phe393-->Leu), inhibited the biological activity of RET by impairing the correct maturation of the RET protein and its transport to the cell surface.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angrist M., Bolk S., Thiel B., Puffenberger E. G., Hofstra R. M., Buys C. H., Cass D. T., Chakravarti A. Mutation analysis of the RET receptor tyrosine kinase in Hirschsprung disease. Hum Mol Genet. 1995 May;4(5):821–830. doi: 10.1093/hmg/4.5.821. [DOI] [PubMed] [Google Scholar]
- Angrist M., Kauffman E., Slaugenhaupt S. A., Matise T. C., Puffenberger E. G., Washington S. S., Lipson A., Cass D. T., Reyna T., Weeks D. E. A gene for Hirschsprung disease (megacolon) in the pericentromeric region of human chromosome 10. Nat Genet. 1993 Aug;4(4):351–356. doi: 10.1038/ng0893-351. [DOI] [PubMed] [Google Scholar]
- Asai N., Iwashita T., Matsuyama M., Takahashi M. Mechanism of activation of the ret proto-oncogene by multiple endocrine neoplasia 2A mutations. Mol Cell Biol. 1995 Mar;15(3):1613–1619. doi: 10.1128/mcb.15.3.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attie T., Pelet A., Sarda P., Eng C., Edery P., Mulligan L. M., Ponder B. A., Munnich A., Lyonnet S. A 7 bp deletion of the RET proto-oncogene in familial Hirschsprung's disease. Hum Mol Genet. 1994 Aug;3(8):1439–1440. doi: 10.1093/hmg/3.8.1439. [DOI] [PubMed] [Google Scholar]
- Attié T., Pelet A., Edery P., Eng C., Mulligan L. M., Amiel J., Boutrand L., Beldjord C., Nihoul-Fékété C., Munnich A. Diversity of RET proto-oncogene mutations in familial and sporadic Hirschsprung disease. Hum Mol Genet. 1995 Aug;4(8):1381–1386. doi: 10.1093/hmg/4.8.1381. [DOI] [PubMed] [Google Scholar]
- Califano D., Monaco C., de Vita G., D'Alessio A., Dathan N. A., Possenti R., Vecchio G., Fusco A., Santoro M., de Franciscis V. Activated RET/PTC oncogene elicits immediate early and delayed response genes in PC12 cells. Oncogene. 1995 Jul 6;11(1):107–112. [PubMed] [Google Scholar]
- Carlson K. M., Dou S., Chi D., Scavarda N., Toshima K., Jackson C. E., Wells S. A., Jr, Goodfellow P. J., Donis-Keller H. Single missense mutation in the tyrosine kinase catalytic domain of the RET protooncogene is associated with multiple endocrine neoplasia type 2B. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1579–1583. doi: 10.1073/pnas.91.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessio A., De Vita G., Calì G., Nitsch L., Fusco A., Vecchio G., Santelli G., Santoro M., de Franciscis V. Expression of the RET oncogene induces differentiation of SK-N-BE neuroblastoma cells. Cell Growth Differ. 1995 Nov;6(11):1387–1394. [PubMed] [Google Scholar]
- D'Arcangelo G., Halegoua S. A branched signaling pathway for nerve growth factor is revealed by Src-, Ras-, and Raf-mediated gene inductions. Mol Cell Biol. 1993 Jun;13(6):3146–3155. doi: 10.1128/mcb.13.6.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore P. P., Pierce J. H., Kraus M. H., Segatto O., King C. R., Aaronson S. A. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 1987 Jul 10;237(4811):178–182. doi: 10.1126/science.2885917. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Dou S., Chi D., Carlson K. M., Toshima K., Lairmore T. C., Howe J. R., Moley J. F., Goodfellow P., Wells S. A., Jr Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet. 1993 Jul;2(7):851–856. doi: 10.1093/hmg/2.7.851. [DOI] [PubMed] [Google Scholar]
- Edery P., Lyonnet S., Mulligan L. M., Pelet A., Dow E., Abel L., Holder S., Nihoul-Fékété C., Ponder B. A., Munnich A. Mutations of the RET proto-oncogene in Hirschsprung's disease. Nature. 1994 Jan 27;367(6461):378–380. doi: 10.1038/367378a0. [DOI] [PubMed] [Google Scholar]
- Eng C., Smith D. P., Mulligan L. M., Nagai M. A., Healey C. S., Ponder M. A., Gardner E., Scheumann G. F., Jackson C. E., Tunnacliffe A. Point mutation within the tyrosine kinase domain of the RET proto-oncogene in multiple endocrine neoplasia type 2B and related sporadic tumours. Hum Mol Genet. 1994 Feb;3(2):237–241. doi: 10.1093/hmg/3.2.237. [DOI] [PubMed] [Google Scholar]
- Fazioli F., Minichiello L., Matoskova B., Wong W. T., Di Fiore P. P. eps15, a novel tyrosine kinase substrate, exhibits transforming activity. Mol Cell Biol. 1993 Sep;13(9):5814–5828. doi: 10.1128/mcb.13.9.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco M., Santoro M., Berlingieri M. T., Melillo R. M., Donghi R., Bongarzone I., Pierotti M. A., Della Porta G., Fusco A., Vecchio G. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell. 1990 Feb 23;60(4):557–563. doi: 10.1016/0092-8674(90)90659-3. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hofstra R. M., Landsvater R. M., Ceccherini I., Stulp R. P., Stelwagen T., Luo Y., Pasini B., Höppener J. W., van Amstel H. K., Romeo G. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. 1994 Jan 27;367(6461):375–376. doi: 10.1038/367375a0. [DOI] [PubMed] [Google Scholar]
- Itoh F., Ishizaka Y., Tahira T., Yamamoto M., Miya A., Imai K., Yachi A., Takai S., Sugimura T., Nagao M. Identification and analysis of the ret proto-oncogene promoter region in neuroblastoma cell lines and medullary thyroid carcinomas from MEN2A patients. Oncogene. 1992 Jun;7(6):1201–1206. [PubMed] [Google Scholar]
- Iwamoto T., Taniguchi M., Asai N., Ohkusu K., Nakashima I., Takahashi M. cDNA cloning of mouse ret proto-oncogene and its sequence similarity to the cadherin superfamily. Oncogene. 1993 Apr;8(4):1087–1091. [PubMed] [Google Scholar]
- Janssen-Timmen U., Lemaire P., Mattéi M. G., Revelant O., Charnay P. Structure, chromosome mapping and regulation of the mouse zinc-finger gene Krox-24; evidence for a common regulatory pathway for immediate-early serum-response genes. Gene. 1989 Aug 15;80(2):325–336. doi: 10.1016/0378-1119(89)90296-5. [DOI] [PubMed] [Google Scholar]
- Kwok J. B., Gardner E., Warner J. P., Ponder B. A., Mulligan L. M. Structural analysis of the human ret proto-oncogene using exon trapping. Oncogene. 1993 Sep;8(9):2575–2582. [PubMed] [Google Scholar]
- Lanzi C., Borrello M. G., Bongarzone I., Migliazza A., Fusco A., Grieco M., Santoro M., Gambetta R. A., Zunino F., Della Porta G. Identification of the product of two oncogenic rearranged forms of the RET proto-oncogene in papillary thyroid carcinomas. Oncogene. 1992 Nov;7(11):2189–2194. [PubMed] [Google Scholar]
- Lemaire P., Revelant O., Bravo R., Charnay P. Two mouse genes encoding potential transcription factors with identical DNA-binding domains are activated by growth factors in cultured cells. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4691–4695. doi: 10.1073/pnas.85.13.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Ceccherini I., Pasini B., Matera I., Bicocchi M. P., Barone V., Bocciardi R., Käriäinen H., Weber D., Devoto M. Close linkage with the RET protooncogene and boundaries of deletion mutations in autosomal dominant Hirschsprung disease. Hum Mol Genet. 1993 Nov;2(11):1803–1808. doi: 10.1093/hmg/2.11.1803. [DOI] [PubMed] [Google Scholar]
- Lyonnet S., Bolino A., Pelet A., Abel L., Nihoul-Fékété C., Briard M. L., Mok-Siu V., Kaariainen H., Martucciello G., Lerone M. A gene for Hirschsprung disease maps to the proximal long arm of chromosome 10. Nat Genet. 1993 Aug;4(4):346–350. doi: 10.1038/ng0893-346. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987 Nov 6;238(4828):797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Mulligan L. M., Kwok J. B., Healey C. S., Elsdon M. J., Eng C., Gardner E., Love D. R., Mole S. E., Moore J. K., Papi L. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993 Jun 3;363(6428):458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- Pachnis V., Mankoo B., Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993 Dec;119(4):1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- Possenti R., Di Rocco G., Nasi S., Levi A. Regulatory elements in the promoter region of vgf, a nerve growth factor-inducible gene. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3815–3819. doi: 10.1073/pnas.89.9.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puffenberger E. G., Hosoda K., Washington S. S., Nakao K., deWit D., Yanagisawa M., Chakravart A. A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung's disease. Cell. 1994 Dec 30;79(7):1257–1266. doi: 10.1016/0092-8674(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Romeo G., Ronchetto P., Luo Y., Barone V., Seri M., Ceccherini I., Pasini B., Bocciardi R., Lerone M., Käriäinen H. Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung's disease. Nature. 1994 Jan 27;367(6461):377–378. doi: 10.1038/367377a0. [DOI] [PubMed] [Google Scholar]
- Santoro M., Carlomagno F., Romano A., Bottaro D. P., Dathan N. A., Grieco M., Fusco A., Vecchio G., Matoskova B., Kraus M. H. Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science. 1995 Jan 20;267(5196):381–383. doi: 10.1126/science.7824936. [DOI] [PubMed] [Google Scholar]
- Santoro M., Wong W. T., Aroca P., Santos E., Matoskova B., Grieco M., Fusco A., di Fiore P. P. An epidermal growth factor receptor/ret chimera generates mitogenic and transforming signals: evidence for a ret-specific signaling pathway. Mol Cell Biol. 1994 Jan;14(1):663–675. doi: 10.1128/mcb.14.1.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. The human protooncogene ret: a communicative cadherin? Trends Biochem Sci. 1992 Nov;17(11):468–469. doi: 10.1016/0968-0004(92)90490-z. [DOI] [PubMed] [Google Scholar]
- Schuchardt A., D'Agati V., Larsson-Blomberg L., Costantini F., Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994 Jan 27;367(6461):380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Schuchardt A., Srinivas S., Pachnis V., Costantini F. Isolation and characterization of a chicken homolog of the c-ret proto-oncogene. Oncogene. 1995 Feb 16;10(4):641–649. [PubMed] [Google Scholar]
- Smith D. P., Eng C., Ponder B. A. Mutations of the RET proto-oncogene in the multiple endocrine neoplasia type 2 syndromes and Hirschsprung disease. J Cell Sci Suppl. 1994;18:43–49. doi: 10.1242/jcs.1994.supplement_18.6. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Asai N., Iwashita T., Isomura T., Miyazaki K., Matsuyama M. Characterization of the ret proto-oncogene products expressed in mouse L cells. Oncogene. 1993 Nov;8(11):2925–2929. [PubMed] [Google Scholar]
- Takahashi M., Buma Y., Iwamoto T., Inaguma Y., Ikeda H., Hiai H. Cloning and expression of the ret proto-oncogene encoding a tyrosine kinase with two potential transmembrane domains. Oncogene. 1988 Nov;3(5):571–578. [PubMed] [Google Scholar]
- Takahashi M., Buma Y., Taniguchi M. Identification of the ret proto-oncogene products in neuroblastoma and leukemia cells. Oncogene. 1991 Feb;6(2):297–301. [PubMed] [Google Scholar]
- Takahashi M., Ritz J., Cooper G. M. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985 Sep;42(2):581–588. doi: 10.1016/0092-8674(85)90115-1. [DOI] [PubMed] [Google Scholar]
- van den Pol A. N., Decavel C., Levi A., Paterson B. Hypothalamic expression of a novel gene product, VGF: immunocytochemical analysis. J Neurosci. 1989 Dec;9(12):4122–4137. doi: 10.1523/JNEUROSCI.09-12-04122.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]