Abstract
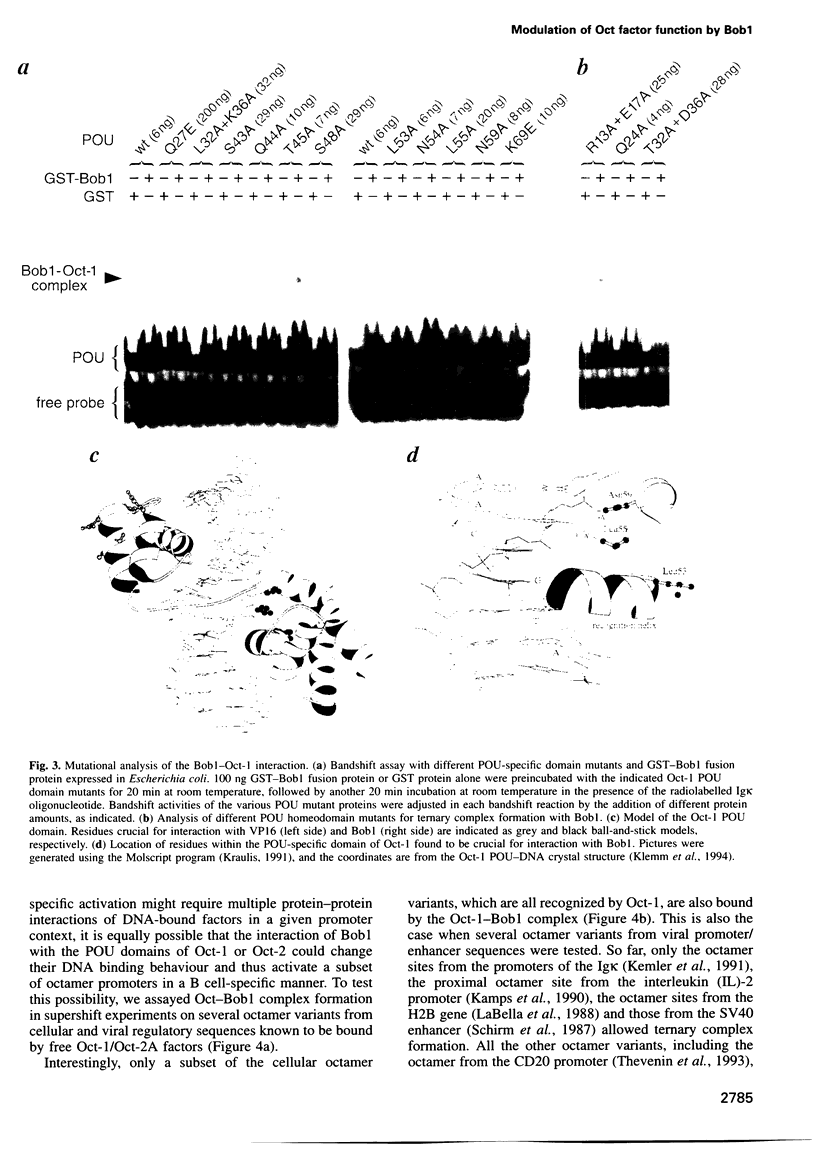
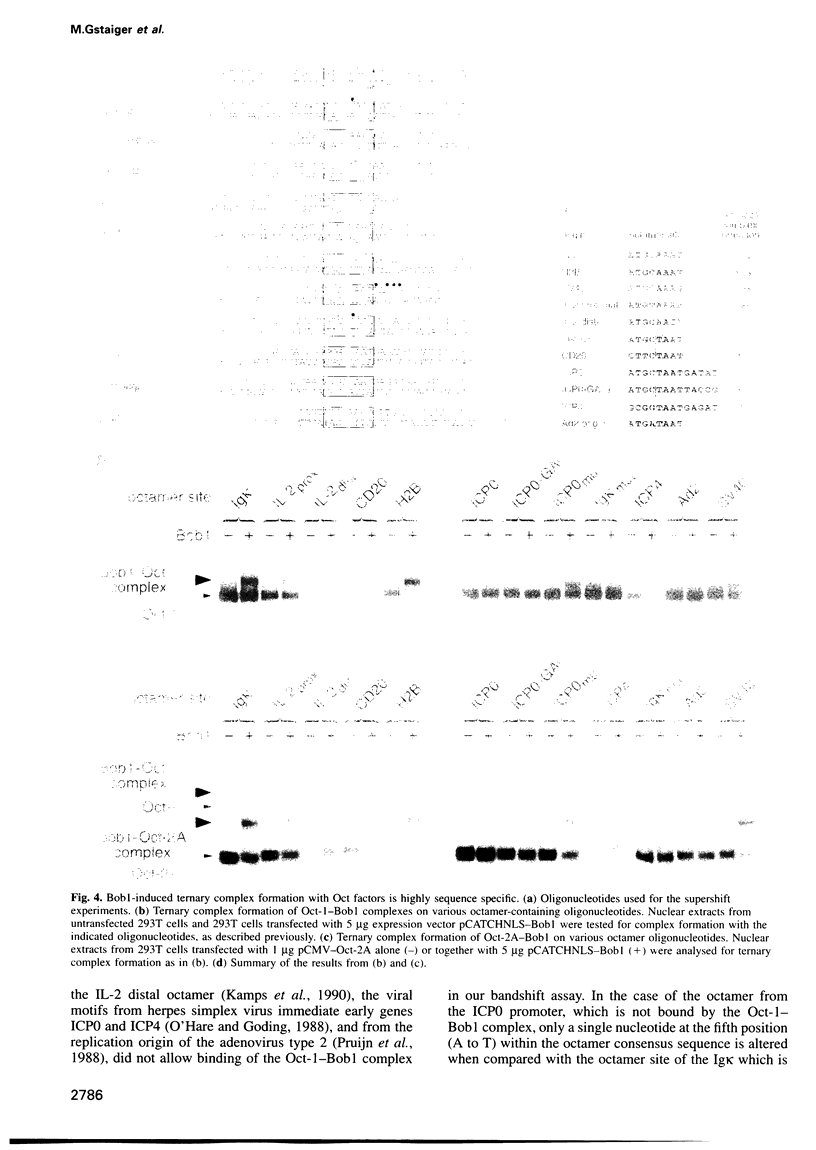
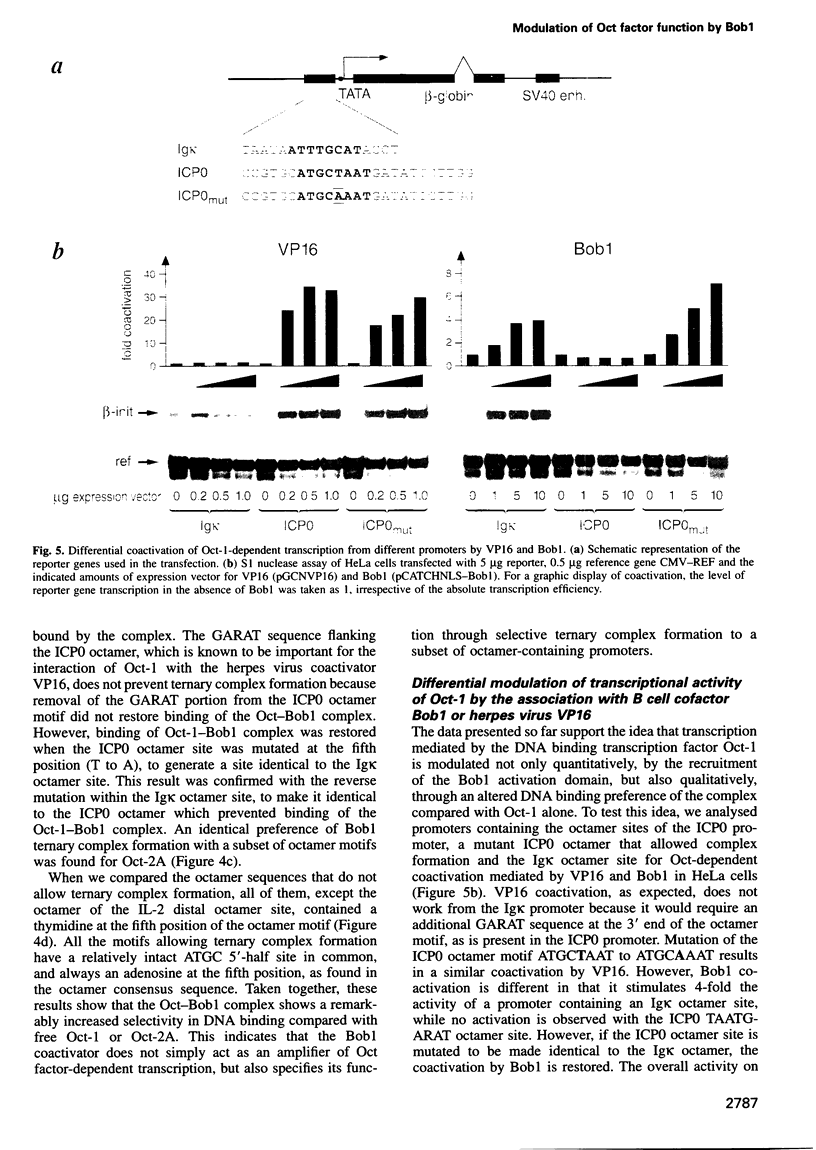
We have shown previously that both octamer binding transcription factors, namely the ubiquitous Oct-1 and the B cell-specific Oct-2A protein, can be enhanced in transcriptional activity by their association with the B cell-specific coactivator protein Bob1, also called OBF-1 or OCA-B. Here we study the structural requirements for ternary complex formation of DNA-Oct-Bob1 and coactivation function of Bob1. In analogy to DNA-bound transcription factors, Bob1 has a modular structure that includes an interaction domain (amino acids 1-65) and a C-terminal domain (amino acids 65-256), both important for transcriptional activation. A mutational analysis has resolved a region of seven amino acids (amino acids 26-32) in the N-terminus of Bob1 that are important for contacting the DNA binding POU domain of Oct-1 or Oct-2. In contrast to the viral coactivator VP16 (vmw65), which interacts with Oct-1 via the POU homeosubdomain, Bob1 association with Oct factors requires residues located in the POU-specific subdomain. Because the same residues are also involved in DNA recognition, we surmised that this association would affect the DNA binding specificity of the Oct-Bob1 complex compared with free Oct factors. While Oct-1 or Oct-2 bind to a large variety of octamer sequences, Bob1 ternary complex formation is indeed highly selective and occurs only in a subset of these sequences, leading to the differential coactivation of octamer-containing promoters. The results uncover a new level in selectivity that furthers our understanding in the regulation of cell type-specific gene expression.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Annweiler A., Müller-Immerglück M., Wirth T. Oct2 transactivation from a remote enhancer position requires a B-cell-restricted activity. Mol Cell Biol. 1992 Jul;12(7):3107–3116. doi: 10.1128/mcb.12.7.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnera E., Xu L., Schaffner W., Arnosti D. N. POU-specific domain of Oct-2 factor confers 'octamer' motif DNA binding specificity on heterologous Antennapedia homeodomain. FEBS Lett. 1992 Dec 21;314(3):361–365. doi: 10.1016/0014-5793(92)81506-h. [DOI] [PubMed] [Google Scholar]
- Cleary M. A., Herr W. Mechanisms for flexibility in DNA sequence recognition and VP16-induced complex formation by the Oct-1 POU domain. Mol Cell Biol. 1995 Apr;15(4):2090–2100. doi: 10.1128/mcb.15.4.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenjaerts F. E., van Oosterhout J. A., van der Vliet P. C. The Oct-1 POU domain stimulates adenovirus DNA replication by a direct interaction between the viral precursor terminal protein-DNA polymerase complex and the POU homeodomain. EMBO J. 1994 Nov 15;13(22):5401–5409. doi: 10.1002/j.1460-2075.1994.tb06875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran L. M., Karvelas M., Nossal G. J., Ye Z. S., Jacks T., Baltimore D. Oct-2, although not required for early B-cell development, is critical for later B-cell maturation and for postnatal survival. Genes Dev. 1993 Apr;7(4):570–582. doi: 10.1101/gad.7.4.570. [DOI] [PubMed] [Google Scholar]
- Danzeiser D. A., Urso O., Kunkel G. R. Functional characterization of elements in a human U6 small nuclear RNA gene distal control region. Mol Cell Biol. 1993 Aug;13(8):4670–4678. doi: 10.1128/mcb.13.8.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douville P., Hagmann M., Georgiev O., Schaffner W. Positive and negative regulation at the herpes simplex virus ICP4 and ICP0 TAATGARAT motifs. Virology. 1995 Feb 20;207(1):107–116. doi: 10.1006/viro.1995.1056. [DOI] [PubMed] [Google Scholar]
- Georgiev O., Bourquin J. P., Gstaiger M., Knoepfel L., Schaffner W., Hovens C. Two versatile eukaryotic vectors permitting epitope tagging, radiolabelling and nuclear localisation of expressed proteins. Gene. 1996 Feb 12;168(2):165–167. doi: 10.1016/0378-1119(95)00764-4. [DOI] [PubMed] [Google Scholar]
- Gietz D., St Jean A., Woods R. A., Schiestl R. H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992 Mar 25;20(6):1425–1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves R. F., O'Hare P. Structural requirements in the herpes simplex virus type 1 transactivator Vmw65 for interaction with the cellular octamer-binding protein and target TAATGARAT sequences. J Virol. 1990 Jun;64(6):2716–2724. doi: 10.1128/jvi.64.6.2716-2724.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gstaiger M., Knoepfel L., Georgiev O., Schaffner W., Hovens C. M. A B-cell coactivator of octamer-binding transcription factors. Nature. 1995 Jan 26;373(6512):360–362. doi: 10.1038/373360a0. [DOI] [PubMed] [Google Scholar]
- Herr W., Cleary M. A. The POU domain: versatility in transcriptional regulation by a flexible two-in-one DNA-binding domain. Genes Dev. 1995 Jul 15;9(14):1679–1693. doi: 10.1101/gad.9.14.1679. [DOI] [PubMed] [Google Scholar]
- Hinkley C., Perry M. Histone H2B gene transcription during Xenopus early development requires functional cooperation between proteins bound to the CCAAT and octamer motifs. Mol Cell Biol. 1992 Oct;12(10):4400–4411. doi: 10.1128/mcb.12.10.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T., Grosschedl R. Complex pattern of immunoglobulin mu gene expression in normal and transgenic mice: nonoverlapping regulatory sequences govern distinct tissue specificities. Genes Dev. 1991 Jun;5(6):932–943. doi: 10.1101/gad.5.6.932. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Corcoran L., LeBowitz J. H., Baltimore D. The promoter of the human interleukin-2 gene contains two octamer-binding sites and is partially activated by the expression of Oct-2. Mol Cell Biol. 1990 Oct;10(10):5464–5472. doi: 10.1128/mcb.10.10.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler I., Bucher E., Seipel K., Müller-Immerglück M. M., Schaffner W. Promoters with the octamer DNA motif (ATGCAAAT) can be ubiquitous or cell type-specific depending on binding affinity of the octamer site and Oct-factor concentration. Nucleic Acids Res. 1991 Jan 25;19(2):237–242. doi: 10.1093/nar/19.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler I., Schaffner W. Octamer transcription factors and the cell type-specificity of immunoglobulin gene expression. FASEB J. 1990 Mar;4(5):1444–1449. doi: 10.1096/fasebj.4.5.2407588. [DOI] [PubMed] [Google Scholar]
- Klemm J. D., Rould M. A., Aurora R., Herr W., Pabo C. O. Crystal structure of the Oct-1 POU domain bound to an octamer site: DNA recognition with tethered DNA-binding modules. Cell. 1994 Apr 8;77(1):21–32. doi: 10.1016/0092-8674(94)90231-3. [DOI] [PubMed] [Google Scholar]
- LaBella F., Sive H. L., Roeder R. G., Heintz N. Cell-cycle regulation of a human histone H2b gene is mediated by the H2b subtype-specific consensus element. Genes Dev. 1988 Jan;2(1):32–39. doi: 10.1101/gad.2.1.32. [DOI] [PubMed] [Google Scholar]
- Lai J. S., Cleary M. A., Herr W. A single amino acid exchange transfers VP16-induced positive control from the Oct-1 to the Oct-2 homeo domain. Genes Dev. 1992 Nov;6(11):2058–2065. doi: 10.1101/gad.6.11.2058. [DOI] [PubMed] [Google Scholar]
- Luo Y., Fujii H., Gerster T., Roeder R. G. A novel B cell-derived coactivator potentiates the activation of immunoglobulin promoters by octamer-binding transcription factors. Cell. 1992 Oct 16;71(2):231–241. doi: 10.1016/0092-8674(92)90352-d. [DOI] [PubMed] [Google Scholar]
- Luo Y., Roeder R. G. Cloning, functional characterization, and mechanism of action of the B-cell-specific transcriptional coactivator OCA-B. Mol Cell Biol. 1995 Aug;15(8):4115–4124. doi: 10.1128/mcb.15.8.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M. M., Ruppert S., Schaffner W., Matthias P. A cloned octamer transcription factor stimulates transcription from lymphoid-specific promoters in non-B cells. Nature. 1988 Dec 8;336(6199):544–551. doi: 10.1038/336544a0. [DOI] [PubMed] [Google Scholar]
- Müller M. M., Schreiber E., Schaffner W., Matthias P. Rapid test for in vivo stability and DNA binding of mutated octamer binding proteins with 'mini-extracts' prepared from transfected cells. Nucleic Acids Res. 1989 Aug 11;17(15):6420–6420. doi: 10.1093/nar/17.15.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Goding C. R. Herpes simplex virus regulatory elements and the immunoglobulin octamer domain bind a common factor and are both targets for virion transactivation. Cell. 1988 Feb 12;52(3):435–445. doi: 10.1016/s0092-8674(88)80036-9. [DOI] [PubMed] [Google Scholar]
- Pierani A., Heguy A., Fujii H., Roeder R. G. Activation of octamer-containing promoters by either octamer-binding transcription factor 1 (OTF-1) or OTF-2 and requirement of an additional B-cell-specific component for optimal transcription of immunoglobulin promoters. Mol Cell Biol. 1990 Dec;10(12):6204–6215. doi: 10.1128/mcb.10.12.6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz J. L., Kristie T. M., Sharp P. A. Recognition of the surface of a homeo domain protein. Genes Dev. 1992 Nov;6(11):2047–2057. doi: 10.1101/gad.6.11.2047. [DOI] [PubMed] [Google Scholar]
- Pruijn G. J., van Miltenburg R. T., Claessens J. A., van der Vliet P. C. Interaction between the octamer-binding protein nuclear factor III and the adenovirus origin of DNA replication. J Virol. 1988 Sep;62(9):3092–3102. doi: 10.1128/jvi.62.9.3092-3102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. G. POU-domain transcription factors: pou-er-ful developmental regulators. Genes Dev. 1991 Jun;5(6):897–907. doi: 10.1101/gad.5.6.897. [DOI] [PubMed] [Google Scholar]
- Rost B., Sander C., Schneider R. PHD--an automatic mail server for protein secondary structure prediction. Comput Appl Biosci. 1994 Feb;10(1):53–60. doi: 10.1093/bioinformatics/10.1.53. [DOI] [PubMed] [Google Scholar]
- Schirm S., Jiricny J., Schaffner W. The SV40 enhancer can be dissected into multiple segments, each with a different cell type specificity. Genes Dev. 1987 Mar;1(1):65–74. doi: 10.1101/gad.1.1.65. [DOI] [PubMed] [Google Scholar]
- Schöler H. R. Octamania: the POU factors in murine development. Trends Genet. 1991 Oct;7(10):323–329. doi: 10.1016/0168-9525(91)90422-m. [DOI] [PubMed] [Google Scholar]
- Seipel K., Georgiev O., Schaffner W. Different activation domains stimulate transcription from remote ('enhancer') and proximal ('promoter') positions. EMBO J. 1992 Dec;11(13):4961–4968. doi: 10.1002/j.1460-2075.1992.tb05603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive H. L., Heintz N., Roeder R. G. Multiple sequence elements are required for maximal in vitro transcription of a human histone H2B gene. Mol Cell Biol. 1986 Oct;6(10):3329–3340. doi: 10.1128/mcb.6.10.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive H. L., Roeder R. G. Interaction of a common factor with conserved promoter and enhancer sequences in histone H2B, immunoglobulin, and U2 small nuclear RNA (snRNA) genes. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6382–6386. doi: 10.1073/pnas.83.17.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt L. M., Lenardo M. J. Immunoglobulin gene transcription. Annu Rev Immunol. 1991;9:373–398. doi: 10.1146/annurev.iy.09.040191.002105. [DOI] [PubMed] [Google Scholar]
- Staudt L. M., Singh H., Sen R., Wirth T., Sharp P. A., Baltimore D. A lymphoid-specific protein binding to the octamer motif of immunoglobulin genes. Nature. 1986 Oct 16;323(6089):640–643. doi: 10.1038/323640a0. [DOI] [PubMed] [Google Scholar]
- Stern S., Herr W. The herpes simplex virus trans-activator VP16 recognizes the Oct-1 homeo domain: evidence for a homeo domain recognition subdomain. Genes Dev. 1991 Dec;5(12B):2555–2566. doi: 10.1101/gad.5.12b.2555. [DOI] [PubMed] [Google Scholar]
- Stern S., Tanaka M., Herr W. The Oct-1 homoeodomain directs formation of a multiprotein-DNA complex with the HSV transactivator VP16. Nature. 1989 Oct 19;341(6243):624–630. doi: 10.1038/341624a0. [DOI] [PubMed] [Google Scholar]
- Strubin M., Newell J. W., Matthias P. OBF-1, a novel B cell-specific coactivator that stimulates immunoglobulin promoter activity through association with octamer-binding proteins. Cell. 1995 Feb 10;80(3):497–506. doi: 10.1016/0092-8674(95)90500-6. [DOI] [PubMed] [Google Scholar]
- Thévenin C., Lucas B. P., Kozlow E. J., Kehrl J. H. Cell type- and stage-specific expression of the CD20/B1 antigen correlates with the activity of a diverged octamer DNA motif present in its promoter. J Biol Chem. 1993 Mar 15;268(8):5949–5956. [PubMed] [Google Scholar]
- Triezenberg S. J., Kingsbury R. C., McKnight S. L. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 1988 Jun;2(6):718–729. doi: 10.1101/gad.2.6.718. [DOI] [PubMed] [Google Scholar]
- Walker S., Hayes S., O'Hare P. Site-specific conformational alteration of the Oct-1 POU domain-DNA complex as the basis for differential recognition by Vmw65 (VP16). Cell. 1994 Dec 2;79(5):841–852. doi: 10.1016/0092-8674(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Westin G., Gerster T., Müller M. M., Schaffner G., Schaffner W. OVEC, a versatile system to study transcription in mammalian cells and cell-free extracts. Nucleic Acids Res. 1987 Sep 11;15(17):6787–6798. doi: 10.1093/nar/15.17.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwilling S., König H., Wirth T. High mobility group protein 2 functionally interacts with the POU domains of octamer transcription factors. EMBO J. 1995 Mar 15;14(6):1198–1208. doi: 10.1002/j.1460-2075.1995.tb07103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- apRhys C. M., Ciufo D. M., O'Neill E. A., Kelly T. J., Hayward G. S. Overlapping octamer and TAATGARAT motifs in the VF65-response elements in herpes simplex virus immediate-early promoters represent independent binding sites for cellular nuclear factor III. J Virol. 1989 Jun;63(6):2798–2812. doi: 10.1128/jvi.63.6.2798-2812.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen H. C., Strating M. J., Cox M., Kaptein R., van der Vliet P. C. Mutation of the Oct-1 POU-specific recognition helix leads to altered DNA binding and influences enhancement of adenovirus DNA replication. Nucleic Acids Res. 1995 Aug 25;23(16):3189–3197. doi: 10.1093/nar/23.16.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]







