Abstract
ErbB-2 is associated with several solid tumours of which breast cancer is the commonest cancer in women worldwide. Though anti-ErbB-2 antibody appears to play a significant role in prevention and therapy, naturally occurring anti-ErbB-2 antibody associated with the cleaved ectodomain of overexpressed ErbB-2 self antigen is detectable in patients. It is therefore essential to understand the course of antibody mediated protection during disease progression. 100% of FVB/Nneu mice expressing mutated, constitutively active ErbB-2 develop mammary carcinoma. It has been shown that vaccination with ErbB-2 associated with a T helper cell epitope P30 can offer protection against transplantable tumour but it is unclear whether the same vaccine protects against naturally developing tumour. We have analysed the course of the disease following prophylactic, and therapeutic vaccination in this spontaneous, eutopic mammary carcinoma model that more closely resembles the human disease. 100% protection against tumour development was observed subsequent to prophylactic immunisation but disease progression was unaffected by therapeutic vaccination. The antibody response exhibited restricted expansion of the Immunoglobulin (Ig) variable (V)-gene repertoire by ErbB-2 specific B cells compared with the non-antigen specific B cell pool and control mice. The serum antibody profile was similar in therapeutically injected mice without any effect on tumour burden.
Keywords: Breast cancer, ErbB-2, Tolerance, Mouse model, Antibody, V-genes, Repertoire
Introduction
Breast cancer is the commonest cancer in women worldwide and also affects men, albeit with c.100-fold lower incidence. ErbB-2 (Neu/HER2) is overexpressed at high transcript and protein levels in 20-30% of primary human breast cancers and is associated with tumour progression, invasion, metastases and poor prognosis [1]. The 185 kD transmembrane ErbB2 protein, is also associated with other human malignancies, including ovarian cancer, and often results in significantly poor prognosis compared to their non-ErbB-2 expressing counterparts [2]. It is one of the four members of the Epidermal growth Factor Receptor (EGFR) tyrosine kinase family [3] and is expressed in moderate to low concentration in healthy adult tissue [4]. Ligand mediated heterodimerisation with other members of the family triggers intrinsic tyrosine phosphorylation of ErbB-2 leading to activation and downstream effects of cell proliferation, survival and transformation [5] although no natural ligand of ErbB-2 has been identified so far. Overexpression in malignancies promotes spontaneous dimerisation even in the absence of ligands and leads to constitutive activation of the signalling pathways [6]. Apparently, the partner of dimerisation directs differential downstream effects on tumour cells. Heregulin ligand-mediated complex of ErbB-2 with ErbB3 or ErbB4 inhibits tumour proliferation but increases invasiveness [7].
ErbB-2 ectodomain is cleaved by metalloproteases leaving the constitutively active, truncated, intracellular tyrosine kinase receptor in the tumour tissue [8]. This circulating serum ErbB-2, a 97 to 115 kD oncoprotein, is detectable in patients as a prognostic disease marker [9]. In ErbB-2 associated breast cancer patients, a spontaneous anti-ErbB-2 immune response against this autologous, native ErbB-2 ectodomain has been documented in both early and late stage metastatic diseases [10-12]. Though there is coexistence of ErbB-2 antigen and antibody in patients, monoclonal antibody therapy has proven to be effective against disease progression in ErbB-2-expressing human breast cancer and transplantable animal models. The humanised anti-ErbB-2 recombinant monoclonal antibody trastuzumab (Herceptin®) is already in clinical practice. Moreover, in transgenic mice, ErbB-2 vaccination induced anti-tumour immunity mediated by both humoral and cellular arms [13] along with a modulating effect on oncogenic activity of tumour associated ErbB-2 [4]. The down modulating effect on ErbB-2 expression by anti-ErbB-2 monoclonal antibody is also evident in transformed cell lines[14]. Whereas most previous studies have relied on ectopic transplanted tumour models, to reproduce the human breast cancer scenario it is important to study the effects of the anti-ErbB-2 immune response on spontaneous development of eutopic mammary carcinoma.
Several genetic and non-genetic vaccination strategies targeted against ErbB-2 exhibited various degrees of success in animal models. Peptide vaccines like E75, GP2, AE37, and Ii-Key [15] and peptide-pulsed autologous dendritic cell vaccine (NCT00923143) are in clinical trials (www.clinicaltrials.gov). These peptides evoke cytotoxic T cell mediated immunity and are restricted by the requirement for HLA matching. Naked DNA (NCT00393783) and vaccine formulations (NCT00197522, NCT00485277) containing autologous dendritic cells transfected with ErbB-2 DNA are under clinical investigation in the hope of circumventing HLA restriction, with limited success to date. In a transplanted tumour animal model, vaccination with the ErbB-2 protein ectodomain fused with a T helper cell epitope was successful in overcoming tolerance and retarding progression of transplantable tumours [16]. However, the immune response to tumours transplanted at ectopic sites differs qualitatively from the response to spontaneously arising natural tumours[17]. In order to mimick human breast cancer, we therefore studied the effects of prophylactic and therapeutic immunisation with ErbB-2 on a transgenic mouse model of spontaneously developing mammary carcinoma.
Materials and methods
Animals
The FVB/Nneu transgenic mouse colony was established from founder breeding pairs of mice purchased from Jackson Laboratories, USA. The animals were maintained in the Central Research Facility, University of Glasgow. Blood samples were collected by tail vein sampling, once a month after immunisation and animals were culled at the end of the procedure by CO2 inhalation. Tumour volumes were calculated from the mean diameter obtained by measuring the tumour diameter in two directions at right angles with Digital Vernier Callipers (Maplin) and total tumour volumes added together in each individual mouse. All animal experiments were conducted under UK Home Office Project Licence No. PPL 60/3495 and Personal Licences PIL 60/2619 and PIL 60/10883.
ErbB-2 protein preparation
The external domain of rat ErbB-2 alone and conjugated with a tetanus toxin epitope (ErbB-2-P30 hereafter) were produced in recombinant S2 insect cell cultures as described elsewhere [16]. The culture supernatant was dialysed into 0.1 M sodium phosphate buffer pH 7.5 and concentrated using an Amicon Stir Cell concentrator. The protein was purified by immobilised metal (Zn++) ion chromatography, exploiting the affinity of Zn++ for the included His tag. Eluted fractions containing the purified protein were combined and analysed by SDS-PAGE, Coomassie staining and mass spectrometry followed by western blot analysis using anti-ErbB-2 mAb-7.16.4 against the ectodomain of rat ErbB-2 (kindly donated by Dr. Hongtao Zhang, Dept of Pathology and Lab medicine, University of Pennsylvania). As the recombinant protein was expressed as a secretory protein from the Drosophila melanogaster S2 cell line by CdCl2 induction, the wild type S2 cell line (Kindly donated by Professor Julian Dow, Integrative & Systems Biology, University of Glasgow) was induced in the same way and the cell supernatant mock purified as above for the control.
Animal immunisation
Rat ErbB-2-P30 protein and mock purified wild type supernatant in 0.1 M sodium phosphate buffer at pH 7.5 were mixed with an equal volume of adjuphos (Rehydraphos kindly donated by Professor James Brewer, Institute of Infection, Immunity & Inflammation, University of Glasgow) and three groups of 15 mice were injected subcutaneously in the neck region with either the test protein 1) 50 μg of rat ErbB-2-P30 protein in 100μl, or for control immunisation with 2) 100μl of wild type supernatant preparation or 3) adjuphos, as an adjuvant only control. The mice were injected three times at 8, 10 and 12 weeks of age. Sera were collected at monthly interval after the third injection by tail bleeding and finally exsanguinated by cardiac puncture. To examine the therapeutic effect of the rat ErbB-2-P30 protein, 10 animals were injected with the rat ErbB-2-P30 protein when the tumour was palpable (mean diameter on detection was 2.5 mm) along with 10 control animals injected with the wild type supernatant preparation. The injection schedule was the same as described before.
Antibody response
Serum antibody responses of the immunised mice were tested using an ELISA “Ensemble” kit (Alpha Diagnostics International) following the manufacturer’s instructions. 96 well plates were coated overnight with recombinant rat ErbB-2 in coating buffer (6 μg/ml, 100 μl/well) or a similar amount of diafiltrated wild type S2 cell supernatant. Diluted serum samples were added in triplicate in different dilutions and read on a Tecan Sunrise™ ELISA plate reader supported by Magellan™ data analysis software. Anti-ErbB-2 mAb-7.16.4 was used as an IgG standard defined as 2 U/ml initial concentration. For IgA ELISA, Ammonium sulphate precipitated total immunoglobulin was used as a positive control. Sera from all the animals were tested by ELISA and the concentrations calculated from standard curves.
Primer design for immunoglobulin variable gene analysis
Immunoglobulin heavy and light chain sequences were compiled from the international ImMunoGeneTics database (IMGT) [18] and primers are designed accordingly. (Supplementary Material Table 1). The junctional primers were used with some modification from elsewhere [19] The degenerate primers were added in different concentrations depending on their diversity. Constant region primers for all Heavy (H) chains isotypes were used as described elsewhere [20]. A semi-nested PCR system was used for amplification from the cDNA template.
Fluorescence Activated Cell Sorting (FACS) of single B-cells
Spleen cells were harvested and washed in PBS containing 1% FCS. Haemolysis was performed using 0.15 M ammonium chloride at pH 7.2 for 10 minutes at room temperature. Antigen specific B cells were stained with biotinylated ErbB-2 [linkage agent: (Long Arm) N-hydroxysuccinimide ester – water soluble (Vector Laboratories)] and captured by streptavidin APC. Biotinylated BSA was used as a negative control. Cells were stained with FITC conjugated rat anti-mouse B220 (BD Pharmingen™) and PE conjugated rat anti-mouse CD138 antibodies (BD Pharmingen™). Cells were dispersed through nitex to prepare single cell suspension and sorted through a BD FACSAria™ I system. The antigen-specific and non-specific pools were retained and samples re-analysed; 85 – 95% of the cells in the antigen-specific pool were specific for the recombinant antigen.
RNA extraction, reverse transcription (RT) and PCR
RNA was isolated from equal numbers of antigen specific and non-specific sorted cells using Qiagen-RNeasy Mini Kit. Complementary DNA (cDNA) was produced from total RNA using the following reaction mixture: 2-4 μl of RNA template, 4 mM dithiothrietol, 2.5 mM MgCl2, 40 U of RNaseOUT™ (Invitrogen) recombinant RNAse inhibitor, 1 U of DNAse™ (Invitrogen) in diethylpyrocarbonate treated water and incubated for 30 minutes at room temperature. The DNAse was heat inactivated at 70°C for 5 minutes, then 200 ng of random hexamer (Invitrogen) was added and the template was denatured by heating at 70°C for 5 minutes. Finally, dNTP was added to a final concentration of 0.8 mM and the mix was divided into two aliquots. To one aliquot, 200 U of Superscript III™ (Invitrogen) was added and the other was used as a RT control. Reactions were incubated at 50°C for 45 minutes for reverse transcription and cDNA synthesis.
2 μl of the cDNA template was amplified by Expand Hi-Fidelity polymerase enzyme (Roche) using 0.2 mM dNTP mix in 1.5 μM/2.0 μM of Mg2+ in enzyme specific buffer with 0.2 μM of reverse primers and the forward primers as described in Table 1. A typical amplification programme was three minutes at 94°C for initial denaturation followed by 34 cycles of 30 seconds, annealing between 52°C to 55°C for 45 seconds and extension at 55°C to 62°C for one minute, ending with a final extension of 10 minutes at 72°C. Amplified PCR products from the cDNA were purified by agarose gel electrophoresis, extracted using Gel Extraction Kit (Qiagen), cloned into the pGEM-T Easy vector (Promega) and sequenced from both directions by the DNA Sequencing Service (Dundee University using T7 and SP6 primers. Sequences were assembled using the Contig Express programme of the Vector NTI suite 9 and 10.
Analysis of Ig V-gene sequences and replacement mutations
IMGT-V-QUEST software for mouse was used to align the query sequence with the highest match in the IMGT database and for statistical analysis of replacement and silent mutations.
Statistical Analysis
Student’s 2-tailed T test with unequal variance was used for comparison of antibody titres. The Chi square test was used for statistical analysis of immunologlobulin repertoire.
Results
Purification of ErbB-2-P30
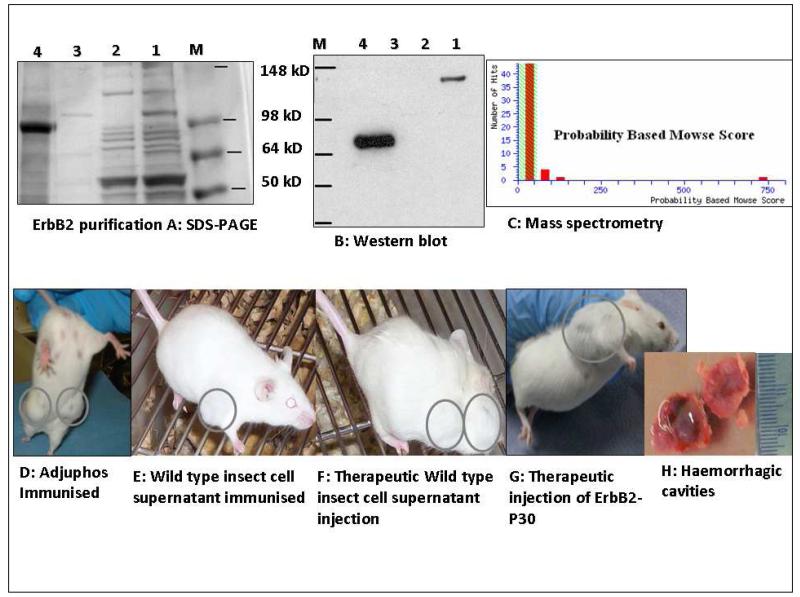
ErbB-2-P30 protein purified from transfected S2 insect cells was analysed by SDS-PAGE (Figure 1A). A principal 77kD band of rat ErbB-2-P30 protein was observed after purification with a small number of additional faint bands. Further analysis by mass spectrometry revealed a single peak corresponding to the extracellular region of rat ErbB-2 protein (gi∣28948771) (Figure 1C) and western blot analysis showed a single band of purified protein of 77kD (Figure 1B).
Figure 1. Purification of rat ErbB-2-P30 protein and development of tumours in control and immunised mice.
SDS-PAGE and western blot analysis of purified of ErbB-2-p30. (A) Lane 1, pre-adsorbtion insect cell supernatant; Lane 2, flow through; Lane 3, wash fluid; Lane 4, eluted protein containing a predominant 77 kD protein band with some faint additional bands; Western blotting revealed a single band of 77 kD (B), and a single peak was observed by mass spectrometry of the purified protein (C). The high molecular weight band in B1 is probably aggregated ErbB-2-P30. 100% of unimmunised transgenic mice and mice injected with adjuphos (D) or wild type insect cell supernatant (E & F) developed mammary tumours. A similar disease profile was observed in animals injected with ErbB-2 –P30 after the detection of tumours (G). The tumours often grew rapidly due to intratumour haemorrhage which was evident after dissection (H).
Tumour development and the antibody response to ErbB-2
The FVB/Nneu transgenic animals developed multifocal tumours in the mammary and salivary glands, extending from the neck to groin. The solid tumours often showed sudden haemorrhage and expansion; blood filled cavities were observed during dissection (Figure 1H). The tumour phenotype was similar in unimmunised animals, control immunised groups and animals immunised after tumour onset (Figure 1D-G).
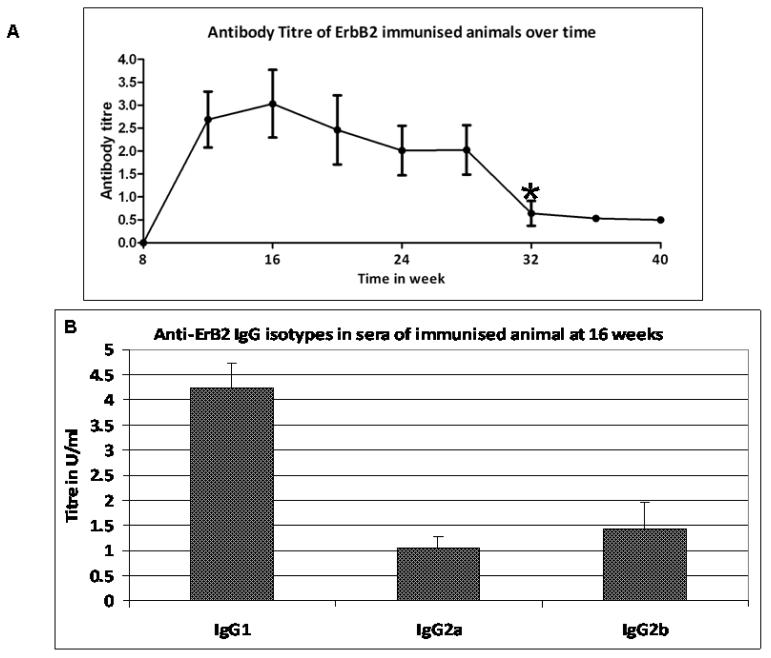
Transgenic mice carrying the rat ErbB-2 oncogene were prophylactically immunised with identical rat ErbB-2-P30 protein vaccine before development of overt tumours and the effect on spontaneous development of mammary carcinoma assessed. Both proteins possess an activating T to A mutation in position 661 that results in constitutive activation of ErbB-2 and development of mammary carcinoma in 100% of these animals by the age of 28 weeks [21]. This mutation is necessary for malignancy in the rodent model whereas overexpression of native ErbB-2 in humans is responsible, in many cases, for the development of breast cancer. However, in both instances, the causative protein can be regarded as a self antigen and it has previously been shown that ErbB-2 alone induces only a very weak antibody response in breast cancer patients [16]. Therefore, a tetanus toxin epitope (P30) was linked to ErbB-2 to facilitate Th-cell help. All the ErbB-2-P30 immunised animals mounted high titres of antibody against ErbB-2 and did not show any cross reactivity with wild type insect cell supernatant, showing that immunological tolerance was broken. The average antibody titres from all the immunised animals are represented in Figure 2A.
Figure 2. Anti-ErbB-2 antibody titre and isotype in ErbB-2-P30 immunised animals.
All 15 of the ErbB-2-P30 immunised animals showed high titres of anti-ErbB-2 antibody up to 28 weeks of age, diminishing significantly by week 32. IgG1 was significantly higher compared with IgG2a at the age of 16 weeks when the titre was at its highest.
The antibody titres decreased significantly (p <0.005) in all the animals after 28 weeks of age (16 weeks after last injection) though the animals remained tumour free until 40 weeks of age. Tumour-associated rat ErbB-2 has 95.7% total amino acid identity with mouse native ErbB-2 but there was no detectable immune response in the unimmunised or control immunised animals. This is in contrast to human breast cancer where anti-ErbB-2 antibodies are often detected.
The IgG1 isotype was significantly higher (p<0.005) compared with the IgG2a & IgG2b isotypes as shown in the 32nd week bleed (Figure 2B), consistent with the known Th2 bias of adjuphos [22]. There was no detectable serum IgA response in these animals and the IgM response was only detectable at 12 weeks.
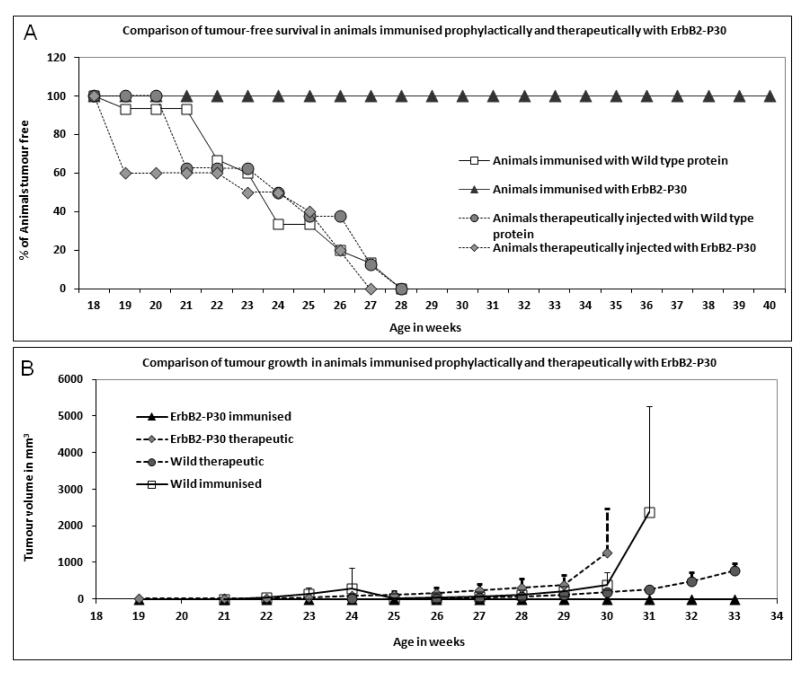
Prophylactic immunisation with ErbB-2-P30 protects against spontaneous tumour development
All the animals immunised with ErbB-2-P30 before the onset of tumour development were perfectly healthy and tumour free throughout their life span (40 weeks), while both control groups developed tumours by 28 weeks of age with a mean age of onset at 23.5 weeks (Figure 3A). The tumours often had haemorrhagic complications and attained the maximum size permitted by the licence of 12 mm diameter within a few weeks from the appearance of a visible tumour. There was no significant difference in response between adjuvant and mock purified protein (p=0.3), although the appearance of tumours was consistently delayed in the adjuphos group from 22-27 weeks, which may be due to non-specific stimulation of the innate response. All the control animals had one or more tumours of the maximum permitted size by the age of 32 weeks. The tumours in the control animals did not show any significant B or T cell infiltration by immunohistology.
Figure 3. Comparison of tumour-free survival and tumour growth in mice immunised prophylactically or therapeutically with ErbB-2-P30.
A: All animals immunised prophylactically with ErbB-2-P30 remained tumour free to at least 40 weeks of age, whereas all animals injected therapeutically with ErbB-2-P30, adjuphos or wild type insect cell supernatant developed the maximum permitted size of tumours by the age of 28 weeks. There was no significant difference between adjuphos and wild type supernatant immunised groups (p=0.3). Therapeutic immunisation with ErbB-2-P30 did not improve survival compared with the wild type supernatant treated group (p=0.6)
B: Tumour growth in therapeutically immunised mice compared with prophylactically immunised animals. Mean tumour volume for each group was plotted against the age of the animals revealing a similar profile for control immunised mice and animals immunised after the onset of tumour growth. 100% protection from tumour growth was observed in prophylactically vaccinated animals only.
Tumour growth is not inhibited by therapeutic ErbB-2-P30 immunisation
For transgenic animals to represent a suitable model for treatment of human breast cancer, it was important to investigate the consequences of therapeutic immunisation with the tumour–associated antigen following spontaneous inception of tumours. For this purpose we injected groups of animals as soon as they had measurable tumours (average 2.5 mm) with the same ErbB-2-P30 used for prophylactic vaccination. The animals were investigated either for 40 weeks when they were tumour free or when the tumour size was 12 mm. ErbB-2-P30 immunised animals demonstrated a similar profile of tumour growth compared to animals injected with wild type supernatant at the onset of tumour development (p=0.6, Figure 3B). The difference between the therapeutic control and the prophylactically immunised control animals at 31 weeks of age is due to greater haemorrhagic complications in the latter resulting in shorter survival by 2-3 weeks depending on the tumour size, as shown in Figure 1. These mice had to be euthanized at this time due to the legal requirement of maximal permissible tumour size. This phenotype is in striking contrast to the 100% disease free-state observed in prophylactically vaccinated animals, although the therapeutically immunised group mounted comparable anti-ErbB-2 immune responses with significantly high IgG1 antibody titres (p<0.005 compared with IgG2a (see supplementary material). Maximum survival of therapeutically treated animals after the last booster injection was 7 weeks and all the animals maintained high IgG titres until death.
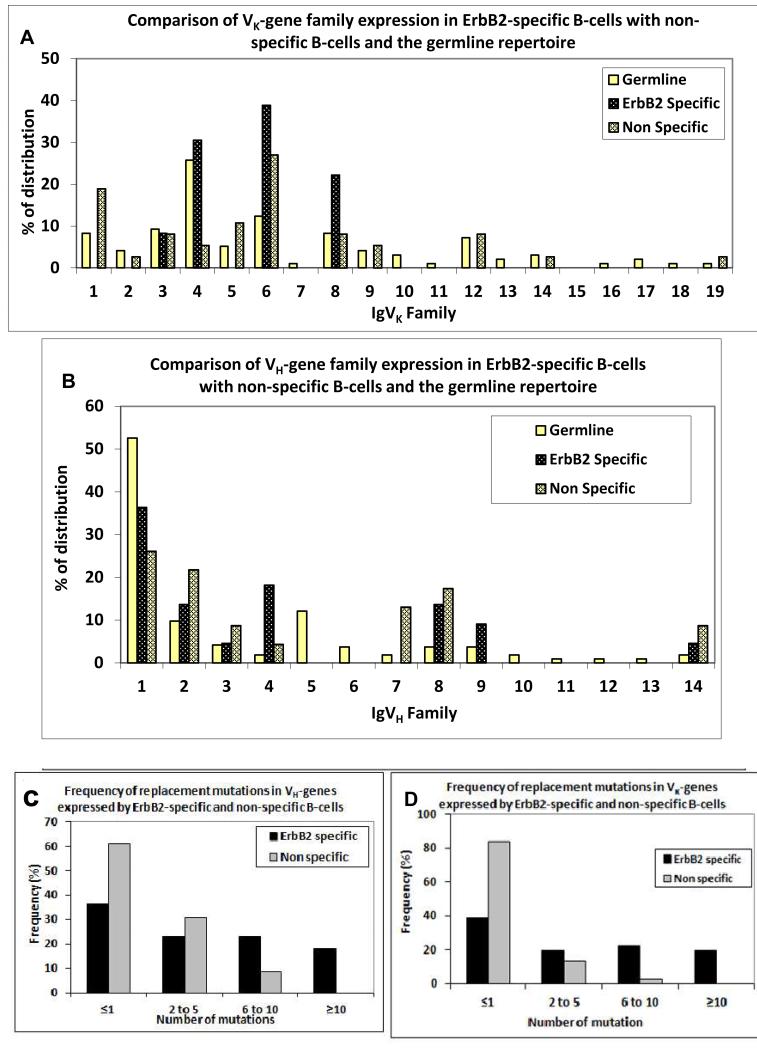
ErbB-2-specific B-cells express a restricted Ig V-gene repertoire
The spleens from all animals were assayed for the presence of ErbB-2 antigen specific B cells. No antigen specific B cells were detected in spleens of the control immunised groups. 0.5 - 1.5% of FITC-B220 positive spleen cells from the immunised animals were ErbB-2 specific 20 to 28 weeks after the last booster dose (given at 12 weeks). As large numbers of FITC-B220 positive cells were not specific for ErbB-2, the number of antigen specific and non-specific cells was equalised prior to RNA isolation for immunoglobulin repertoire analysis. Comparison of the functionally rearranged Ig V-genes expressed by ErbB-2-specific B-cells and the germline Ig V-gene repertoire, as documented in the IMGT database [18] revealed different expression patterns of V kappa chain genes. The non-ErbB-2-specific B-cell pool showed an expression pattern that did not differ significantly from the germ line profile (n=37, Chi square p=0.27), whereas the ErbB-2 specific, splenic B cells expressed genes from the Vκ3, 4, 6 & 8 families only, with a significantly different profile from the germline repertoire (Figure 4A, n=36, Chi square p=<0.05), especially the Vκ8 family, and there was evidence of selection against expression of Vκ1 compared with both the non-ErbB-2-specific and germline repertoires. There was no preferential expression observed in the control immunised B cells (n=49, Chi square p=0.4). The antigen-specific VH-gene family repertoire did not differ significantly overall from either the control immunised group or the germline heavy chain families (n=36, p=0.12) but VH4 and VH9 showed evidence of preferential expression compared with both control groups. There also appeared to be selection against VH5 compared with the germline and against VH7 compared with the non-specific group (Figure 4B). ErbB-2-specific B cells expressed a high proportion of replacement mutations in both VH and Vκ-genes compared with the non-specific B cell pool (n=36 for each group) indicating an antigen driven response in these cells (Fig. 4C & D).
Figure 4. Expression of IgVκ and IgVH gene families and somatic hypermutation in anti-ErbB-2 antibodies.
(A & B) Relative distribution of expression of IgVκ and IgVH families among ErbB-2 specific compared with non-specific, splenic B cells and the germline repertoire; (C & D) Comparative distribution of replacement mutations in IgVH & IgVκ genes expressed by ErbB-2 specific and non-specific, B cells in ErbB-2-P30 immunised mice. There is restricted expression of V-gene families in ErbB-2 specific B cells compared with the number of members in germline genes and the non-specific repertoire, especially Vκ4, Vκ8 and VH4. A high level of replacement mutations was observed in both VH and Vκ-genes from ErbB-2 specific B cells compared with non-specific cells.
Discussion
There have been many studies on the T cell response to tumour antigens in animal models of breast cancer using whole cells, recombinant proteins, glycoproteins (MUC-1) and, recently, DNA vaccines [17], with varying degrees of success in inhibiting tumour growth, reviewed in [23-26]. However, there have been very few studies on the B-cell response to breast cancer, possibly because B-cells have been implicated in exacerbation of tumour development via the inflammatory response [27]. Despite these reservations, it was clearly shown in two independent studies, using different sources of antigen, that immunisation of ErbB-2 transgenic (FVB/Nneu) mice with different immunogenic forms of ErbB-2 inhibits growth of transplanted, syngeneic mammary tumour cells and is dependent on the B-cell response [28;29]. In both cases, elimination of tumours was dependent on B-cells and antibody, but not on CD8+ T-cells, nor even on CD4+ T-cells after allowing time for T-cell help. The effectiveness of anti-ErbB-2 in inhibiting tumour growth was clearly demonstrated by passive transfer of anti-ErbB-2 antibodies induced by the same ErbB-2-P30 fusion protein as used here, into the same FVB/Nneu mice [16]. Furthermore, we have shown that B-cells respond to tumour-associated antigens, including HER-2, in active ectopic germinal centres within human breast tumours [30-32]. In vivo and in vitro studies have demonstrated major roles for antibody-dependent inhibition of signalling and antibody-dependent cell mediated cytotoxicity (ADCC) in tumour growth inhibition and tumour cell killing, including mouse models of breast cancer [33];[34];[35]. ADCC was shown to be dependent on antibody affinity for both antigen and the Fc receptor [36;37].
Most animal studies of the immune response to mammary carcinoma have employed syngeneic transplanted tumours. Unfortunately, the immune response to transplanted tumours has been shown to differ quantitatively and qualitatively from the response to spontaneous, autochthonous tumours [17]. The mechanism of angiogenesis has also been shown to operate by different pathways in autochthonous and transplanted tumours [38] resulting in marked differences in drug responses [39]. We therefore investigated the B-cell response in FVB/Nneu mice spontaneously developing autochthonous mammary carcinomas to prophylactic and therapeutic immunisation with an ErbB-2-P30 fusion protein.
The results demonstrate that prophylactic immunisation with ErbB-2-P30 breaks tolerance, induce a humoral anti-ErbB-2 response and completely prevent spontaneous tumour development in 100% of the transgenic mice. During the development of mammary carcinoma, expansion of tumour antigen-expressing tissue can augment the TReg response at tumour sites [40] and studies have shown that growing autochthonous tumours do not provide a suitable milieu for dendritic cell activation and can induce tolerance [41]. In our model, none of the control animals elicited a spontaneous antibody response against ErbB-2 expressed on the tumour cell surface, whereas immunisation with ErbB-2-P30 induced humoral immunity, by-passing T-cell tolerance. There was significant expansion of ErbB-2-specific B-cells with 0.5 - 1.5% of splenic B-cells specific for ErbB-2 as late as 28 weeks after the last booster immunisation. Early immunisation before any overt tumour growth induced a high titre antibody response and provided complete protection from tumour development compared with mice that were not immunised with ErB2-P30. Although the antibody titre declined later in life, the mice remain tumour free for the rest of their lives (40 weeks). Immunisation with a mammary tissue-specific antigen (α-lactalbumin) has also been shown to suppress development of tumours in mice, although tumour suppression was less effective in this system than with our prophylactic vaccination regime [42]. In view of the immunisation regime employed and evidence from the tumour transplant model using the same transgenic mouse model and tumour [29], it seems reasonable to conclude that protection from tumour growth in this system is largely B-cell mediated.
Interestingly, the same antigen can elicit an antibody response even at a later stage of the disease as evident in our therapeutically treated animals, though circulating antibodies paralleled disease progression in this case, very similar to human breast cancer where a spontaneous anti-ErbB-2 immune response against the autologous ErbB-2 ectodomain has also been documented during early and late stage metastatic disease [10-12]. It will be interesting to compare the epitope specificity and avidity of antibody during disease progression. Thus, a breech in tolerance and induction of immunity against the ErbB-2 autoantigen can be induced at any stage of tumour development but early vaccination is crucial for clearance of tumour antigen at an early stage.
Analysis of the Ig V-gene repertoires of ErbB-2-specific and non-specific B-cells revealed selection for Vκ-gene families Vκ4, 6 & 8 and VH-gene families 4 & 9, with especially strong selection for Vκ8 and VH4, compared with the non-specific and germline V-gene repertoires. No individual Vκ or VH-genes dominated the response, implying that no single B-cell clone expanded at the expense of others, although we cannot exclude the possibility that such a dominant clone or clones were missed when sampling the B-cell pool.
Conclusion
ErbB-2-P30 immunisation of ErbB-2 transgenic mice can break self-tolerance against this oncogenic protein and elicits Th2-type, anti-ErbB-2-specific humoral immunity. Immunisation before overt tumour development completely abrogated tumour growth in all immunised animals for the life of the animals, whereas high circulating antibody titres were unable to prevent disease progression once tumour is established suggesting the importance of early immunisation in breast cancer prevention.
Supplementary Material
Acknowledgements
We are grateful to Dana Leach for help in facilitating provision of the ErbB-2-P30 vaccine reagent and thank Deepak Ipe for preparing the wild type insect cell supernatant for immunisation. The project was funded by Cancer Research UK grant no. C12046/A6927 to D. I. Stott.
Abbreviations
- (Ig)
Immunoglobulin
- (V)
variable
- (H)
Heavy
Footnotes
Conflicts of interest: The authors have no financial or commercial conflicts of interest.
Reference List
- [1].Slamon DJ. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. 1987. [DOI] [PubMed]
- [2].Berchuck A, Kamel A, Whitaker R, Kerns B, Olt G, Kinney R, et al. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990;50(13):4087–91. [PubMed] [Google Scholar]
- [3].Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam AW, et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309(5967):418–25. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- [4].Whittington PJ, Radkevich-Brown O, Jacob JB, Jones RF, Weise AM, Wei WZ. Her-2 DNA versus cell vaccine: immunogenicity and anti-tumor activity. Cancer Immunol Immunother. 2009;58(5):759–67. doi: 10.1007/s00262-008-0599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Olayioye MA. Update on HER-2 as a target for cancer therapy: intracellular signaling pathways of ErbB2/HER-2 and family members. Breast Cancer Res. 2001;3(6):385–9. doi: 10.1186/bcr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198(2-3):165–84. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- [7].Xu FJ, Stack S, Boyer C, O’Briant K, Whitaker R, Mills GB, et al. Heregulin and agonistic anti-p185(c-erbB2) antibodies inhibit proliferation but increase invasiveness of breast cancer cells that overexpress p185(c-erbB2): increased invasiveness may contribute to poor prognosis. Clin Cancer Res. 1997;3(9):1629–34. [PubMed] [Google Scholar]
- [8].Codony-Servat J, Albanell J, Lopez-Talavera JC, Arribas J, Baselga J. Cleavage of the HER2 ectodomain is a pervanadate activable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer Res. 1999;59(6):1196–201. [PubMed] [Google Scholar]
- [9].Luftner D, Luke C, Possinger K. Serum HER-2/neu in the management of breast cancer patients. Clin Biochem. 2003;36(4):233–40. doi: 10.1016/s0009-9120(03)00026-2. [DOI] [PubMed] [Google Scholar]
- [10].Bei R, Masuelli L, Moriconi E, Visco V, Moretti A, Kraus MH, et al. Immune responses to all ErbB family receptors detectable in serum of cancer patients. Oncogene. 1999;18(6):1267–75. doi: 10.1038/sj.onc.1202442. [DOI] [PubMed] [Google Scholar]
- [11].Disis ML, Calenoff E, McLaughlin G, Murphy AE, Chen W, Groner B, et al. Existent T-cell and antibody immunity to HER-2/neu protein in patients with breast cancer. Cancer Res. 1994;54(1):16–20. [PubMed] [Google Scholar]
- [12].Pupa SM, Menard S, Andreola S, Colnaghi MI. Antibody response against the c-erbB-2 oncoprotein in breast carcinoma patients. Cancer Res. 1993;53(24):5864–6. [PubMed] [Google Scholar]
- [13].Reilly RT, Machiels JP, Emens LA, Ercolini AM, Okoye FI, Lei RY, et al. The collaboration of both humoral and cellular HER-2/neu-targeted immune responses is required for the complete eradication of HER-2/neu-expressing tumors. Cancer Res. 2001;61(3):880–3. [PubMed] [Google Scholar]
- [14].Drebin JA, Link VC, Stern DF, Weinberg RA, Greene MI. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell. 1985;41(3):697–706. doi: 10.1016/s0092-8674(85)80050-7. [DOI] [PubMed] [Google Scholar]
- [15].Jones KL, Buzdar AU. Evolving novel anti-HER2 strategies. The Lancet Oncology. 2009;10(12):1179–87. doi: 10.1016/S1470-2045(09)70315-8. [DOI] [PubMed] [Google Scholar]
- [16].Renard V, Sonderbye L, Ebbehoj K, Rasmussen PB, Gregorius K, Gottschalk T, et al. HER-2 DNA and protein vaccines containing potent Th cell epitopes induce distinct protective and therapeutic antitumor responses in HER-2 transgenic mice. J Immunol. 2003;171(3):1588–95. doi: 10.4049/jimmunol.171.3.1588. [DOI] [PubMed] [Google Scholar]
- [17].Rovero S, Amici A, Carlo ED, Bei R, Nanni P, Quaglino E, et al. DNA Vaccination Against Rat Her-2/Neu p185 More Effectively Inhibits Carcinogenesis Than Transplantable Carcinomas in Transgenic BALB/c Mice. J Immunol. 2000;165:5133–42. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]
- [18].Lefranc MP, Giudicelli V, Ginestoux C, Bodmer J, Muller W, Bontrop R, et al. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 1999;27(1):209–12. doi: 10.1093/nar/27.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Barbas CF. Generation of Antibody Libraries: PCR Amplification and Assembly of Light- and Heavy-chain Coding Sequences. In: Burton DR, Scott JK, Silverman GJ, editors. Phage Display A Laboratory Mannual. Cold Spring Harbour Laboratory Press; New York: 2001. [Google Scholar]
- [20].Wang Z, Raifu M, Howard M, Smith L, Hansen D, Goldsby R, et al. Universal PCR amplification of mouse immunoglobulin gene variable regions: the design of degenerate primers and an assessment of the effect of DNA polymerase 3′ to 5′ exonuclease activity. J Immunol Methods. 2000;233(1-2):167–77. doi: 10.1016/s0022-1759(99)00184-2. [DOI] [PubMed] [Google Scholar]
- [21].Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54(1):105–15. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- [22].Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol. 1999;163(12):6448–54. [PubMed] [Google Scholar]
- [23].Lollini PL, Cavallo F, Nanni P, Forni G. Vaccines for tumour prevention. Nat Rev Cancer. 2006;6:204–16. doi: 10.1038/nrc1815. [DOI] [PubMed] [Google Scholar]
- [24].Calogero RA, Musiani P, Forni G, Cavallo F. Towards a long-lasting immune prevention of HER2 mammary carcinomas: directions from transgenic mice. Cell Cycle. 2004;3:704–6. [PubMed] [Google Scholar]
- [25].Mukherjee P, Tinder TL, Basu GD, Pathangey LB, Chen L, Gendler SJ. Therapeutic efficacy of MUC1-specific cytotoxic T lymphocytes and CD137 co-stimulation in a spontaneous breast cancer model. Breast Dis. 2004;20:53–63. doi: 10.3233/bd-2004-20107. [DOI] [PubMed] [Google Scholar]
- [26].Miller F, Jones RF, Jacob J, Kong YC, Wei WZ. From breast cancer immunobiology to her-2 DNA vaccine and autoimmune sequelae. Breast Dis. 2004;20:43–51. doi: 10.3233/bd-2004-20106. [DOI] [PubMed] [Google Scholar]
- [27].de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- [28].Renard V, Leach DR. Perspectives on the development of a therapeutic HER-2 cancer vaccine. Vaccine. 2007;25(Suppl 2):B17–B23. doi: 10.1016/j.vaccine.2007.05.060. [DOI] [PubMed] [Google Scholar]
- [29].Renard V, Sonderbye L, Ebbehoj K, Rasmussen PB, Gregorius K, Gottschalk T, et al. HER-2 DNA and Protein Vaccines Containing Potent Th Cell Epitopes Induce Distinct Protective and Therapeutic Antitumor Responses in HER-2 Transgenic Mice. J Immunol. 2003;171:1588–95. doi: 10.4049/jimmunol.171.3.1588. [DOI] [PubMed] [Google Scholar]
- [30].Nzula S, Going JJ, Stott DI. Antigen-driven clonal proliferation, somatic hypermutation and selection of B-lymphocytes infiltrating human ductal breast carcinoma. Cancer Res. 2003;63:3275–80. [PubMed] [Google Scholar]
- [31].Nzula S, Going JJ, Stott DI. The role of B lymphocytes in breast cancer: a review and current status. Cancer Therapy. 2003;1:353–62. [Google Scholar]
- [32].Stott DI, McIntyre D. The ectopic germinal centre response in autoimmune disease and cancer. In: Huang F-P, editor. Autoimmune Disorders - Current Concepts and Advances from Bedside to Mechanistic Insights. Intech; Rijeka: 2011. pp. 395–432. [Google Scholar]
- [33].Hubert P, Heitzmann A, Viel S, Nicolas A, Sastre-Garau X, Oppezzo P, et al. Antibody-dependent cell cytotoxicity synapses form in mice during tumor-specific antibody immunotherapy. Cancer Res. 2011;71(15):5134–43. doi: 10.1158/0008-5472.CAN-10-4222. [DOI] [PubMed] [Google Scholar]
- [34].Overdijk MB, Verploegen S, van den Brakel JH, Lammerts van Bueren JJ, Vink T, van de Winkel JGJ, et al. Epidermal Growth Factor Receptor (EGFR) Antibody-Induced Antibody-Dependent Cellular Cytotoxicity Plays a Prominent Role in Inhibiting Tumorigenesis, Even of Tumor Cells Insensitive to EGFR Signaling Inhibition. J Immunol. 2011;187:3383–90. doi: 10.4049/jimmunol.1003926. [DOI] [PubMed] [Google Scholar]
- [35].Triulzi C, Vertuani S, Curcio C, Antognoli A, Seibt J, Akusjarvi G, et al. Antibody-dependent natural killer cell-mediated cytotoxicity engendered by a kinase-inactive human HER2 adenovirus-based vaccination mediates resistance to breast tumors. Cancer Res. 2010;70(19):7431–41. doi: 10.1158/0008-5472.CAN-10-0493. [DOI] [PubMed] [Google Scholar]
- [36].Tang Y, Lou J, Alpaugh RK, Robinson MK, Marks JD, Weiner LM. Regulation of Antibody-Dependent Cellular Cytotoxicity by IgG Intrinsic and Apparent Affinity for Target Antigen. J Immunol. 2007;179:2815–23. doi: 10.4049/jimmunol.179.5.2815. [DOI] [PubMed] [Google Scholar]
- [37].Stavenhagen JB, Gorlatov S, Tuaillon N, Rankin CT, Li H, Burke S, et al. Fc Optimization of Therapeutic Antibodies Enhances Their Ability to Kill Tumor Cells In vitro and Controls Tumor Expansion In vivo via Low-Affinity Activating Fc{gamma} Receptors. Cancer Res. 2007;67:8882–90. doi: 10.1158/0008-5472.CAN-07-0696. [DOI] [PubMed] [Google Scholar]
- [38].Sikder H, Huso DL, Zhang H, Wang B, Ryu B, Hwang ST, et al. Disruption of Id1 reveals major differences in angiogenesis between transplanted and autochthonous tumors. Cancer Cell. 2003;4:291–9. doi: 10.1016/s1535-6108(03)00245-9. [DOI] [PubMed] [Google Scholar]
- [39].Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog Signaling Enhances Delivery of Chemotherapy in a Mouse Model of Pancreatic Cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ambrosino E, Spadaro M, Iezzi M, Curcio C, Forni G, Musiani P, et al. Immunosurveillance of Erbb2 carcinogenesis in transgenic mice is concealed by a dominant regulatory T-cell self-tolerance. Cancer Res. 2006;66(15):7734–40. doi: 10.1158/0008-5472.CAN-06-1432. [DOI] [PubMed] [Google Scholar]
- [41].Lyman MA, Aung S, Biggs JA, Sherman LA. A spontaneously arising pancreatic tumor does not promote the differentiation of naive CD8+ T lymphocytes into effector CTL. J Immunol. 2004;172(11):6558–67. doi: 10.4049/jimmunol.172.11.6558. [DOI] [PubMed] [Google Scholar]
- [42].Jaini R, Kesaraju P, Johnson JM, Altuntas CZ, Jane-Wit D, Tuohy VK. An autoimmune-mediated strategy for prophylactic breast cancer vaccination. Nat Med. 2010;16(7):799–803. doi: 10.1038/nm.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.