Abstract
Proteins that are imported from the cytosol into mitochondria cross the mitochondrial membranes in an unfolded conformation and then fold in the matrix. Some of these proteins require the chaperonin hsp60 for folding. To test whether hsp60 is required for the folding of all imported matrix proteins, we monitored the folding of four monomeric proteins after import into mitochondria from wild-type yeast or from a mutant strain in which hsp60 had been inactivated. The four precursors included two authentic matrix proteins (rhodanese and the mitochondrial cyclophilin Cpr3p) and two artificial precursors (matrix-targeted variants of dihydrofolate reductase and barnase). Only rhodanese formed a tight complex with hsp60 and required hsp60 for folding. The three other proteins folded efficiently without, and showed no detectable binding to, hsp60. Thus, the mitochondrial chaperonin system is not essential for the folding of all matrix proteins. These data agree well with earlier in vitro studies, which had demonstrated that only a subset of proteins require chaperones for efficient folding.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker J., Craig E. A. Heat-shock proteins as molecular chaperones. Eur J Biochem. 1994 Jan 15;219(1-2):11–23. doi: 10.1007/978-3-642-79502-2_2. [DOI] [PubMed] [Google Scholar]
- Blond-Elguindi S., Cwirla S. E., Dower W. J., Lipshutz R. J., Sprang S. R., Sambrook J. F., Gething M. J. Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell. 1993 Nov 19;75(4):717–728. doi: 10.1016/0092-8674(93)90492-9. [DOI] [PubMed] [Google Scholar]
- Bolliger L., Deloche O., Glick B. S., Georgopoulos C., Jenö P., Kronidou N., Horst M., Morishima N., Schatz G. A mitochondrial homolog of bacterial GrpE interacts with mitochondrial hsp70 and is essential for viability. EMBO J. 1994 Apr 15;13(8):1998–2006. doi: 10.1002/j.1460-2075.1994.tb06469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M. Y., Hartl F. U., Martin J., Pollock R. A., Kalousek F., Neupert W., Hallberg E. M., Hallberg R. L., Horwich A. L. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 1989 Feb 16;337(6208):620–625. doi: 10.1038/337620a0. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Kramer J., Kosic-Smithers J. SSC1, a member of the 70-kDa heat shock protein multigene family of Saccharomyces cerevisiae, is essential for growth. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4156–4160. doi: 10.1073/pnas.84.12.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. S., Becker A., Heitman J., Hall M. N., Brennan M. B. A yeast cyclophilin gene essential for lactate metabolism at high temperature. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11169–11173. doi: 10.1073/pnas.89.23.11169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson C. M., Evans P. A., Radford S. E. Understanding how proteins fold: the lysozyme story so far. Trends Biochem Sci. 1994 Jan;19(1):31–37. doi: 10.1016/0968-0004(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Ellis R. J. Molecular chaperones: the plant connection. Science. 1990 Nov 16;250(4983):954–959. doi: 10.1126/science.250.4983.954. [DOI] [PubMed] [Google Scholar]
- Fersht A. R. The sixth Datta Lecture. Protein folding and stability: the pathway of folding of barnase. FEBS Lett. 1993 Jun 28;325(1-2):5–16. doi: 10.1016/0014-5793(93)81405-o. [DOI] [PubMed] [Google Scholar]
- Flynn G. C., Pohl J., Flocco M. T., Rothman J. E. Peptide-binding specificity of the molecular chaperone BiP. Nature. 1991 Oct 24;353(6346):726–730. doi: 10.1038/353726a0. [DOI] [PubMed] [Google Scholar]
- Gambill B. D., Voos W., Kang P. J., Miao B., Langer T., Craig E. A., Pfanner N. A dual role for mitochondrial heat shock protein 70 in membrane translocation of preproteins. J Cell Biol. 1993 Oct;123(1):109–117. doi: 10.1083/jcb.123.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B. S., Brandt A., Cunningham K., Müller S., Hallberg R. L., Schatz G. Cytochromes c1 and b2 are sorted to the intermembrane space of yeast mitochondria by a stop-transfer mechanism. Cell. 1992 May 29;69(5):809–822. doi: 10.1016/0092-8674(92)90292-k. [DOI] [PubMed] [Google Scholar]
- Glick B. S. Can Hsp70 proteins act as force-generating motors? Cell. 1995 Jan 13;80(1):11–14. doi: 10.1016/0092-8674(95)90444-1. [DOI] [PubMed] [Google Scholar]
- Glick B. S., Wachter C., Reid G. A., Schatz G. Import of cytochrome b2 to the mitochondrial intermembrane space: the tightly folded heme-binding domain makes import dependent upon matrix ATP. Protein Sci. 1993 Nov;2(11):1901–1917. doi: 10.1002/pro.5560021112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray T. E., Fersht A. R. Refolding of barnase in the presence of GroE. J Mol Biol. 1993 Aug 20;232(4):1197–1207. doi: 10.1006/jmbi.1993.1471. [DOI] [PubMed] [Google Scholar]
- Haid A., Suissa M. Immunochemical identification of membrane proteins after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Methods Enzymol. 1983;96:192–205. doi: 10.1016/s0076-6879(83)96017-2. [DOI] [PubMed] [Google Scholar]
- Hallberg E. M., Shu Y., Hallberg R. L. Loss of mitochondrial hsp60 function: nonequivalent effects on matrix-targeted and intermembrane-targeted proteins. Mol Cell Biol. 1993 May;13(5):3050–3057. doi: 10.1128/mcb.13.5.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannavy K., Rospert S., Schatz G. Protein import into mitochondria: a paradigm for the translocation of polypeptides across membranes. Curr Opin Cell Biol. 1993 Aug;5(4):694–700. doi: 10.1016/0955-0674(93)90142-d. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Hlodan R., Langer T. Molecular chaperones in protein folding: the art of avoiding sticky situations. Trends Biochem Sci. 1994 Jan;19(1):20–25. doi: 10.1016/0968-0004(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Heitman J., Movva N. R., Hiestand P. C., Hall M. N. FK 506-binding protein proline rotamase is a target for the immunosuppressive agent FK 506 in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1948–1952. doi: 10.1073/pnas.88.5.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz P. M., Butler M. Interactive intermediates are formed during the urea unfolding of rhodanese. J Biol Chem. 1993 Feb 5;268(4):2500–2504. [PubMed] [Google Scholar]
- Horwich A. L., Low K. B., Fenton W. A., Hirshfield I. N., Furtak K. Folding in vivo of bacterial cytoplasmic proteins: role of GroEL. Cell. 1993 Sep 10;74(5):909–917. doi: 10.1016/0092-8674(93)90470-b. [DOI] [PubMed] [Google Scholar]
- Hurt E. C., Goldschmidt-Clermont M., Pesold-Hurt B., Rochaix J. D., Schatz G. A mitochondrial presequence can transport a chloroplast-encoded protein into yeast mitochondria. J Biol Chem. 1986 Sep 5;261(25):11440–11443. [PubMed] [Google Scholar]
- Höhfeld J., Hartl F. U. Role of the chaperonin cofactor Hsp10 in protein folding and sorting in yeast mitochondria. J Cell Biol. 1994 Jul;126(2):305–315. doi: 10.1083/jcb.126.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda E., Yoshida S., Mitsuzawa H., Uno I., Toh-e A. YGE1 is a yeast homologue of Escherichia coli grpE and is required for maintenance of mitochondrial functions. FEBS Lett. 1994 Feb 21;339(3):265–268. doi: 10.1016/0014-5793(94)80428-1. [DOI] [PubMed] [Google Scholar]
- Jaenicke R. Folding and association of proteins. Prog Biophys Mol Biol. 1987;49(2-3):117–237. doi: 10.1016/0079-6107(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Kang P. J., Ostermann J., Shilling J., Neupert W., Craig E. A., Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990 Nov 8;348(6297):137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Koll H., Guiard B., Rassow J., Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Antifolding activity of hsp60 couples protein import into the mitochondrial matrix with export to the intermembrane space. Cell. 1992 Mar 20;68(6):1163–1175. doi: 10.1016/0092-8674(92)90086-r. [DOI] [PubMed] [Google Scholar]
- Kronidou N. G., Oppliger W., Bolliger L., Hannavy K., Glick B. S., Schatz G., Horst M. Dynamic interaction between Isp45 and mitochondrial hsp70 in the protein import system of the yeast mitochondrial inner membrane. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12818–12822. doi: 10.1073/pnas.91.26.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübrich M., Dietmeier K., Pfanner N. Genetic and biochemical dissection of the mitochondrial protein-import machinery. Curr Genet. 1995 Apr;27(5):393–403. doi: 10.1007/BF00311207. [DOI] [PubMed] [Google Scholar]
- Laloraya S., Gambill B. D., Craig E. A. A role for a eukaryotic GrpE-related protein, Mge1p, in protein translocation. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6481–6485. doi: 10.1073/pnas.91.14.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry S. J., Jordan R., McMacken R., Gierasch L. M. Different conformations for the same polypeptide bound to chaperones DnaK and GroEL. Nature. 1992 Jan 30;355(6359):455–457. doi: 10.1038/355455a0. [DOI] [PubMed] [Google Scholar]
- Liberek K., Marszalek J., Ang D., Georgopoulos C., Zylicz M. Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2874–2878. doi: 10.1073/pnas.88.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Todd M. J., Viitanen P. V. Chaperonins and protein folding: unity and disunity of mechanisms. Philos Trans R Soc Lond B Biol Sci. 1993 Mar 29;339(1289):297–304. doi: 10.1098/rstb.1993.0028. [DOI] [PubMed] [Google Scholar]
- Manning-Krieg U. C., Scherer P. E., Schatz G. Sequential action of mitochondrial chaperones in protein import into the matrix. EMBO J. 1991 Nov;10(11):3273–3280. doi: 10.1002/j.1460-2075.1991.tb04891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J., Langer T., Boteva R., Schramel A., Horwich A. L., Hartl F. U. Chaperonin-mediated protein folding at the surface of groEL through a 'molten globule'-like intermediate. Nature. 1991 Jul 4;352(6330):36–42. doi: 10.1038/352036a0. [DOI] [PubMed] [Google Scholar]
- Matouschek A., Kellis J. T., Jr, Serrano L., Bycroft M., Fersht A. R. Transient folding intermediates characterized by protein engineering. Nature. 1990 Aug 2;346(6283):440–445. doi: 10.1038/346440a0. [DOI] [PubMed] [Google Scholar]
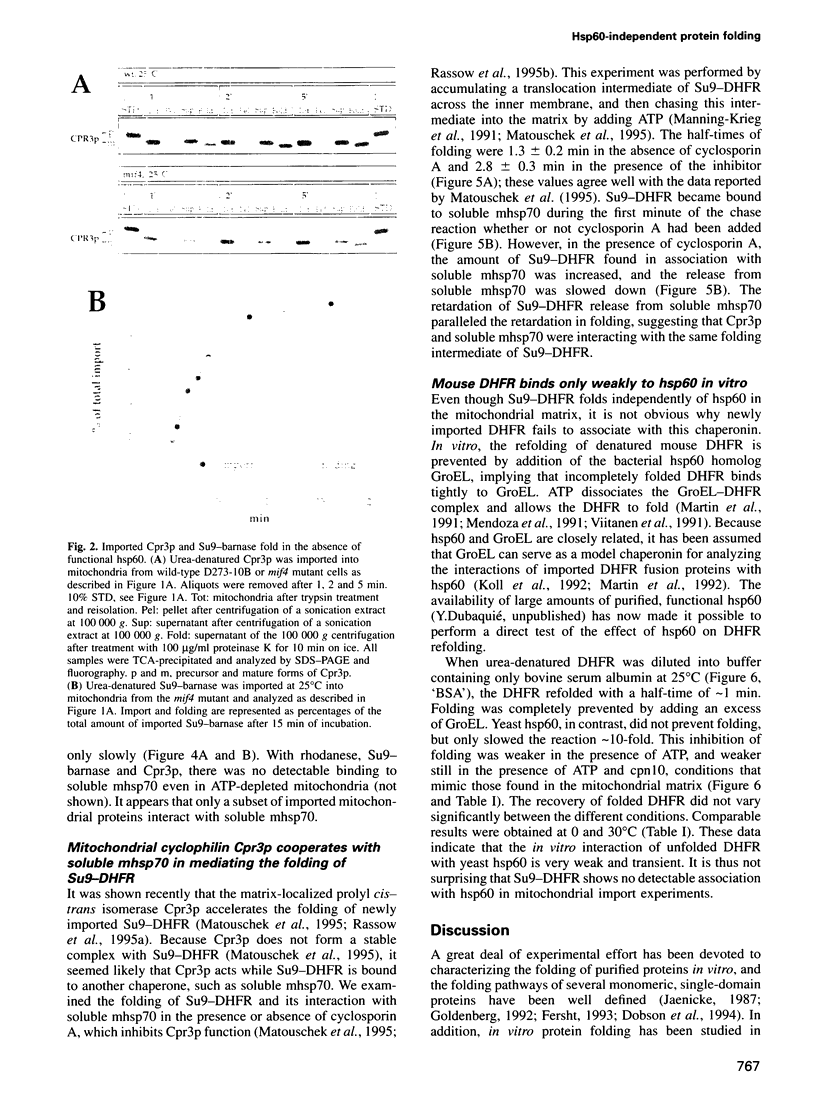
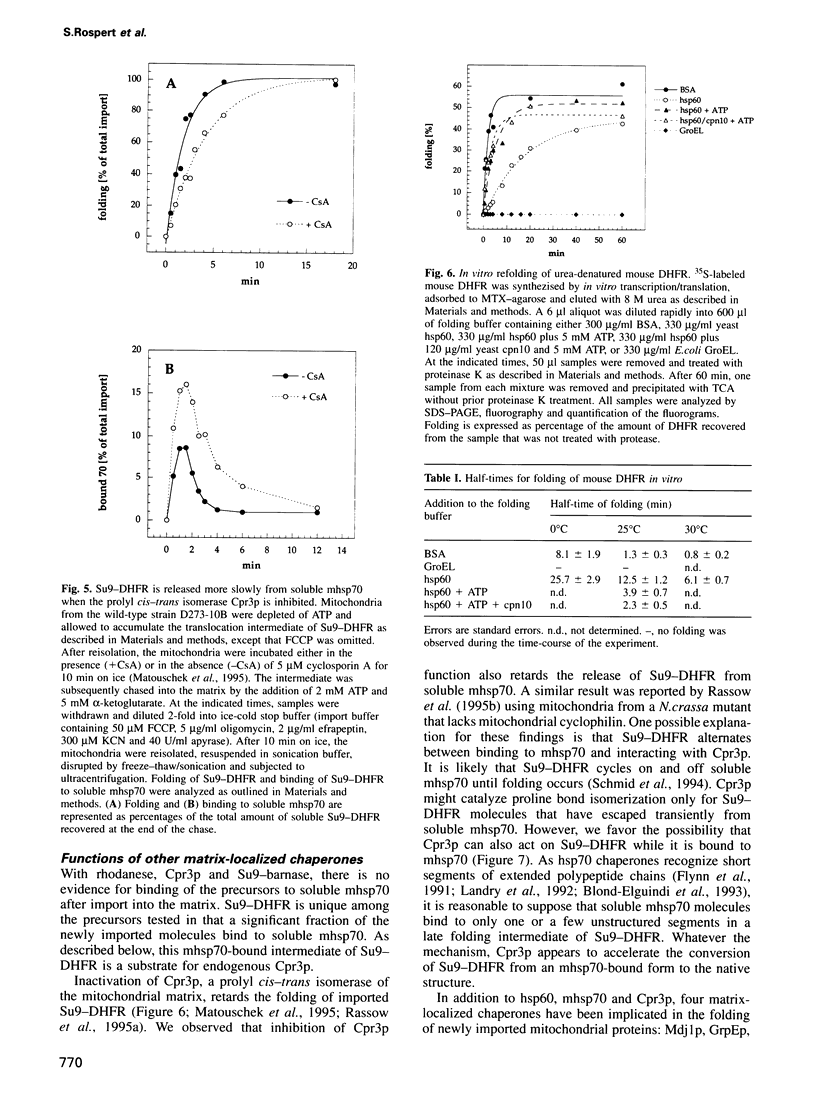
- Matouschek A., Rospert S., Schmid K., Glick B. S., Schatz G. Cyclophilin catalyzes protein folding in yeast mitochondria. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6319–6323. doi: 10.1073/pnas.92.14.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza J. A., Rogers E., Lorimer G. H., Horowitz P. M. Chaperonins facilitate the in vitro folding of monomeric mitochondrial rhodanese. J Biol Chem. 1991 Jul 15;266(20):13044–13049. [PubMed] [Google Scholar]
- Miller D. M., Kurzban G. P., Mendoza J. A., Chirgwin J. M., Hardies S. C., Horowitz P. M. Recombinant bovine rhodanese: purification and comparison with bovine liver rhodanese. Biochim Biophys Acta. 1992 Jun 24;1121(3):286–292. doi: 10.1016/0167-4838(92)90158-a. [DOI] [PubMed] [Google Scholar]
- Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989 Sep 14;341(6238):125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Müller H. K., Harmey M. A., Neupert W. Mitochondrial protein import: involvement of the mature part of a cleavable precursor protein in the binding to receptor sites. EMBO J. 1987 Nov;6(11):3449–3454. doi: 10.1002/j.1460-2075.1987.tb02668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassow J., Maarse A. C., Krainer E., Kübrich M., Müller H., Meijer M., Craig E. A., Pfanner N. Mitochondrial protein import: biochemical and genetic evidence for interaction of matrix hsp70 and the inner membrane protein MIM44. J Cell Biol. 1994 Dec;127(6 Pt 1):1547–1556. doi: 10.1083/jcb.127.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassow J., Mohrs K., Koidl S., Barthelmess I. B., Pfanner N., Tropschug M. Cyclophilin 20 is involved in mitochondrial protein folding in cooperation with molecular chaperones Hsp70 and Hsp60. Mol Cell Biol. 1995 May;15(5):2654–2662. doi: 10.1128/mcb.15.5.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassow J., Voos W., Pfanner N. Partner proteins determine multiple functions of Hsp70. Trends Cell Biol. 1995 May;5(5):207–212. doi: 10.1016/s0962-8924(00)89001-7. [DOI] [PubMed] [Google Scholar]
- Rospert S., Glick B. S., Jenö P., Schatz G., Todd M. J., Lorimer G. H., Viitanen P. V. Identification and functional analysis of chaperonin 10, the groES homolog from yeast mitochondria. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10967–10971. doi: 10.1073/pnas.90.23.10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rospert S., Hallberg R. Interaction of HSP 60 with proteins imported into the mitochondrial matrix. Methods Enzymol. 1995;260:287–292. doi: 10.1016/0076-6879(95)60145-7. [DOI] [PubMed] [Google Scholar]
- Rospert S., Junne T., Glick B. S., Schatz G. Cloning and disruption of the gene encoding yeast mitochondrial chaperonin 10, the homolog of E. coli groES. FEBS Lett. 1993 Dec 13;335(3):358–360. doi: 10.1016/0014-5793(93)80419-u. [DOI] [PubMed] [Google Scholar]
- Rospert S., Müller S., Schatz G., Glick B. S. Fusion proteins containing the cytochrome b2 presequence are sorted to the mitochondrial intermembrane space independently of hsp60. J Biol Chem. 1994 Jun 24;269(25):17279–17288. [PubMed] [Google Scholar]
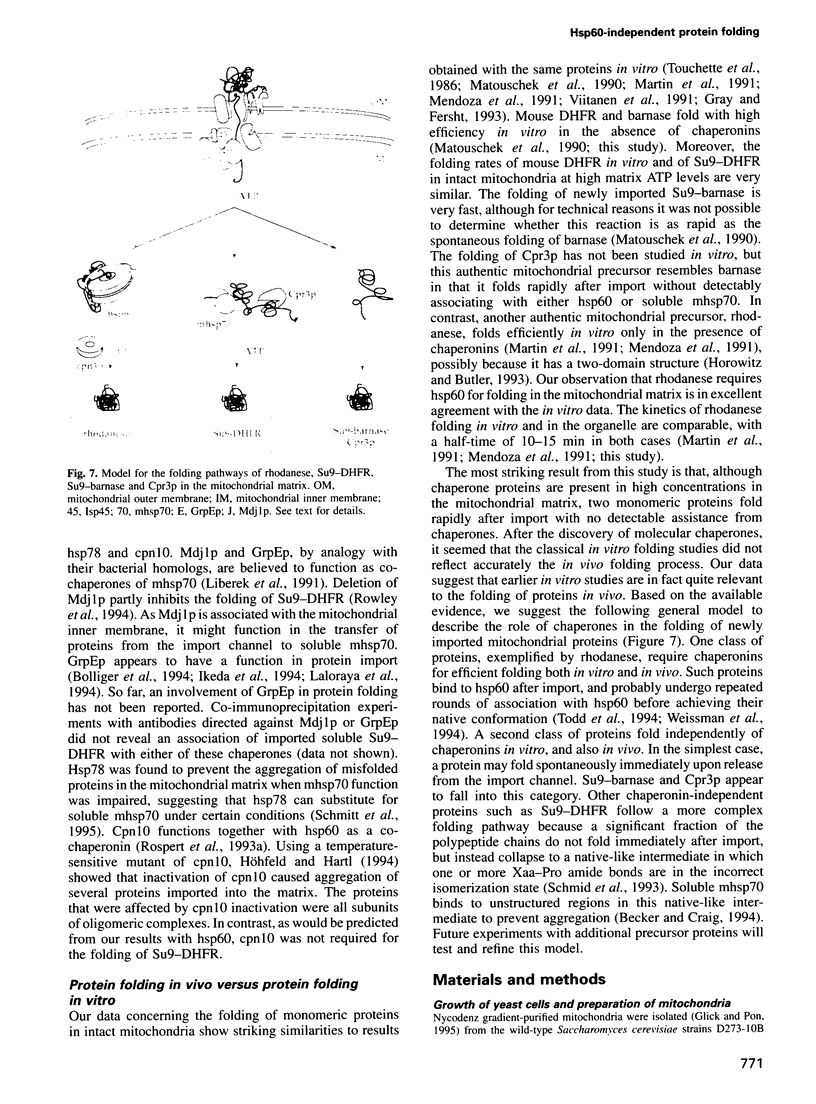
- Rowley N., Prip-Buus C., Westermann B., Brown C., Schwarz E., Barrell B., Neupert W. Mdj1p, a novel chaperone of the DnaJ family, is involved in mitochondrial biogenesis and protein folding. Cell. 1994 Apr 22;77(2):249–259. doi: 10.1016/0092-8674(94)90317-4. [DOI] [PubMed] [Google Scholar]
- Saijo T., Welch W. J., Tanaka K. Intramitochondrial folding and assembly of medium-chain acyl-CoA dehydrogenase (MCAD). Demonstration of impaired transfer of K304E-variant MCAD from its complex with hsp60 to the native tetramer. J Biol Chem. 1994 Feb 11;269(6):4401–4408. [PubMed] [Google Scholar]
- Schmid D., Baici A., Gehring H., Christen P. Kinetics of molecular chaperone action. Science. 1994 Feb 18;263(5149):971–973. doi: 10.1126/science.8310296. [DOI] [PubMed] [Google Scholar]
- Schmid F. X., Mayr L. M., Mücke M., Schönbrunner E. R. Prolyl isomerases: role in protein folding. Adv Protein Chem. 1993;44:25–66. doi: 10.1016/s0065-3233(08)60563-x. [DOI] [PubMed] [Google Scholar]
- Schmitt M., Neupert W., Langer T. Hsp78, a Clp homologue within mitochondria, can substitute for chaperone functions of mt-hsp70. EMBO J. 1995 Jul 17;14(14):3434–3444. doi: 10.1002/j.1460-2075.1995.tb07349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H. C., Berthold J., Bauer M. F., Dietmeier K., Guiard B., Brunner M., Neupert W. Mitochondrial Hsp70/MIM44 complex facilitates protein import. Nature. 1994 Oct 27;371(6500):768–774. doi: 10.1038/371768a0. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Stuart R. A., Cyr D. M., Craig E. A., Neupert W. Mitochondrial molecular chaperones: their role in protein translocation. Trends Biochem Sci. 1994 Feb;19(2):87–92. doi: 10.1016/0968-0004(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Stueber D., Ibrahimi I., Cutler D., Dobberstein B., Bujard H. A novel in vitro transcription-translation system: accurate and efficient synthesis of single proteins from cloned DNA sequences. EMBO J. 1984 Dec 20;3(13):3143–3148. doi: 10.1002/j.1460-2075.1984.tb02271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd M. J., Viitanen P. V., Lorimer G. H. Dynamics of the chaperonin ATPase cycle: implications for facilitated protein folding. Science. 1994 Jul 29;265(5172):659–666. doi: 10.1126/science.7913555. [DOI] [PubMed] [Google Scholar]
- Touchette N. A., Perry K. M., Matthews C. R. Folding of dihydrofolate reductase from Escherichia coli. Biochemistry. 1986 Sep 23;25(19):5445–5452. doi: 10.1021/bi00367a015. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Schatz G. Point mutations destabilizing a precursor protein enhance its post-translational import into mitochondria. EMBO J. 1988 Apr;7(4):1147–1151. doi: 10.1002/j.1460-2075.1988.tb02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viitanen P. V., Donaldson G. K., Lorimer G. H., Lubben T. H., Gatenby A. A. Complex interactions between the chaperonin 60 molecular chaperone and dihydrofolate reductase. Biochemistry. 1991 Oct 8;30(40):9716–9723. doi: 10.1021/bi00104a021. [DOI] [PubMed] [Google Scholar]
- Viitanen P. V., Gatenby A. A., Lorimer G. H. Purified chaperonin 60 (groEL) interacts with the nonnative states of a multitude of Escherichia coli proteins. Protein Sci. 1992 Mar;1(3):363–369. doi: 10.1002/pro.5560010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter C., Schatz G., Glick B. S. Protein import into mitochondria: the requirement for external ATP is precursor-specific whereas intramitochondrial ATP is universally needed for translocation into the matrix. Mol Biol Cell. 1994 Apr;5(4):465–474. doi: 10.1091/mbc.5.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman J. S., Kashi Y., Fenton W. A., Horwich A. L. GroEL-mediated protein folding proceeds by multiple rounds of binding and release of nonnative forms. Cell. 1994 Aug 26;78(4):693–702. doi: 10.1016/0092-8674(94)90533-9. [DOI] [PubMed] [Google Scholar]