Abstract
It has been validated that c-kit positive (c-kit+) cells in infarcted myocardium are from bone marrow (BM). Given the recent study that in the heart, estrogen receptor alpha (ERα) is involved in adaptive mechanisms by supporting cardiomyocytes survival via post-infarct cardiac c-kit+ cells, we tested a novel hypothesis that membrane ERα (mERа) supports survival of BM c-kit+ cells and enhance protective paracrine function for cardiac repair. Our data showed that myocardial infarction (MI) leads to an increase in c-kit+ first in bone marrow and then specifically within the infarcted myocardium. Also up-regulated mERа in post-infarct BM c-kit+ cells was found in day 3 post MI. In vitro co-culture system, mERа+ enhances the beneficial effects of BM c-kit+ cells by increasing their viability and reducing apoptosis. Post-infarct c-kit+ mERа+ cells population expresses predominant ERα and holds self-renewal as well as cardiac differentiation potentials after MI. In vivo, BM c-kit+ cells reduced infarct size, fibrosis and improved cardiac function. In conclusion, BM c-kit+ mERа+ exerted significantly cardiac protection after MI. A potential important implication of this study is that the manipulation of BM c-kit+ stem cells with ERа-dependent fashion may be helpful in recovering functional performance after cardiac tissue injury.
Keywords: Acute myocardial infarction, stem cells, estrogen receptor alpha, cardiac repair
Introduction
After acute myocardial infarction (AMI), heart function is impaired and cardiomyocytes in the AMI-effected region lose their functionality [1]. Bone marrow contains a varied assortment of stem cells with putative cardiac potential, opening the potential for the use of these cells in stem cell therapy [2]. Within the bone marrow, c-kit is the precursor to haemopoietic stem cells (HSCs) as well as the endothelial progenitor cells (EPCs), and is expressed on hemangioblasts. Emerging evidence suggests that BM c-kit+ cells can improve cardiac function in animals and humans after injury [3], however, definite identification of the cell types and the underling cellular mechanisms relevant to cardiomyogenesis are still unclear.
Estrogens are complex hormones with pleiotropic effects that alter target gene transcription in both reproductive and non-reproductive tissues including the cardiovascular system [4]. E2, as the most potent estrogen found in humans, has a wide range of effects on the heart, including preventing apoptosis and reducing damage after cardiac ischemia and reperfusion [5]. The biological effects of estrogen are mainly mediated by estrogen receptors (ER). Studies have shown that ERs, including ERα, ERβ, and GPR30, confer cardioprotective effects both in genomic and non-genomic mechanisms [6]. Patten et al. have shown that [7] acute E2 treatment reduces cardiomyocyte apoptosis and elicits cardioprotection via activation of PI3K/Akt signaling. Also the estrogen effects depend on the relative amount of ERα and ERβ in both blood vessels and myocardium. Many studies have shown that ERα, but not ERβ, is responsible for estrogen-mediated cardio-protection in rodent models [8]. However, most researches focused on estrogen-dependent ER modulation for cardiac repair.
After myocardial infarction, ERа can mediate contribution of Bone Marrow-derived Endothelial progenitor cells to functional recovery [9]. Even in the absence of estrogen, ERα-dependent activation, molecular regulation, and organ protection may occur [10]. Recent study has shown that many organs require membrane ERα to collaborate with nuclear ERα for normal development and function through specific signal transduction [11]. The previous study has showed that in response to acute myocardial infarction (MI), activation of ERα facilitates survival of adult cardiomyocytes through post-infarct cardiac c-kit+ cells. This may have a valid explanation for protective actions of ERα against cardiac injury [12]. Hence, it is possible that ERа mediate cell behaviors of BM c-kit+ cells through Estrogen-dependent way.
In this study, we tested a novel hypothesis that membrane ERα supports survival of BM c-kit+ cells and enhance protective cardiac repair function. We here provide evidence that post-infarct BM c-kit+ mERа+ cell population expresses predominant ERα expression and holds self-renewal, proliferation as well as cardiac differentiation potentials after acute ischemic injury. c-kit+ mERа+ cells are easily survived with low apoptosis in vitro and reduce the post-MI deterioration of cardiac function in vivo. These results have implication in promoting BM c-kit+ stem cells-mediated cardiac repair through ERа-dependent fashion after MI.
Materials and methods
Induction of myocardial infarction in adult mice
Myocardial infarction (MI) was induced in male C57BL/6 wild-type mice at 8-10 weeks of age (22-25 g) by coronary artery ligation as described previously [13]. Briefly, mice were anesthetized with gaseous isoflurane and then the left anterior descending coronary artery was tightened with a suture after thoracotomy. Sham-operated mouse underwent the same surgical procedure without coronary ligation.
In vitro isolation of post-infarcted bone marrow, spleen and myocyte cells
C57BL/6 mice were sacrificed, and their femurs and tibias were collected. Animals were perfused with phosphate buffered saline (PBS) under anaesthesia to remove blood from the heart. Cardiac cell populations were isolated using a Worthington neonatal cardiomyocyte isolation system [12]. Spleen was collected and spleen cells were isolated with the same way with Bone marrow cells.
The bones of each mouse were flushed with phosphate-buffered saline (PBS) containing 10% Fetal Bovine Serum (FBS, Lot. S1810, Biowest). Then, the resulting cell aggregates were filtered through a 40 µm cell strainer and resuspended in PBS. BM-MNCs were isolated using Ficoll Lymphocyte Separation Medium (Lot. L0560, Biowest). A portion of the BM-MNCs were resuspended in Iscove’s Modified Dulbecco’s Medium (IMDM, Lot. 12440-053, Life technology) containing 10% FBS. All of the bone marrow, cardiac and spleen cells were collected and resuspended for use.
Flow Cytometry (FCM) analysis
Mouse BM-MNCs, spleen cells and myocyte cells were washed using 0.5% Bovine Serum Albumin (BSA) in PBS containing 2mM EDTA (Staining Buffer) and blocked with 1% BSA in PBS. Cell pellets were incubated with phycoerythrin (PE)-conjugated c-kit anti-mouse antibodies (1:100, Cat. 130-097-978; BD); and Fluorescein isothiocyanate (FITC)-conjugated anti-CD45 (1:200; Cat.553079, BD), anti-mouse CD34 FITC (1:200; Cat.553079, BD), FITC-conjugated ERalpha antibodies (1:100, Milipore, 04-227) at 4°C for 30 minutes in the dark. Cells were then washed, re-suspended in PBS, and purity of the cells was determined via flow cytometric analysis using a BD Accuri C6 flow cytometer and the data subsequently analyzed using C-Flow Plus1.0 software.
Isolation and culture of post-infarct bone marrow c-kit+ membrane ERalpha (+) cells
Mouse BM-MNCs were washed and blocked similarly as that in FCM. Cells were resuspended and then incubated with phycoerythrin (PE)-conjugated c-kit anti-mouse antibodies (1:100, Cat. 130-097-978; BD) and FITC-conjugated ERalpha antibodies (1:100, Milipore, 04-227) at 4°C for 20 minutes. BM c-kit+, membrane ER alpha+ cells and BM c-kit+ membrane ER alpha- cells were selectively collected was sorted and selectively collected by BD FACS Canto.
Then, A portion of the BM cells were co-cultured with post-infarct myocytes (1000 cells/well) in 96-well plates, re-suspended in Iscove’s Modified Dulbecco’s Medium (IMDM, Lot. 12440-053, Life technology) containing 10% FBS. Then, cell culture was performed in 96-well culture plates for 2 days. All of the media contained 1% Penicillin Streptomycin (P/S, Lot. 1266328, Gibco). Cell behaviors were recorded by Nikon microscope.
Cell transplantation and immunohistochemistry (IHC) staining
After day 3 of MI, BM c-kit+ membrane ER alpha (mERа)– cells, BM c-kit+ mERа+ cells were isolated and suspended in IMDM culture medium. Immediately after MI injury, 1×105 donor BM cells suspended in 50 μL IMDM culture medium were injected through caudal vein.
After 7 days of AMI, the infarcted heart were collected as detailed in previous part and fixed. The heart was excised and cut into five 1-mm-thick transverse slices, parallel to the atrioventricular groove. Each slice was incubated in a 1% solution of TTC at 37°C for 15 minutes to differentiate infarct area (pale) from viable (brick red) myocardial area.
Formalin fixed hearts were processed, embedded in paraffin and cut as 5 μm-thick sections. After deparaffinization and blocking, Masson Trichrome staining, and Sirius red staining were done on the same series of heart sections.
Apoptotic myocytes were evaluated by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) kit (Roche) and counterstained with HE staining. The percentage of TUNEL positive cells was evaluated by viewing 500-700 cells in 10 randomly chosen fields from each well at ×10 magnification.
Immunofluorescence staining
Paraffin slides and cultured cells were fixed by 4% paraformaldehyde for 20 min at room temperature. Fixed cells were incubated with blocking buffer containing 3% BSA for 30 min, treated with Rabbit c-kit antibody (Santa Cruz, SC5535), mouse ERα antibody (Santa Cruz, sc-787) overnight at 4°C. Then FITC-conjugated Goat-anti-rabbit IgG secondary antibody (1:500, Chemicon) or TRITC-conjugated Goat-anti-mouse IgG (1:500, Gibico) were added for 1 h at 37 °C in a humidified atmosphere of 5% CO2, then washed three times with 1 ml PBS for 5 min. To stain the cell nuclei, the cells were incubated in 2 μg/mL Hoechst 33258, followed by rinsing with PBS. The images were recorded with a fluorescence microscope (Eclipse TE2000-E, Nikon).
Cell survival/apoptosis assay
After 1,3, 5,11 days co-culture, 4 kinds of cells(c-kit+ mERа+ cells, c-kit+ mERа- cells, c-kit- mERа+ cells, and c-kit- mERа- cells) were collected respectively and analyzed by annexin V/PI staining( Invitrogen, V13241) for cell survival and apoptosis. After staining a cell population with Alexa Fluor® 488 annexin V and PI in the provided binding buffer, apoptotic cells show green fluorescence, dead cells show red and green fluorescence, and live cells show little or no fluorescence. The rates of viable cells and apoptotic cells were determined via flow cytometric analysis using a BD Accuri C6 flow cytometer and the data subsequently analyzed using C-Flow Plus1.0 software.
Reverse transcription-polymerase chain reaction (RT-PCR) and real-time PCR
Total RNAs were extracted using TRIzol (RiboPure kit, Ambion, AM1924) following the manufacturer’s instruction. One microgram of total RNA per 20 μl of reaction volume was reverse transcribed into cDNA using the PrimeScript RT reagent Kit (Takara, RR037A). Real-time PCR reactions were performed and monitored using the SYBR Green PCR Mastermix and PCR apparatus (Takara, TP800). cDNA samples (2 μl for total volume of 25 μl per reaction) were analyzed for gene of interest and for the reference gene glyceraldehyde-3-phosphatedehydrogenase (GAPDH). The level of expression of each target gene was then calculated as previously described. The results were normalized to those of BM c-kit- ERalpha- cells. Each sample was repeated at least three times for each gene of interest. RT-PCR was performed at 95°C for 2 min followed by 34 cycles of 30 s denaturation at 95°C, 30 s annealing at the primer specific temperature, and 1 min elongation at 72°C. The PCR primers were synthesized by Shanghai Sangon Biotechnology co., LTD. The primers information is detailed in Supplementary Table 1.
Echocardiography analysis
Echocardiography was performed to determine cardiac structure and function in conscious mice. Hearts were viewed in the short-axis between the two papillary muscles and each measurement was obtained with M-mode by averaging results from three consecutive heart beats. Fractional shortening (%FS) was calculated as follows: %FS = (LVIDd – LVIDs)/LVIDd × 100, where LVIDd is diastolic LV internal diameter and LVIDs is systolic LV internal diameter. Left ventricular ejection fraction (EF) was automatically calculated by the echocardiography software according to the Teicholz formula. Parameters including LVIDd were measured to determine structural changes in cardiac morphology.
Statistical analysis
Results are expressed as mean ± SEM. The two-tailed Student’s unpaired t test was used to analyze two-group comparisons with the software Graphpad Prism 5. Differences were considered to be significant at *P values <0.05 for all procedures (*P values <0.05, **P values <0.01, *** P values <0.001).
Results
c-kit+ cells increase in infarcted myocardium and post-infarct BM cells
Acute myocardial infarction is associated with an increased metabolic activity and increased levels of progenitor cells within days after AMI. The extent of the apoptosis and myocardial infarction was first determined by TUNEL staining of paraffinembedded sections as shown in Supplementary Figure 1. TUNEL-positive cardiomyocytes were clearly detected in the infarcted mouse heart. TUNEL+ cardiomyocyte nucleus number was moderately increased in infarcted hearts and more significantly increased in hearts at day-7 post-AMI. These results indicate that apoptotic cardiomyocyte death is essentially taking place in the early stage of AMI. Apoptosis is thus suggested to be involved in the pathophysiology of heart failure. That is to say, in order to restore the cardiac functions post-AMI, the cell behaviors in early stage of AMI (1-7 days) is more important.
It has been validated that c-kit+ cells in infarcted myocardium are from bone marrow [14]. However, the time point when BM cells started to migrate to infarcted myocardium are little evaluated. In this study, the dynamic expression of c-kit in BM, heart and spleen in day-1, day-3, day-5, day-7, day-11, and day-21 after AMI was stated in Figure 1. In general, the time evolution of c-kit+ cells rate are different among BM, heart and spleen cells over different days after AMI. ~3% expressed c-kit in spleen cells and kept alomost unchanged along with the time. While, in early stage of AMI (1 days, 3days), c-kit expression was up-regulated in BM cells, and then slightly decreased from 5-days to 11 days, finally a little increased in day-21 after AMI. As shwon in Figure 2, the percentage of c-kit+ cells in BM cells was as follows: day-1 (5.8±0.45%), day-3 (14.8±1.5%), day-5 (12.11±0.32%), day-7 (10.91±1.25%), day-11 (7.59±0.82%), day-21 (10.77%±1.8%) respectively. In heart cells, the percentage of c-kit+ cells consistently increases in the first week after AMI, while at later time, the c-kit expression decreased over time. As shown in Figure 2, the frequency of c-kit+ cells in post-infarcted myocytes was: day-1 (6.5±0.66%), day-3 (8.6±0.7%), day-5 (12.4±0.6%), day7 (20.33±1.6%), day-11 (15.56±1.0%), day-21 (10.1±1.1%) respectively. As is shown above, MI leads to an increase in c-kit+ first in bone marrow and then specifically within the infarcted myocardium, and BM c-kit+ cells is possible to paly the cardiac repair functions in the time before 7 days post-AMI.
Figure 1.
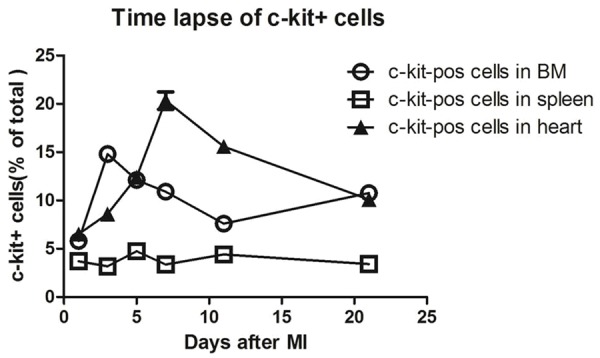
The time lapse of c-kit-positive cells in mouse BM, spleen and heart after MI. Quantification of the number of c-kit+ cells over a time course after MI in wild-type mice showed that MI leads to an increase in c-kit+ first in bone marrow and then specifically within the infarcted myocardium.
Figure 2.
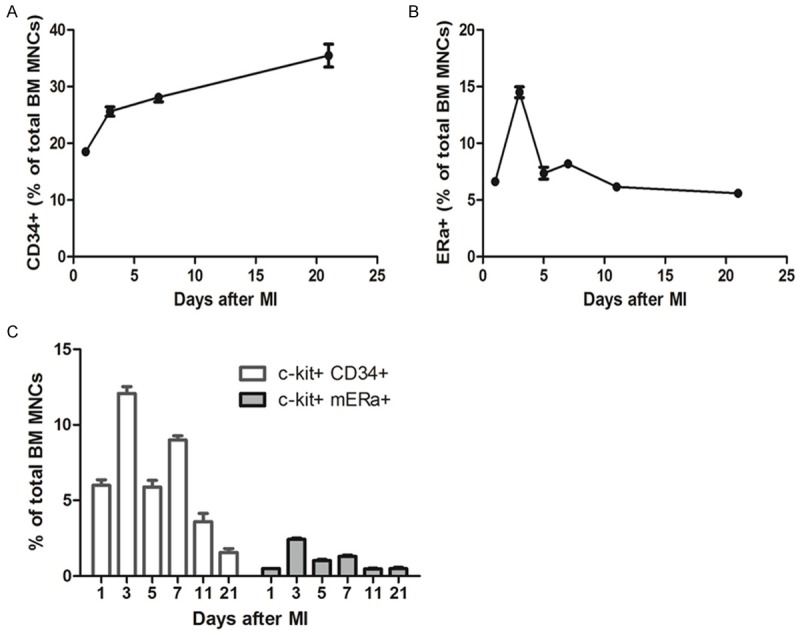
FCM analysis for BM cells showed that double positive of c-kit and membrane ER alpha (mERа) is a new portion of BM stem cells. A. CD34+ cells in BM MNCs after different days of AMI. B. mERа+ cells in BM MNCs after different days of AMI. C. Quantification of c-kit+ CD34+ cells and c-kit+ mERа+ in total BM MNCs after different days post MI. The ratios show that after day 3 post MI, the double positive of c-kit and mERа is more than other days.
Up-regulated membrane ER alpha (mERа) in post-infarct BM c-kit+ cells
Given the recent study that ERα-involved adaptive mechanisms in the heart by supporting cardiomyocytes survival via post-infarct cardiac c-kit+ cells [12], it is also possible that ER may be involved in regulating the BM c-kit+ cells performance. According to literature data, cardiac c-kit+ cells were also positive for CD105 and a majority of them was positive for CD31 and CD34 (83.7±8.6 and 75.7±11.4%, respectively) [15]. In this study, we first evaluated the kinetics of CD34, mERа expression in BM cells and BM c-kit+ cells over time evolution. As shown in Figure 2, hematopoietic CD34+ rate increased gradually in BM cells along with the days post-AMI; while mERа expression levels increased dramatically from day-1 (6.6%±0.4) to day 3 (14.5±0.82%) post-AMI, over the period from day 5 to day 21, the mERа remained level at ~5% in BM cells. It is interesting to note that, mERа accounted for not a great deal of difference between BM cells and BM c-kit+ cells. FCM analysis for BM cells (Figure 2C) show that double positive of membrane ER alpha (mERа) and c-kit is a new portion of BM stem cells. Moreover, the double positive cells collected from the mouse BM in early stage of AMI (especially day-3 post AMI) were better than that from the later time. The existence of double positive c-kit and mERа cells in BM-MNCs was confirmed by immunofluorescence staining (Supplementary Figure 2). These results show that double positive c-kit+ mERа+ cells isolated from day-3 post-MI mouse will be capable of functional BM stem cell sub-population.
c-kit+ mERа+ cells are easily survived with low apoptosis in vitro
A major obstacle associated with adult BM stem cells expansion is the loss of “stemness”, or regenerative capacity, of freshly isolated cells, presumably due to the absence of the native cellular niches [16]. In this study, after loading the initially sorted c-kit+ mERа+ cells, c-kit+ mERа- cells, c-kit- mERа+ cells, and c-kit- mERа- cells onto in vitro co-culture system with post-infarct myocardium, the effects of c-kit and mERа on the cells behavior, survival, and apoptosis were studied.
Figure 3 shows the time course of changes in cells morphologies when co-cultured with post-infarct myocardium in vitro. After 1-day in culture, the cells kept round and suspended in c-kit+ mERа+ cells, c-kit+ mERа- cells, c-kit- mERа+ cells; however, in c-kit- mERа- cells, cell adhesion was obvious. After 3-days in culture, the cell clusters were only found in c-kit+ mERа+ cells. In c-kit+ mERа- cells, most of the survived cells were attached to the cell culture plate. After 5-days in culture, cell proliferation without no obvious adhesion and spreading was still found in c-kit+ mERа+ cells, while cells spreading are found in other 3 kinds of cells. During additional time in culture, cell apoptosis without obvious cell proliferation were found in c-kit+ mERа- cells, c-kit- mERа+ cells, and c-kit- mERа- cells. In contrast, c-kit+ mERа+ cells has been reached over 70% confluency and passaged successfully.
Figure 3.
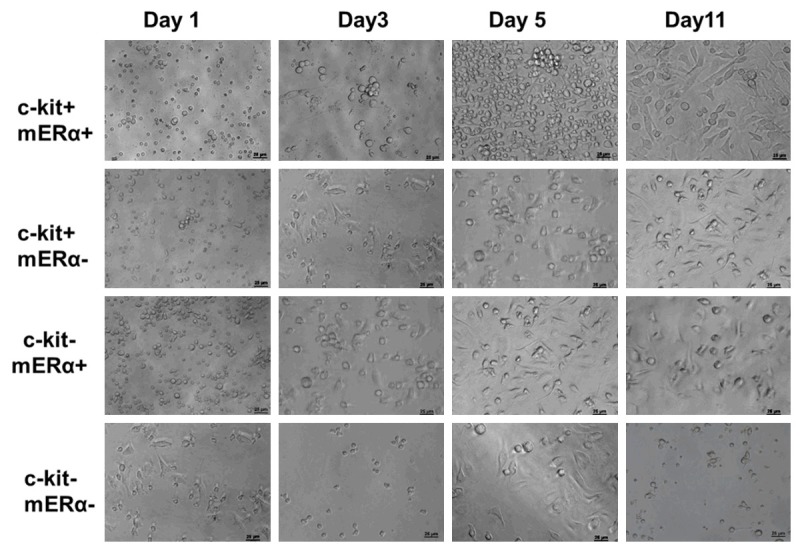
Time evolution of 4 kinds of BM cell portions co-cultured with post-infarct mouse adult myocytes: c-kit+ mERа+ cells, c-kit+ mERа- cells, c-kit- mERа+ cells, and c-kit- mERа- cells. All of the cells were collected from 1 day after AMI mouse. The proliferation of c-kit+ mERа+ cells is highly obvious, while c-kit- mERа- cells failed to proliferate in vitro. Scale bar =50 μm.
After 1, 3, 5, 11 days co-culture, 4 kinds of cells were collected respectively and analyzed by annexin V/PI staining for cell survival and apoptosis. As shown in Figure 4A, the viable BM cells ratio were deceased over the culture time in c-kit+ mERа- cells, c-kit- mERа+ cells, and c-kit- mERа- cells, except in c-kit+ mERа+ cells. The survived cells ratio is 82.46±3.23%, 66.83±2.74%, 82.67±3.0%, and 75.33±3.0%% after 1, 3, 5, 11 days culture respectively. Meanwhile, the cell apoptosis rate increased in c-kit+ mERа- cells, c-kit- mERа+ cells, and c-kit- mERа-cells over culture time. After 11-days culture, apoptotic cells accounted for more than 50%; while cell apoptosis was kept steady in double positive c-kit+ mERа+ cells and lower than 20% as shown Figure 4B. Because of high survival and low apoptosis characteristics of the special cells population in vitro, it is assumed that BM c-kit+ mERа+ stem cells will be new seeds cells for cardiac repair after AMI.
Figure 4.
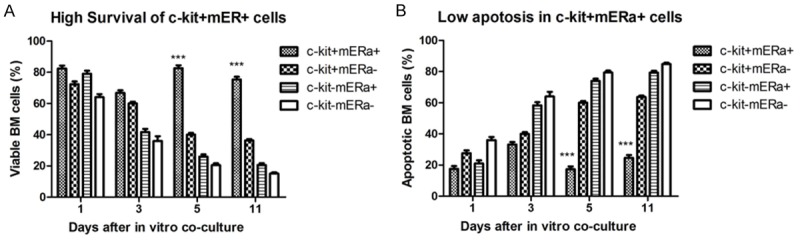
Cell survival and apoptosis analysis for 4 kinds of BM cells (c-kit+ mERа+ cells, c-kit+ mERа- cells, c-kit- mERа+ cells, and c-kit- mERа- cells) in different culture time. c-kit+ mERа+ cells are easily survived with low apoptosis, which indicated that this special portion of BM c-kit+ stem cells will be new candidate seeds cells for cardiac repair after MI.
Post-infarct BM c-kit+ mERа+ cells population with ERα predominance and self-renewal/differentiation potentials
To determine whether the dynamic morphological differences in c-kit+ mERа+ cells, c-kit+ mERа- cells, c-kit- mERа+ cells, and c-kit- mERа- cells corresponded to changes in the patterns of gene or protein expression, we firstly examined ERα and ERβ mRNA expression of these 4 kinds of BM cells after 5 days culture, ERα mRNA was up-regulated 2.8 folds in BM c-kit+ mERа+ cells, compared to BM c-kit- mERа- cells, but ERβ mRNA remained unaltered in BM c-kit+ mERа+ cells population as shown by real-time PCR analysis (Figure 5A).
Figure 5.
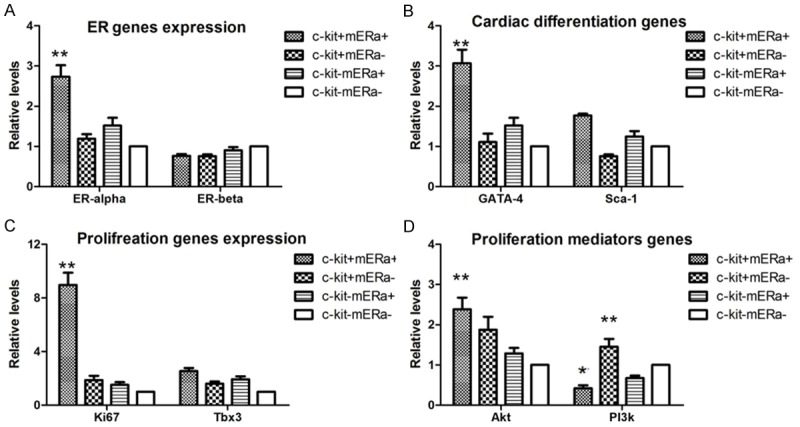
Post-infarct BM c-kit+ mERа+ cells population with ERα predominance and self-renewal/differentiation potentials. Gene expression of ERs, self-renewal and differentiation markers in 4 kinds of BM cells (c-kit+ mERа+ cells, c-kit+ mERа- cells, c-kit- mERа+ cells, and c-kit- mERа- cells) after 5 days culture were analyzed by real-time PCR. A. ERα and ERβ. B. GATA-4 and Sca-1 (transcription factors for cardiacdifferentiation). C. Tbx3 and ki67 (transcription factors for self-renewal and proliferation). D. Akt and Notch 1 (mediators of proliferation and survival). Expression levels were normalized to the expression of β-actin housekeeping gene. n=3; *P<0.05, **P<0.01 vs. c-kit- mERа- cell population.
Furthermore, we evaluated the regulation of transcription factors implicated in cardiogenic fate decision of mesodermal cells (GATA-4, Sca-1) and genes required for survival and proliferation (Ki67, Tbx3, Akt, Notch1) in ex vivo post-infarct c-kit+ mERа+ cells, c-kit+ mERа- cells, c-kit- mERа+ cells, in comparison to c-kit- mERа- cells. As shown in Figure 5B, expression of GATA4, a critical transcription factor for proper mammalian cardiac development was up-regulated in all 3 kinds of cells, significantly induced 3.1-fold in c-kit+ mERа+ cells, compared to BM c-kit- mERа- cells. Expression of Sca-1 was slightly up-regulated on c-kit+ mERа+, c-kit- mERа+ cells, while showed slight down-regulation on c-kit+ mERа- cells.
Figure 5C and 5D shows the expression of Ki67, Tbx3, and Akt was significantly induced 9.5-fold, 2.8-fold, and 2.5-fold, respectively, in these post-infarct c-kit+ mERа+ cells, with the exception of Notch 1, which remained unchanged. These results demonstrated that c-kit+ mERа+ cells can favor cell survival and decrease cells apoptosis.
These data provided ex vivo data that post-infarct c-kit+ mERа+ cells population express predominant ERα and holds self-renewal as well as cardiac differentiation potentials after acute ischemic injury.
Effects of transplantation post-infarct BM c-kit+ mERа+ cells on myocardial infarct size and cardiac function
The extent of the myocardial infarction and fibrosis were evaluated at day 7 after cell transplantation using TTC staining, Masson’s trichrome staining and Sirus red staining (Figure 6). A reduction in infarct area was noted among mice treated with c-kit+ mERа+ cells as compared to PBS and c-kit+ cell alone. Masson’s trichrome staining showed that there were increased islands of viable cardiac muscle in the peri-infarct regions at day 7 after cell transplantation. The fibrotic area reduced in BM cells treated hearts, and BM c-kit+ mERа+ cells further reduced it from both Masson’s trichrome staining and Sirius Red staining results.
Figure 6.
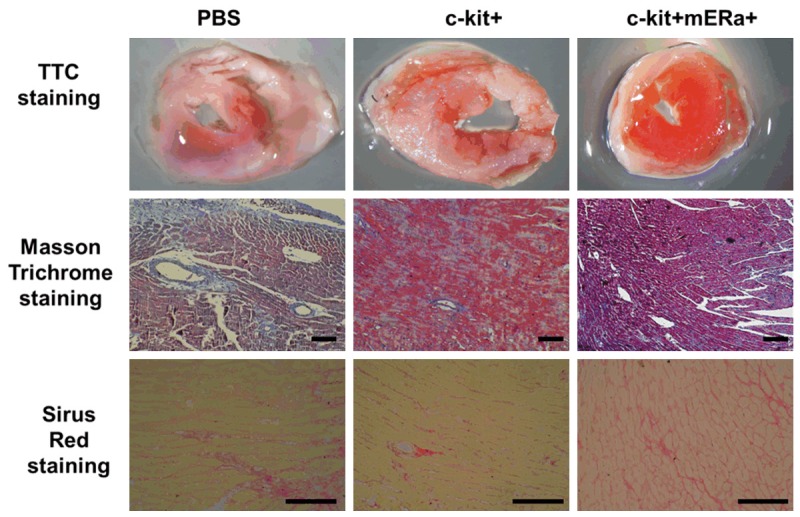
Transplantation of BM c-kit+ mERа+ cells reduces infarct size and fibrosis. A. Representative images of TTC-stained heart sections obtained from MI+PBS, MI+BM c-kit+ cells, and MI+ c-kit+ mERа+ cells groups at day 7 after transplantation. B. Representative images of fibrotic area in infarct border zone by Masson’s trichrome staining at day 7 post-MI. scale bar =50 μm. C. Collagen-specific Sirius Red stained heart sections obtained from MI+PBS, MI+BM c-kit+ cells, and MI+ c-kit+ mERа+ cells groups at day 7 after transplantation. Scale bar =50 μm.
Cardiac function was assessed using echocardiography at 21 d after induction of myocardial infarction and cell transplantation (Figure 7). Left ventricle rational shortening (FS) and ejection fraction (EF) were greater in post-MI hearts at 3 weeks treated with BM c-kit+ mERа+ cells than in post-MI hearts treated with BM c-kit+ cells or PBS. Moreover, BM c-kit+ mERа+ cells significantly limited the increase of diastolic left ventricle internal diameter (LVIDd) induced by MI, comparing with BM c-kit+ cells or PBS. These results show that BM c-kit+ cells with mERа reduce the post-MI deterioration of cardiac function, and this cardioprotective effect is significantly greater than that of BM c-kit+ without mERа.
Figure 7.
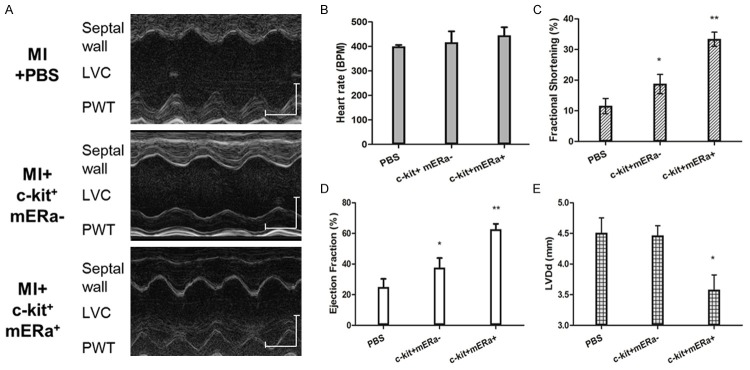
Transplantation of BM c-kit+ mERа+ cells improved improves cardiac function as comparison to BM c-kit+ cells or PBS. (A) Representative M-mode images showing cardiac function in each groups: hearts with MI at 3 weeks after PBS, BM c-kit+ cells and BM c-kit+ mERа+ cells injection. Scale bar: X axis: 0.1 second; Y axis: 0.2 cm. (B) Heart rates were controlled to be similar in different groups. (C-E) Fraction Shortening (C), Ejection Fraction (D) and LVIDd (E) at 3 weeks after cell transplantation. n=6 for each group. *P<0.05 versus MI+PBS group; **P<0.05 versus MI+PBS group.
Discussions
Numerous Bone marrow-derived cell populations, including unselected bone marrow-derived mononuclear Cells (BM-MNCs) and selected BM-MNCs (eg. CD34+ cells) have been suggested to enhance cardiac repair in experimental animal models [17]. Emerging evidence suggests that BM c-kit+ cells can improve cardiac function in animals and humans after injury [3], however, definite identification of the cell types and the underling cellular mechanisms relevant to cardiomyogenesis are still unclear. A recent study has suggested that cardiac ERα supports survival of cardiomyocytes through post-infarct cardiac c-kit+ cells [12]. In this study, we tested a novel hypothesis that membrane ERα supports survival of BM c-kit+ cells and enhance protective cardiac repair.
Apoptosis has been implicated as the main contributor to the massive loss of donor cells [18]. Also, Apoptosis is recognized to participate in the pathophysiology of various cardiac diseases in adults including ischemia-reperfusion, myocarditis, cardiomyopathy [19]. In our study, we are now trying to understand the cellular mechanisms of heart failure difference by using animal models of myocardial infarction in adult mice. The results showed that mouse at day-7 post-AMI exhibit a higher incidence of cardiomyocyte apoptosis than sham group and other post-AMI mouse. This implies that period of acute myocardial infarction (AMI) is more susceptible for apoptosis than those with other periods. In other words, in order to restore the cardiac functions post-AMI, the cell behaviors in early stage of AMI (1-7 days) is more important.
There is little study about the time evolution of the influence of AMI on the progenitor cells residing in the BM, heart, and Spleen. Our data suggest that AMI increases the number of c-kit+ cells in BM, and heart within 7 days, while the c-kit+ cells in spleen was kept almost unchanged along with the time. The results are consistent with before [14] and confirmed that MI leads to an increase in c-kit+ first in bone marrow ane then specifically within the infarcted myocardium, and BM c-kit+ cells is possible to paly the cardiac repair functions in the time before 7 days post-AMI.
Although various studies have been confirmed that AMI stimulate various types of BM-drevided cells mobilization, the influence of AMI on the specified progenitor cells residing in the BM has not been clearly evaluated. In this study, CD34, hematopoietic and vascular-associated markers, and membrane ERα expressions were evaluated in mouse BM post-AMI. We observed that although hematopoietic CD34+ rate increased gradually in BM cells along with the days post-AMI, CD34 accounted for the overall decrease tendency in BM c-kit+ cells. The results is different from previous study in human that circulating CD34+ cell levels significantly increase in patients with AMI, peaking around 7 days after ischaemic injury [20]. In contrast, mERа accounted for not a great deal of difference between BM cells and BM c-kit+ cells. The percentage of mERа+ cells in BM cells and BM c-kit+ cells upregulated in mouse post-AMI, peaking around 3 day after AMI. In this study, we further sorted a new BM derived cell subtype with double positive of membrane ER alpha (mERа) and c-kit. The identification of mERа on BM cells was confirmed by immunofluorescence and FCM analysis.
Previous studies have shown that membrane ERα on structures of cardiomyocytes have a critical role for the rapid (non-genomic) E2 effects in heart [21]. After myocardial infarction, ERа can mediate contribution of Bone Marrow-derived Endothelial progenitor cells to functional recovery [9]. Even in the absence of estrogen, ERα-dependent activation, molecular regulation, and organ protection may occur [10]. Hence, it is possible that mERа mediate cell behaviors of BM c-kit+ cells. In this study, we compared c-kit+ mERа+ cells, c-kit+ mERа- cells, c-kit- mERа+ cells, and c-kit- mERа- cells behaviors onto in vitro co-culture system with post-infarct myocardium. The co-culture system mimics the in vivo hypoxia micro-environment of heart post AMI. The paracrine factors secreted by infarcted myocardium also stimulated the cardiac repair function of BM cells. The observed high proliferation and low apoptosis were particularly obvious in double positive of BM c-kit+ mERа+ stem cells. The enhanced ERα expression in proliferating post-infarct BM c-kit+ cells suggests that mERα may participate in cardiac adaptive mechanisms involving post-infarct BM c-kit+ cells in response to ischemic injury. Interplay between the disease condition, microenvironment, and types of stem cells will ultimately determine the functional benefits.
Bone marrow contains a varied assortment of stem cells with putative cardiac potential, opening the potential for the use of these cells in stem cell therapy. One of the limitations of stem cell therapy for heart disease is the low survival rate of transplanted stem cells [22]. mERа+ enhances the beneficial effects of BM c-kit+ cells by increasing their viability and paracrine function in vitro and in vivo. Transplanted BM c-kit+ with or without mERа reduce infarct size and fibrosis in post-MI hearts, likely by secreting paracrine factors that may protect cardiomyocytes. Hence, it is assumed that BM c-kit+ mERа+ stem cells will be new seeds cells for cardiac repair after AMI. However, the nature of the mobilizing, migration and homing signals for mERа+ and the mechanism of differentiation and incorporation into the target tissues need to be explored further.
We here provide evidence that post-infarct BM c-kit+ mERа+ cell population expresses predominant ERα expression and holds self-renewal, proliferation as well as cardiac differentiation potentials after acute ischemic injury. Mechanistically, activation of the PI3K/Akt signaling cascade plays an important common role in ER-mediated acute signaling in cardioprotection after MI, which would lead to downstream activation of NOS/NO/SNO signaling [7]. Further studies will be necessary to investigate whether the mER-mediated beneficial effects on post-infarction remodeling and function involve BM c-kit+ cells, and to determine the mechanism whereby these double positive cells home to the infarcted myocardium in an ERа-dependent fashion.
In conclusion, acute myocardial infarction is associated with an increased metabolic activity and increased mERа levels of BM c-kit+ cells within 3 days after MI. Post-infarct c-kit+ mERа+ cells population express predominant ERα and holds self-renewal as well as cardiac differentiation potentials after acute ischemic injury. BM c-kit+ cells with mERа reduce the post-MI deterioration of cardiac function, and this cardioprotective effect is significantly greater than that of BM c-kit+ without mERа. A potential important implication of this study is that the manipulation of BM c-kit+ stem cells with ERа-dependent fashion may be helpful in recovering functional performance after cardiac tissue injury.
Acknowledgements
We thank Prof. Jun Li and Ben He’s help on the study. This study was supported by the grants from the National Science Foundation of China (81300091 to JFL), the Ph.D. Programs Foundation of Ministry of Education of China (20120073120110 to JFL), Foundation of Committee on health and family planning in Shanghai City (20134334 to FS), and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (group 46, to JFL).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Rosen MR, Myerburg RJ, Francis DP, Cole GD, Marban E. Translating stem cell research to cardiac disease therapies: pitfalls and prospects for improvement. J Am Coll Cardiol. 2014;64:922–37. doi: 10.1016/j.jacc.2014.06.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J, Zhang Z, Liu Y, Guo C, Gong Y, Yang S, Ma M, Li Z, Gao WQ, He Z. Generation, characterization, and potential therapeutic applications of cardiomyocytes from various stem cells. Stem Cells Dev. 2012;21:2095–110. doi: 10.1089/scd.2012.0031. [DOI] [PubMed] [Google Scholar]
- 3.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–98. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knowlton AA, Lee AR. Estrogen and the cardiovascular system. Pharmacol Ther. 2012;135:54–70. doi: 10.1016/j.pharmthera.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beer S, Reincke M, Kral M, Callies F, Stromer H, Dienesch C, Steinhauer S, Ertl G, Allolio B, Neubauer S. High-dose 17beta-estradiol treatment prevents development of heart failure post-myocardial infarction in the rat. Basic Res Cardiol. 2007;102:9–18. doi: 10.1007/s00395-006-0608-1. [DOI] [PubMed] [Google Scholar]
- 6.Deschamps AM, Murphy E, Sun J. Estrogen receptor activation and cardioprotection in ischemia reperfusion injury. Trends Cardiovasc Med. 2010;20:73–8. doi: 10.1016/j.tcm.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, Michael A, Haq S, Nuedling S, Grohe C, Force T, Mendelsohn ME, Karas RH. 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res. 2004;95:692–9. doi: 10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- 8.Booth EA, Obeid NR, Lucchesi BR. Activation of estrogen receptor-alpha protects the in vivo rabbit heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2005;289:H2039–47. doi: 10.1152/ajpheart.00479.2005. [DOI] [PubMed] [Google Scholar]
- 9.Hamada H, Kim MK, Iwakura A, Ii M, Thorne T, Qin G, Asai J, Tsutsumi Y, Sekiguchi H, Silver M, Wecker A, Bord E, Zhu Y, Kishore R, Losordo DW. Estrogen receptors alpha and beta mediate contribution of bone marrow-derived endothelial progenitor cells to functional recovery after myocardial infarction. Circulation. 2006;114:2261–70. doi: 10.1161/CIRCULATIONAHA.106.631465. [DOI] [PubMed] [Google Scholar]
- 10.Barton M, Meyer MR, Prossnitz ER. Estrogen-independent activation of estrogen receptors. Hypertension. 2011;57:1056–7. doi: 10.1161/HYPERTENSIONAHA.111.173427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedram A, Razandi M, Lewis M, Hammes S, Levin ER. Membrane-localized estrogen receptor alpha is required for normal organ development and function. Dev Cell. 2014;29:482–90. doi: 10.1016/j.devcel.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinckmann M, Kaschina E, Altarche-Xifro W, Curato C, Timm M, Grzesiak A, Dong J, Kappert K, Kintscher U, Unger T, Li J. Estrogen receptor alpha supports cardiomyocytes indirectly through post-infarct cardiac c-kit+ cells. J Mol Cell Cardiol. 2009;47:66–75. doi: 10.1016/j.yjmcc.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Gao E, Koch WJ. A novel and efficient model of coronary artery ligation in the mouse. Methods Mol Biol. 2013;1037:299–311. doi: 10.1007/978-1-62703-505-7_17. [DOI] [PubMed] [Google Scholar]
- 14.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–77. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matuszczak S, Czapla J, Jarosz-Biej M, Wisniewska E, Cichon T, Smolarczyk R, Kobusinska M, Gajda K, Wilczek P, Sliwka J, Zembala M, Zembala M, Szala S. Characteristic of c-Kit+ progenitor cells in explanted human hearts. Clin Res Cardiol. 2014;103:711–8. doi: 10.1007/s00392-014-0705-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elnakish MT, Hassan F, Dakhlallah D, Marsh CB, Alhaider IA, Khan M. Mesenchymal stem cells for cardiac regeneration: translation to bedside reality. Stem Cells Int. 2012;2012:646038. doi: 10.1155/2012/646038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakob P, Landmesser U. Current status of cell-based therapy for heart failure. Curr Heart Fail Rep. 2013;10:165–76. doi: 10.1007/s11897-013-0134-z. [DOI] [PubMed] [Google Scholar]
- 18.Bai X, Yan Y, Coleman M, Wu G, Rabinovich B, Seidensticker M, Alt E. Tracking long-term survival of intramyocardially delivered human adipose tissue-derived stem cells using bioluminescence imaging. Mol Imaging Biol. 2011;13:633–45. doi: 10.1007/s11307-010-0392-z. [DOI] [PubMed] [Google Scholar]
- 19.Kang PM, Izumo S. Apoptosis and heart failure: A critical review of the literature. Circ Res. 2000;86:1107–13. doi: 10.1161/01.res.86.11.1107. [DOI] [PubMed] [Google Scholar]
- 20.Wojakowski W, Tendera M, Michalowska A, Majka M, Kucia M, Maslankiewicz K, Wyderka R, Ochala A, Ratajczak MZ. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–20. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 21.Ropero AB, Eghbali M, Minosyan TY, Tang G, Toro L, Stefani E. Heart estrogen receptor alpha: distinct membrane and nuclear distribution patterns and regulation by estrogen. J Mol Cell Cardiol. 2006;41:496–510. doi: 10.1016/j.yjmcc.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Terrovitis JV, Smith RR, Marban E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res. 2010;106:479–94. doi: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


