Abstract
Spontaneous intracerebral hemorrhage (ICH) is a common and fatal subtype of stroke, with hypertension the most common cause of this disorder. Bone marrow derived mesenchymal stem cells (BM-MSCs) have been shown to elicit protective properties in stroke models. In the present study, male spontaneously hypertensive rats (SHR) were subjected to ICH by intracerebral injection with autologous blood, Wistar-Kyoto (WKY) rats were employed as control. The neurological function outcomes and blood-brain barrier (BBB) were assessed after BM-MSCs transplantation. Our results showed that BM-MSCs grafts via the tail vein significantly decreased the modified neurological severity score (mNSS) and the modified limb placing test (MLPT) score at 14 days after ICH, and the scores were gradually lowered till the end of test. Furthermore, BM-MSCs transplantation effectively attenuated the BBB permeability compared with the vehicle only group, as evidenced by the low level of Evans blue leakage in the BM-MSC group. In addition, we found that BM-MSCs grafts elevated the levels of tight junction associated protein occludin, and type IV collagen. Taken together, our results suggest that intravenously transplanted BM-MSCs exert therapeutic effects on ICH in spontaneously hypertensive rats. The underlying mechanisms are associated with the enhanced neurological function recovery and increased integrity of BBB. Our results provide the increased understanding of the underlying mechanisms and perspective of BMSCs in treatment for stroke.
Keywords: Bone marrow mesenchymal stem cell, blood-brain barrier, neurological function, intracerebral hemorrhage, spontaneously hypertensive rats
Introduction
Stroke is a leading cause of death and associated with long-term disability for survivors. Spontaneous intracerebral hemorrhage (ICH) is a common and fatal subtype of stroke, accounting for about 10-15% of all strokes worldwide [1], however, it can lead to more devastating outcomes than the ischemic stroke. Previous studies have showed that a variety of etiologies attribute to this event, among which hypertension is the most common cause of ICH. In recent decades, despite considerable progress made in animal and preclinical studies, there are still no effective therapeutic strategies for clinical practice. Thus, it is very important to further understand the mechanisms and develop novel therapy strategies for stroke. Spontaneously hypertensive rat (SHR) is a good animal model of human primary hypertension and extensively used in cardiovascular and neurological diseases. SHR obtains from Wistar-Kyoto (WKY) rat and begins to increase blood pressure at about 5-6 weeks of age. It has been shown that SHR models with intracerebral hemorrhage could induce more severe neurological deficits than WKY rat [2], and the efficacy of treatment in experiments with health animals are usually overstated when compared with animals with comorbidities, like hypertension [3]. SHR model based studies may provide much precise data for pre-clinical trials as recommendations [4].
Bone marrow mesenchymal stem cells (BM-MSCs) are self-renewing and multipotent cells, which can differentiate into various cell types, such as osteoblasts [5], adipocytes [6], chondroblasts, vascular smooth muscle cells, etc [7,8]. Previous studies have shown that BM-MSCs can exert anti-inflammatory, anti-apoptosis and regenerative properties in many diseases, including stroke [9-11]. It has been reported that BM-MSCs could pass through the blood-brain barrier (BBB) without disrupting the host brain architecture [12]. Transplantated BM-MSCs via intracerebral or intravenous route can migrate to the injured sites and differentiate into neurons or secrete various neurotrophic factors and promote functional improvement [13,14]. Recently, Ito M and colleagues demonstrated that transplantation of BM-MSCs protected neurovascular units and ameliorated brain damage in SHR model [15]. Moreover, the transplanted BM-MSCs exerted its protective effects in SHR model through suppressing oxidative stress and apoptosis [16]. However, whether MSCs could improve neurologic function and ameliorate BBB dysfunction after ICH in SHR still poorly understood.
To better understand the effects of BM-MSCs transplantation on stroke, in the present study, we used autologous blood injection to induce ICH in SHR, long-term effects of BM-MSCs transplantation on ICH evoked neurological deficits and blood-brain barrier dysfunction in SHR were investigated.
Methods
Isolation and culture of bone marrow-derived BM-MSCs
All animal experiments were conducted with the approval of the Experimental Animal Ethics Committee of Harbin Medical University. Bone marrow stromal cells BM-MSCs were harvested from femurs and tibias of 8-week-old male SHR, as described previously [15,17]. Briefly, SHR was sacrificed and placed in 70% alcohol for disinfection. Both femurs and tibias were detached from muscle and soft tissue. Bone marrow cells were flushed out from bones with 2 ml of DMEM medium (Gibco, Carlsbad, CA, USA). The resulted cell suspension was centrifuged and depleted the red blood cells with Red Blood Cell Lysis Buffer (Solarbio science, Beijing, China), After that, the cells were resuspended, seeded at a density of 1×105/ml in a cell culture flask and cultured at 37°C with 5% CO2. The medium containing non-adherent cells was discarded 24 hours later, and fresh medium was added. Every 4-5 days, the cultured cells were digested with 0.25% trypsin for passage when 80% confluence. Cells through the third passages were collected and preserved for the following experiments.
Identification and labeling of BM-MSCs
BM-MSCs were collected following the third passage, after centrifugation, a suspension containing 1×106 single cells was incubated with one of the primary antibodies against CD90 (1:100, Ebioscience, Headquarters, San Diego), CD29 (1:100, Ebioscience), and CD45 (1:100, Ebioscience) for 40 min, all of which were conjugated to APC. Cell identification was performed via flow cytometry (Becton-Dickinson, San Jose, CA).
Prior to transplantation, BM-MSCs were labeled with PKH26 red fluorescence cell linker kit (Sigma-Aldrich, Saint Louis, MO, USA) according to the manufacturer’s instructions [3]. In brief, 2×107 cells were resuspended in 1 ml of diluent C and added into the dye solution; after incubating at 25°C for 5 min, 2 ml of FCS was added to stop the reaction, Cells were supplemented with 4 ml of complete medium and washed three times. The pre-labeled BMSCs were recultured for further study. At 7, 14, 28, and 42 days after injury, the brain tissue were removed for frozen section, tracking of the PKH26 labeling BMSCs were visualized using a fluorescence microscope.
Animals and study design
Male SHR and WKY rats were purchased from Vital River (Vital River Laboratory Animal Technology Co., Ltd, Beijing, China). SHR were divided into BM-MSC+SHR and SHR + vehicle only (n=24 in each group) groups, WKY rats were employed as control group (n=12). The surgery was performed as previously described with slight modification [18]. Briefly, rats were anesthetized with 10% of Chloral hydrate (3 ml/kg, intraperitoneally) and immobilized in a stereotaxic frame. A midline skin incision was made and the skull was perforated using a 0.5 mm dental drill (2.9 mm lateral to midline). 50 μL of autologous blood collected from right femoral artery was infused over 5 min, the needle was left in place for 3 min, and withdrawn slowly. The bone hole was sealed with bone wax, the scalp wound was sutured, and the animal was placed in a cage with free access to food and water. WKY rats only subjected to the surgery without blood injection. For experiment design, each animal in BM-MSC+SHR group was injected with 1×106 PKH26 pre-labeled BM-MSCs (100 μl) via the tail vein. Animals in the SHR group received an equal volume of vehicle. The body weight and blood pressure were measured at 7, 14, 28, 42 days post ICH using the tail-cuff method as previously described. The brain tissues were collected at 14 days after ICH.
Assessment of neurological function
The neurological function of rats was assessed using the modified Neurological Severity Score (mNSS) [19] and Modified limb placing test (MLPT) [20]. Neurologic functions were scored by two investigators blind to the experimental conditions. The tests were performed before and 1, 3, 7, 14, 28, and 42 days post-ICH. Neurologic function was graded 0-18 scores (0, normal; 18, maximal deficit) and 0-10 (0, normal; 10, maximal deficit), respectively.
Evans blue staining
BBB integrity was assessed by Evans blue staining. Evans blue (2% solution 2 mL/kg) was injected into the tail vein 6 h before sacrifice at 7, 14, 28, 42 days post- injury. The rats were transcardially perfused with heparinized phosphate-buffered saline to remove the intravascular dye. For qualitative examinations, brains were snap-frozen, then sectioned into 20 μm slices, the extravasation of Evans blue dye was observed under a fluorescence microscope (excitation 620 nm, emission 680 nm).
Western blotting
The brain samples from each group were homogenized and total proteins from the homogenate were extracted by RIPA lysis buffer (Beyotime), total protein concentrations were determined using the Bradford assay. Equal amounts of protein (30 μg) were separated by 8% SDS-PAGE and the separated proteins were then transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). After that, the membrane was blocked with 5% skim milk at room temperature for 1 h, followed by incubation with primary Occludin antibody (1:1000 dilution, Wanleibio) at 4°C overnight. Subsequently, after three washes with TBST, the membrane incubated with horseradish peroxidase-conjugated secondary antibody at room temperature for 1 h. The targeted proteins were visualized using the enhanced chemiluminescence (ECL) substrate, and analyzed by Image J software. The β-actin protein served as an internal control.
Immunofluorescence staining
The expression of occludin and collagen IV was determined by immunofluorescence analyses. In brief, frozen sections of brain (5-7 μm) were fixed in acetone at 4°C for 15 min, followed by antigen retrieval for 10 min. After blocking nonspecific sites with blocking solution at room temperature for 30 min, the sections were incubated with the corresponding primary antibodies After three washes with PBS, the (occludin 1:100 dilution, Wanleibio, Shenyang, China; collagen IV 1:50 dilution, Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight. The sections were then washed three times with PBS and incubated with corresponding FITC- labeled secondary antibodies (Beyotime, Haimen, China) for 30 min. After a nuclear staining step with DAPI (4,6-diamino-2-phenylindole) (Sigma-Aldrich), the sections were mounted in buffered glycerin and visualized under a fluorescent microscope (Olympus IX53, Tokyo, Japan).
Statistical analysis
All data were expressed as mean ± standard deviation. One-way analysis of variance was used for the analyses between the groups; Bonferroni’s post hoc test was used for multiple comparisons. Values of P<0.05 were considered statistically significant.
Results
Characterization of BM-MSCs and distribution in the brain
The morphology of BM-MSCs was photographed after the third passage, as shown in Figure 1A, BMSCs exhibited typical spindle-shaped morphology as previously described [21]. It has been reported that BM-MSCs are characterized for the presence of mesenchymal surface antigens (such as CD90 and CD29), and the absence of hematopoietic markers (such as CD45). To identify the cultured cells, the cell surface markers of BM-MSCs were determined by flow cytometry. As shown in Figure 1B, More than 90% of the cells expressed the mesenchymal stem cell markers CD29 and CD90, whereas less than 7% of the cells expressed CD45. The results indicated that the cultured cells were mesenchymal stem cells, can be used for further study. BM-MSCs pre-labeled with PKH26 were used for in vivo transplantation, the temporal distribution of BM-MSCs in the brain were determined at 7, 14, 28, and 42 days after injury. As shown in Figure 1C, amounts of BM-MSCs labeled with PKH26 were present in the brain tissue, and the cells gradually decreased at the end of experiment.
Figure 1.
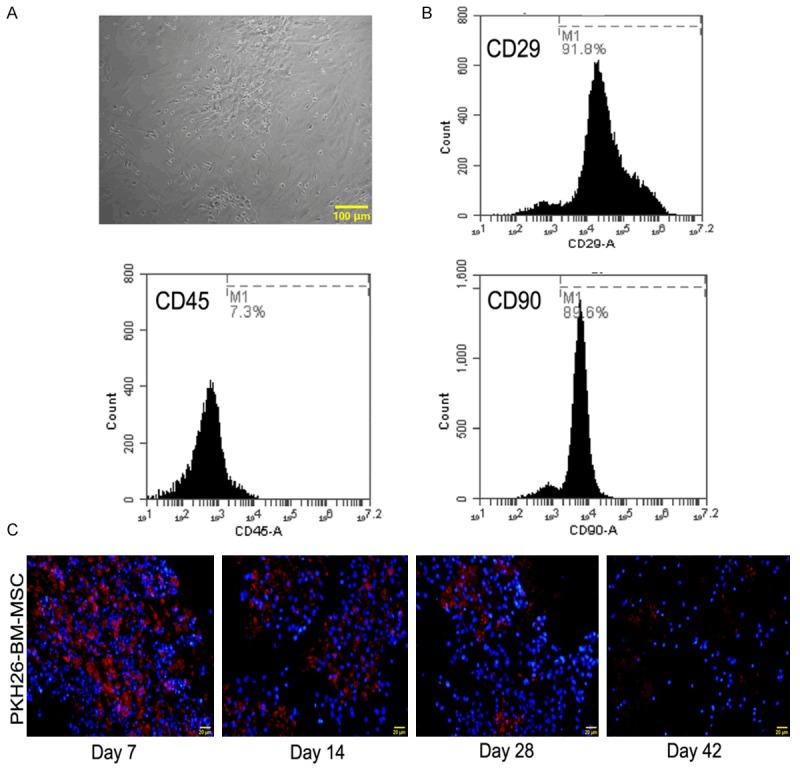
Characterization of BM-MSC and temporal distribution in the brain (A) Morphology of MSCs (the third passage, magnification 100×) BM-MSCs exhibited fibroblast-like morphology (B) Phenotypes of BM-MSCs. The present (CD29, CD90) or absent (CD45) surface markers of BM-MSCs were determined by flow cytometry. (C) Distribution of BM-MSCs pre-labeled with PKH26 in the brain. BM-MSCs pre-labeled with PKH26 were transplanted in SHR via the tail vein, the frozen brain section from 7, 14, 28, 42 days after ICH were measured by immunofluorescence, double staining of PKH26 (red fluorescence) and DAPI (blue fluorescence) was shown, magnification 200×, scale bar 50 μm.
Effects of BM-MSC transplantation on blood pressure and neurologic function of SHR
The sytolic artery blood pressure (SBP) and diastolic blood pressure (DBP) from each group rats were measured at 7, 14, 28, and 42 days after ICH. Figure 2A, 2B showed that the SBP and MBP of BM-MSC or vehicle transplanted SHR were significantly higher than WKY group. For example, at 42 days post-ICH, the SBP/DBP in BMSCs grafts group was 181.53±5.1/142.67±4.48 mmHg; the vehicle group was 184.6±6.8/147.8±7 mmHg, the WKY group was 109.23±5/84.8±4.29 mmHg (BM-MSC+SHR or SHR vs. the WKY, P<0.01). The blood pressure of WKY rats did not change during the experiment. The effect of BM-MSC grafts on neurologic function recovery was evaluated using mNSS and MLPT scores before and 1, 3, 7, 14, 28, and 42 days post-ICH. Figure 2C, 2D showed that the mNSS scores and MLPT scores in the BM-MSC group were significantly decreased at 14 days post-ICH compared to those of the vehicle transplanted group (P<0.01). Both scores showed a sharp fall after 14 days and maintained to the end of test. There were no significant differences between BM-MSC and vehicle transplanted group during the first seven days after ICH (P>0.05).
Figure 2.
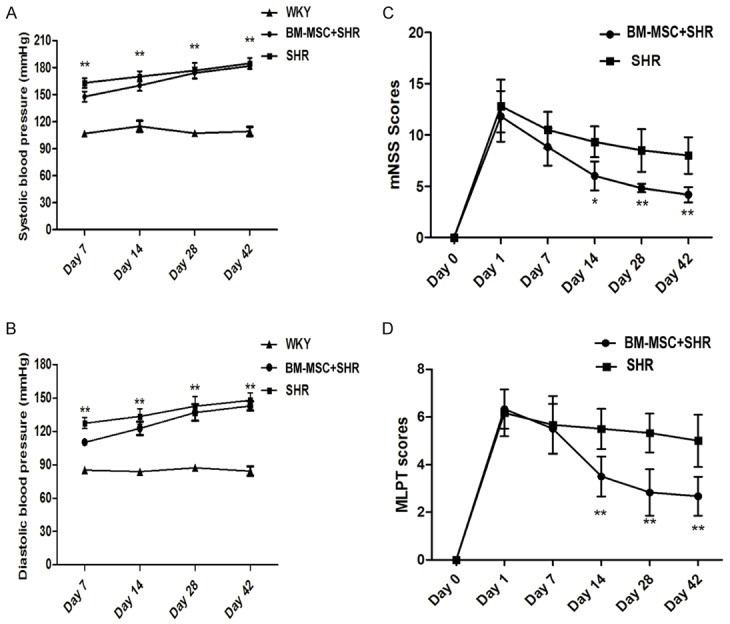
Effects of MSCs transplantation on blood pressure and neurologic function of SHR. (A) Sytolic artery blood pressure (SBP) and (B) diastolic blood pressure (DBP) of rats from each group were determined at 7, 14, 28, and 42 days post-ICH. The neurological function of rats was assessed using the (C) modified Neurological Severity Score (mNSS) and (D) Modified limb placing test (MLPT) were performed for neurological function before and after ICH. All of the experiments were performed in triplicate, data are presented as the mean ± standard deviation; (A, B) compared with the WKY group, **P<0.01; (C, D) compared with the SHR group, *P<0.05 or **P<0.01.
BM-MSCs grafts ameliorates ICH induced blood-brain barrier dysfunction in SHR
Previous studies have shown that ICH causes primary and secondary BBB dysfunction. Evans blue dye is a well used marker of BBB leakage, because it has a very high affinity for serum albumin, which cannot pass through the BBB in normal circumstances. The effect of BM-MSCs on BBB integrity was assessed by Evans blue extravasation measurement. As shown in Figure 3, ICH caused significantly high levels of Evans blue extravasation. Whereas, BM-MSCs treatment group presented lower fluorescence compared with the SHR group. There was no fluorescence in the WKY group at 14 days post-ICH.
Figure 3.
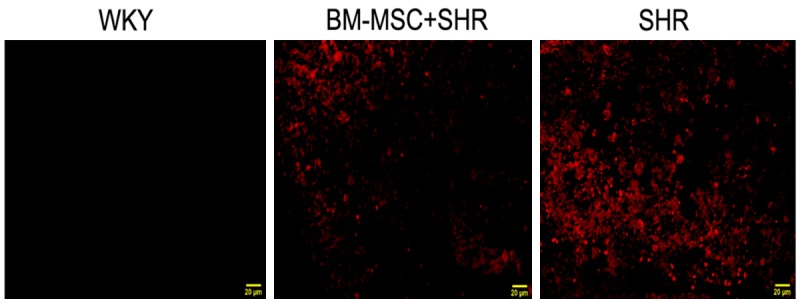
Effect of BM-MSCs grafts on ICH induced blood-brain barrier dysfunction in SHR. Frozen brain sections from 14 days post-ICH were observed under a fluorescence microscope, magnification 400×, scale bar 20 μm.
BMSCs grafts increases the expression of occludin and collagen IV in SHR
To investigate the further effects of BM-MSCs grafts on BBB integrity, we examined the expression of tight junctional protein occludin and the component of basement membrane, collagen IV. Immunofluorescence staining results showed that a large number of collagen IV positive cells presented in the brain tissue of WKY rat, while the expression of collagen IV significantly decreased in ICH rats compared to the WKY group, BM-MSCs grafts reversed the decrease (Figure 4). Simultaneously, the expression of occludin was also up-regulated after BMSCs grafts compared with the vehicle group (Figure 5).
Figure 4.
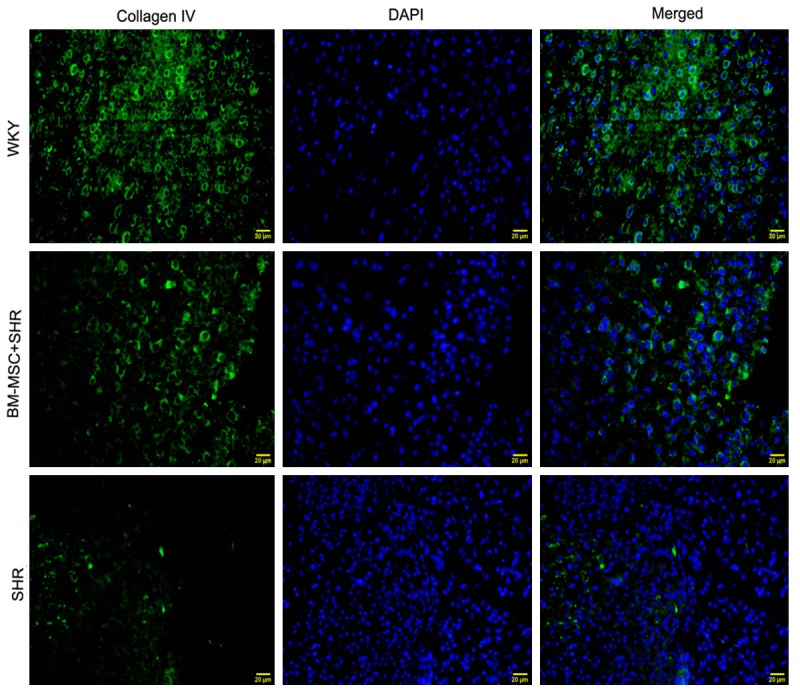
BM-MSCs increases the expression of collagen IV in the brain. Images showing immunofluorescence staining for collagen IV (green) and DAPI for nucleus (blue) in the WKY, BM-MSC+SHR, and SHR groups. The brain tissues were collected at 14 days after ICH, magnification 400×, scale bar 20 μm.
Figure 5.
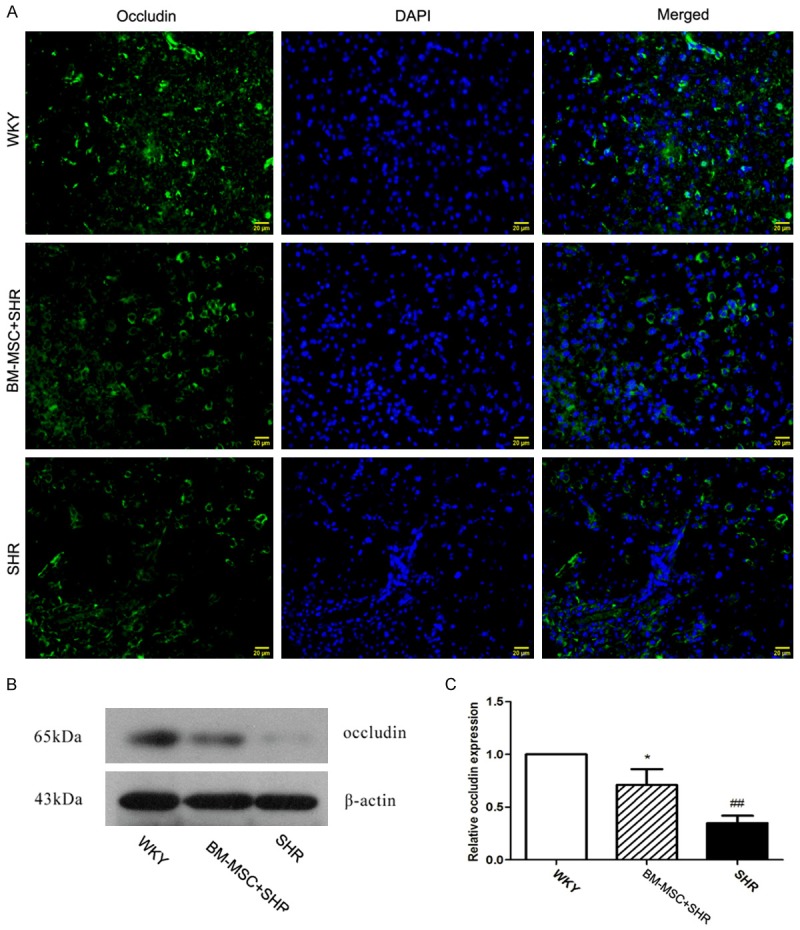
BM-MSCs increases the expression of occludin in the brain. A. Images showing immunofluorescence staining for occludin (green) and DAPI for nucleus (blue) in the WKY, BM-MSC (1×106) +SHR, and SHR groups. The brain tissues were collected at 14 days after ICH, magnification 400×, scale bar 20 μm. B. The expression of occludin in the brain was detected by Western blot; C. β-actin was used as an internal reference to normalize occludin expression. All of the data are presented as mean ± standard deviation. Compared with the WKY group, ##P<0.01, and compared with the SHR group, *P<0.05.
Discussion
The current study was undertaken to investigate the impact of intravenous delivery of BM-MSCs on ICH induced neurological deficits and BBB disruption in SHR. Our results indicate that intravenously transplantation of BM-MSCs improved the neurological function and BBB restoration which were impaired by ICH. To the best of our knowledge, we provide the first evidence that systemically transplanted BM-MSCs exert long-term neuroprotective properties in SHR with intracerebral hemorrhage.
BM-MSCs are emerging as therapeutic candidate in a variety of disease including stroke [8]. Because the multipotent cells are easily isolated from bone marrow and expanded rapidly in vitro [22]. Previous studies have shown that BMSCs are lack of the hematopoietic markers like CD34, CD45, CD14, CD19, HLA-DR, or CD79α, but positively express CD73, CD90, CD105, CD29, CD44 [23-26]. In the present study, we found that the BM-MSCs cultures exhibited typical fibroblast-like morphology as previously reported [16,17]. For identification of BMSCs, the presence or absence of markers of BM-MSCs were further determined at the third passage. Our results showed that the expanded BMSCs were uniformly positive for CD29 and CD90, while negative for CD45. Subsequently, we investigated the temporal distribution of the transplanted BM-MSCs in the brain tissues. Our results revealed that PKH26-labeled BM-MSCs were long-term present in the brain of SHR, and the results were in line with a recent study, which reported that the BM-MSCs presented in the brain until 30 days in a spontaneous stroke model [16].
Previously, Minnerup et al [3] reported that the bone marrow-derived mononuclear cells (BM-MNCs) did not improved the functional outcome at early stage of stroke in SHR. Consistent with this finding, our results showed that there were no significant differences between the BM-MSCs and the vehicle groups at 1 and 7 days after ICH. However, BM-MSCs grafts significantly improved neurological recovery at 14 days post- ICH, and the mNSS and MLPT scores were much lower than that of the vehicle group until 42 days post-ICH. Results from our study indicate that BM-MSCs may exert long-term neuroprotective effects during ICH in SHR model.
Previous studies have demonstrated that disruption of the BBB plays an important role in the development of neurological dysfunction in stroke [27,28]. The BBB is a dynamic, complex structure that plays an important role in protecting the neuronal microenvironment [29]. It is formed by the basement membranes, cerebral endothelial cells (pericytes, astrocyte end feet) and tight junctions [30]. Collagen type IV is one of the major functional components of the basement membranes, which provide scaffold for cerebral endothelial cells interacting with each other[31]. Occludin is described one main component of tight junctions [32,33]. Altered or loss of occludin and collagen IV expression were frequently observed in the compromised BBB in stroke models [15,34-36]. In the present study, we found that BM-MSCs transplantation reduced the BBB permeability, as indicated by the low level of Evans blue extravasation. Moreover, the expression of occludin and collagen IV was decreased after ICH, BM-MSCs grafts up-regulated the collagen IV and occludin protein levels in the injured brain. Our results indicate that BM-MSCs grafts restore ICH- induced BBB disruption, closely related with the up-regulation of tight junction protein occludin and the collagen IV.
In conclusion, results from the present study suggest that intravenously transplanted BM-MSCs exerts therapeutic effects in Spontaneously hypertensive rats with ICH, the underlying mechanisms involve enhanced neurological function recovery and improved integrity of the blood brain barrier. Our results may provide increased understanding of the underlying mechanisms and perspective of BM-MSCs in treatment for stroke.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of Heilongjiang Province (No.: H2015061), the Health Department of Heilongjiang Province (No.: 2014-278), the State Key Laboratory of Neuroscience, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (No.: SKLN-201402), and the National Key Clinical Specialist Construction Program of Department of Neurology.
Disclosure of conflict of interest
None.
References
- 1.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11:720–731. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu G, Bao X, Xi G, Keep RF, Thompson BG, Hua Y. Brain injury after intracerebral hemorrhage in spontaneously hypertensive rats. J Neurosurg. 2011;114:1805–1811. doi: 10.3171/2011.1.JNS101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minnerup J, Wagner DC, Strecker JK, Posel C, Sevimli-Abdis S, Schmidt A, Schilling M, Boltze J, Diederich K, Schabitz WR. Bone marrow-derived mononuclear cells do not exert acute neuroprotection after stroke in spontaneously hypertensive rats. Front Cell Neurosci. 2014;7:288. doi: 10.3389/fncel.2013.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stem Cell Therapies as an Emerging Paradigm in Stroke Participants. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40:510–515. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]
- 5.Ashton BA, Allen TD, Howlett CR, Eaglesom CC, Hattori A, Owen M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop Relat Res. 1980:294–307. [PubMed] [Google Scholar]
- 6.Pereira RF, Halford KW, O’Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 8.Seo JH, Cho SR. Neurorestoration induced by mesenchymal stem cells: potential therapeutic mechanisms for clinical trials. Yonsei Med J. 2012;53:1059–1067. doi: 10.3349/ymj.2012.53.6.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, Hong NH, Kim JH, Ban JJ, Park HK, Kim SU, Park CG, Lee SK, Kim M, Roh JK. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131:616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- 10.van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47:1634–1643. doi: 10.1002/hep.22236. [DOI] [PubMed] [Google Scholar]
- 11.Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, Kocsis JD. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129:2734–2745. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wislet-Gendebien S, Hans G, Leprince P, Rigo JM, Moonen G, Rogister B. Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem Cells. 2005;23:392–402. doi: 10.1634/stemcells.2004-0149. [DOI] [PubMed] [Google Scholar]
- 15.Ito M, Kuroda S, Sugiyama T, Maruichi K, Kawabori M, Nakayama N, Houkin K, Iwasaki Y. Transplanted bone marrow stromal cells protect neurovascular units and ameliorate brain damage in stroke-prone spontaneously hypertensive rats. Neuropathology. 2012;32:522–533. doi: 10.1111/j.1440-1789.2011.01291.x. [DOI] [PubMed] [Google Scholar]
- 16.Calio ML, Marinho DS, Ko GM, Ribeiro RR, Carbonel AF, Oyama LM, Ormanji M, Guirao TP, Calio PL, Reis LA, Simoes Mde J, Lisboa-Nascimento T, Ferreira AT, Bertoncini CR. Transplantation of bone marrow mesenchymal stem cells decreases oxidative stress, apoptosis, and hippocampal damage in brain of a spontaneous stroke model. Free Radic Biol Med. 2014;70:141–154. doi: 10.1016/j.freeradbiomed.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Tsai MJ, Tsai SK, Hu BR, Liou DY, Huang SL, Huang MC, Huang WC, Cheng H, Huang SS. Recovery of neurological function of ischemic stroke by application of conditioned medium of bone marrow mesenchymal stem cells derived from normal and cerebral ischemia rats. J Biomed Sci. 2014;21:5. doi: 10.1186/1423-0127-21-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue M, Del Bigio MR. Intracerebral injection of autologous whole blood in rats: time course of inflammation and cell death. Neurosci Lett. 2000;283:230–232. doi: 10.1016/s0304-3940(00)00971-x. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 20.Puurunen K, Jolkkonen J, Sirvio J, Haapalinna A, Sivenius J. An alpha(2)-adrenergic antagonist, atipamezole, facilitates behavioral recovery after focal cerebral ischemia in rats. Neuropharmacology. 2001;40:597–606. doi: 10.1016/s0028-3908(00)00182-9. [DOI] [PubMed] [Google Scholar]
- 21.Kunter U, Rong S, Djuric Z, Boor P, Muller-Newen G, Yu D, Floege J. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol. 2006;17:2202–2212. doi: 10.1681/ASN.2005080815. [DOI] [PubMed] [Google Scholar]
- 22.Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 23.Teixeira FG, Carvalho MM, Sousa N, Salgado AJ. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell Mol Life Sci. 2013;70:3871–3882. doi: 10.1007/s00018-013-1290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 25.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 26.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keep RF, Zhou N, Xiang J, Andjelkovic AV, Hua Y, Xi G. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS. 2014;11:18. doi: 10.1186/2045-8118-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36:437–449. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- 30.Bernacki J, Dobrowolska A, Nierwinska K, Malecki A. Physiology and pharmacological role of the blood-brain barrier. Pharmacol Rep. 2008;60:600–622. [PubMed] [Google Scholar]
- 31.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 32.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 35.Summermann E, Huwer H, Seitz G. Carcinosarcoma of the lung, a tumour which has a poor prognosis and is extremely rarely diagnosed preoperatively. Thorac Cardiovasc Surg. 1990;38:247–250. doi: 10.1055/s-2007-1014027. [DOI] [PubMed] [Google Scholar]
- 36.Veltkamp R, Siebing DA, Sun L, Heiland S, Bieber K, Marti HH, Nagel S, Schwab S, Schwaninger M. Hyperbaric oxygen reduces blood-brain barrier damage and edema after transient focal cerebral ischemia. Stroke. 2005;36:1679–1683. doi: 10.1161/01.STR.0000173408.94728.79. [DOI] [PubMed] [Google Scholar]


