Abstract
Dopaminergic neurons are suggested to be a critical physiopathology substrate for addiction disorders. It is not well known whether the clonal mesencephalic dopaminergic cell line MN9D cells can be applied to study morphine addiction. Immunofluorescence staining and reverse transcription-polymerase chain reaction (RT-PCR) were used to detect protein and mRNA expression of the μ, κ, and δ-opioid receptors in MN9D cells. Immunofluorescence staining of TH was applied to quantify the number of dopaminergic neurons. The results showed that the μ, κ, and δ-receptors were all expressed in MN9D cells, and the number of TH-positive cells was significantly greater in the MN9D cells than SH-SY5Y cells. The data suggest that MN9D cells can be used as an in vitro models in future studies to explore the mechanisms of morphine addiction related to dopaminergic neurons.
Keywords: MN9D cells, opioid receptors, tyrosine hydroxylase, SH-SY5Y cells
Introduction
The mesencephalic dopamine system is one of the key brain regions of drug addiction, and it is thought to be the final pathway for the reward system [1,2]. And approaches for in vitro studies are essential to study this disease. Because morphine binds to brain opioid receptors [3], it is necessary to have the same receptors present in a morphine-dependent cell model. At present, dopaminergic SH-SY5Y cells, which possess mid-level activity of the dopamine-β-hydroxy enzyme [4], are widely used to study the mechanism of dopaminergic addiction at the cellular level. Another dopaminergic cell line, the MN9D cells, which also exhibit dopamine-β-hydroxy enzyme activity [5], are harvested from primary, mouse, embryonic ventral mesencephalic cells and neuroblastomas. MN9D cells are widely used to study Parkinson’s disease (PD) [6,7]. The previous studies have shown that MN9D cells contain NURR1 protein, which is closely associated with differentiation and survival of dopaminergic neurons [8-10]. Prostaglandin E-2 rapidly and markedly activates expression of endogenous NURR1 in MN9D cells to protect the neurons [11]. The present study proved that the μ, κ, and δ opioid receptor expression in MN9D cells at the protein and RNA levels, suggesting that MN9D cells could serve as an ideal cell model of morphine dependent and morphine addiction. Additionally, there were significantly more dopaminergic neurons in the MN9D cell population than those in the SH-SY5Y cell population, indicating that MN9D cells could be more suitable than SH-SY5Y cells for studying mechanisms of dopaminergic addiction at the cellular level.
Materials and methods
Materials and reagents
MN9D cells were provided by the Institution of Neuroscience Research at Capital Medical University. The culture medium was DMEM/F12 (Gibco, Grand Island, USA) containing 10% fetal bovine serum. Mouse monoclonal anti-μ, κ, and δ receptor antibodies and mouse monoclonal anti-tyrosine hydroxylase (TH) antibody were purchased from Abcam (Hong Kong, China). Dylight 594-goat anti-mouse IgG was purchased from EarthOx Life Sciences (Millbrae, California, USA). The primers for the μ, κ, and δ receptors were synthesized by Sangon Biotech (Shanghai, China).
MN9D cell culture
MN9D cells were cultured in Dulbecco’s-Modified Eagle Media/F12 (DMEM/F12) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. After two to three cell passages, the MN9D cells were applied to the experiment. The cells were digested, passaged, and seeded at a density of 2 × 105/L in six-well plates pre-coated with poly-D-lysine and maintained at 37°C in a 5% CO2 incubator.
Immunofluorescence labeling
MN9D cells were pretreated with 4% paraformaldehyde for 30 min, 3% H2O2 in methanol for 20 min, and 1% bovine serum albumin in phosphate-buffered saline for 30 min. The primary antibodies specific to the μ, κ, and δ receptors were used at 1:150, and the primary antibody specific to TH was used at 1:200. Dylight 594-goat anti-mouse IgG (1:800) was used as the secondary antibody for all stains. Cell nuclei were stained by DAPI (1:10,000).
RNA extraction and reverse transcription (RT)
(1) Preparation of samples: cell lysates were placed at room temperature for 5 min until completely dissolved. (2) Extraction of total RNA: the total RNA extraction kit (TIANGEN BIOTECH CO. LTD) was used to extract total RNA. Total RNA was extracted from cell lysates during (1-1.5) h according to instruction manual. (3) 0.5 µg total RNA was reversetranscribed into first-strand cDNA using 0.5 µL Oligo (dT) primer, 2 µL 5× Primescript Buffer, 0.5 µL Primescript RT Enzyme Mix I, and 0.5 µL random 6-mers in a final volume of 10 µL. The first-strand cDNA was stored at -80°C until further use.
Semi-quantitative RT-polymerase chain reaction (RT-PCR)
(1) Primers were synthesized by Sangon Biotech. The primers used were as follows: μ recptor: upstream primer: 5’-TCTAATGTATTGTCTGGTTTGCCGTATTG-3’; downstream primer: 5’-AACTCTTCATGTAAGGTGACTAGGTGCTTC-3’; the length of products was 150 bp. κ recptor: Upstream primer: 5’-AGTAGAACCAGAAAGGTGGCTGAGTGATTAA-3’; downstream primer: 5’-TGCCCACAGTTGTTGGAAAGCATTAGAT-3’; the length of products was 200 bp. δ recptor: Upstream primer: 5’-GGCACATTAGTGATCTAGCTGAGTAGGA-3’; downstream primer: 5’-AGAGTGTTGTCTTTGTTTCTCAAGGGAT-3’; The length of products was 200 bp.
(2) For PCR amplification, 2 µL cDNA was combined with 0.4 µL (10 µM) upstream and downstream primers of the μ receptor, κ receptor, and δ receptor, 10 µL SYBR Premix ExTaq (2×), 0.4 µL Rox Reference Dye II (50×), and 6.8 µL distilled water in a final volume of 20 µL. PCR was performed in a DNA thermal cycler (Tgradient 48, Biometra) after first denaturation at 95°C for 5 min. Each cycle consisted of denaturation at 95°C for 35 s, annealing at 40°C for 34 s, and extension at 72°C for 60 s. The total number of PCR cycles was 35.
(3) For semi-quantitative analysis, 25 µL of PCR products were examined by electrophoresis on a 2% agarose gel containing 0.5 µL/mg ethidium bromide. Unvitec BTS-20Mas used to photograph and analyze optical density.
Cell quantification
The number of TH-positive cells and the total cells in the MN9D cell and SH-SY5Y cell populations was quantified (per 400× field of view) in five fields of view, and the rate of TH positive cells (%) was calculated. Two independent observers, who were blinded to the experimental conditions, performed quantifications and calculated the average ratio of TH-positive cells.
Statistical methods
Statistical analysis was performed using one-way analysis of variance. Results are presented as mean ± standard error of the mean. The threshold for statistical significance was defined as P < 0.05.
Results
Results of MN9D cell culture
Observed with an inverted microscope, MN9D cells had typical morphological characteristics of neurons with plentiful cytoplasm, large nuclei and two to three synapses (Figure 1). The round cells with short synapses were undifferentiated, while the polygonal cells with different length synapse were differentiated.
Figure 1.
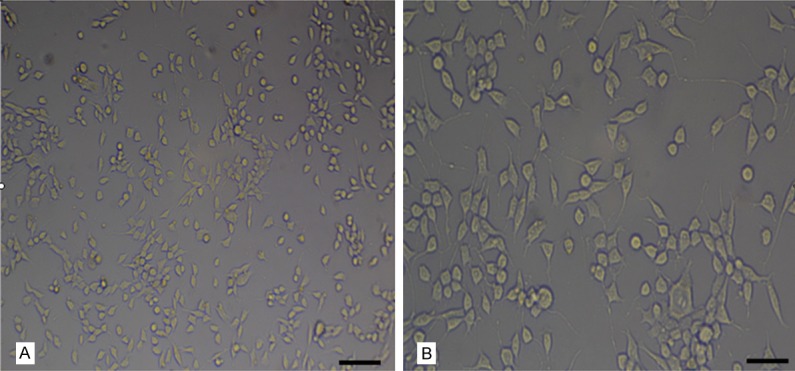
Morphological characteristics of MN9D cells. MN9D cells, large and rich in cytoplasm, rounded or polygonal, presented a normal shape and uniform distribution. Bar = 100 μm in (A); bar = 50 μm in (B).
Expression of the μ, κ, and δ receptors in MN9D cells
The μ, κ, and δ receptor proteins were extensively expressed in the cytoplasm of MN9D cells with immunofluorescence staining (Figure 2A-C). The κ and δ receptors were strongly expressed, while expression of the µ receptor was weaker.
Figure 2.
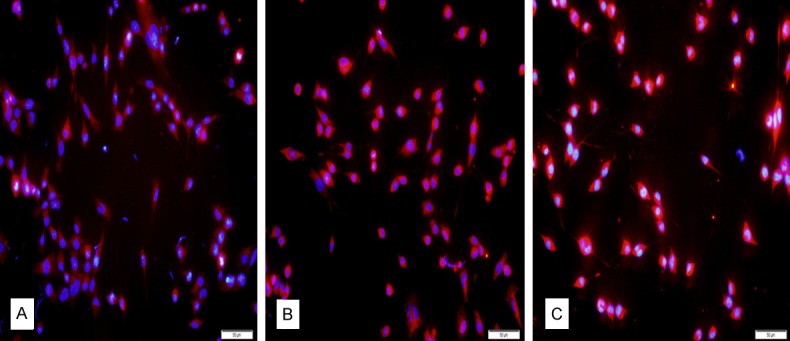
Protein expressions of opioid receptors in MN9D cells. (A-C) showed the μ, κ, and δ opioid receptor expression in MN9D cell cytoplasm, respectively. Red: opioid receptors. Blue: DAPI. Bars = 50 μm.
The mRNA of the three receptors was successfully obtained from the MN9D cells. PCR amplification products of the receptors were separated by agarose gel electrophoresis, revealing that the μ receptor mRNA was 150 bp, and the κ and δ receptor mRNA were both 200 bp (Figure 3).
Figure 3.
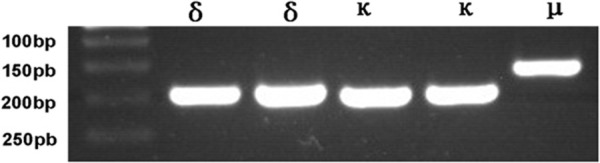
RNA Expressions of the μ, κ, and δ receptor in MN9D cells.
Immunofluorescence of TH in MN9D cells and SH-SY5Y cells
TH expression was not homogeneous in both MN9D cells and SH-SY5Y cells. TH-positive cells of the two cell lines, presenting large and polygon cell bodies, had no significant morphological difference. Compared with SH-SY5Y cells, MN9D cells owned significantly more TH-positive cells (Figure 4A, 4B). The ratio of TH was 20.4% ± 5.8% in MN9D cells and 6.2% ± 2.6% in SH-SY5Y cells, with significant differences between the groups (P < 0.05) (Figure 5).
Figure 4.
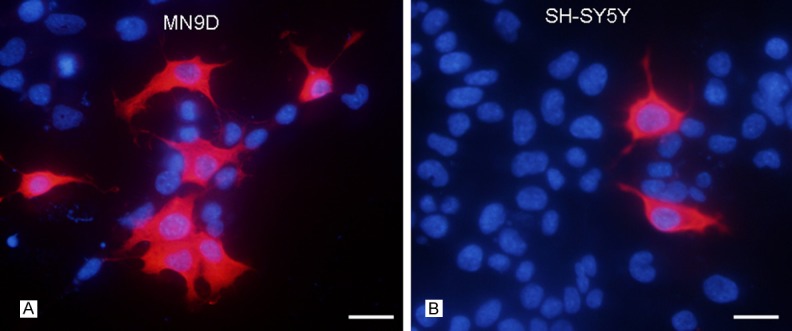
TH-positive cells expression in MN9D cells (A) and SH-SY5Y cells (B). Compared with SH-SY5Y cells, TH-positive cells were significantly more in MN9D cells. Red: TH, Blue: DAPI. Bars = 25 μm.
Figure 5.
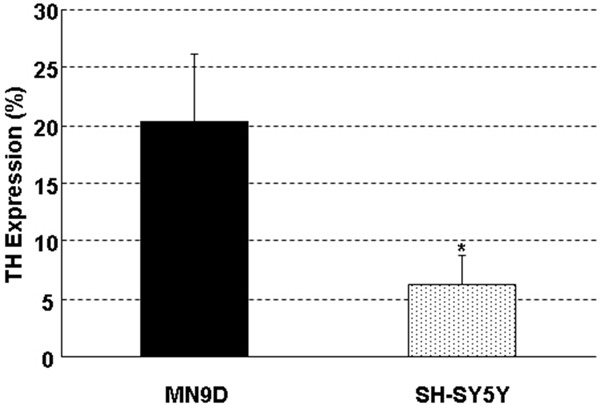
Expression rate of TH-positive cells in MN9D and SH-SY5Y cells. The positive rate was 20.4% ± 5.8% in MN9D cells, and 6.2% ± 2.6% in SH-SY5Y, with significant differences between the groups (*P < 0.05).
Discussion
Opiate addiction is manifested as compulsive drug use [12-14]. In terms of opioid drugs, the abuse of morphine has led to more serious public health and social issues [15]. The effects of morphine on neural plasticity are closely related to the mechanisms of addiction [16,17]. The mesencephalic dopamine system is known to take part in the reward aspect of all drugs of dependence, and is considered to be the last pathway of this response [2]. Previous studies have indicated that long duration of morphine addiction can result in pathological changes to mesencephalic dopaminergic neurons [18-20]. Cell culture of high quality is essential for further studying the mechanisms of addiction. Morphine binds to opioid receptors in the brain [3]. Therefore, the expression of at least one of the three opioid receptors (μ, κ, and δ) is needed to establish an in vitro morphine-dependent model. Using immunofluorescence and RT-PCR technology, the present study initially determined that the μ, κ, and δ opioid receptors were expressed in MN9D cells, suggesting that MN9D cells could serve as an appropriate morphine-dependent cell model.
The dopaminergic MN9D cell line originates from a compound of primary, mouse, embryonic ventral mesencephalic cells and neuroblastoma. The cells synthesize, release, and absorb dopamine and express TH [21,22]. The dopamine in MN9D cells does not easily convert to norepinephrine; additionally, MN9D cells exhibit lower absorption characteristics of catecholamine compared with SH-SY5Y cells [23]. Therefore, MN9D cells are widely used in the cellular level studies of dopamine nerve injury, such as the mechanism and therapy studies associated with PD [24]. At present, SH-SY5Y cells are commonly used to study the mechanism of dopaminergic addiction. SH-SY5Y cells, constructed in 1970, are derived from metastases of a neuroblastoma patient. The SH-SY5Y cells express mid-level dopamine-β-hydroxy enzyme activity [4]. However, some reports in recent years have indicated that there is not abundant dopamine or norepinephrine stored in the SH-SY5Y cells, and dopamine can effectively convert to norepinephrine. These results have illustrated that these cells are more suitable for studies related to noradrenergic neurons. According to the origin of MN9D cells and the characteristics of higher dopamine levels, we speculated that MN9D cells could be more suitable for studying the mechanisms of dopaminergic addiction at the cellular level. Consistent with our hypothesis, MN9D cells express the μ, κ, and δ opioid receptors at high levels, and have a significantly greater ratio of dopaminergic neurons than SH-SY5Y cells. Taken together, our data reveal that MN9D cells are the ideal cell line for further studying the mechanisms of dopaminergic addiction of morphine.
Acknowledgements
This study was supported by the national college students’ innovation and entrepreneurship training program (Grant NO. 201210089007).
Disclosure of conflict of interest
None.
References
- 1.D’Ardenne K, Lohrenz T, Bartley KA, Montague PR. Computational heterogeneity in the human mesencephalic dopamine system. Cogn Affect Behav Neurosci. 2013;13:747–56. doi: 10.3758/s13415-013-0191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt EF, Sutton MA, Schad CA, Karanian DA, Brodikn ES, Self DW. Extinction training regulates tyrosine hydroxylase during withdrawal from cocaine self-administration. J Neurosci. 2001;21:RC137. doi: 10.1523/JNEUROSCI.21-07-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollé P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 4.Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1998;38:3751–3757. [PubMed] [Google Scholar]
- 5.Choi HK, Won L, Roback JD, Wainer BH, Heller A. Specific modulation of dopamine expression in neuronal hybrid cells by primary cells from different brain regions. Proc Natl Acad Sci U S A. 1992;89:8943–8947. doi: 10.1073/pnas.89.19.8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niu HY, Zhang XW, Liu HD, Gao DS. Establishment of injury model of dopaminergic MN9D cells. Acta Academiae Medicinae Xuzhou. 2010;30:301–304. [Google Scholar]
- 7.Spittau B, Zhou X, Ming M, Krieglstein K. IL6 protects MN9D cells and midbrain dopaminergic neurons from MPP+-induced neurodegeneration. Neuromolecular Med. 2012;14:317–27. doi: 10.1007/s12017-012-8189-7. [DOI] [PubMed] [Google Scholar]
- 8.Gil M, McKinney C, Lee MK, Eells JB, Phyillaier MA, Nikodem VM. Regulation of GTP cyclohydrolase I expression by orphan receptor Nurr1 in cell culture and in vivo. J Neurochem. 2007;101:142–150. doi: 10.1111/j.1471-4159.2006.04356.x. [DOI] [PubMed] [Google Scholar]
- 9.Alavian KN, Scholz C, Simon HH. Transcrip tional regulation of mesencephalic dopaminergic neurons: the full circle of life and death. Mov Disord. 2008;23:319–328. doi: 10.1002/mds.21640. [DOI] [PubMed] [Google Scholar]
- 10.García-Yagüe ÁJ, Rada P, Rojo AI, Lastres-Becker I, Cuadrado A. Nuclear import and export signals control the subcellular localization of Nurr1 protein in response to oxidative stress. J Biol Chem. 2013;288:5506–17. doi: 10.1074/jbc.M112.439190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao YM, Zhang HY, Xu QY, Lu FY. Regulation of prostaglandin E2 on orphan nuclear receptor Nurr1 expression in MN9D cells. Chinese Pharmacological Bulletin. 2009;25:26–29. [Google Scholar]
- 12.Vargas-Perez H, Bahi A, Bufalino MR, Ting-A-Kee R, Maal-Bared G, Lam J, Fahmy A, Clarke L, Blanchard JK, Larsen BR, Steffensen S, Dreyer JL, van der Kooy D. BDNF signaling in the VTA links the drug-dependent state to drug withdrawal aversions. J Neurosci. 2014;34:7899–909. doi: 10.1523/JNEUROSCI.3776-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyons D, de Jaeger X, Rosen LG, Ahmad T, Lauzon NM, Zunder J, Coolen LM, Rushlow W, Laviolette SR. Opiate exposure and withdrawal induces a molecular memory switch in the basolateral amygdale between ERK1/2 and CaMIIβ-dependent signaling substrates. J Neurosci. 2013;33:14693–704. doi: 10.1523/JNEUROSCI.1226-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlosburg JE, Vendruscolo LF, Bremer PT, Lockner JW, Wade CL, Nunes AA, Stowe GN, Edwards S, Janda KD, Koob GF. Dynamic vaccine blocks relapse to compulsive intake of heroin. Proc Natl Acad Sci U S A. 2013;110:9036–41. doi: 10.1073/pnas.1219159110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 16.Pitchers KK, Coppens CM, Beloate LN, Fuller J, Van S, Frohmader KS, Laviolette SR, Lehman MN, Coolen LM. Endogenous opioid-induced neuroplasticity of dopaminergic neurons in the ventral tegmental area influences natural and opiate reward. J Neurosci. 2014;34:8825–36. doi: 10.1523/JNEUROSCI.0133-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu J, Cao GF, Dang YH, Chen T. Structural plasticity associated with drugs addiction. Sheng Li Ke Xue Jin Zhan. 2011;42:413–8. [PubMed] [Google Scholar]
- 18.Si JY, Li ZH, Chen Y, Nie SD, Jiang L, Gu SD, Zhang L. Morphological changes of ventral tegmental area DA neurons in morphine withdrawal rats. Chinese Journal of Drug Dependence. 2003;12:180–183. [Google Scholar]
- 19.Mazei-Robison MS, Nestler EJ. Opiate-induced molecular and cellular plasticity of ventral tegmental area and locus coeruleus catecholamine neurons. Cold Spring Harb Perspect Med. 2012;2:a012070. doi: 10.1101/cshperspect.a012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci U S A. 1996;93:11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heller A, Price S, Won L. Glial-derived neurotrophic factor (GDNF) induced morphological differentiation of an immortalized monoclonal hybrid dopaminergic cell line of mesencephalic neuronal origin. Brain Res. 1996;725:132–136. doi: 10.1016/0006-8993(96)00345-9. [DOI] [PubMed] [Google Scholar]
- 22.Han BS, Hong HS, Choi WS, Markelonis GJ, Oh TH, Oh YJ. Caspase-dependent and -independent cell death pathways in primary cultures of mesencephalic dopaminergic neurons after neurotoxin treatment. J Neurosci. 2003;23:5069–5078. doi: 10.1523/JNEUROSCI.23-12-05069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balasooriya IS, Wimalasena K. Are SH-SY5Y and MN9D Cell Lines Truly Dopaminergic? The FASEB Journal. 2007;21:912.9. [Google Scholar]
- 24.Rick CE, Ebert A, Virag T, Bohn MC, Surmeier DJ. Differentiated dopaminergic MN9D cells only partially recapitulate the electrophysiological properties of midbrain dopaminergic neurons. Dev Neurosci. 2006;28:528–537. doi: 10.1159/000095115. [DOI] [PubMed] [Google Scholar]


