Abstract
Background: Histone H2AX phosphorylation is a sensitive marker for DSB which contributes to both genomic instability and cancer treatment. Monitoring its formation may be a sensitive means to monitor cancer progression and treatment effect. Objective: To define the role of phospho-H2AX (pH2AX) expression in development and prognosis of epithelial ovarian cancer (EOC). Methods: The expression of pH2AX in 87 EOC samples and 28 samples of normal ovarian tissues were examined by immunohistochemistry (IHC). The results were semi-quantitatively scored and analyzed by chi-square test. The overall survival time (OS) and disease free interval (DFI) were collected by follow-up and analyzed by Kaplan-Meier analysis. Results: The expression level of pH2AX protein in EOC were higher than that in normal tissues (P<0.001). Among the sensitive cases, high expression of pH2AX was found in 53.2% cases while for resistant cases, high expression rate was 80% (P=0.025). However, pH2AX expression was not significantly correlated with age, histopathological type, tumor differentiation, lymph node metastasis or FIGO stages. Kaplan-Meier analysis found that DFI was negatively correlated with the pH2AX expression, where higher expression of pH2AX resulted in shorter DFI while no OS difference was detected in our study. Conclusion: pH2AX may be used to detect EOC at an early stage and identify women at higher risk for relapse.
Keywords: Histone H2AX, epithelial ovarian cancer, immunohistochemistry, overall survival, disease free interval, Kaplan-Meier analysis
Introduction
The DNA double-strand break (DSB) is a serious lesion that can initiate genomic instability which may lead to cancer [1]. H2AX is rapidly phosphorylated at serine 139 by members of the phosphatidyl inositol 3-kinase family of kinases in response to DSB [2]. H2AX is a member of the histone H2A family, one of the five families of histones that package and organize eukaryotic DNA into chromatin. The basic subunit of chromatin, the nucleosome contains two H2A molecules, of which the percentage of H2AX varies from 2% to 20% in different cell types [3]. Histone H2AX phosphorylation is a sensitive marker for DSB which contributes to both genomic instability and cancer treatment, so monitoring their formation may be a sensitive means to monitor cancer progression and treatment effect [4].
So far, H2AX has generated much scientific interest because of its localization in highly vulnerable cytogenetic regions, such as 11q23, which is known to undergo frequent alteration in most human cancers, including lymphomas, leukaemia and breast cancer [5,6].
As a consensus, mutations in BRCA1 and BRCA2 genes confer an increased risk for breast and ovarian cancer. Study found that pH2AX nuclear staining levels were significantly higher in BRCA1/2 mutation-positive ovarian epithelium compared with the control ovarian epithelium [7]. Ovarian cancer patients with mutations of BRCA1 or BRCA2 have increased sensitivity to platinum chemotherapy, thus improving their overall survival outcome [8,9]. Thus, the objective of this article is to find out some clues of relationship between pH2AX expression and epithelial ovarian cancer (EOC), especially aims to define the role of pH2AX expression in development and prognosis of EOC.
Materials and methods
Patients and tissue specimens
Archival formalin fixed paraffin-embedded (FFPE) specimens of 87 EOC patients admitted to West China Second Hospital of Sichuan University from 2001 to 2013 were included into this retrospective study. 87 cases with clinical data were available for immunohistochemical studies (Table 1). 18 of the 87 EOC patients with normal peritumoral tissue, together with another 10 specimens from normal ovarian tissues were obtained to be the control group. The tumors were confirmed as malignant after surgery by pathologists from West China Second Hospital of Sichuan University. Information of age, histopathological type, stage of disease, resident lesion, lymph node metastasis and response to the first-line chemotherapy were retrieved from the medical records. Disease free interval (DFI) and overall survival time (OS) were obtained through follow-up by phone or mail. In the end, 24 cases lost to follow-up and 63 cases got the complete survival data. The study was approved by the Ethics Committees of West China Second Hospital of Sichuan University. Informed consent was obtained from all participating patients.
Table 1.
Clinical characteristics of included EOC patients
| Characteristics | Cases (N/%) |
|---|---|
| n | 87 |
| Age | |
| ≤50 y | 37/42.5 |
| >50 y | 50/57.5 |
| Histology | |
| serous | 43/49.4 |
| mucous | 6/6.9 |
| Clear cell | 14/16.1 |
| endometrioid | 9/10.3 |
| other | 15/17.3 |
| FIGO stages | |
| I | 21/24.1 |
| II | 6/6.9 |
| III | 59/67.8 |
| IV | 1/1.2 |
| Differentiation | |
| Grade 1/2 | 17/19.5 |
| Grade 3 | 55/63.2 |
| unknown | 15/17.3 |
| Lymph node metastasis | |
| negative | 39/44.8 |
| positive | 15/17.3 |
| unknown | 33/37.9 |
| Resident lesion | |
| none | 41/47.2 |
| ≤0.5 cm | 6/6.9 |
| ≤1 cm | 14/16.1 |
| >1 cm | 19/21.8 |
| unknown | 7/8.0 |
Immunohistochemistry
For immunohistochemistry, 3 μm-thick sections cut from the FFPE tissue blocks were deparaffinized and rehydrated using xylene and a graded series of ethanol (absolute, 95%, 85%, 75%), followed by two 3 min washes in phosphate buffered saline (PBS). Antigen retrieval was performed in EDTA (10 mmol/L, pH 9.0), which was microwaved at 90-100°C for 20 min and washed in PBS for 3×3 min. The sections were then incubated for 10 min in 3% (v/v) hydrogen peroxide to block endogenous peroxidase activity, washed in PBS for 3×3 min, blocked at room temperature for 10 min by using 2% normal goat serum, and incubated in a humidified chamber overnight at 4°C with the primary antibodies anti- phospho-histone H2AX (1:50 dilution, CST, US). The sections were then washed in PBS (3×5 min) and incubated at 37°C for 30 min with the secondary antibodies (goat-anti-rabbit, SP-9001, Zhong-shan Golden Bridge Inc, China). After a wash with PBS (3×5 min), the sections were incubated with ready-to-use streptavidin peroxidase (SP-9001, Zhong-shan Golden Bridge Inc, China) at 37°C for 15 min and well rinsed with PBS (3×5 min). Colors were developed with a DAB kit (Boster Inc, China). The sections were then counterstained with hematoxylin, dehydrated, and mounted. Negative controls were prepared by substituting PBS for the primary antibodies.
Immunoreactivity scoring
For evaluation of pH2AX protein expression, a reproducible semi-quantitative method that takes both staining intensity and area scores into account was adopted.
The staining intensity was scored as follows: 0 (negative), 1 (weak staining=light yellow), 2 (moderate staining=yellow brown) and 3 (strong staining=brown or black). The staining area was the percentage of positive tumor cells, which was scored as follows: 0 (0-9% tumor cells stained), 1 (10%-30% positive tumor cells), 2 (31%-50% positive tumor cells), 3 (51%-80% positive tumor cells), 4 (81%-100% positive tumor cells) [10]. Five photos (×400) were taken randomly for each specimen and the final immunoreactivity score (IS) for each photo was obtained by adding the staining intensity and area scores. The IS for each specimen was the average value of the five photos. Using the value 4 of IS as a cut-off value, pH2AX expression was divided into pH2AX-high (IS≥4) and pH2AX-low (IS<4) groups. Each section was assessed by two histopathologists independently, who were blinded to patients’ information. Positive samples were defined as those showing brown signals in the nuclei and/or cytoplasm of cells.
Statistical analysis
As the study endpoints, DFI refers to the period of time without disease recurrence or progression after achieving complete clinical remission (CCR) following debulking surgery and first-line chemotherapy. Overall survival time (OS) is the time from surgery until the date of death or last follow-up. To assess correlations of prognostic and clinical variables with pH2AX expression, chi-square test and Fisher’s exact test were used for categorical variables. Chi-square test was also performed to compare the expression of pH2AX between tumor tissues and normal ovarian tissues. DFI and OS were estimated with Kaplan-Meier analysis with a log-rank score for determining statistical significance. All P values were two-sided. A P≤0.05 was considered statistically significant. All statistical analysis was performed using SPSS19.0 for Windows (IBM, US).
Results
Increased pH2AX expression in EOC
According to the IS, we compared 87 samples of EOC with 28 samples from benign tissues (18 from adjacent part of tumor and 10 from normal ovaries). pH2AX immunoreactivity was predominantly localized in the nuclei of the tumor cell; however, cytoplasm staining was also evident in some cancer cells especially in the mucous carcinoma (Figure 1D). Most benign cells were not stained while high and moderate staining could be easily found in the cancer cells (Figure 1). High expression of pH2AX was found in 64.4% (56/87) of EOC tissues while low expression was found in 75% of normal tissues (21/28). The expression level of pH2AX protein in EOC were higher than that in normal tissues (P<0.001, Table 2).
Figure 1.
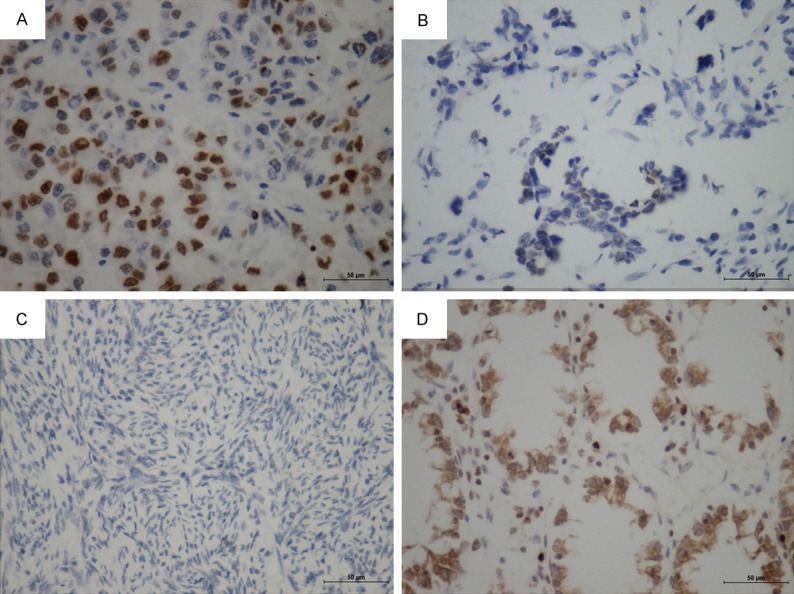
Expression of pH2AX in EOC and normal ovarian tissue. A. High expression of pH2AX in clear cell carcinoma (×400). B. Low expression of pH2AX in serous carcinoma (×400). C. Negative expression of pH2AX in normal ovarian tissue (×400). D. Cytoplasm staining was also evident in some cancer cells (mucous carcinoma, x400).
Table 2.
Comparison of pH2AX expression between EOC tissues and normal ovarian tissues
| Tissue | pH2AX high expression (N) | pH2AX low expression (N) | P value |
|---|---|---|---|
| EOC | 56 | 31 | P<0.001 |
| Normal ovary | 7 | 21 |
Association of expression level of pH2AX with the clinicopathological parameters of EOC
In order to assess the clinical role of pH2AX in EOC, the correlations between its expression level and clinicopathological parameters, including age, histopathological type, tumor differentiation, lymph node metastasis, FIGO stage, size of resident lesion and response to chemotherapy were analyzed (Table 3). Among 87 cases examined, statistical analysis showed that pH2AX expression was not significantly correlated with age, histopathological type, tumor differentiation, lymph node metastasis, FIGO stage or size of resident lesion in EOC patients (P>0.05). Interestingly, significant correlation was noted in the response to the chemotherapy (P=0.025). Among the sensitive cases (CCR lasts for more than 6 months after first-line chemotherapy), high expression of pH2AX was found in 53.2% (25/47) cases while for resistant cases (CCR lasts for less than 6 months or disease could not be controlled during chemotherapy), high expression rate was 80% (20/25).
Table 3.
Relationship between pH2AX expression and different clinicopathological parameters
| pH2AX expression | P value | |||
|---|---|---|---|---|
|
|
||||
| High (%) | Low (%) | |||
| Age | ≤50 y | 27 (72.9) | 10 (27.1) | 0.149 |
| >50 y | 29 (58) | 21 (42) | ||
| Histology | Serous | 28 (65.1) | 15 (35.9) | 0.497 |
| Mucos | 3 (50) | 3 (50) | ||
| Clear cell | 11 (78.6) | 3 (21.4) | ||
| Endometrioid | 4 (44.4) | 5 (55.6) | ||
| Others | 10 (76.9) | 3 (23.1) | ||
| FIGO stage | I+II | 18 (66.7) | 9 (23.3) | 0.764 |
| III+IV | 38 (63.3) | 22 (26.7) | ||
| Differentiation | Grade 1&2 | 12 (70.6) | 5 (29.4) | 0.359 |
| Grade 3 | 32 (58.2) | 23 (41.8) | ||
| Lymph node metastasis | negative | 24 (61.5) | 15 (38.5) | 0.917 |
| positive | 9 (60) | 6 (40) | ||
| Resident lesions | none | 23 (56.1) | 18 (43.9) | 0.229 |
| ≤1 cm | 13 (65) | 7 (35) | ||
| >1 cm | 15 (78.9) | 4 (21.1) | ||
| Chemotherapy response | sensitive | 25 (53.2) | 22 (46.8) | 0.025 |
| resistant | 20 (80) | 5 (20) | ||
Expression of pH2AX for different survival outcome in EOC patients
Expression level of pH2AX was evaluated for correlations with OS and DFI by using Kaplan-Meier analysis. The results showed that patients’ DFI was negatively correlated with the pH2AX expression, where higher expression of pH2AX resulted in shorter DFI (Figure 2A). Of the available 63 EOC patients, the median DFI was 9.21 months and 31.56 months in pH2AX high expression and low expression group respectively (P=0.007). However, it did not show any significant correlation with the OS (Figure 2B). The median survival time was 24.96 months in pH2AX high expression group and 30.58 months in low expression group (P=0.341).
Figure 2.
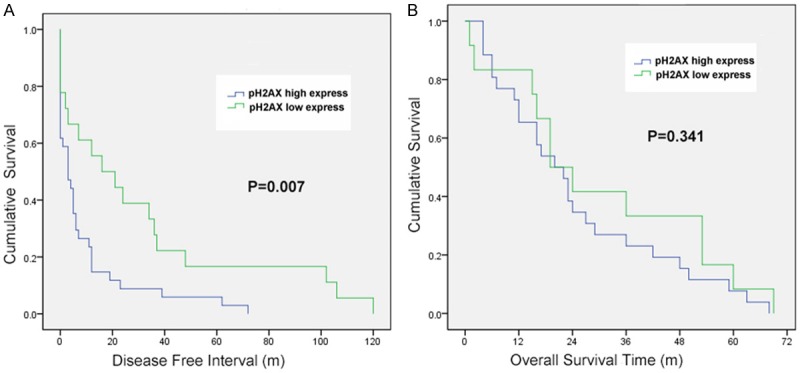
A. Disease free survival curves of patients with EOC, subdivided according to pH2AX expression. B. Overall survival curves of patients with EOC, subdivided according to pH2AX expression.
Discussion
Ovarian cancer has the highest mortality rate of all gynecological cancers. There were an estimated 23, 8719 new cases, resulting in 15,1917 deaths in 2012 [11]. EOC accounts for approximately 90% of all cases of ovarian cancer and debulking surgery following first-line chemotherapy is the standard treatment which results in CCR in up to 75% [12]. Despite high response rates, the recurrence and mortality rates are high, thus, to find an early diagnostic index and reliable prognostic marker for EOC is very essential during the tumor treatment.
A previous study [13] has demonstrated increased levels of DSBs in tumour cells in clinical specimens from various tissues, as well as in tumour cell cultures. Histone H2AX phosphorylation is a sensitive marker for DSB and in our study, pH2AX expression level is significantly higher in EOC than in normal ovarian tissue, which indicates higher endogenous genomic instability in cancer tissues. Though there has not been any precancerous lesion found in ovarian cancer, pH2AX level may serve to detect cancer lesions at the early stage. Studies found that the 5-year overall survival for early stage of EOC ranges from 50-95% while only 20-30% for advanced EOC [14], so the earlier we detect the tumor, the better prognosis the patients may have.
The H2AX gene is not essential, but its absence shows increased genomic instability and sensitivity to DNA damaging agents [15]. A previous study found H2AX gene was regulated by miR-24-2 which affected apoptotic and proliferation pathways of cancer cells. Overexpression of miR-24-2 induces apoptosis by downregulating the expression of H2AX gene [16]. Our study used semi-quantitative IHC assessment to test the different expression levels of pH2AX in platinum-sensitive and platinum-resistant cases, which showed the sensitive group had lower expression level of pH2AX than resistant group. It might be caused by absence of H2AX gene or downregulation of micro RNAs or other pathways, which needs to be further studied.
Tumor with high expression level of pH2AX is associated with a high risk of recurrence. Kaplan-Meier analysis showed that over 80% of patients recurring within the first 6 months after primary treatment had significantly elevated expression of this protein. This subgroup of patients with particularly difficult to treat has not been previously identified by protein or other molecular. Thus, this result allows both identification of women at higher risk for relapse and suggest potential therapeutic targets.
Our study has several limitations. First, though we examined 87 patients, this is still a limited sample size for all clinical correlations examined, which may also be the reason for no OS difference detected in our research. Secondly, IHC is just a qualitative method and it can only provide some clues for the further researches which should be quantitative and more thorough.
Disclosure of conflict of interest
None.
References
- 1.Jeggo PA, Lobrich M. DNA double-strand breaks: their cellular and clinical impact? Oncogene. 2007;26:7717–19. doi: 10.1038/sj.onc.1210868. [DOI] [PubMed] [Google Scholar]
- 2.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo . J Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Driscoll M, Gennery AR, Seidel J, Concannon P, Jeggo PA. An overview of three new disorders associated with genetic instability: LIG4 syndrome, RS-SCID and ATR–Seckel syndrome. DNA Repair (Amst) 2004;3:1227–1235. doi: 10.1016/j.dnarep.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. γH2AX and Cancer. Nat Rev Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newsham IF. The long and short of chromosome 11 in breast cancer. Am J Pathol. 1998;153:5–9. doi: 10.1016/S0002-9440(10)65538-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novik KL, Spinelli JJ, Macarthur AC, Shumansky K, Sipahimalani P, Leach S, Lai A, Connors JM, Gascoyne RD, Gallagher RP, Brooks-Wilson AR. Genetic variation in H2AFX contributes to risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16:1098–1106. doi: 10.1158/1055-9965.EPI-06-0639. [DOI] [PubMed] [Google Scholar]
- 7.Staff S, Tolonen T, Laasanen SL, Mecklin JP, Isola J, Mäenpää J. Quantitative analysis of γ-H2AX and p53 nuclear expression levels in ovarian and fallopian tube epithelium from risk-reducing salpingo-oophorectomies in BRCA1 and BRCA2 mutation carriers. Int J Gynecol Pathol. 2014;33:309–16. doi: 10.1097/PGP.0b013e31829c673b. [DOI] [PubMed] [Google Scholar]
- 8.Cass I, Baldwin RL, Varkey T, Moslehi R, Narod SA, Karlan BY. Improved survival in women with BRCA associated ovarian carcinoma. Cancer. 2003;97:2187–2195. doi: 10.1002/cncr.11310. [DOI] [PubMed] [Google Scholar]
- 9.Tan DS, Rothermundt C, Thomas K, Bancroft E, Eeles R, Shanley S, Ardern-Jones A, Norman A, Kaye SB, Gore ME. “BRCAness” syndrome in ovarian cancer: A case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J. Clin. Oncol. 2008;26:5530–5536. doi: 10.1200/JCO.2008.16.1703. [DOI] [PubMed] [Google Scholar]
- 10.Brenda JB, Tiffany MH, Julie EG, Yfantis HG, Lee DH, Chanock SJ, Ambs S. Association of breast cancer outcome with status of p53 and MDM2 SNP309. J Natil Can Inst. 2006;98:911–919. doi: 10.1093/jnci/djj245. [DOI] [PubMed] [Google Scholar]
- 11.GLOBOCAN 2012: EStimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Default.aspx.
- 12.Mei L, Chen H, Wei DM, Fang F, Liu GJ, Xie HY, Wang X, Zou J, Han X, Feng D. Maintenance chemotherapy for ovarian cancer. Cochrane Database Syst Rev. 2013:CD007414. doi: 10.1002/14651858.CD007414.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sedelnikova OA, Bonner WM. γH2AX in cancer cells: a potential biomarker for cancer diagnostics, prediction and recurrence. Cell Cycle. 2006;5:2909–2913. doi: 10.4161/cc.5.24.3569. [DOI] [PubMed] [Google Scholar]
- 14.Yong WK, Su MB, Hyunsun L, Yoon JK, Woonq SA. Development of multiplexed bead-based immunoassays for the detection of early stage ovarian cancer using a combination of serum biomarkers. PLoS One. 2012;7:e44960. doi: 10.1371/journal.pone.0044960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassing CH, Chua KF, Sekiguchi J, Suh H, Whitlow SR, Fleming JC, Monroe BC, Ciccone DN, Yan C, Vlasakova K, Livingston DM, Ferguson DO, Scully R, Alt FW. Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc Natl Acad Sci U S A. 2002;99:8173–78. doi: 10.1073/pnas.122228699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srivastava N, Manvati S, Srivastava A, Pal R, Kalaiarasan P, Chattopadhyay S, Gochhait S, Dua R, Bamezai RN. miR-24-2 controls H2AFX expression regardless of gene copy number alteration and induces apoptosis by targeting antiapoptotic gene BCL-2: a potential for therapeutic intervention. Breast Cancer Res. 2011;13:R39. doi: 10.1186/bcr2861. [DOI] [PMC free article] [PubMed] [Google Scholar]


