Abstract
Vesicle-associated membrane protein 8 (VAMP8) gene plays an important role in biological functions like endosomal fusion, sequential granule-to-granule fusion and autophagy. The current research identified VAMP8 acted as a novel oncogene by promoting cell proliferation and therapeutic resistance in glioma. Nevertheless, the association between VAMP8 genes polymorphism and glioma patients has not been well studied. In our study, to explore the association between single nucleotide polymorphisms (SNPs) of VAMP8 gene with glioma risk in the Chinese Han population, we performed a hospital based case-control study (992 cases and 1008 controls). Eight common tagging SNPs of VAMP8 gene were genotyped, while no significant difference in allele or genotype frequency was found between glioma patients and healthy controls. No positive linkage disequilibrium (LD) was detected either. No haplotype distribution was positive. Accordingly, our study suggested that VAMP8 gene variants might not contribute to glioma susceptibility and associated with glioma in the Chinese Han population.
Keywords: VAMP8 gene, polymorphisms, genetic susceptibility, molecular biology, glioma
Introduction
Glioma, arising from glial or precursor cells, is one of the most common brain tumors with the incidence rate of 6.02 per 100,000 [1]. The broad category glioma accounted for nearly 28% of all brain tumors and 80% for malignant brain tumors [1]. Malignant gliomas, the most frequent and deadly malignant brain tumors, are second only to stroke as the leading cause of death from neurological diseases. Despite the development of aggressive surgery, radiation and chemotherapy, and the improvement of diagnosis and detection ability in recent years, the prognosis of glioma still remains dismal and the survival from malignant gliomas remains poor with a median survival time on average 14 months for patients with glioblastoma following diagnosis [2]. Previous studies of gliomas, as well as of other types of cancer, suggested that gliomas might arise from a combination of inherited genetic variation and differential environmental exposures. Previous candidate-gene association studies have revealed several loci that are associated with glioma risk. These include the DNA repair genes PRKDC (also known as XRCC7) [3], XRCC1 [4], PARP1 [5], MGMT [6], ERCC1 and ERCC2 [7]; the cell cycle gene EGFt61 [8]; and the inflammation gene IL13 [9]. However, as the complexity nature of glioma, it still needs the discoveries of more susceptible regions within human genome to provide references for clinical prediction, diagnose and treatment of glioma.
Soluble NSF (nethylmaleimide-sensitive factor) receptor (SNARE) is a superfamily of small proteins with different sizes and primary structures [10]. As an essential mechanism for cellular activities, it’s observed SNARE involved in the progression of various tumors, such as non-small cell lung cancer [11,12]. Vesicle-associated membrane protein 8 (VAMP8) was first identified as an endosomal SNARE and has been revealed to participate in biological functions like endosomal fusion [13], the exocytosis of GLUT4 and insulin [14,15], sequential granule-to-granule fusion [16] and autophagy [17]. More important, Chen et al recently identified VAMP8 acted as a novel oncogene in glioma by promoting cell proliferation and therapeutic resistance [18]. While, the association between VAMP8 gene polymorphisms and glioma has not been well studied.
To date, fewer studies have examined the susceptibility of VAMP8 gene with diseases, and no association studies have been done between VAMP8 polymorphisms and glioma. Therefore, to identify the association between genetic polymorphism of VAMP8 and glioma risk, the authors performed a hospital-based case-control study in a Chinese Han population.
Methods and materials
Patients selection
992 patients histopathologically diagnosed with glioma were from the Department of Neurosurgery at Huashan Hospital (Shanghai, China) between October 2007 and July 2013. And, pathological diagnoses and demographic data of all eligible cases were validated by trained abstractors. All control subjects were randomly selected from trauma outpatients and annual check-up visitors at the same hospital during the similar time period and frequency matched to cases by sex, age (±5 years) and residence (urban or rural areas). The controls with a self-reported history of cancer or central nervous system-related diseases or previously receiving radiotherapy\chemotherapy for certain diseases were excluded. All cases and controls in this study were genetically from Shanghai and its surrounding regions (Jiangsu, Anhui and Zhejiang provinces) in eastern China with a Han Chinese ethnic background.
At recruitment, written informed consent was obtained from each subject and the detailed demographic information, including demographic factors, smoking status, family history of cancer and other lifestyle factors, were collected by trained nurses. For childhood glioma patients under the age of 18 years, baseline data were collected from their parents or other relatives. After interview, ~3-5 ml peripheral venous blood sample were collected from each subject for DNA extraction and genotyping. Our research protocol was approved by the Ethics Committee for Human Subject research of Fudan University.
Selection of tagging SNPs and genotyping assays
We based the r2 statistic to find tagging SNPs according to the HapMap database (http://www.hapmap.org/, phase III Aug10, on NCBI B36 assembly, dbSNP b132; population: Han Chinese in Beijing, China) on the basis of pairwise linkage disequilibrium (LD) r2 threshold of 0.8. A total of eight tagging SNPs in this region were selected for this study.
We used the white blood cell fractions of the whole blood samples for extraction of genomic DNA using the Qiagen Blood Kit (Qiagen, Chatsworth, CA), according to the manufacturer’s instructions. Fragments containing polymorphisms were amplified by the PCR and genotyped with Sequenom MassARRAY iPLEX platform using allele-specific matrix-assisted laser desorption ionization-time-of-flight mass spectrometry assay [19]. We examined genotyping quality by means of a detailed quality control procedure that ensured over 95% successful call rate with duplicate calling of genotypes, internal positive control samples.
Statistical analysis
SPSS software (SPSS, Chicago, IL) was used for statistical analyses. The χ2 test was used to compare the differences in demographic characteristics including sex, smoking statues, family history of cancers, and also frequency distributions of genotypes and alleles between cases and controls. Unconditional logistic regression analysis was performed to calculate ORs and 95% CIs as estimates of the relative risk for each SNP, with adjustment for family history of cancers. All SNPs were required genotyping rate ≥0.95. All P-values presented were two-sided test, and the level of P≤0.05 was considered significant. Haploview software V4.2 was used to assess P-value of HWE and genotyping rate, HWE was performed only among controls, genotyping rate was performed both cases and controls.
The pairwise linkage disequilibrium (LD) among the SNPs was examined using Lewontin’s standardized coefficient D’ and LD coefficient r2 [20], and Haplotype blocks were assessed by the Haploview software given by Gabriel et al [21]. To account for haplotype ambiguity, the statistical significance of the performed global and haplotype-specific tests (haplo.score) was expressed as a permutation P value (minimal simulation: 10,000 with a significance level <0.05) [22].
Results
The distribution of demographic characteristics of the 992 cases and 1008 controls were presented in Table 1. The mean ± SD of age was 44.25±15.73 for cases and 45.18±18.62 for controls. Approximately 60% of both cases and controls were male, corresponding to the previous study [23]. Cases were more likely to report a family history of cancer (among first-degree relatives) than controls (P=0.003), and the smoke status between cases and controls were no significant difference. The histological subtypes were also presented.
Table 1.
Distribution of demographic variables in glioma cases and controls
| No. of Cases | % | No. of Controls | % | P a | |
|---|---|---|---|---|---|
| Demographics | |||||
| Total | 992 | 1008 | |||
| Sex | 0.99 | ||||
| Male | 593 | 59.8 | 603 | 60.9 | |
| Female | 394 | 39.7 | 401 | 38.7 | |
| Missing | 5 | 0.5 | 4 | 0.4 | |
| Age | 0.64b | ||||
| Mean ± SD | 44.25±15.73 | 45.18±18.62 | |||
| Smoke status | 0.99 | ||||
| Never | 586 | 59.1 | 593 | 58.8 | |
| Ever | 209 | 20.7 | 213 | 21.1 | |
| Current | 188 | 19.0 | 192 | 19.1 | |
| Missing | 9 | 1.0 | 10 | 1.0 | |
| Family History of Cancer | 0.003 | ||||
| No | 723 | 72.9 | 781 | 77.5 | |
| Yes | 163 | 16.4 | 120 | 11.9 | |
| Missing | 106 | 10.7 | 107 | 10.6 | |
| Histological types | |||||
| Glioblastoma | 326 | 32.9 | |||
| Other gliomasc | 666 | 67.1 |
The significant differences are indicated in bold.
Two-sided χ2 test.
Independent-samples T-test.
Other gliomas including oligodendrogliomas, ependymomas or mixed glioma.
Our statistics analysis found the allele and genotype distributions show no any significant difference in all the eight SNPs between control and patients. The difference was also not observed in the dominant model with multivariate logistic regression analysis, which is shown in Tables 2 and 3. All SNPs were in Hardy-Weinberg equilibrium in control subjects, the P value were more than 0.05.
Table 2.
Information of 8 genotyped SNPs of gene VAMP8
| SNP ID | Chromosome | Chromosome position | SNP locationa | Base change | Minor allele frequency | Genotyping rate (%) | P-value for HWEc | P-value for χ2 | OR (95% CI)d | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Databaseb | Control | Case | |||||||||
| rs3770098 | 2 | 85658878 | intron | G/A | 0.325 | 0.305 | 0.315 | 97.6% | 0.31 | 0.536 | 0.95 (0.81-1.11) |
| rs7579147 | 2 | 85659165 | intron | A/G | 0.292 | 0.262 | 0.268 | 100% | 0.88 | 0.711 | 0.97 (0.83-1.34) |
| rs3731828 | 2 | 85659777 | synonymous | A/G | 0.244 | 0.262 | 0.268 | 99.9% | 0.92 | 0.711 | 0.97 (0.83-1.34) |
| rs1348818 | 2 | 85660593 | intron | A/G | 0.307 | 0.305 | 0.315 | 100% | 0.16 | 0.536 | 0.95 (0.82-1.11) |
| rs13421434 | 2 | 85661121 | intron | A/G | 0.033 | 0.043 | 0.047 | 99.9% | 0.44 | 0.555 | 0.90 (0.64-1.27) |
| rs1009 | 2 | 85662248 | synonymous | A/G | 0.305 | 0.305 | 0.316 | 100% | 0.15 | 0.536 | 0.95 (0.82-1.11) |
| rs1058588 | 2 | 85662382 | 3’ UTR | A/G | 0.305 | 0.305 | 0.316 | 100% | 0.15 | 0.536 | 0.95 (0.82-1.11) |
| rs1010 | 2 | 85662493 | 3’ UTR | A/G | 0.304 | 0.305 | 0.315 | 99.9% | 0.17 | 0.562 | 0.96 (0.82-1.13) |
Abbreviations: VAMP8, vesicle-associated membrane protein 8: SNP, single nucleotide polymorphism; HWE, Hardy-Weinberg equilibrium. The significant differences are indicated in bold.
SNP location in National Center for Biotechnology Information Genome build 37.3 (http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?).
Minor allele frequency from dbSNP databases.
HWE P-value in control group.
Adjusted for gender, age, smoke and family history of cancer.
Table 3.
Genotype frequencies of SNPs between cases and controls and their associations with glioma risk
| SNP ID | Genotype | No. of cases | % | No. of controls | % | P-value for χ2 testa | Logistic regressiona | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| OR (95% CI) | P-valuea | |||||||
| rs3770098 | CC | 358 | 36.1 | 370 | 36.71 | 0.98 | 1.00 (reference) | |
| AC | 472 | 47.6 | 475 | 47.12 | 1.03 (0.85-1.25) | 0.79 | ||
| AA | 135 | 13.6 | 143 | 14.19 | 0.98 (0.74-1.29) | 0.86 | ||
| Dominant | CC/AC+AA | 607 | 61.2 | 618 | 61.31 | 0.87 | 1.02 (0.85-1.22) | 0.87 |
| rs7579147 | GG | 429 | 43.2 | 434 | 43.06 | 0.97 | 1.00 (reference) | |
| AG | 441 | 44.46 | 457 | 45.34 | 0.98 (0.81-1.18) | 0.80 | ||
| AA | 121 | 12.20 | 117 | 11.61 | 1.05 (0.79-1.39) | 0.76 | ||
| Dominant | GG/GA+AG | 562 | 56.65 | 574 | 56.94 | 0.92 | 0.99 (0.83-1.18) | 0.92 |
| rs3731828 | CC | 429 | 43.25 | 433 | 42.96 | 0.98 | 1.00 (reference) | |
| TC | 441 | 44.46 | 457 | 45.34 | 0.97 (0.81-1.17) | 0.78 | ||
| TT | 120 | 12.10 | 117 | 11.61 | 1.04 (0.78-1.38) | 0.81 | ||
| Dominant | CC/TC+TT | 561 | 56.55 | 574 | 56.94 | 0.88 | 0.99 (0.83-1.19) | 0.88 |
| rs1348818 | TT | 361 | 36.39 | 370 | 36.71 | 0.99 | 1.00 (reference) | |
| AT | 490 | 49.40 | 492 | 48.81 | 1.02 (0.84-1.24) | 0.83 | ||
| AA | 141 | 14.21 | 146 | 14.48 | 0.99 (0.75-1.30) | 0.94 | ||
| Dominant | TT/AT+AA | 631 | 63.61 | 638 | 63.29 | 0.88 | 1.01 (0.85-1.22) | 0.88 |
| rs13421434 | CC | 903 | 91.03 | 916 | 90.87 | 0.97 | 1.00 (reference) | |
| AC | 85 | 8.57 | 91 | 9.03 | 0.95 (0.70-1.29) | 0.73 | ||
| AA | 1 | 0.10 | 1 | 0.10 | 1.01 (0.06-16.2) | 0.99 | ||
| Dominant | CC/AC+AA | 86 | 8.67 | 92 | 9.13 | 0.74 | 0.95 (0.70-1.29) | 0.74 |
| rs1009 | AA | 361 | 36.39 | 369 | 36.61 | 0.99 | 1.00 (reference) | |
| GA | 490 | 49.40 | 492 | 48.81 | 1.02 (0.84-1.23) | 0.86 | ||
| GG | 141 | 14.21 | 146 | 14.48 | 0.99 (0.75-1.30) | 0.93 | ||
| Dominant | AA/GA+GG | 631 | 63.61 | 638 | 63.29 | 0.91 | 1.01 (0.84-1.21) | 0.91 |
| rs1058588 | CC | 361 | 36.39 | 369 | 36.61 | 0.99 | 1.00 (reference) | |
| TC | 490 | 49.40 | 492 | 48.81 | 1.02 (0.84-1.23) | 0.86 | ||
| TT | 141 | 14.21 | 146 | 14.48 | 0.99 (0.75-1.30) | 0.93 | ||
| Dominant | CC/TC+TT | 631 | 63.61 | 638 | 63.29 | 0.91 | 1.01 (0.85-1.21) | 0.91 |
| rs1010 | TT | 361 | 36.39 | 369 | 36.61 | 0.99 | 1.00 (reference) | |
| CT | 490 | 49.40 | 490 | 48.61 | 1.02 (0.84-1.24) | 0.82 | ||
| CC | 141 | 14.21 | 146 | 14.48 | 0.99 (0.75-1.30) | 0.93 | ||
| Dominant | TT/CT+CC | 631 | 63.61 | 636 | 63.10 | 0.88 | 1.01 (0.85-1.22) | 0.88 |
Abbreviations: SNP, single nucleotide polymorphism; OR, odds ratio; CI, confidence interval. The significant differences are indicated in bold.
Adjusted for gender, age, smoke and family history of cancer.
The LD plot was shown in Figure 1. One block was defined, the logistic regression analysis revealed the haplotype was not significant associated with glioma risk either, even after 10000 time permutation test. The results were shown in Table 4. All these results suggested that the VAMP8 polymorphisms were not associated with the glioma risk.
Figure 1.
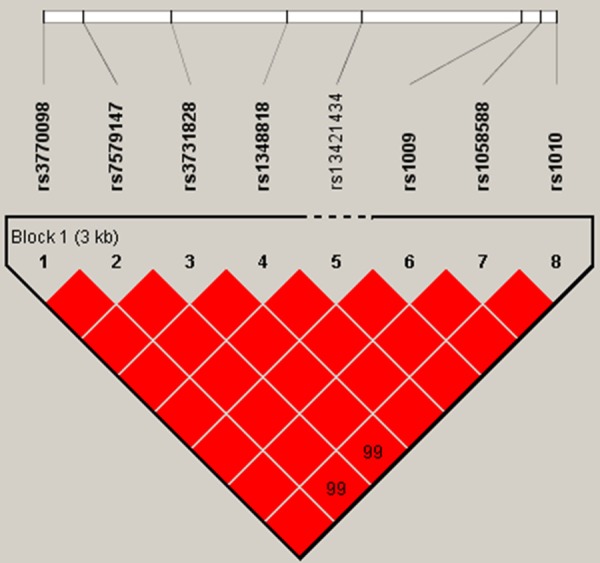
A schematic view of associations between SNPs and glioma risk.
Table 4.
Frequency distributions of haplotypes in each block in VAMP8, and association with glioma risk
| Haplotypea | Overall | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Case (%) | Control (%) | P-value for χ2 testb | Logistic regression | Global score testc | ||
|
| ||||||
| OR (95% CI)b | P-valueb | |||||
| Block | 0.99 | χ2 = 0.1120, df = 2, | ||||
| CGCTACT | 606 (0.611) | 616 (0.611) | 1.00 (reference) | p-value = 0.912, | ||
| AATAGTC | 341 (0.344) | 346 (0.343) | 1.00 (0.83-1.21) | 0.99 | simb = 0.940 | |
| AGCAGTC | 45 (0.045) | 46 (0.046) | 0.99 (0.65-1.52) | 0.97 | ||
Abbreviations: OR, odds ratio; CI, confidence interval.
Polymorphic bases were in 5’-3’ order listed in Table 1.
Adjusted for gender, age, smoke and family history of cancer.
Generated by permutation test with 10000 times simulation.
Discussion
In this case-control study, we examined eight SNPs (rs3770098, rs7579147, rs3731828, rs1348818, rs13421434, rs1009, rs1058588 and rs1010) from 992 glioma patients and 1,008 controls in Chinese population. According to the observed results, there was no positive association between VAMP8 gene and glioma.
VAMP8 is a member of SNARE family, which is a superfamily of small proteins with different sizes and primary structures. VAMP8 has been reported to play multiple roles of in diverse cellular activities like endocytosis [Endobrevin, a novel synaptobrevin/VAMP-like protein preferentially associated with the early endosome], exocytosis [Dual role of VAMP8 in regulating insulin exocytosis and islet beta cell growth], granule fusion [VAMP8 is a SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) selectively required for sequential granule-to-granule fusion] and autophagy [The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes]. Loss of VAMP8 could suppress the normal physiological activities of cells. More importantly, the recent study demonstrated its roles in the prediction of the prognosis and chemotherapy treatment efficacy for glioma patients as well as its regulatory functions in the progression and temozolomide sensitivity of glioma cells. Therefore, we tried to find whether there were any SNPs within the gene region of VAMP8 that were responsible for glioma risk.
In our study, all of these SNPs were in Hardy-Weinberg equilibrium in our samples, but we failed to observe any statistical significance neither in allele and genotypes frequencies of the SNPs nor in the frequency distributions of haplotypes between cases and controls in VAMP8 gene and glioma risk. Among the genotyped SNPs, the current knowledge about the first seven is rather limited since there are no reports indicating their possible roles in any biological activities and diseases. Nevertheless, as to rs1010, the eighth one we detected in our study, there are pieces of evidences showing that it is significantly associated with the risk of noncardioembolic stroke [24], coronary heart disease [24,25], preeclampsia [26] and platelet reactivity [27]. However, there are also controversies arguing against its roles in platelet functions [28] and association with heart disease risk in the elderly [29], which render its precise functions in the aforementioned disorders elusive.
Probably, the following limitations and reasons might be responsible for the results in this study: First, the sample size in this study is not big enough. Despite the number of samples is 2,000, larger number of samples should be incorporated into the study to reach more statistical power. Moreover, samples from different races and meta-analyses are also needed to further test the association; second, the SNP coverage in the present study is also limited. Genetic analyses with more functional SNPs and saturated SNP coverage of the gene region of VAMP8 are necessary to fully reflect the contributions of VAMP8 to glioma risk; third, these results might imply that VAMP8 does not participate in the initiation process of glioma. Despite previous reported functions of VAMP8 in glioma development and temozolomide sensitivity regulation, it does not demonstrate its role in the initiation of glioma. It is possible that VAMP8 itself does not involve in the onset but in the maintenance and progression of glioma, which makes it less likely to exhibit any significant association in glioma risk between the case and control samples. In addition, although our result does not support the association of VAMP8 with glioma, it does not exclude the possibility that this gene confers genetic susceptibility to glioma in other population.
In conclusion, our study found no significant association between the SNPs in VAMP8 gene region and glioma risk, indicating that these variants might not contribute to glioma susceptibility in the Chinese Han population. Although the negative results, the studies based on more SNPs of VAMP8 or other important members of SNARE in glioma as well as other diseases would be worthwhile. And our data may provide a reference for further studies on the role of the gene in other populations.
Acknowledgements
We thank all staff of the Department of Neurosurgery of Huashan Hospital for epidemiology data collection. We further thank all volunteers recruited in this study. This work was partially supported by grants from the National Natural Science Foundation of China (No. 30600577).
Disclosure of conflict of interest
None.
References
- 1.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15:1–56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKean-Cowdin R, Barnholtz-Sloan J, Inskip PD, Ruder AM, Butler M, Rajaraman P, Razavi P, Patoka J, Wiencke JK, Bondy ML, Wrensch M. Associations between polymorphisms in DNA repair genes and glioblastoma. Cancer Epidemiol Biomarkers Prev. 2009;18:1118–1126. doi: 10.1158/1055-9965.EPI-08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiuru A, Lindholm C, Heinävaara S, Ilus T, Jokinen P, Haapasalo H, Salminen T, Christen-sen HC, Feychting M, Johansen C, Lönn S, Malmer B, Schoemaker MJ, Swerdlow AJ, Auvinen A. XRCC1 and XRCC3 variants and risk of glioma and meningioma. J Neurooncol. 2008;88:135–142. doi: 10.1007/s11060-008-9556-y. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Scheurer ME, El-Zein R, Cao Y, Do KA, Gilbert M, Aldape KD, Wei Q, Etzel C. Association and interactions between DNArepair gene polymorphisms andadult glioma. Cancer Epidemiol Biomarkers Prev. 2009;18:204–214. doi: 10.1158/1055-9965.EPI-08-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felini MJ, Olshan AF, Schroeder JC, North KE, Carozza SE, Kelsey KT, Liu M, Rice T, Wiencke JK, Wrensch MR. DNA repair polymorphisms XRCC1 and MGMT and risk of adult gliomas. Neuroepidemiology. 2007;29:55–58. doi: 10.1159/000108919. [DOI] [PubMed] [Google Scholar]
- 7.Wrensch M, Kelsey KT, Liu M, Miike R, Moghadassi M, Sison JD, Aldape K, McMillan A, Wiemels J, Wiencke JK. ERCC1 and ERCC2 polymorphisms and adult glioma. Neuro Oncol. 2005;7:495–507. doi: 10.1215/S1152851705000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa BM, Ferreira P, Costa S, Canedo P, Oliveira P, Silva A, Pardal F, Suriano G, Machado JC, Lopes JM, Reis RM. Association between functional EGFt61 polymorphism and glioma risk. Clin Cancer Res. 2007;13:2621–2626. doi: 10.1158/1078-0432.CCR-06-2606. [DOI] [PubMed] [Google Scholar]
- 9.Amirian E, Liu Y, Scheurer ME, El-Zein R, Gilbert MR, Bondy ML. Genetic variants in inflammation pathway genes and asthma in glioma susceptibility. Neuro Oncol. 2010;12:444–452. doi: 10.1093/neuonc/nop057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng RW, Abellan E, Fussenegger M. Differential effect of exocytic SNAREs on the production of recombinant proteins in mammalian cells. Biotechnol Bioeng. 2011;108:611–620. doi: 10.1002/bit.22986. [DOI] [PubMed] [Google Scholar]
- 11.Lau SK, Boutros PC, Pintilie M, Blackhall FH, Zhu CQ, Strumpf D, Johnston MR, Darling G, Keshavjee S, Waddell TK, Liu N, Lau D, Penn LZ, Shepherd FA, Jurisica I, Der SD, Tsao MS. Three-gene prognostic classifi er for early-stage non small-cell lung cancer. J. Clin. Oncol. 2007;25:5562–5569. doi: 10.1200/JCO.2007.12.0352. [DOI] [PubMed] [Google Scholar]
- 12.Hasan N, Hu C. Vesicle-associated membrane protein 2 mediates traffi cking of alpha5beta1 integrin to the plasma membrane. Exp Cell Res. 2010;316:12–23. doi: 10.1016/j.yexcr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Wong SH, Zhang T, Xu Y, Subramaniam VN, Griffi ths G, Hong W. Endobrevin, a novel synaptobrevin/VAMP-like protein preferentially associated with the early endosome. Mol Biol Cell. 1998;9:1549–1563. doi: 10.1091/mbc.9.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu D, Zhang Y, Lam PP, Dolai S, Liu Y, Cai EP, Choi D, Schroer SA, Kang Y, Allister EM, Qin T, Wheeler MB, Wang CC, Hong WJ, Woo M, Gaisano HY. Dual role of VAMP8 in regulating insulin exocytosis and islet beta cell growth. Cell Metab. 2012;16:238–249. doi: 10.1016/j.cmet.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Zhao P, Yang L, Lopez JA, Fan J, Burchfi eld JG, Bai L, Hong W, Xu T, James DE. Variations in the requirement for v-SNAREs in GLUT4 traffi cking in adipocytes. J Cell Sci. 2009;122:3472–3480. doi: 10.1242/jcs.047449. [DOI] [PubMed] [Google Scholar]
- 16.Behrendorff N, Dolai S, Hong W, Gaisano HY, Thorn P. Vesicle-associated membrane protein 8 (VAMP8) is a SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) selectively required for sequential granule-to-granule fusion. J Biol Chem. 2011;286:29627–29634. doi: 10.1074/jbc.M111.265199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itakura E, Kishi-Itakura C, Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell. 2012;151:1256–1269. doi: 10.1016/j.cell.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Meng D, Wang H, Sun R, Wang D, Wang S, Fan J, Zhao Y, Wang J, Yang S, Huai C, Song X, Qin R, Xu T, Yun D, Hu L, Yang J, Zhang X, Chen H, Chen J, Chen H, Lu D. VAMP8 facilitates cellular proliferation and temozolomide resistance in human glioma cells. Neuro Oncol. 2014 doi: 10.1093/neuonc/nou219. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Donnell M, Little D, Braun A. MassArray as an enabling technology for the industrial-scale analysis of DNA. Genetic Engineering News. 1997;17:39–43. [Google Scholar]
- 20.Lewontin R. On measures of gametic disequilibrium. Genetics. 1988;120:849–852. doi: 10.1093/genetics/120.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 22.Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA. Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet. 2002;70:425–434. doi: 10.1086/338688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Zhang H, Zhou K, Chen L, Xu Z, Zhong Y, Liu H, Li R, Shugart YY, Wei Q, Jin L, Huang F, Lu D, Zhou L. Tagging SNPs in non-homologous end-joining pathway genes and risk of glioma. Carcinogenesis. 2007;28:1906–1913. doi: 10.1093/carcin/bgm073. [DOI] [PubMed] [Google Scholar]
- 24.Luke MM, Lalouschek W, Rowland CM, Catanese JJ, Bolonick JI, Bui ND, Greisenegger S, Endler G, Devlin JJ, Mannhalter C. Polymorphisms associated with both noncardioembolic stroke and coronary heart disease: vienna stroke registry. Cerebrovasc Dis. 2009;28:499–504. doi: 10.1159/000236914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bare LA, Morrison AC, Rowland CM, Shiffman D, Luke MM, Iakoubova OA, Kane JP, Malloy MJ, Ellis SG, Pankow JS, Willerson JT, Devlin JJ, Boerwinkle E. Five common gene variants identify elevated genetic risk for coronary heart disease. Genet Med. 2007;9:682–689. doi: 10.1097/gim.0b013e318156fb62. [DOI] [PubMed] [Google Scholar]
- 26.Lykke JA, Bare LA, Olsen J, Lagier R, Tong C, Arellano A, Paidas MJ, Langhoff-Roos J. Vascular associated gene variants in patients with preeclampsia: results from the Danish National Birth Cohort. Acta Obstet Gynecol Scand. 2012;91:1053–1060. doi: 10.1111/j.1600-0412.2012.01479.x. [DOI] [PubMed] [Google Scholar]
- 27.Kondkar AA, Bray MS, Leal SM, Nagalla S, Liu DJ, Jin Y, Dong JF, Ren Q, Whiteheart SW, Shaw C, Bray PF. VAMP8/endobrevin is overexpressed in hyperreactive human platelets: suggested role for platelet microRNA. J Thromb Haemost. 2010;8:369–378. doi: 10.1111/j.1538-7836.2009.03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaussem P, Ishida BY, Fontana P, Pullinger CR, Khane JP, Aiach M, Bachelot-Loza C, Gandrille S. No influence of the VAMP8 rs1010 single nucleotide polymorphism on platelet functions in vitro. J Cell Mol Med. 2009;13:601–603. doi: 10.1111/j.1582-4934.2009.00500_2.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akao H, Polisecki E, Kajinami K, Trompet S, Robertson M, Ford I, Jukema JW, de Craen AJ, Westendorp RG, Shepherd J, Packard C, Buckley BM, Schaefer EJ. KIF6, LPA, TAS2R50, and VAMP8 genetic variation, low density lipoprotein cholesterol lowering response to pravastatin, and heart disease risk reduction in the elderly. Atherosclerosis. 2012;220:456–462. doi: 10.1016/j.atherosclerosis.2011.11.037. [DOI] [PubMed] [Google Scholar]


