Abstract
Laryngeal squamous cell carcinoma is a common malignant tumor of otolaryngeal region. At present, effective treatment of laryngeal squamous cell carcinoma still depends on surgery and radiotherapy. In recent years, application of CO2 laser resection in the treatment of stage T1 glottic carcinoma can remove the tumor completely and reduce the injury of laryngeal tissues. But recurrence still happened in some postoperative patients. Here, we selected 131 patients to compare the therapeutic effects of CO2 laser resection and traditional split laryngeal surgery on the early laryngeal cancer, examined the expression of p27 and PTEN by immunohistochemistry in early laryngeal squamous cell carcinoma tissues in correlation to clinical outcome. After two years follow-up 14/85 (16.5%) of CO2 laser treatment group presented with local recurrence (recurrent group), while that of split laryngeal surgery group was 6/46 (13.0%). There was no statistical significance in recurrence rate between the two groups (P > 0.05). 10 of all the 111 (9.0%) non-recurrent patients did not follow the doctor’s advice to quit smoking after the operation, while 12 in the 20 (60.0%) recurrent patients did not; the difference between the two groups was statistically significant (P < 0.01). The positive rates of p27 were 80.2% (105/131) and 43.5% (57/131), and that of PTEN were 83.2% (109/131) and 48.9% (64/131) in the cancer adjacent tissues (negative surgical margin tissues) and in laryngeal carcinoma tissues, respectively (P < 0.001). The expression rates of p27 and PTEN in laryngeal carcinoma tissues of the recurrent group were 20.0% (4/20), 10.0% (2/20) and that in non recurrent group were 47.7% (53/111) and 55.9% (62/111), respectively, with a significant difference (P < 0.001). In addition, the expression of p27 and PTEN in tumor resected marginal tissues of the recurrence group was 50.0% (10/20), 40.0% (8/20) and that in non recurrence group was 85.6% (95/111) and 91.0% (101/111), respectively; the difference was also statistically significant between both groups (P < 0.001). In conclusion, there is no statistically significant difference in tumor recurrence rate between CO2 laser surgery and traditional split laryngeal surgery. Postoperative recurrence is closely related to resume smoking. The recurrence rate of p27 and/or PTEN-negative patients was higher than that of the positive ones,that should be followed up closely after treatment.
Keywords: CO2 laser resection, Laryngeal squamous cell carcinoma, p27, PTEN, recurrence
Introduction
Supraglottic cancer is the most common type of laryngeal squamous cell carcinoma [1]. In recent years the application of CO2 laser in treating stage T1 cancer of the vocal cords can completely resect the tumor and reduce the occurrence of laryngeal dysfunction, but there are still some patients with recurrence, and the recurrence rate and cervical lymph node metastasis rate hover between 10-50% [2]. The main factor affecting the prognosis of patients with laryngeal cancer with CO2 laser resection has not been fully elucidated yet. Thus, to figure out the postoperative recurrence factors of the tumor became a very important issue for its early intervention.
Here, we analyzed the clinical factors and tumor markers that may indicate cancer recurrence in order to select the rational treatment of laryngeal squamous cell carcinoma and guide clinicians to choose the appropriate treatment for prevention of the recurrence and improvement of the prognosis of the disease.
Material and methods
Cases description
Our study involved 85 patients who underwent CO2 laser resection surgery at Tangshan Union Hospital from February, 2004 to October, 2010, including 78 males and 7 females, aged 48 to 75 years, with mean (59.3 ± 14.7) years, according to the AJCC Cancer Staging, they were all belonged to stage T1 that was subdivided into T1a and T1b, including 58 cases in stage T1a and 27 cases in stage T1b. Another 46 patients who underwent laryngeal split surgery were also selected at Tangshan Union Hospital from January, 2000 to December, 2004, including 41 males and 5 females, aged 45 to 70 years, with mean (52.3 ± 14.7) years, among which 30 cases were in stage T1a and 16 cases in stage T1b. All the 131 patients involved in the study have been informed research content and signed the informed consent voluntarily. The study was approved by the Ethics Committee of Tangshan Union Hospital. All specimens were retained 5 mm as adjacent tissues (negative surgical margins). After surgery all patients were advised to quit smoking.
Tumor-associated molecular protein expression by immunohistochemistry
All the surgically removed tissue specimens were fixed in 10% neutral formalin, embedded in paraffin, and one representative block from each patient was sectioned at 4 μm, stained with hematoxylin and eosin (HE) and evaluated by immunohistochemistry according to the protocol described in the manufacturer’s guide accompanying the kit. Invasive breast cancer samples with p27 and PTEN expression were used as positive immunostaining controls. For the negative control, the primary antibodies were replaced with phosphate-buffered saline (PBS). The mouse monoclonal antibody against human p27 (clone number: SX53GB, 1:100) and PTEN (clone number: ZA-0251, 1:40) were all purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. SP immunohistochemistry kit was purchased from Maxim Biotechnology Development Co, Fuzhou, China.
Evaluation of immunohistochemical analysis
Positive reaction of the immunohistochemical staining showed brown or brownish yellow in color. PTEN expressed in the nucleus of cancer cells. Positive signals of p27 located in nucleus, no cytoplasmic staining and part of the inflammatory cells around cancer cells may be stained occasionally. 10 high magnification visions were selected randomly in each stained section and 10 high power field representatives was observed, the brown nuclear staining cells were observed and counted. Positive staining in more than 10% of the cells was considered positive, while less than 10% or colorless were defined as negative.
Statistical analysis
The statistical analyses were performed with PASW Statistics 18.0 (SPSS Inc., Chicago, IL, U S A). Recurrence rates of the two surgical methods, the relationships between the expression of p27, PTEN and the various clinicopathological findings were evaluated using the Chi-square test, and the Spearman Chi-square test rank to evaluate correlation between the expression of two proteins. P values less than 0.05 were considered to be statistically significant.
Results
Difference between the CO2 laser resection and split laryngeal surgery
During follow-up, 20 patients (recurrence group) presented with recurrence that underwent CO2 laser resection or split laryngeal surgery of the total 131 patients. 111 cases were without recurrence (non-recurrence group). Among them, 14 cases presented with local recurrence in 85 patients treated with CO2 laser resection surgery, the recurrence rate was 16.5%, while 6 cases presented with local recurrence in 46 patients underwent routine split laryngeal surgery, the recurrence rate was 13.0%. There was no statistical difference between the two surgical methods (χ2 = 0.271, P > 0.05). 10 of the 111 (9.0%) non recurrence group patients addicted to smoking after the surgery without following the doctors’ advice, while that of the recurrence group was 12 cases (60.0%), with statistically significant difference (χ2 = 31.533, P < 0.001).
Correlation of the expression of p27 and PTEN with outcomes of patients with laryngeal carcinoma
The positive rates of p27 expression were 43.5% (57/131) and 80.2% (105/131), and that of PTEN were 48.9% (64/131) and 83.2% (109/131) in the cancer adjacent tissues (negative surgical margin tissues) and in laryngeal carcinoma tissues, respectively (P < 0.001) (Figure 1A-D; Table 2). The expression rates of p27 and PTEN in laryngeal carcinoma tissues of the recurrent group were 20.0% (4/20), 47.7% (53/111) and that in non recurrent group were 10.0% (2/20) and 55.9% (62/111), respectively, with a significant difference (P < 0.001). In addition, the expression of p27 and PTEN in tumor resected marginal tissues of the recurrence group was 50.0% (10/20), 40.0% (8/20) and that in non recurrence group was 85.6% (95/111) and 91.0% (101/111), respectively; the difference was also statistically significant between the two groups (P < 0.001, Tables 1, 3). No correlation between the expression of the two proteins and other clinicopathological features was found in this study (Table 1).
Figure 1.
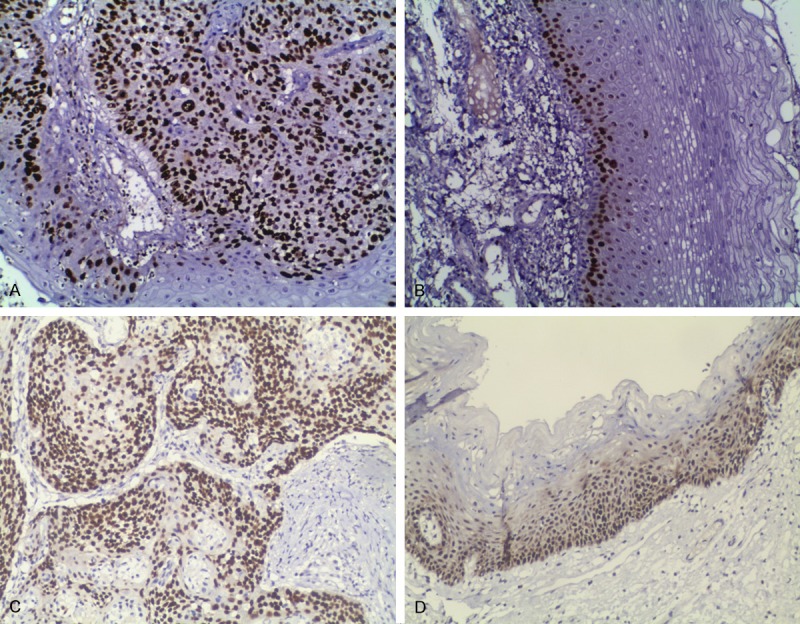
Expression of PTEN and p27 in laryngeal squamous cell carcinoma and its adjacent tissues. A. Immunohistochemical detection of PTEN in laryngeal carcinoma tissue. B. Immunohistochemical detection of PTEN in laryngeal carcinoma adjacent tissue. C. Immunohistochemical detection of p27 in laryngeal carcinoma tissue. D. Immunohistochemical detection of p27 in laryngeal carcinoma adjacent tissue. SP method, original magnification × 100.
Table 2.
Expression of p21, p27 in laryngeal squamous carcinoma and tumor resection marginal tissues (No. of patients)
| Tissue source | PTEN+ | χ2 | P | p27+ | χ2 | P |
|---|---|---|---|---|---|---|
| Carcinoma tissues | 64 | 34.458 | 0.000 | 57 | 37.262 | 0.000 |
| Tumor surgical marginal tissues | 109 | 105 |
Table 1.
Correlation between expression of PTEN, p27 and tumor recurrence (No. of patients)
| Clinical features | Total No. | PTEN+ | χ2 | P | p27+ | χ2 | P |
|---|---|---|---|---|---|---|---|
| Total No. | 131 | 64 | 57 | ||||
| Differentiation | |||||||
| Well-moderately Differentiated | 100 | 46 | 1.378 | 0.240 | 40 | 2.120 | 0.145 |
| Poorly-differentiated | 31 | 18 | 17 | ||||
| TNM staging | |||||||
| Stage T1a | 88 | 39 | 2.208 | 0.137 | 40 | 0.412 | 0.521 |
| Stage T1b | 43 | 25 | 17 | ||||
| Prognosis | |||||||
| Recurrence | 20 | 2 | 12.485 | 0.000 | 4 | 4.240 | 0.039 |
| Non recurrence | 111 | 62 | 53 |
Table 3.
Correlation between expression of PTEN, p27 in the tumor resection marginal tissues with tumor recurrence (No. of patients)
| Prognosis | PTEN+ | χ2 | P | p27 + | χ2 | P |
|---|---|---|---|---|---|---|
| Recurrence | 8 | 31.533 | 0.000 | 10 | 13.490 | 0.000 |
| Non recurrence | 101 | 95 |
Discussion
Supraglottic cancer is the most common type of laryngeal squamous cell carcinoma, mostly invasive growth and well-differentiated. Growth characteristics of supraglottic cancer depend on its unique anatomical features. Due to the rare lymphatic drainage, early lesions rarely present metastasis. Therefore, CO2 laser surgery for laryngeal cancer became one of the minimally invasive surgeries for the treatment of laryngeal cancer in recent years. CO2 laser surgery can remove the tumor through natural channels without neck incision and tracheotomy, with less damage and bleeding. Combined with microscopes, it can judge resected margins of the tumor accurately, resect precisely and retain organ’s function in a great degree, recover soon, shorten the hospital stay and reduce the medical expenses. Therefore, CO2 laser resection surgery has obvious advantages compared with radiation therapy and traditional split laryngeal surgery [3]. In recent years, due to the application of laryngeal microsurgery techniques in the treatment of supraglottic cancer, early diagnosis and CO2 laser resection became easier and possible [4]. Under the laryngoscope, CO2 laser in treatment of early laryngeal cancer can accurately remove the tumor, stop bleeding effectively and reduce recurrence after surgery, thus prior to the traditional split laryngeal surgery [5]. Studies have shown that CO2 laser resection in treating the early glottic cancer has the same therapeutic effect with radiation therapy, throat cut or partial split laryngectomy; the 5-year survival rate was above 80%, local control rate was 77% to 100% and throat retention rate was more than 90% [6]. It was reported that after CO2 laser surgery of non-selective stage T1 glottic cancer cases (including T1a, T1b) and some cases of stage T2 of patients, 5-year local control rate was 88% (81-93%), larynx retention rate was 95%, while the patients of stage T1a glottic cancer exposed satisfactory to the CO2 laser surgery by laryngoscope supporting, the 5-year local control rate was 92% and larynx preservation rate was 98% [7-9]. Our study included all those untreated stage T1 patients (T1a, T1b) were subjected to CO2 laser surgery alone that can expose previously to laryngoscope, the local control rate was 83.5% (71/85), larynx preservation rate was 95.3% (81/85) and the recurrence rate was 16.5% (14/85), with no significant difference compared with split throat surgery, similar to that reported in the literature [10-12]. Smoking is another risk factor that is closely correlated with many tumors including throat cancer [13]. More than 90% of laryngeal cancer patients have a history of smoking at different times [14], so quit smoking after tumor resection is critical for avoiding recurrence. Our research indicated that recurrence after surgery was correlated with addicted to smoking again.
Tumorigenesis is a complex process of multifactors and multi-stage. Compared to their normal original cell, the tumor cells contain not only some proteins that are not expressed in the normal cells, but also some proteins of different expression levels that have same nature with normal cells [15]. Thus, by comparing differences of protein expression in tumor and normal tissues of patients, may be found with the tumor-associated proteins, these proteins can be used as prognostic tumor markers [16]. Animal experiments showed that almost every organ or tissue may express p27 protein. As a cyclin-dependent kinase inhibitor (CDKI), it played a pivotal role in promoting self-balancing in the organization [17]. Studies have shown that, p27 protein can be detected in human benign and malignant lesions, but the expression in benign lesions and normal tissues was significantly higher than that of malignant tissues, suggesting that p27 protein expression in the most quiescent cells, down-regulation of p27 gene will result in abnormal proliferation of cells [18]. As a member of CDKI family p27 can inhibit G1-S phase of cell cycle by acting on CDK directly or indirectly [19]. p27 gene-transfected cells and its overexpression afterwards could strongly inhibit DNA synthesis and prevent their entry into S phase from G1 phase, so S-phase cells decreased. Therefore, p27 gene was considered to be a member of tumor suppressor gene family [20]. Study showed that lack or decreased expression of p27 was closely associated with patients’ poor prognosis [18], which was consistent with our findings that the positive rate of p27 in laryngeal cancer tissues was significantly lower than that in the adjacent tissues. Meanwhile, the positive rate of p27 in laryngeal cancer recurrence group was significantly lower than the non-recurrence group, suggesting that p27 played a negative role of regulating the growth of laryngeal cancer.
PTEN has been shown to act as a tumor suppressor whose function includes suspending the cell cycle in G phase,accelerating cell apoptosis, regulating cell growth ,maintaining normal metabolism and internal environment homeostasis, inhibiting cell proliferation, tumor angiogenesis and invasiveness in tumor cells. Currently it was found that the expression of PTEN was strongly relevance to recurrence and prognosis of head and neck squamous cell carcinoma. Decreased and even loss of expression of PTEN protein in tumor tissues, including laryngeal carcinoma, was significantly correlated with tumor recurrence and metastasis [21]. Previous study showed that PTEN gene played an important role in occurrence and development of laryngeal cancer [22]. Our studies found that the positive rate of PTEN in 131 surgical margins of laryngeal cancer was 83.2%, which was significantly higher than that of cancer tissues. Meanwhile, the positive expression rate of PTEN in recurrence group was significantly lower than that of the non-recurrence group, suggesting that decreased or absent PTEN expression in laryngeal surgical margin tissues is closely related to the recurrence of tumor. Studies have shown that the performance of PTEN in its tumor suppressor role in the cell cycle regulated by its upregulation of p27 expression. Cheney et al [23] found that PTEN may induce the combination of p27 and cyclin E/cyclin-dependent kinase 2 (cyclinE/CDK2) complex that significantly inhibits CDK2 kinase activity, resulting in growth inhibition of cell cycle. Sun et al [24] and other studies have shown that PTEN could inhibit degradation of p27 in the post-transcriptional level through the PI3K pathway. These may be the reasons to explain in our study that low expression of PTEN often accompanies with p27 simultaneously in the vocal carcinoma tissues.
In summary, there was no significant difference between CO2 laser surgery and traditional split throat surgery in postoperative recurrence rate. With little surgical trauma and retaining much of laryngeal function, CO2 laser surgery is the preferred surgical modality in treating early laryngeal cancer. However, for those negative margins after surgery did not mean that there were no molecular biology changes to some extent, such as some tumor suppressor genes’ mutation, deletion or decreased activity including PTEN and p27, which played key roles in promoting the recurrence of laryngeal squamous cell carcinoma. For those patients with negative expression of p27 and PTEN should be followed-up closely in order to take measures timely before tumor recurrence.
Disclosure of conflict of interest
None.
References
- 1.Aaltonen LM, Rautiainen N, Sellman J, Saarilahti K, Mäkitie A, Rihkanen H, Laranne J, Kleemola L, Wigren T, Sala E, Lindholm P, Grenman R, Joensuu H. Voice quality after treatment of early vocal cord cancer: a randomized trial comparing laser surgery with radiation therapy. Int J Radiat Oncol Biol Phys. 2014;90:255–260. doi: 10.1016/j.ijrobp.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 2.Marcotullio D, de Vincentiis M, Iannella G, Bigelli C, Magliulo G. Surgical treatment of T1b glottic tumor, 10-years follow-up. Eur Rev Med Pharmacol Sci. 2014;18:1212–1217. [PubMed] [Google Scholar]
- 3.Remmelts AJ, Hoebers FJ, Klop WM, Balm AJ, Hamming-Vrieze O, van den Brekel MW. Evaluation of laser surgery and radiotherapy as treatment modalities in early stage laryngeal carcinoma: tumour outcome and quality of voice. Eur Arch Otorhinolaryngol. 2013;270:2079–2087. doi: 10.1007/s00405-013-2460-x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang SY, Lu ZM, Luo XN, Chen LS, Ge PJ, Song XH, Chen SH, Wu YL. Retrospective analysis of prognostic factors in 205 patients with laryngeal squamous cell carcinoma who underwent surgical treatment. PLoS One. 2013;8:e60157. doi: 10.1371/journal.pone.0060157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hakeem AH, Tubachi J, Pradhan SA. Significance of anterior commissural involvement in early glottic squamous cell carcinoma treated with trans-oral CO2 laser microsurgery. Laryngoscope. 2013;123:1912–1917. doi: 10.1002/lary.24012. [DOI] [PubMed] [Google Scholar]
- 6.Peretti G, Piazza C, Ansarin M, De Benedetto L, Cocco D, Cattaneo A, Nicolai P, Chiesa F. Transoral CO2 laser microsurgery for Tis-T3 supraglottic squamous cell carcinomas. Eur Arch Otorhinolaryngol. 2010;267:1735–1742. doi: 10.1007/s00405-010-1284-1. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Zhou J. Local recurrence of CO2 laser surgery for patients with early glottic carcinoma: a systematic review and meta-analysis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;26:783–792. [PubMed] [Google Scholar]
- 8.Schrijvers ML, van Riel EL, Langendijk JA, Dikkers FG, Schuuring E, van der Wal JE, van der Laan BF. Higher laryngeal preservation rate after CO2 laser surgery compared with radiotherapy in T1a glottic laryngeal carcinoma. Head Neck. 2009;31:759–764. doi: 10.1002/hed.21027. [DOI] [PubMed] [Google Scholar]
- 9.Galletti B, Freni F, Cammaroto G, Catalano N, Gangemi G, Galletti F. Vocal outcome after CO2 laser cordectomy performed on patients affected by early glottic carcinoma. J Voice. 2012;26:801–805. doi: 10.1016/j.jvoice.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Yang C, Sun Q, Chen L, Luo Y, Zhang S, Liu H, Liu Y. Observation of postoperative recovery time with different surgical procedures for treatment of vocal polyps. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;28:564–567. [PubMed] [Google Scholar]
- 11.Özdemir S, Tuncer Ü, Tarkan Ö, Kara K, Sürmelioğlu Ö. Carbon dioxide laser endoscopic posterior cordotomy technique for bilateral abductor vocal cord paralysis: a 15-year experience. JAMA Otolaryngol Head Neck Surg. 2013;139:401–404. doi: 10.1001/jamaoto.2013.41. [DOI] [PubMed] [Google Scholar]
- 12.Lee HS, Chun BG, Kim SW, Kim ST, Oh JH, Hong JC, Lee KD. Transoral laser microsurgery for early glottic cancer as one-stage single-modality therapy. Laryngoscope. 2013;123:2670–2674. doi: 10.1002/lary.24080. [DOI] [PubMed] [Google Scholar]
- 13.Narwani V, Harries M. Treatment modality: a predictor of continued tobacco use after treatment in patients with laryngeal cancer. J Laryngol Otol. 2014;128:153–158. doi: 10.1017/S0022215113003344. [DOI] [PubMed] [Google Scholar]
- 14.Mackiewicz-Nartowicz H, Sinkiewicz A, Piwczyński D, Betlejewski S, Owczarek A. Prospective factors of T1 and T2 glottic carcinoma. Otolaryngol Pol. 2007;61:921–925. doi: 10.1016/S0030-6657(07)70554-1. [DOI] [PubMed] [Google Scholar]
- 15.Lionello M, Staffieri A, Marioni G. Potential prognostic and therapeutic role for angiogenesis markers in laryngeal carcinoma. Acta Otolaryngol. 2012;132:574–582. doi: 10.3109/00016489.2011.652308. [DOI] [PubMed] [Google Scholar]
- 16.Pich A, Chiusa L, Navone R. Prognostic relevance of cell proliferation in head and neck tumors. Ann Oncol. 2004;15:1319–1329. doi: 10.1093/annonc/mdh299. [DOI] [PubMed] [Google Scholar]
- 17.Breitenstein A, Akhmedov A, Camici GG. p27 (Kip1) inhibits tissue factor expression. Biochem Biophys Res Commun. 2013;439:559–563. doi: 10.1016/j.bbrc.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Pereira SS, Morais T, Costa MM, Monteiro MP, Pignatelli D. The emerging role of the molecular marker p27 in the differential diagnosis of adrenocortical tumors endocrine connections. Endocr Connect. 2013;2:137–145. doi: 10.1530/EC-13-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Yang WT, Zheng PS. Msi1 promotes tumor growth and cell proliferation by targeting cell cycle checkpoint proteins p21, p27 and p53 in cervical carcinomas. Oncotarget. 2014;5:10870–10885. doi: 10.18632/oncotarget.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keles N, Erdamar B, Kaur A, Değer K. p21, p53, and p27 Kip1 alterations in benign and malignant tumors of sinonasal epithelium. Otolaryngol Head Neck Surg. 2003;129:77–84. doi: 10.1016/S0194-59980300520-5. [DOI] [PubMed] [Google Scholar]
- 21.Zhu XL, Wang ZF, Lei WB, Zhuang HW, Hou WJ, Wen YH, Wen WP. Tumorigenesis role and clinical significance of DJ-1, a negative regulator of PTEN, in supraglottic squamous cell carcinoma. J Exp Clin Cancer Res. 2012;31:94. doi: 10.1186/1756-9966-31-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Q, Li Y. Expression of survivin and PTEN in laryngeal squamous cell carcinoma transplanted on the back sides of nude mice treated by gold throat tablets. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;26:1134–1143. [PubMed] [Google Scholar]
- 23.Cheney IW, Neuteboom ST, Vaillancourt MT, Ramachandra M, Bookstein R. Adenovirus mediated gene transfer of MMACl/PTEN to glioblastom cells inhibits S phase entry by the recruitment of p27KIP1 into cyclin E/CDK2 complexes. Cancer Res. 1999;59:2318–2323. [PubMed] [Google Scholar]
- 24.Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H. PTEN modulates cell cycle progression and cell survival by regulaing phosphatidylinositol 3, 4, 5, trisphosprate and Akt/protein kinase signaling pathway. Proc Natl Acad Sci U S A. 1999;96:6199–6304. doi: 10.1073/pnas.96.11.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]


