Abstract
Background
Chemoprevention is an option for women who are at increased risk of breast cancer (five year risk ≥1.7%). It is uncertain, however, how often women accept and complete five years of therapy and whether clinical or demographic factors predict completion.
Methods
Medical records were abstracted for 219 women whose five year risk of breast cancer was ≥ 1.7% and who were offered chemoprevention while attending a high risk breast clinic at the Moffitt Cancer Center. We examined the likelihood of accepting chemoprevention and completing five years of therapy, and potential clinical and demographic predictors of these outcomes, using multivariable logistic regression and survival analysis models.
Results
There were 118/219 women (54.4%) who accepted a recommendation for chemoprevention and began therapy. The likelihood of accepting chemoprevention was associated with lifetime breast cancer risk and was higher for women with specific high risk conditions (lobular carcinoma in situ and atypical ductal hyperplasia). Women with osteoporosis and those that consumed alcohol were also more likely to accept medication. There were 58/118 (49.2%) women who stopped medication at least temporarily after starting therapy. Based on survival curves, an estimated 60% of women who begin chemoprevention will complete five years of therapy.
Conclusions
A substantial percentage of women at increased risk of breast cancer will decline chemoprevention and among those that accept therapy, approximately 40% will not be able to complete five years of therapy because of side effects.
Keywords: Breast Cancer, Breast cancer prevention, atypical ductal hyperplasia, atypical lobular hyperplasia, lobular carcinoma in situ, tamoxifen, raloxifene, chemoprevention
Introduction
It is estimated that 235,000 women will be diagnosed with breast cancer in 2014.1 Several medications have been shown to reduce the incidence of breast cancer, including the selective estrogen receptor modulators (SERM) tamoxifen,2, 3 and raloxifene,3, 4 and more recently, aromatase inhibitors including exemestane5 and anastrozole.6
The use of medications to reduce breast cancer incidence (chemoprevention) has been recommended for women at increased risk of breast cancer7, 8 and are generally taken over a five year time period. It is estimated that more than 10 million women are eligible for chemoprevention.9 Despite these recommendations, acceptance of chemoprevention among women has been limited.10
Previous studies that have examined uptake and adherence to chemoprevention have had important limitations. Many studies have assessed women’s likelihood of accepting chemoprevention when posed as a theoretical decision, rather than their actual acceptance in real clinical settings.11, 12 In addition, most studies have not assessed rates of chemoprevention adherence among women who begin therapy.13
To address these limitations, we examined acceptance and adherence to chemoprevention among women attending a high risk breast clinic within an NCI Comprehensive Cancer Center. We hypothesized that acceptance and adherence to chemoprevention would be related to the woman’s individual risk of breast cancer, as estimated by the Gail Model, or by SEER population estimates (for women with lobular carcinoma in situ).
Material and Methods
The H. Lee Moffitt Cancer Center Breast Surveillance Clinic provides care to women at increased risk of breast cancer because of family history (excluding those with known deleterious mutations in BRCA or other risk conferring genes) or a risk-conferring condition demonstrated by biopsy (e.g. lobular carcinoma in situ, atypical ductal hyperplasia, atypical lobular hyperplasia). The clinic provides comprehensive risk assessment, counseling on risk reduction options, and ongoing screening systematically to all women who attend the clinic. Recommendations for chemoprevention are made on the patient’s initial visit to the breast surveillance clinic. For patients that elect to begin chemoprevention, prescriptions are provided by the breast surveillance clinic and are not managed by referring physicians or primary care providers.
For most women, breast cancer risk was estimated using the Gail model, providing 5-year and lifetime risk estimates.14 The Gail model has been validated in several settings15 but may underestimate breast cancer risk in women with atypical hyperplasia16 and women with family history of breast cancer in second degree relatives.17 For women with LCIS (for whom the Gail model has not been validated), 5-year and lifetime breast cancer risks were estimated using SEER population estimates.18 Women were generally followed every six months (regardless of whether chemoprevention is being used) with imaging modalities selected based on the woman’s level of risk. Most women (94%) were referred to the clinic from other providers within the Moffitt Cancer Center.
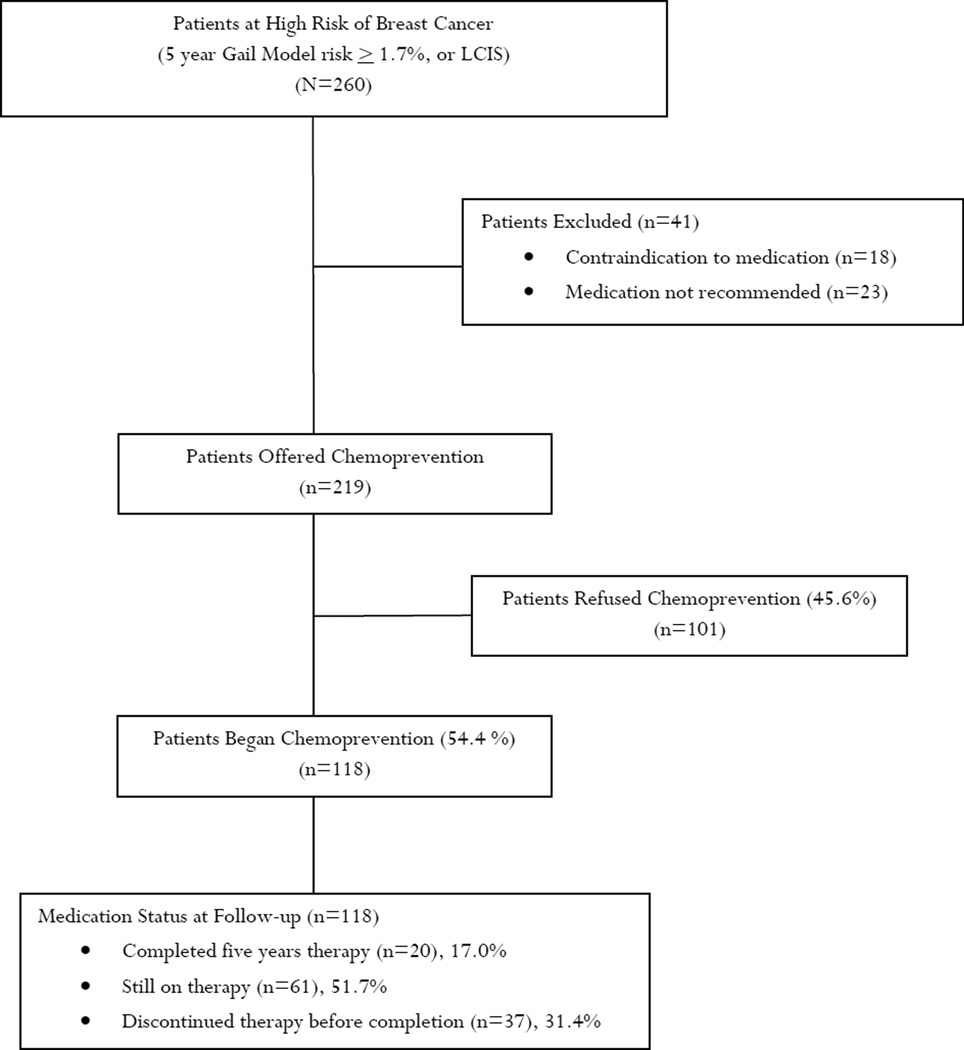
In March 2011, the patient scheduling database was used to identify all patients seen in the breast surveillance clinic during the interval 12/1/2006 through 03/14/2011. The scheduling system identified 387 women that had been seen at least one time during that interval. From this group we identified 260 women that had sufficiently elevated risk to consider chemoprevention (5-year Gail Model risk ≥1.7%, or lobular carcinoma in situ). There were 41 women excluded (Figure 1) because of either 1) a contraindication to medication (n=18) or 2) no evidence in the medical record that chemoprevention had been recommended (n=23). The remaining 219 women who were offered chemoprevention constituted the study sample of interest.
Figure 1.
Summary of High Risk Breast Cohort and Use of Chemoprevention
The dates of initial appointment for this group ranged from 4/26/04 to 3/9/2011 and the dates of last recorded visit in the medical record ranged from 1/2/2008 to 11/08/2012. Women had on average 5.8 (SD 3.5) visits in the clinic and the average length of follow up for the cohort was 33.3 months (SD 21.2).
Medical records of this patient cohort were abstracted by two trained and experienced research abstractors. Data abstracted included breast cancer risk factors (age, age at menarche, age at first live birth, family history of breast cancer in first degree relatives, prior biopsies, alcohol use, body mass index (BMI), exercise habits, mammographic density), five year and lifetime risk of breast cancer, menopausal status, and use of chemoprevention (tamoxifen, raloxifene). We also assessed selected comorbid illnesses that could potentially influence recommendations and acceptance of tamoxifen or raloxifene because of concerns for thrombotic complications (hypertension, diabetes, cardiovascular disease) or because of potential secondary benefits (osteoporosis). Sample sizes were large enough to examine HTN (n=74) and Diabetes (n=14) individually but sample size was too small to examine heart disease individually (n=3).
Adherence to chemoprevention was assessed solely by documentation in the medical record (e.g. clinic notes indicating patient was taking chemoprevention, medication reconciliation by nurse indicating chemoprevention, prescriptions provided to patient for chemoprevention). We did not verify adherence in other ways (pill counts, assessing pharmacy records, etc.). We examined whether women discontinued chemoprevention prematurely (i.e. prior to five years of therapy) either temporarily (clinic records indicate chemoprevention was restarted at some point during follow up) or permanently (chemoprevention was not restarted during follow up period).
We examined the relationship between accepting the offer to begin chemoprevention and patient characteristics using the Wilcoxon Rank Sum Test for continuous variables (used because of skewed, non-normal data) and the Chi-Square test using exact method for categorical variables. We examined candidate clinical predictors of chemoprevention acceptance with multivariable logistic regression and used a backwards elimination algorithm (significance level to stay, alpha=0.05) to select the final multivariable model. Variables that are unrelated to outcomes in bivariate analysis may in fact be important independent outcome predictors in multivariable analysis because of confounding. For this reason all clinical variables were eligible for inclusion in the initial multivariable logistic model. Because of the small sample size we did not explore interaction terms in logistic models.
For women who began chemoprevention, we examined the length of time women were able to remain on therapy (up to a maximum of five years) using the Kaplan-Meier method. The starting point for the survival analysis was defined as the point that medical records indicated the patient had initiated chemoprevention. Patients who later switched from one drug to the other (switching from tamoxifen to raloxifene for example) were considered adherent to therapy in the survival analysis. We examined predictors of discontinuing therapy prematurely using the Cox proportional hazards models. Patients who were still on chemoprevention at last follow up and those who completed treatment were treated as censored observations for the analysis. All p-values are two-tailed.
Our sampling strategy may have introduced bias by sampling persons having an office visit during a specified time period (12/1/06 through 3/14/11). It is possible that patients originally seen before this time interval would be more likely to be sampled if they accepted an offer for chemoprevention or if they were more likely to remain on therapy. To examine this possibility we compared the proportion of women accepting an offer for chemoprevention and the proportion of women who stopped chemoprevention for women who were first seen before 12/01/06 (n=29) and those who were first seen after this date (n=190). As a sensitivity analysis, we also examined these outcomes including and excluding persons whose first visit occurred before 12/1/06.
This study was approved by the U. of South Florida Institutional Review Board which waived informed consent for the subjects in this study.
Results
The average age was 56.0 years (range 37 – 94); the patient cohort was primarily white and non-Hispanic (Table 1). Most women had a history of a prior breast biopsy demonstrating a high risk lesion, most commonly atypical hyperplasia. Other potential risk factors for breast cancer (e.g. family history, mammographic breast density) were also common. Women in the sample were at substantially elevated breast cancer risk with an average five year risk of 4.0% (range 1.0% – 18.2%), and average lifetime risk of 22.9% (range 2.4% – 59.6%).
Table 1.
Characteristics of Women at High Risk of Breast Cancer
| N=260 | ||
|---|---|---|
| Age, mean (years, SD) | 56.0 (9.7) | |
| Race/Ethnicity (n, %) | ||
| White, non-Hispanic | 230 | 88.5 |
| Black, non-Hispanic | 7 | 2.7 |
| Hispanic | 16 | 6.2 |
| Other | 7 | 2.7 |
| Marital Status (n, %) | ||
| Married | 189 | 72.7 |
| Not married | 71 | 27.3 |
| Smoking Status (n, %) | ||
| Never smoker | 183 | 70.4 |
| Prior smoker | 61 | 23.5 |
| Current smoker | 16 | 6.2 |
| Alcohol Use (n, %) | ||
| No | 152 | 58.7 |
| Yes | 107 | 41.3 |
| Regular Exercise (n, %) | ||
| No | 129 | 50.2 |
| Yes | 128 | 49.8 |
| Health Insurance Status (n, %) | ||
| Insured | 240 | 92.3 |
| Uninsured | 20 | 7.7 |
| Comorbid Illness (n, %) | ||
| No | 159 | 61.2 |
| Yes | 101 | 38.9 |
| Menopausal Status (n, %) | ||
| Premenopausal | 80 | 30.9 |
| Post menopausal | 179 | 69.1 |
| Prior Hysterectomy (n, %) | ||
| No | 160 | 61.5 |
| Yes | 100 | 38.5 |
| BMI (mean, SD) | 27.0 (5.9) | |
| Gail Model Risk (mean, SD) | ||
| Five year risk (%) | 4.0 (2.3) | |
| Lifetime risk (%) | 22.9 (9.9) | |
| Breast Cancer First Degree Relatives (n, %) | ||
| 0 | 143 | 55.2 |
| 1 | 91 | 35.1 |
| 2 or more | 25 | 9.7 |
| Prior Biopsies (n, %) | ||
| None | 23 | 8.8 |
| Lobular carcinoma in situ | 26 | 10.0 |
| Atypical ductal hyperplasia | 121 | 46.5 |
| Atypical lobular hyperplasia | 57 | 21.9 |
| Flat epithelia atypia | 24 | 9.2 |
| Other | 9 | 3.5 |
| Estrogen Use (n, %) | ||
| None | 235 | 90.2 |
| Systemic | 16 | 6.2 |
| Vaginal | 6 | 2.3 |
| Other | 3 | 1.3 |
| Mammographic Breast Density (n, %) | ||
| Entirely fat | 18 | 7.0 |
| Scattered densities | 60 | 23.4 |
| Heterogeneously dense | 115 | 44.8 |
| Extremely dense | 64 | 24.9 |
There were 219 women eligible to receive chemoprevention and for whom the medical record documented a recommendation for therapy. There were 118 women (54.4%) who accepted this recommendation and began therapy and 101 women (45.6%) who declined. Of the 118 women beginning chemoprevention, 73 women (61.9%) took only tamoxifen, 34 women (28.8%) took only raloxifene, and 11 women (9.3%) took some combination of the two drugs. There was no difference in the average age (accepted therapy 54.2 years vs. declined therapy 55.0 years, p=0.46), estimated five year risk (accepted therapy 4.0% vs. declined therapy 3.9%, p=0.81) or lifetime risk of breast cancer (accepted therapy 23.7% vs. declined therapy 22.0%, p=0.48), BMI (accepted therapy 26.9 vs. declined therapy 27.1, p=0.51) or number of prior breast biopsies (accepted therapy 1.6 vs. declined therapy 1.7, p=0.75) among those who accepted therapy and those who declined.
With the exception of having been diagnosed with lobular carcinoma in situ, other demographic and clinical characteristics were not associated with accepting medication in bivariate analysis (Table 2). Neither combined comorbidity, nor specific comorbid conditions (i.e. hypertension or diabetes) were related to medication acceptance. In multivariable logistic analysis, five patient characteristics were independently associated with greater odds of accepting chemoprevention (Table 3). The likelihood of accepting chemoprevention was associated with lifetime breast cancer risk, with the odds of accepting medication increasing four percent for each one percent increase in lifetime risk. Breast conditions identified by biopsy also impacted medication acceptance with women diagnosed with lobular carcinoma in situ having more than seven times greater odds of accepting medication and women having atypical ductal hyperplasia having more than twice the odds of accepting medication. A history of alcohol consumption or osteoporosis also increased the odds of accepting medication.
Table 2.
Bivariate Analysis of Accepting Chemoprevention
| N=219 | |||
|---|---|---|---|
| Accepted Medication n (%) |
Declined Medication n (%) |
p-value* | |
| Race Ethnicity | 0.37 | ||
| White, non-Hispanic | 108 (56.0) | 85 (44.0) | |
| Black, non-Hispanic | 2 (40.0) | 3 (60.0) | |
| Hispanic | 6 (42.9) | 8 (57.1) | |
| Other | 2 (28.6) | 5 (71.4) | |
| Marital Status | 0.75 | ||
| Married | 29 (51.8) | 27 (48.2) | |
| Not married | 89 (54.6) | 74 (45.4) | |
| Smoking Status | 0.68 | ||
| Never smoker | 84 (53.2) | 74 (46.8) | |
| Prior smoker | 26 (53.1) | 23 (46.9) | |
| Current smoker | 8 (66.7) | 4 (33.3) | |
| Alcohol Use | 0.10 | ||
| No | 62 (48.8) | 65 (51.2) | |
| Yes | 56 (60.9) | 36 (39.1) | |
| Regular Exercise | 0.79 | ||
| No | 56 (54.9) | 46 (45.1) | |
| Yes | 62 (53.0) | 55 (47.0) | |
| Health Insurance Status | 0.13 | ||
| Insured | 112 (55.4) | 90 (44.6) | |
| Uninsured | 6 (35.3) | 11 (64.7) | |
| Comorbid Illness | 0.49 | ||
| No | 78 (55.7) | 62 (44.3) | |
| Yes | 40 (50.6) | 39 (49.4) | |
| Menopausal Status | 0.78 | ||
| Premenopausal | 42 (55.3) | 34 (44.7) | |
| Post menopausal | 75 (52.8) | 67 (47.2) | |
| Prior Hysterectomy | 0.16 | ||
| No | 68 (50.0) | 68 (50.0) | |
| Yes | 50 (60.2) | 33 (39.8) | |
| Breast Cancer First Degree Relatives | 0.53 | ||
| 0 | 66 (55.9) | 52 (44.1) | |
| 1 | 43 (53.8) | 37 (46.3) | |
| 2 or more | 9 (42.9) | 12 (57.1) | |
| Lobular Carcinoma In Situ | 0.02 | ||
| No | 99 (50.8) | 96 (49.2) | |
| Yes | 18 (78.3) | 5 (21.7) | |
| Atypical Ductal Hyperplasia | 0.07 | ||
| No | 56 (48.3) | 60 (51.7) | |
| Yes | 61 (61.0) | 39 (39.0) | |
| Atypical Lobular Hyperplasia | 0.26 | ||
| No | 86 (51.8) | 80 (48.2) | |
| Yes | 31 (60.8) | 20 (39.2) | |
| Flat Epithelial Atypia | 0.83 | ||
| No | 104 (53.3) | 91 (46.7) | |
| Yes | 13 (56.5) | 10 (43.5) | |
| Systemic Estrogen Use | 1.00 | ||
| No | 111 (53.6) | 96 (46.4) | |
| Yes | 6 (54.5) | 5 (45.5) | |
| Mammographic Breast Density | 0.09 | ||
| Entirely fat | 7 (50.0) | 7 (50.0) | |
| Scattered densities | 34 (70.8) | 14 (29.2) | |
| Heterogeneously dense | 50 (50.0) | 50 (50.0) | |
| Extremely dense | 27 (49.1) | 28 (50.9) | |
p-values were obtained using Chi-Square test using exact method.
Table 3.
Multivariable Logistic Regression Analysis of Accepting Chemoprevention
| (N=219) | ||
|---|---|---|
| Predictor | Adjusted Odds Ratio (95% CI) |
P-value |
| Lifetime Breast Cancer Risk | 1.04 (1.002 – 1.08) | 0.04 |
| Osteoporosis | ||
| No | 1.00 | 0.003 |
| Yes | 3.43 (1.54 – 7.65) | |
| Lobular Carcinoma in Situ | ||
| No | 1.00 | 0.02 |
| Yes | 7.65 (1.48 – 39.5) | |
| Atypical Ductal Hyperplasia | ||
| No | 1.00 | 0.004 |
| Yes | 2.76 (1.37 – 5.54) | |
| Alcohol Consumption | ||
| None | 1.00 | 0.007 |
| Some use | 2.6 (1.30 – 5.22) | |
There were 58/118 (49.2%) women who stopped medication at least temporarily after starting therapy. The most common reasons for discontinuing therapy were; hot flashes (27/58 women, 46.6%), vaginal bleeding or change in periods (12/58 women, 20.7%), vaginal dryness (9/58 women, 15.5%), fear of potential side effects (7/58 women, 12.1%), changes in mood (6/58 women, 10.3%) and musculoskeletal pains (6/58 women, 10.3%). There was only one occurrence of uterine cancer (a post-menopausal woman taking tamoxifen) and no episodes of deep venous thrombosis, pulmonary embolus, or stroke. For 37 women who discontinued therapy, some intervention strategy was attempted, most often temporarily stopping the drug (29/37 women), switching to a different chemopreventive agent (9/37 women), or adding an additional medication to treat hot flashes or vaginal dryness (9/37 women). Women who attempted some strategy to deal with side effects were less likely to prematurely discontinue therapy (19/37 women, 51.4% vs. 17/20 women 85.0%, p=0.005).
Among the 118 women who began therapy, data was available for 109 women regarding the total length of time they remained on therapy. Twenty women completed five years of therapy, 34 women discontinued therapy before completing five years, and the remaining 55 women were still on therapy at last follow up. For those women who permanently discontinued therapy, more than half did so in the first year (21/34 61.8%) and more than three quarters did so within the first two years (29/34, 85.3%).
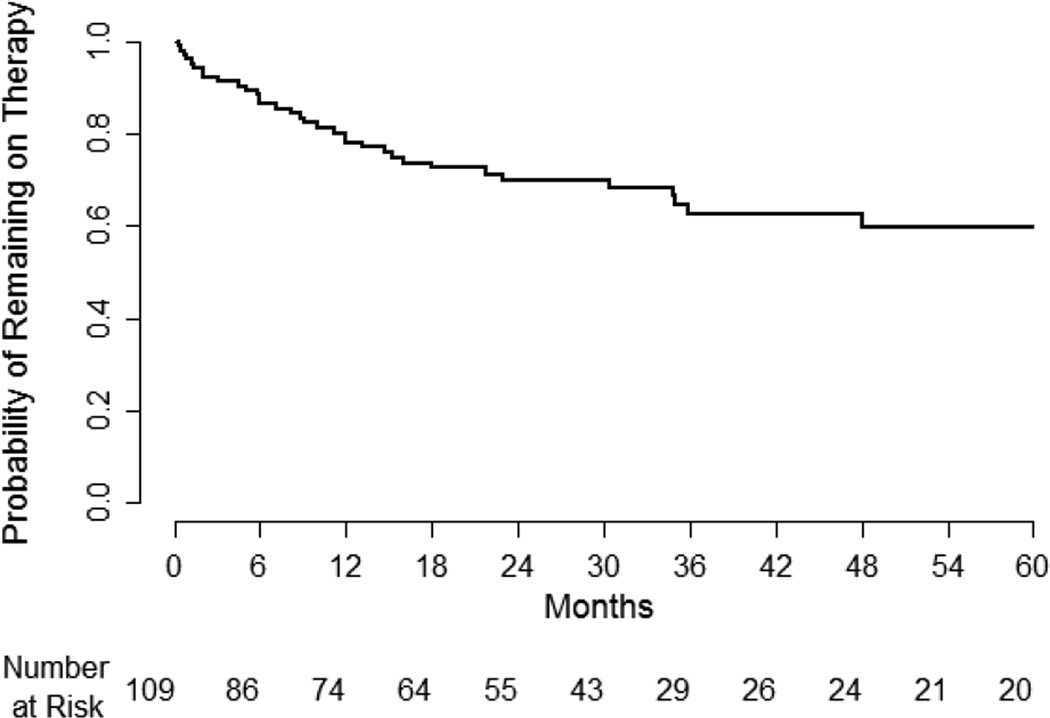
The probability of remaining on therapy for the recommended duration of 60 months is shown in Figure 2. Based on the survival probability curve, 60% of women (95% CI 47% – 70%) who began therapy would be expected to complete the recommended five years of therapy. In a Cox multivariable proportional hazards model, the only clinical/demographic characteristic that predicted higher rates of discontinuation was family history of breast cancer (adjusted hazard rate 3.2, 95% CI 1.07 – 9.61, p=0.04). Rates of discontinuing medication were not higher for tamoxifen compared to raloxifene (hazard rate 1.2, 95% CI 0.5 – 2.8, p=0.66).
Figure 2.
Probability of Remaining on Chemoprevention
There were 54 women that had at least one breast biopsy during their follow-up and 8 women were diagnosed with breast cancer (2 women DCIS, 2 women invasive lobular carcinoma, 4 women invasive ductal carcinoma). Among women having used chemoprevention, 2/118 (1.7%) were diagnosed with breast cancer while 6/142 (4.2%) of women not on chemoprevention were diagnosed with breast cancer (p=0.24). Use of chemoprevention had no impact on the likelihood of women undergoing biopsy (chemoprevention used: 27/118, 22.9% women with biopsies, chemoprevention not used: 27/142, 19.0% women with biopsies, p=0.44).
There was no difference between persons whose first visit was before 12/1/06 and those whose first visit was after this date in the likelihood of accepting an offer to begin chemoprevention (65.5% vs. 52.1%, p=0.17) or in the likelihood of stopping chemoprevention once started (44.4% vs. 45.5%, p=0.94). Furthermore, the outcomes of acceptance and stopping of chemoprevention were not substantively different when women whose first visit was before 12/1/06 were included in the analysis versus when this group was excluded (accept offer of chemoprevention 53.9% vs. 52.1%; stopped chemoprevention once started 45.3% vs. 45.4%).
Conclusions
Among women eligible for chemoprevention, we found that about half began medication when offered, and an estimated 60 percent were expected to complete five years of recommended therapy. A meta-analysis of five studies found generally lower acceptance rates of chemoprevention (14.8% on average).13 A more recent study of high risk women also found modest acceptance of chemoprevention (10.6%).19
The higher rate of acceptance in our study may have been influenced by its setting within a high risk breast clinic. In a similar study of high risk patients attending a university breast clinic, Rahman and colleagues reported that 46% of women were offered tamoxifen and 31% accepted the offer.20 Other studies conducted in similar settings reported that about 50% of women accept an offer to begin tamoxifen.21, 22 Uptake of tamoxifen in the setting of clinical trials, however, has been lower, ranging from 5–14%.23, 24
Studies of tamoxifen uptake in primary care practice have also reported much lower acceptance rates (2 – 6%).25, 26 Decision making about chemoprevention is complex and there are numerous barriers to addressing this topic in primary care settings.27 Although decision support tools have been advocated to address this, paradoxically interest in chemoprevention tends to decline as women receive more information about the drugs’ effects.28
We found that acceptance of chemoprevention was related to patients’ overall lifetime breast cancer risk. Breast cancer risk has been an inconsistent predictor of acceptance of chemoprevention in other studies.13 We also found that acceptance of chemoprevention was more likely for women with a prior history of osteoporosis. For post-menopausal women, tamoxifen and raloxifene would be expected to provide additional benefit for osteoporosis which may have made these medications more attractive.
Several specific high risk conditions (LCIS, atypical ductal hyperplasia) increased the likelihood of chemoprevention acceptance independent of the patient’s overall lifetime risk of breast cancer. Chemoprevention with tamoxifen has shown greater benefit among women with atypical hyperplasia2 and this may have persuaded some women to begin therapy. It is unclear, however, why a history of LCIS would so strongly impact treatment decisions, above and beyond its impact on estimated lifetime risk of breast cancer. Even controlling for the estimated lifetime risk of breast cancer, women with LCIS had seven times greater odds of accepting therapy compared to other high risk women. Although all women were informed of their estimated lifetime risk of breast cancer, it is possible that the perception of risk was higher for women with LCIS. Including the term “carcinoma” in the nomenclature of LCIS may contribute to the perception of risk. Acceptance of medication has been more strongly tied to perceived risk of breast cancer than to actual risk.13, 29, 30
Once started, a substantial number of women stopped chemoprevention due to perceived side effects. Most women who discontinue medication did so within the first year. We estimate that about 60% of women are able to complete the full five years of recommended therapy. In randomized trials of chemoprevention, adherence rates ranged from 72%–80% for raloxifene and 60%–72% for tamoxifen.31, 32 Studies that examined tamoxifen use as adjuvant therapy for breast cancer have found that between 31–60% of women discontinue therapy before five years.33
We did not find any strong clinical predictors of discontinuing medication other than having a family history of breast cancer, which was an unexpected finding. It is possible that such women were aware of family member’s experiences with tamoxifen when used to treat breast cancer and were more vigilant with regard to side effects. Chemoprevention adherence has been reported to be lower for younger women, those who smoke or use alcohol, and those with lower education levels.34
Our sample size was too small to draw conclusions about the clinical effectiveness of chemoprevention in regard to the likelihood of subsequent biopsies or breast cancer diagnoses. In addition, women who began chemoprevention were at higher risk for breast cancer than women who chose not to, making the two groups non comparable in regards to these outcomes.
This study has several limitations that should be considered when interpreting the findings. First, the study was conducted at a high risk breast clinic within an NCI Comprehensive Cancer Center and many of the women had high risk conditions identified by biopsy (LCIS, atypical hyperplasia). In addition, our study may over-estimate rates of chemoprevention acceptance because of selection bias (women not interested in risk reduction may not have followed through with referrals). The women in this study, therefore, may have been more motivated to pursue risk reduction than other populations and settings of care. This study did not include women taking aromatase inhibitors so we have no information whether adherence will differ for this class of medications. Our sample did not include women with known BRCA or other risk conferring gene mutations which limits information on this group. We relied on self-report of women to assess medication adherence and did not independently verify these reports. In addition, this study relied solely on data from chart abstractions. Finally, with longer follow up it is possible that some premenopausal women who discontinued therapy with tamoxifen because of side effects may have later taken raloxifene or an aromatase inhibitor after menopause, causing us to underestimate chemoprevention acceptance.
In conclusion, we found that about half of women attending a high risk breast clinic began medication when offered, and an estimated 60 percent were expected to complete the recommended five years of therapy. Our findings are in agreement with others pointing out that chemoprevention is likely to reach only a minority of eligible women at high risk of breast cancer.35, 36 Further research is needed to better understand the barriers preventing wider use of chemoprevention.
Acknowledgments
Funding Statement: This work was self-funded.
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to disclose regarding this work.
Human Subjects Statement: This study was approved by the U. of South Florida Institutional Review Board.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: A Cancer Journal for Clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 3.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. Jama. 2006;295(23):2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 4.Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355(2):125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 5.Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 6.Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomized placebo-controlled trial. Lancet. 2014;383(9922):1041–1048. doi: 10.1016/S0140-6736(13)62292-8. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Preventive Services Task Force. [October 28, 2014];2014 Pages accessed at http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrpv.htm at.
- 8.Visvanathan K, Hurley P, Bantug E, Brown P, Col NF, Cuzick J, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(23):2942–2962. doi: 10.1200/JCO.2013.49.3122. [DOI] [PubMed] [Google Scholar]
- 9.Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95(7):526–532. doi: 10.1093/jnci/95.7.526. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt C. The breast cancer chemoprevention debate. J Natl Cancer Inst. 2011;103(22):1646–1647. doi: 10.1093/jnci/djr479. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan CP, Kim SE, Wong ST, Sawaya GF, Walsh JM, Perez-Stable EJ. Willingness to use tamoxifen to prevent breast cancer among diverse women. Breast Cancer Res Treat. 2012;133(1):357–366. doi: 10.1007/s10549-012-1960-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melnikow J, Paterniti D, Azari R, Kuenneth C, Birch S, Kuppermann M, et al. Preferences of Women Evaluating Risks of Tamoxifen (POWER) study of preferences for tamoxifen for breast cancer risk reduction. Cancer. 2005;103(10):1996–2005. doi: 10.1002/cncr.20981. [DOI] [PubMed] [Google Scholar]
- 13.Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090–3095. doi: 10.1200/JCO.2009.27.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 15.Costantino JP, Gail MH, Pee D, Anderson S, Redmond CK, Benichou J, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91(18):1541–1548. doi: 10.1093/jnci/91.18.1541. [DOI] [PubMed] [Google Scholar]
- 16.Pankratz VS, Hartmann LC, Degnim AC, Vierkant RA, Ghosh K, Vachon CM, et al. Assessment of the accuracy of the Gail model in women with atypical hyperplasia. J Clin Oncol. 2008;26(33):5374–5379. doi: 10.1200/JCO.2007.14.8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amir E, Evans DG, Shenton A, Lalloo F, Moran A, Boggis C, et al. Evaluation of breast cancer risk assessment packages in the family history evaluation and screening programme. J Med Genet. 2003;40(11):807–814. doi: 10.1136/jmg.40.11.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chuba PJ, Hamre MR, Yap J, Severson RK, Lucas D, Shamsa F, et al. Bilateral risk for subsequent breast cancer after lobular carcinoma-in-situ: analysis of surveillance, epidemiology, and end results data. J Clin Oncol. 2005;23(24):5534–5541. doi: 10.1200/JCO.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 19.Donnelly LS, Evans DG, Wiseman J, Fox J, Greenhalgh R, Affen J, et al. Uptake of tamoxifen in consecutive premenopausal women under surveillance in a high-risk breast cancer clinic. Br J Cancer. 2014;110(7):1681–1687. doi: 10.1038/bjc.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Layeequr Rahman R, Crawford S. Chemoprevention Indication Score: a user-friendly tool for prevention of breast cancer - pilot analysis. Breast. 2009;18(5):289–293. doi: 10.1016/j.breast.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Goldenberg VK, Seewaldt VL, Scott V, Bean GR, Broadwater G, Fabian C, et al. Atypia in random periareolar fine-needle aspiration affects the decision of women at high risk to take tamoxifen for breast cancer chemoprevention. Cancer Epidemiol Biomarkers Prev. 2007;16(5):1032–1034. doi: 10.1158/1055-9965.EPI-06-0910. [DOI] [PubMed] [Google Scholar]
- 22.Bober SL, Hoke LA, Duda RB, Regan MM, Tung NM. Decision-making about tamoxifen in women at high risk for breast cancer: clinical and psychological factors. J Clin Oncol. 2004;22(24):4951–4957. doi: 10.1200/JCO.2004.05.192. [DOI] [PubMed] [Google Scholar]
- 23.Evans D, Lalloo F, Shenton A, Boggis C, Howell A. Uptake of screening and prevention in women at very high risk of breast cancer. Lancet. 2001;358(9285):889–890. doi: 10.1016/S0140-6736(01)06039-1. [DOI] [PubMed] [Google Scholar]
- 24.Evans DG, Harvie M, Bundred N, Howell A. Uptake of breast cancer prevention and screening trials. J Med Genet. 2010;47(12):853–855. doi: 10.1136/jmg.2010.082768. [DOI] [PubMed] [Google Scholar]
- 25.Owens WL, Gallagher TJ, Kincheloe MJ, Ruetten VL. Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract. 2011;7(2):85–88. doi: 10.1200/JOP.2010.000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor R, Taguchi K. Tamoxifen for breast cancer chemoprevention: low uptake by high-risk women after evaluation of a breast lump. Ann Fam Med. 2005;3(3):242–247. doi: 10.1370/afm.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravdin PM. The lack, need, and opportunities for decision-making and informational tools to educate primary-care physicians and women about breast cancer chemoprevention. Cancer Prev Res (Phila) 2010;3(6):686–688. doi: 10.1158/1940-6207.CAPR-10-0100. [DOI] [PubMed] [Google Scholar]
- 28.Fagerlin A, Dillard AJ, Smith DM, Zikmund-Fisher BJ, Pitsch R, McClure JB, et al. Women's interest in taking tamoxifen and raloxifene for breast cancer prevention: response to a tailored decision aid. Breast Cancer Res Treat. 2011;127(3):681–688. doi: 10.1007/s10549-011-1450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meiser B, Butow P, Price M, Bennett B, Berry G, Tucker K. Attitudes to prophylactic surgery and chemoprevention in Australian women at increased risk for breast cancer. J Womens Health (Larchmt) 2003;12(8):769–778. doi: 10.1089/154099903322447738. [DOI] [PubMed] [Google Scholar]
- 30.Bastian LA, Lipkus IM, Kuchibhatla MN, Weng HH, Halabi S, Ryan PD, et al. Women's interest in chemoprevention for breast cancer. Arch Intern Med. 2001;161(13):1639–1644. doi: 10.1001/archinte.161.13.1639. [DOI] [PubMed] [Google Scholar]
- 31.Nelson HD, Smith ME, Griffin JC, Fu R. Use of medications to reduce risk for primary breast cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;158(8):604–614. doi: 10.7326/0003-4819-158-8-201304160-00005. [DOI] [PubMed] [Google Scholar]
- 32.Cuzick J, Forbes JF, Sestak I, Cawthorn S, Hamed H, Holli K, et al. Long-term results of tamoxifen prophylaxis for breast cancer--96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99(4):272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 33.Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134(2):459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Land SR, Cronin WM, Wickerham DL, Costantino JP, Christian NJ, Klein WM, et al. Cigarette smoking, obesity, physical activity, and alcohol use as predictors of chemoprevention adherence in the National Surgical Adjuvant Breast and Bowel Project P-1 Breast Cancer Prevention Trial. Cancer Prev Res (Phila) 2011;4(9):1393–1400. doi: 10.1158/1940-6207.CAPR-11-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waters EA, Cronin KA, Graubard BI, Han PK, Freedman AN. Prevalence of tamoxifen use for breast cancer chemoprevention among U.S. women. Cancer Epidemiol Biomarkers Prev. 2010;19(2):443–446. doi: 10.1158/1055-9965.EPI-09-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waters EA, McNeel TS, Stevens WM, Freedman AN. Use of tamoxifen and raloxifene for breast cancer chemoprevention in 2010. Breast Cancer Res Treat. 2012;134(2):875–880. doi: 10.1007/s10549-012-2089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]