Abstract
Objective
We describe the first mouse model of pancreatic intra-epithelial neoplasia (PanIN) lesions induced by alcohol in the presence and absence of chronic pancreatitis.
Methods
Pdx1-Cre; LSL-Kras (KC) mice were exposed to Lieber-DeCarli alcohol diet for 6 weeks with cerulein injections. PanIN lesions and markers of fibrosis, inflammation, histone de-acetylation, epithelial-to-mesenchymal transition (EMT), and cancer stemness were measured by immuno-histochemistry and Western.
Results
Exposure of KC mice to an alcohol diet significantly stimulated fibrosis and slightly, but not significantly, increased the level of PanIN lesions associated with an increase in tumor-promoting M2-macrophages. Importantly, the alcohol diet did not increase activation of stellate cells. Alcohol diet and cerulein injections resulted in synergistic and additive effects on PanIN lesion and M2-Macrophage phenotype induction, respectively. Cerulein-pancreatitis caused stellate cell activation, EMT, and cancer stemness in the pancreas. Pancreatitis caused histone deacetylation which was promoted by the alcohol diet. Pancreatitis increased EMT and cancer stemness markers which not further affected by the alcohol diet.
Conclusion
The results suggest that alcohol has independent effects on promotion of PDAC associated with fibrosis formed through a stellate cell-independent mechanism and that it further promotes early PDAC and M2 macrophage induction in the context of chronic pancreatitis.
Keywords: Alcohol, chronic pancreatitis, pancreatic cancer
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related deaths among both men and women in the United States1 because of a lack of effective therapies. Although PDAC is largely due to environmental factors, there has been a lack of models developed needed to investigate how environmental factors promote the disease.
Chronic pancreatitis, which results from repeated episodes of acute pancreatitis, is the strongest identified risk factor for pancreatic cancer and increases the risk by at least 13.3 fold.2–4 Chronic pancreatitis is a progressive, destructive inflammatory process that ends in total destruction of the pancreas, malabsorption of the nutrients and severe pain.5 By far, the most common etiology of chronic pancreatitis is alcohol abuse, estimated to cause 60–90% of the cases.6, 7 In addition to its effect on chronic pancreatitis, numerous studies showed that heavy alcohol drinking speeds up the process of development of pancreatic cancer and decreases the median age of diagnosis of pancreatic cancer among drinkers.8–10 These results suggest two effects of alcohol abuse on pancreatic cancer promotion. One due to effects of alcohol abuse resulting in chronic pancreatitis; and the other independent of chronic pancreatitis.
Recent studies are demonstrating that educated macrophages (M2 type) play a major role in promotion of many cancers including pancreatic cancer.11, 12 There is a strong association shown between M2 macrophages in the tumors and increased metastasis and poor prognosis in humans.13 The increase in M2 macrophages is also associated with epithelial to mesenchymal transition (EMT) and stemness markers which also are highly associated with metastasis and poor outcome. Such results suggest that M2 macrophages are involved in promoting EMT and stemness in the cancer.
The mechanisms involved in recruiting and supporting M2 macrophage function are not well understood. Our studies focus on the role of interleukin-6 in M2 macrophage function. We find that IL-6 is involved in the conversion of macrophages to the M2 phenotype by stimulating the expression of IL-4 receptors which are known to be critically involved in the activation of M2 macrophages. We further find that IL-6 production is regulated by histone deacetylase (HDAC) enzymes in cancer cells and stellate cells of the tumor. HDAC proteins are critical regulators of fundamental cellular events such as cell cycle, differentiation, and apoptosis.14 HDAC1 has been shown to be involved in regulating epithelial to mesenchymal transition (EMT) in pancreatic cancer by directly interacting and stabilizing the pro-EMT Zeb1 transcription factor.15 Multiple human case-control studies have linked high serum levels of IL-6 with pancreatic cancer and suggested using it as a marker of cancer progression. A strong positive correlation was found among tumor stage, cachexia and decreased survival.16–21
In the present study we developed a mouse model of pancreatic cancer precursors using the Pdx1-Cre;LSL-Kras mice exposed to alcohol diet with and without causing pancreatitis with cerulein injections. We characterized the animal model including pancreatic lesions, fibrosis, inflammation, EMT and cancer stemness. We further, measured the level of histone acetylation in mice pancreatic tissues. The results suggest that alcohol has independent effects on promotion of pancreatic cancer associated with fibrosis formed through a stellate cell-independent mechanism and that it further promotes early pancreatic cancer in the context of chronic pancreatitis which has effects of stellate cell activation, EMT and cancer stemness. The effects of chronic pancreatitis on EMT and cancer stemness did not occur with alcohol treatment alone.
MATERIAL AND METHODS
Reagents
Alpha-smooth muscle actin (αSMA) antibody was purchased from Sigma-Aldrich (St. Louis, MO); Sirius Red/Collagen Staining Kit was from Chondrex (Redmond, WA); F4/80 and vimentin antibodies were from AbCam (Cambrige, MA); Arginase1 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA); and other antibodies were from Cell Signaling (Danvers, MA). Chemicals were from Sigma Aldrich (St. Louis, MO).
Mouse model
The Pdx1-Cre;LSL-Kras mice were generated at Northwestern University, Chicago, IL.22 Mice were housed in a temperature of (20 −/+2°C) room with a 12 hour light-dark cycle.
Mice (at least 10 per group) were fed with Lieber Decarli diet alcohol or regular diet for 6 weeks. During the last 3 weeks of the feeding mice were injected with 7-hourly cerulein injections (50μg/Kg) 2 times per week. The repetitive doses of cerulein cause a histologically verified chronic pancreatitis.23, 24
After 6 weeks, mice were sacrificed 72 hours after the last exposure to cerulein, pancreas collected and preserved adequately for analysis.
All of the mice were housed in Association for Assessment and Accreditation of Laboratory Animal Care-accredited facilities and the experiments were conducted by the Animal Core of the Southern California Research Center for ALPD and Cirrhosis in accordance with the NIH Guide for Care and Use of Laboratory Animals. The IACUC protocol number is 11529.
Immunohistochemistry
Samples of pancreas were fixed in formalin and paraffin embedded. Paraffin-embedded sections of pancreas were stained with hematoxylin and eosin to determine the presence of pancreatic lesions and for standard histological examination. Fibrosis was evaluated by immunostaining with Sirius Red/ Collagen antibody and activated stellate cells identified by αSMA antibody staining. Macrophages were stained by immunofluorescence using antibodies to the general macrophage marker F4/80 and the M2 macrophage specific marker arginase1.
Quantification of the staining was performed using the Leica SCN400 software.
Western blot
Tissue was homogenized and re-suspended in RIPA phosphorylation buffer (50 mM NaCl, 50 mM Tris/HCl pH 7.2, 1% deoxycholic acid, 1% Triton X-100, 0.1% SDS, 10 mM Na2HPO4 + NaH2PO4, 100 mM NaF, 2 mM Na3VO4, 80 μM glycerophosphate, 20% glycerol, 1 mM PMSF, 5 μg/ml each of pepstain, leupeptin, chymostatin, antipain, and aprotinin), sonicated and centrifuged for 15 min at 16,000 x g at 4 °C. Proteins were separated by SDS-PAGE and electrophoretically transferred to nitrocellulose or PVDF membranes. Non-specific binding was blocked for 1 h with 5% bovine serum albumin or non-fat dry milk in Tris-buffered saline (4 mM Tris base, 100 mM NaCl, pH 7.5) containing 0.05% Tween 20. Membranes were incubated with primary antibody overnight at 4°C, and then for 1 h with peroxidase-conjugated secondary antibody. Blots were developed using Supersignal Chemiluminescent Substrate (Pierce, Rockford, IL).
Statistics
Statistical analyses of the immunohistochemical quantifications were performed by using Student’s t test, one-way ANOVA, or Fisher’s exact test with GraphPad Prism (GraphPad Software). A p value < 0.05 was considered statistically significant.
RESULTS
Alcohol feeding and pancreatitis promote PanIN lesions in KC mice
KC mice carry the Kras mutation in the pancreas and spontaneously develop PanIN lesions, which are precursors of PDAC.25 Kras mutation is present in 90% of pancreatic cancer patients.26, 27 The KC mouse pancreas recapitulates characteristics of human disease including a vast desmoplastic reaction and rapid growth, and represents the early stage of the disease.22
We fed the KC mice with Lieber-DeCarli alcohol or regular diet for 6 weeks. During the last 3 weeks of the diet a portion of the mice were injected with 7-hourely injections of cerulein (50μg/Kg) 2 times per week (Fig 1A). This is an established procedure for inducing histologically verified chronic pancreatitis.23, 24
Fig 1. Alcohol further stimulates PanIN lesion formation induced by cerulein in Kras mice.
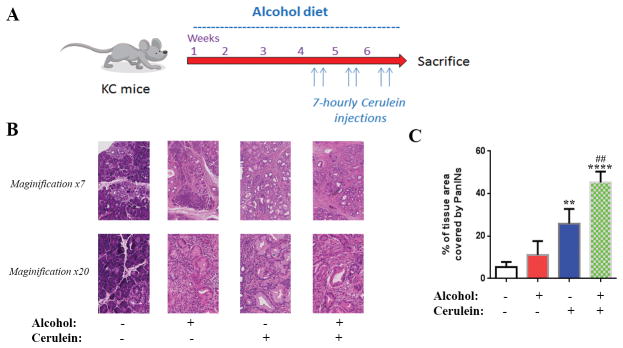
(A) KC mice were exposed to regular or alcohol Lieber-DeCarli diet for 6 weeks. During the last 3 weeks of the diets mice were exposed to 7-hourely injections of cerulein (50μg/Kg) two times a week and sacrificed 72h after the last injection. (B): H&E staining of the pancreas of mice exposed to saline or cerulein injections with and without Lieber-DeCarli alcohol diet. (C) Quantification of tissue area occupied by PanIN lesions. **, p < 0.01 and ****, p<0.001 versus control; ####, p<0.001 versus alcohol diet.
Analysis of the pancreatic tissue showed a 2-fold increase in the percentage of pancreatic tissue occupied by PanIN lesions in mice fed alcohol (10% of the tissue area) compared to regular diet (5% the tissue area) (Fig 1B, C). However, the increase was not significant. Repetitive series of cerulein injections, which are known to induce chronic pancreatitis,23, 24 significantly increased the area of PanIN lesions by 5 fold compared to control mice. The combination of alcohol diet and the repetitive series of cerulein injections had a synergistic effect on inducing PanIN lesions by increasing PanINs from 5% of pancreatic tissue area in control mice to 45% in mice treated with alcohol and cerulein together (Fig 1C).
Alcohol feeding and pancreatitis differentially stimulate fibrosis in KC mice
Next we measured the effects of alcohol feeding and pancreatitis on two characteristics of the microenvironment of pancreatic cancer, namely fibrosis and stellate cell activation. We found that the alcohol diet alone significantly stimulated collagen deposition as measured by Sirius red staining (Fig 2A). Quantification of the staining showed a 2.5-fold increase in collagen deposition (Fig 2B). Cerulein injections also increased Sirius red staining inducing a 3.5-fold increase. The combination of the alcohol diet and cerulein injections did not significantly change the level of collagen deposition compared to each treatment alone (Fig 2B). Differently, stellate cell activation as assessed by measuring the level of its marker α-SMA was not increased by alcohol (Fig 2B, C). Cerulein alone and in combination with alcohol increased α-SMA level by 3 and 4 folds, respectively. These findings suggest that although alcohol feeding and pancreatitis both cause fibrosis of the microenvironment of the developing tumor; the fibrosis is independent of stellate cells in the animals treated with the ethanol diet alone.
Fig 2. Chronic pancreatitis stimulates fibrosis and stellate cells activation; alcohol further stimulates collagen deposition.
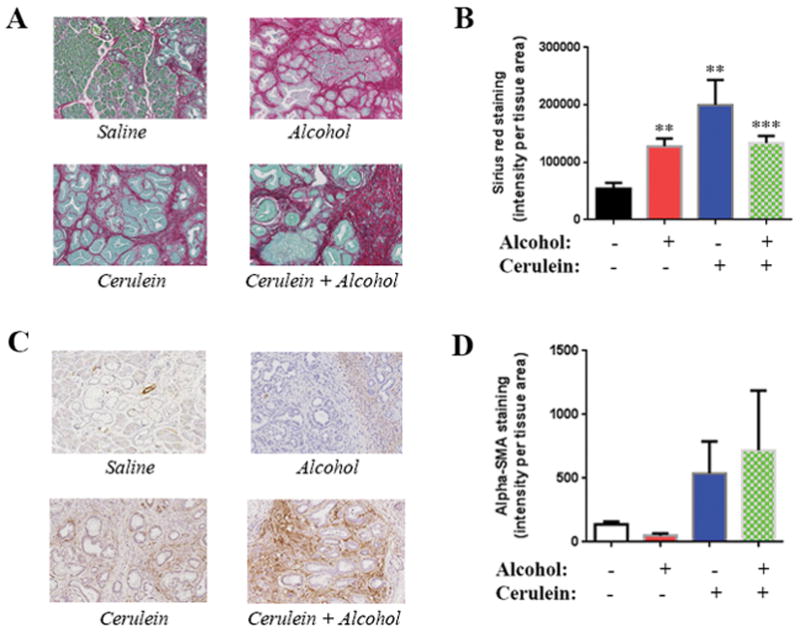
(A) Sirius Red/Collagen staining of the pancreas of KC mice exposed to cerulein with and without alcohol diet; (B) Quantification of collagen staining; (C) α-SMA staining of mice pancreas; (D) Quantification of α-SMA staining. **, p < 0.01 and ***, p<0.005 versus control.
Alcohol feeding and pancreatitis promote M2 macrophages in the KC mouse pancreas
Next, we performed dual staining of macrophages by the general macrophage marker F4/80 and the M2 macrophage specific marker Arginase1 (Fig 3A). We found that alcohol alone had a small effect on the level M2 macrophages (Fig 3B). However, cerulein injections alone increased the level of M2 macrophages by 2 fold. The combination of the alcohol diet and cerulein injections had an additive effect on the level of M2 macrophages (Fig 3B).
Fig 3. Chronic pancreatitis induces the M2 macrophage phenotype; alcohol diet further increases this effect.
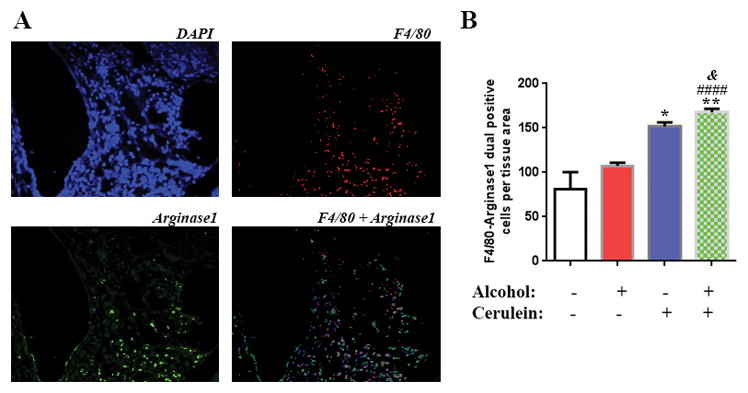
(A) Macrophage dual staining with F4/80 and arginase1 of the pancreas of KC mice exposed to cerulein with and without alcohol diet. (B) Quantification of the macrophage staining. *, p < 0.05 and **, p<0.01 versus control; ####, p<0.001 versus alcohol diet; &, p<0.05 versus cerulein.
Alcoholic chronic pancreatitis stimulates EMT, cancer stemness and HDAC pathway in pancreas of KC mice
EMT is a key step in inducing cancer cell metastasis and cancer stemness is a major contributor to the resistance of cancer cells to treatments.28–30 In our animal model we found that cerulein stimulated EMT as shown by a decrease in E-cadherin levels measured by Western blot (Fig 4A). Whereas, alcohol alone did not affect the E-cadherin level (not shown). Equally important was the up-regulation of the cancer stemness marker Sox2 by cerulein injections. These effects were not further increased by alcohol (Fig 4A).
Fig 4. Alcoholic chronic pancreatitis up-regulates EMT, cancer stemness, and decreases histone acetylation.
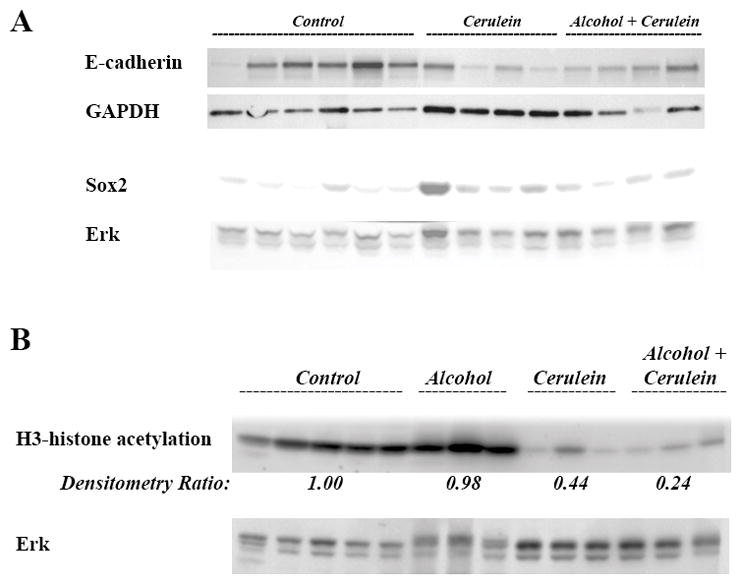
Lysates are made from pancreas of KC mice exposed to cerulein with and without alcohol diet. (A) Protein levels of E-cadherin and Sox2 were measured by Western. (B) Protein levels of H3-histone acetylation were measured by Western. Blots were re-probed for Erk to confirm equal loading.
Our previous results showed a role of HDAC in regulating promotion of pancreatic cancer and stimulation of EMT and cancer stemness. We found that cerulein treatment decreased H3 histone acetylation whereas alcohol feeding alone had no effect (Fig 4B). Importantly, alcohol slightly further decreased the level of histone acetylation in cerulein treated mice from 44% of control level to 24% (Fig 4B).
DISCUSSION
In this study we have developed novel models of early PDAC to determine the differential effects of alcohol feeding and alcoholic chronic pancreatitis on promotion of the disease. The use of repetitive series of cerulein injections causing chronic pancreatitis in alcohol fed mice is an established model of alcoholic pancreatitis.23, 24 This model shows characteristics of the human disease.31 We have used the Pdx1-Cre;LSL-Kras genetically altered mice, which spontaneously develop PanIN lesions,22 the precursors of PDAC. Differently from previously published reports we have exposed these mice to repetitive series of cerulein injections to induce chronic pancreatitis in addition to the environmental factor alcohol.32, 33
A key finding of our study is that the alcohol diet alone increased PanIN size and overall area in the pancreas and stimulated collagen deposition. The combination of the alcohol diet and cerulein injections induced a synergistic increase in PanIN lesion formation. The combined treatment increased the tissue area covered by PanINs by 8.5-folds compared to a 2-fold increase by alcohol diet and a 4-fold increase by cerulein alone. Although the alcohol diet alone stimulated collagen deposition by more than 2 fold, it did not increase stellate cell activation which is thought to be the major source of extracellular matrix production and fibrosis in the pancreas,34, 35 suggesting that alcohol stimulates collagen production by cells other than the stellate cells.
We found that cerulein injections led to stimulation of multiple features found in human PDAC. The effect of cerulein injections was very pronounced on PanIN formation, fibrosis, and stellate cells activation. Although the effect of cerulein on fibrosis and stellate cells activation is similar to what was observed in cerulein models using wild type mice;36 the effect of alcohol in our Kras mice was different as it did not further increase the cerulein-induced stimulation of fibrosis and stellate cells activation.
Associated with PanIN formation, cerulein injections induced multiple other features of human PDAC including activation of macrophages along with stimulation of EMT and cancer stemness. Cerulein pancreatitis induced a significant 2-fold increase in the number of M2 macrophages in the KC mice compared to saline. This effect was further increased by alcohol diet. In fact, although alcohol alone did not show a significant increase in M2 macrophages induction, combination of cerulein and alcohol diet showed an additive effect on inducing activated M2 macrophages.
Recent data shows markers of EMT and metastasis at the early stages of pancreatic cancer.37 In our model we found that cerulein stimulated EMT as measured by the level of E-cadherin. This is very important knowing that EMT is associated with higher level of metastasis and cancer stemness.38, 39 This effect was not further increased by alcohol diet.
HDAC has been shown to mediate EMT in pancreatic cancer cells.15 Our data show strong inhibition of histone acetylation level suggesting it mediates the early EMT observed in this model. In fact, alcohol alone did not decrease histone acetylation level; whereas, cerulein alone or in combination with alcohol induced a significant decrease in histone acetylation. Alcohol further enhanced the effect of cerulein treatment on histone acetylation. This is very important as recently published data from Hartman et al. showed that inhibition of HDAC decreased markers of pancreatitis and protected from tissue injury of the pancreas in C57Bl/6 mice subjected to taurocholic acid model of pancreatitis.40
Several models of acute pancreatitis and mild chronic pancreatitis have been developed in transgenic Kras mice.32, 33 However, in our model we induced a more aggressive exposure to cerulein and combined it with alcohol diet, which is the major reason for chronic pancreatitis.
Alcohol appears to promote PanIN lesions formation independently of pancreatitis through an unknown mechanism; most likely independently of stellate cells activation or recruitment of M2 macrophages. However, when combined with cerulein, alcohol further stimulates fibrosis, and activation of macrophages.
In sum, we have developed an animal model of pancreatic cancer precursors induced by a combination of a genetic mutation and exposure to cerulein chronic pancreatitis with and without alcohol diet. An important finding of the study is the effect of alcohol alone on stimulating PanIN lesion formation and a stellate cells-independent fibrosis, and the stimulation of macrophage M2 activation in the context of chronic pancreatitis.
Acknowledgments
This work was supported by the NIAAA grant K01AA019996 (to ME), the P50AA011999 grant (to HT), the NCI grant P01CA163200 (to SJP), and Merit Review awards from the Department of Veterans Affairs (to SJP and HT).
We thank Dr. Arkadiusz Gertych and Dr. Kolja A. Wawrowsky for their help in scanning and analyzing the IHC slides. We thank Steven Swartwood and Dr. Beatrice Knudsen for their help in the IHC work.
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–71. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Whitcomb DC, Pogue-Geile K. Pancreatitis as a risk for pancreatic cancer. Gastroenterol Clin North Am. 2002;31:663–78. doi: 10.1016/s0889-8553(02)00004-3. [DOI] [PubMed] [Google Scholar]
- 3.Raimondi S, Lowenfels AB, Morselli-Labate AM, et al. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24:349–58. doi: 10.1016/j.bpg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–61. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682–707. doi: 10.1053/gast.2001.22586. [DOI] [PubMed] [Google Scholar]
- 6.Singer MV. Effect of ethanol and alcoholic beverages on the gastrointestinal tract in humans. Rom J Gastroenterol. 2002;11:197–204. [PubMed] [Google Scholar]
- 7.Dufour MC, Adamson MD. The epidemiology of alcohol-induced pancreatitis. Pancreas. 2003;27:286–90. doi: 10.1097/00006676-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Anderson MA, Zolotarevsky E, Cooper KL, et al. Alcohol and tobacco lower the age of presentation in sporadic pancreatic cancer in a dose-dependent manner: a multicenter study. Am J Gastroenterol. 2012;107:1730–9. doi: 10.1038/ajg.2012.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiao L, Silverman DT, Schairer C, et al. Alcohol use and risk of pancreatic cancer: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2009;169:1043–51. doi: 10.1093/aje/kwp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucenteforte E, La Vecchia C, Silverman D, et al. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4) Ann Oncol. 2012;23:374–82. doi: 10.1093/annonc/mdr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho HJ, Jung JI, Lim do Y, et al. Bone marrow-derived, alternatively activated macrophages enhance solid tumor growth and lung metastasis of mammary carcinoma cells in a Balb/C mouse orthotopic model. Breast Cancer Res. 2012;14:R81. doi: 10.1186/bcr3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu CY, Xu JY, Shi XY, et al. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest. 2013;93:844–54. doi: 10.1038/labinvest.2013.69. [DOI] [PubMed] [Google Scholar]
- 13.Kurahara H, Shinchi H, Mataki Y, et al. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res. 2011;167:e211–9. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Marks P, Rifkind RA, Richon VM, et al. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 15.Aghdassi A, Sendler M, Guenther A, et al. Recruitment of histone deacetylases HDAC1 and HDAC2 by the transcriptional repressor ZEB1 downregulates E-cadherin expression in pancreatic cancer. Gut. 2012;61:439–48. doi: 10.1136/gutjnl-2011-300060. [DOI] [PubMed] [Google Scholar]
- 16.Barber MD, Fearon KC, Ross JA. Relationship of serum levels of interleukin-6, soluble interleukin-6 receptor and tumour necrosis factor receptors to the acute-phase protein response in advanced pancreatic cancer. Clin Sci (Lond) 1999;96:83–7. [PubMed] [Google Scholar]
- 17.Ebrahimi B, Tucker SL, Li D, et al. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer. 2004;101:2727–36. doi: 10.1002/cncr.20672. [DOI] [PubMed] [Google Scholar]
- 18.Moses AG, Maingay J, Sangster K, et al. Pro-inflammatory cytokine release by peripheral blood mononuclear cells from patients with advanced pancreatic cancer: relationship to acute phase response and survival. Oncol Rep. 2009;21:1091–5. doi: 10.3892/or_00000328. [DOI] [PubMed] [Google Scholar]
- 19.Mroczko B, Groblewska M, Gryko M, et al. Diagnostic usefulness of serum interleukin 6 (IL-6) and C-reactive protein (CRP) in the differentiation between pancreatic cancer and chronic pancreatitis. J Clin Lab Anal. 2010;24:256–61. doi: 10.1002/jcla.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okada S, Okusaka T, Ishii H, et al. Elevated serum interleukin-6 levels in patients with pancreatic cancer. Jpn J Clin Oncol. 1998;28:12–5. doi: 10.1093/jjco/28.1.12. [DOI] [PubMed] [Google Scholar]
- 21.Wigmore SJ, Fearon KC, Sangster K, et al. Cytokine regulation of constitutive production of interleukin-8 and -6 by human pancreatic cancer cell lines and serum cytokine concentrations in patients with pancreatic cancer. Int J Oncol. 2002;21:881–6. doi: 10.3892/ijo.21.4.881. [DOI] [PubMed] [Google Scholar]
- 22.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 23.Yoo BM, Oh TY, Kim YB, et al. Novel antioxidant ameliorates the fibrosis and inflammation of cerulein-induced chronic pancreatitis in a mouse model. Pancreatology. 2005;5:165–76. doi: 10.1159/000085268. [DOI] [PubMed] [Google Scholar]
- 24.Neuschwander-Tetri BA, Burton FR, Presti ME, et al. Repetitive self-limited acute pancreatitis induces pancreatic fibrogenesis in the mouse. Dig Dis Sci. 2000;45:665–74. doi: 10.1023/a:1005423122127. [DOI] [PubMed] [Google Scholar]
- 25.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 26.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–9. [PubMed] [Google Scholar]
- 27.Pellegata NS, Sessa F, Renault B, et al. K-ras and p53 gene mutations in pancreatic cancer: ductal and nonductal tumors progress through different genetic lesions. Cancer Res. 1994;54:1556–60. [PubMed] [Google Scholar]
- 28.Dissanayake SK, Wade M, Johnson CE, et al. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem. 2007;282:17259–71. doi: 10.1074/jbc.M700075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang FT, Zhuan-Sun YX, Zhuang YY, et al. Inhibition of hedgehog signaling depresses self-renewal of pancreatic cancer stem cells and reverses chemoresistance. Int J Oncol. 2012;41:1707–14. doi: 10.3892/ijo.2012.1597. [DOI] [PubMed] [Google Scholar]
- 30.Zhang AL, Wang QS, Zhong YH, et al. Effect of transcriptional factor snail on epithelial-mesenchymal transition and tumor metastasis. Ai Zheng. 2005;24:1301–5. [PubMed] [Google Scholar]
- 31.Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180–93. doi: 10.1053/j.gastro.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 32.Carriere C, Young AL, Gunn JR, et al. Acute pancreatitis accelerates initiation and progression to pancreatic cancer in mice expressing oncogenic Kras in the nestin cell lineage. PLoS One. 2011;6:e27725. doi: 10.1371/journal.pone.0027725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerra C, Collado M, Navas C, et al. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell. 2011;19:728–39. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Apte MV, Park S, Phillips PA, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–87. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Bachem MG, Schunemann M, Ramadani M, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–21. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 36.Perides G, Tao X, West N, et al. A mouse model of ethanol dependent pancreatic fibrosis. Gut. 2005;54:1461–7. doi: 10.1136/gut.2004.062919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–61. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Battula VL, Evans KW, Hollier BG, et al. Epithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells. Stem Cells. 2010;28:1435–45. doi: 10.1002/stem.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartman H, Wetterholm E, Thorlacius H, et al. Histone Deacetylase Regulates Trypsin Activation, Inflammation, and Tissue Damage in Acute Pancreatitis in Mice. Dig Dis Sci. 2015;60:1284–9. doi: 10.1007/s10620-014-3474-y. [DOI] [PubMed] [Google Scholar]


