Abstract
Background
Recent reports showed that functional control of HIV-1 infection for a prolonged time is possible by early anti-retroviral therapy (ART); however its underlying mechanism needs to be studied with a suitable animal model. Recently, humanized-BLT (bone marrow, liver and thymus) mouse (hu-BLT) was shown to be an excellent model for studying HIV-1 infection. We thus tested the feasibility of studying functional control of HIV-1 infection using hu-BLT mice.
Methods
Animals in three treatment groups (Rx-6h, Rx-24h, Rx-48h) and untreated group were infected with HIV-1, followed by ART initiation at 6, 24 or 48 hours post-infection and continued daily for two weeks. Three weeks after stopping ART, CD8+ T-cells were depleted from all animals. Plasma viral load (PVL) was monitored weekly using droplet digital PCR (ddPCR). Percentage of CD4+ and CD8+ T-cells were measured by flow cytometry. In situ hybridization (ISH) and ddPCR were used to detect viral RNA (vRNA) and DNA.
Results
While control animals had high viremia throughout the study, all Rx-6h animals had undetectable PVL after ART cessation. After CD8+ T-cells depletion, viremia increased and CD4+ T-cells decreased in all animals except the Rx-6h group. Viral DNA was detected in spleens of all animals and a few vRNA+ cells were detected by ISH in one of three Rx-6h animals.
Conclusion
Early ART did not act as prophylaxes, but rather, can control HIV-1 productive infection and prevented CD4+ T-cells depletion in hu-BLT mice. This mouse model can be used to elucidate the mechanism for functional control of HIV-1.
Keywords: Early ART, HIV-1 infection, functional control, hu-BLT mice
Introduction
Anti-retroviral therapy (ART) has greatly reduced mortality and morbidity associated with HIV-1 infection over the last decade1. Early ART treatments not only limited the disease progression in infected infants to enable them to achieve normal growth and development, it also reduced the sexual transmission rates of HIV-1 in adults2,3. Despite these successes, 2.3 million new infections are still occurring annually worldwide4. Recent research breakthroughs suggest the possibility of a functional cure involving several cases of HIV-1 infected individuals. The first is the Berlin acute myeloid leukemia patient who received multiple stem-cell transplantation with donor cells lacking the HIV-1 co-receptor CCR5 (Δ32) after undergoing ablative chemotherapy 5. The patient showed a reduction in replication competent viral reservoir without viral rebound in the absence of ART. In addition, an infant treated with ART at 30 hours postpartum maintained undetectable viral load for 2 years without ART, but viremia rebounded recently6,7. These studies suggest that early treatment could limit the rapid spread of HIV-1, reduce the size of viral reservoirs and achieve a temporary functional control of HIV-1 productive infection.
Despite these recent excitements on functional cure, several critical questions still need to be addressed. One is the mechanisms on how the virus can be suppressed for sustained period without ART and what led to the subsequent viral rebound. Another is the need to determine the timing for initiation and duration of ART after HIV-1 exposure in order to achieve a sustainable functional control of HIV-1 productive infection. Additionally, it is unclear whether the latent reservoirs or low-level productive infected cells could still exist after early ART, which may lead to viral rebound upon ART withdrawal and remain an impediment to a long-term cure. This possibility was demonstrated in two HIV-1 infected individuals who underwent allogeneic hematopoietic stem cell (HSC) transfer and then treated with ART to protect the donor cells 8, 9. While on ART, both individuals had undetectable viremia and HIV-1 DNA in PBMCs after transplantation. Unfortunately, the virus rebounded in both patients several months after ART was stopped, suggesting the re-activation of latently infected reservoirs lead to acute infections. Therefore, it is important to better understand the underlying mechanism of functional control and characterize the timing of early ART treatment and its correlation with the establishment of viral reservoirs.
Unfortunately, studying the effectiveness of early ART initiation and its relationship with the establishment of viral reservoirs will be difficult in patients since the timing of infection cannot be determined accurately. In addition, it will also be challenging to obtain the tissues and specimens needed to determine residual viral reservoirs. One animal model for such studies is the non-human primate (NHP) model. However, NHP is expensive and the number of animals that can be used for these studies is limited. Therefore, alternative animal models are needed.
Recently, a new generation of humanized mouse, the BLT (bone marrow, liver and thymus) mouse (hu-BLT) has been shown to be an alternative model for studying HIV-1 infections. It can be reconstituted with multiple lineages of human immune cells in lymphatic and mucosal tissues, and can elicit antigen specific T cell and humoral responses after viral infection 10-15. Using the hu-BLT mice, this pilot study shows that early ART initiation can functionally control HIV-1 productive infection in the infected animals during the course of study. Whether the virus will eventually rebound and how to compare the time scale between hu-BLT mouse and human remain to be determined. Our result nevertheless indicates that the functional control window through early treatment is small. This model offers an opportunity to decipher the parameters for achieving and maintaining a functional control of HIV-1 infection.
Materials and Methods
Virus preparation
HIV-1 transmitted/founder viruses, HIV-SUMA and HIV-WITO, were generated by transfecting infectious molecular clone pSUMA.c/2821 and pWITO.c/2474 (cat#l 1748 and 11739 from Dr John Kappes via the AIDS Research and Reference Reagent program, Division of AIDS, NIAID, NIH), respectively, into 293T cells. The resulting viruses were expanded by co-culture in pooled PBMC from two healthy human donors. Cell-free supernatant was harvested, filtered and concentrated by ultracentrifugation. Concentrated virus was re-suspended in RPMI and viral titer determined using TZM-bl cells according to standard Reed Muench Method 16.
HIV-1 infection, ART initiation and CD8+ T-cells depletion in hu-BLT mice
Hu-BLT mice were generated by implanting NSG mice (NOD.Cg-Prkdcscid I12rgtm1wj1/SzJ) (Jackson Laboratory, Stock number 005557) with human fetal liver, thymus tissues under left renal capsule and then injecting with autologous human CD34+ HSCs as previously described 14,17. Hu-BLT mice were maintained in micro-isolator cages in BSL-2 animal rooms by following the protocol approved by the Institutional Animal Care and Use Committee at the University of Nebraska-Lincoln.
All animals were inoculated intraperitoneally (IP) with a mixture of HIV-SUMA and HIV-WITO (2.5 × 103 TCID50 each). Daily ART was initiated at 6, 24 or 48 hours post-infection to treatment groups animals by IP injection. ART was continued daily for two weeks and the ART consists of 5mg/mouse of tenofovir disoproxil fumarate (TDF) (Carbosynth Limited) dissolved in 30 μl dimethyl sulfoxide (Fisher Scientific) and 4mg/mouse of lamivudine (Carbosynth Limited) dissolved in 40 μl sterile water (Hospira Inc). The control animals received solvent only. Human CD8+ T-cells of all animals were depleted by IP delivery of two doses of anti-CD8 antibody M-T807R1 (NIH Non-human primate reagent resource) at 5mg/kg at three day interval.
Plasma viral load
Viral RNA was extracted from the animal plasma using QIAamp Viral RNA Mini kit (Qiagen). Plasma viral load (PVL) in copies/ml was determined by droplet digital PCR (ddPCR) as follows: Each reaction consisted of a final concentration of 1X one-step RT-ddPCR supermix (Biorad), 1 mM manganese acetate solution, 900 nM each of forward primer (5′-CAAGCAGCCATGCAAATGTT-3′) and reverses primer (5′-ATGTCACTTCCCCTTGGTTCTC-3′), 250 nM probe (5′-FAM-CCTGGTGCAATAGGCCCTGC-BHQ1-3′), template vRNA and top up to 20 μl with molecular grade water. The primers and probe target the conserved region of HIV-1 gag. Droplet emulsion was generated by QX100 droplet generator (Biorad) and PCR was performed with C1000 Touch Thermal Cycler (Biorad). Raw fluorescence data was quantified using the QX100 droplet reader (Biorad) and analyzed by QuantaSoft version 1.3.2.0 (Biorad). Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software). Background cutoff value of 40 copies/ml was determined with plasma from non-infected hu-BLT mice.
Flow cytometry
The changes in hCD4+ and hCD8+ T-cells in hu-BLT mice during the study were measured by staining the animal PBMC with respective antibodies; mCD45-APC, hCD45-FITC, hCD3-PE, hCD19-PE/Cy5, hCD4-Alexa 700 and hCD8-APC-Cy7 (BioLegend, Cat#103111, 304006, 300408, 302209, 300526 and 301016 respectively). Raw data were quantified by FACSAria III (BD Biosciences) and analyzed with FlowJo version 7.6.4 (TreeStar). Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software).
HIV-1 DNA copy number in tissues
Genomic DNA from animal spleen tissues was extracted using Gentra Puregene kit (Qiagen). The copy number of HIV-1 DNA per million human cells was measured by ddPCR. The protocol is similar to PVL with the exception that the reaction mix consisted of 1X ddPCR supermix for probes (Biorad) and the DNA sample was digested with restriction enzyme Msc I before ddPCR. Digestion of DNA was required to reduce sample viscosity and increase template accessibility, and there is no Msc I sites within the target sequences. Primers and probes are identical to those used for PVL. To determine the number of human cells within the sample analyzed, separate ddPCR reactions were performed with primers and probe against human beta-globin gene. The background cutoff value of 18 copies/106 human cells was determined using genomic DNA extracted from the spleens of uninfected hu-BLT mice.
HIV-1 RNA detection in tissue
Animal spleen tissues were collected during euthanasia and fixed in 4 % paraformaldehyde. In-situ hybridization (ISH) for HIV-1 vRNA in spleen tissues of sacrificed animals were conducted using 35S riboprobes that covered >90% of HIV-1 genome as described previously18. The exposure time of tissue slide radioautography was 7 days.
Results
Experimental design and monitoring of the infected animals
The human immune reconstitution of all hu-BLT mice were measured as the percentage of human cells present in PBMCs by FLOW cytometry. Thirteen adult animals with good immune reconstitution were randomly divided into early treatment (Rx-6h, Rx-24h, Rx-48h, n=3 each) and control (n=4) groups (Supplement Table 1). Kruskal-Wallis nonparametric and ANOVA parametric analysis showed no significant differences (P = 0.1136 and 0.1046, respectively) between the groups in the percentage of reconstituted human CD45+ cells. Similar results (Kruskal-Wallis, P = 0.5874; ANOVA, P = 0.4579) were obtained for the reconstituted human CD4+ cells between the groups.
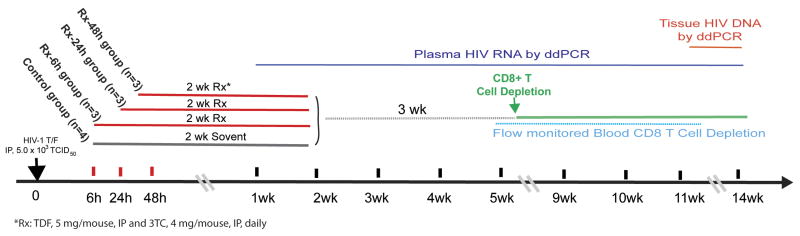
To preclude the possibility that our results are HIV-1 strain specific, all animals were infected intraperitoneally with a mixture of two transmitted/founder HIV-1. The intraperitoneal route was used since it guarantees 100% infection rate compared with either intra-rectal or intra-vaginal inoculation route. The main objectives of this study are to establish an animal model of initiating early ART to functional control of HIV-1 infection, and to determine the most effective treatment time frame needed to achieve functional control and its underlying mechanism (Fig 1). Several studies have shown that ART administration within days of post-infection (p.i.) often resulted in a rebound of viremia during treatment interruption 19,20. In contrast, the Mississippi infant case initiated ART at ∼30 hours after birth was able to suppress viremia for 2 years without ART 6,7. Hence, our study was designed to initiate ART within hours of infection at 6, 24 or 48 hours p.i. (Fig 1). TDF and lamivudine were used in this study.
Figure 1. Schematic of the experimental design.
Thirteen adult hu-BLT mice were randomly divided into early treatment (Rx-6h, Rx-24h, Rx-48h, n=3 each) and control (n=4) groups. All animals were inoculated intraperitoneally (IP) with a mixture of two transmitted/founder HIV-1 viruses (2.5 × 103 TCID50 each). Daily ART was initiated at 6, 24 or 48 hours post-infection for each respective treatment groups. Three weeks after ART was stopped, CD8+ T-cells from all animals were depleted. The levels of CD8+ T-cells, CD4+ T-cells, PVL and vDNA in tissue were monitored as indicated.
Several studies in non-human primate models had shown that CD8+ T-cells can mediate viral suppression and its depletion can dramatically increase viral load21,22. We reasoned that the depletion of CD8+ T-cells may allow previously undetectable residual virus to rebound and enable us to better detect the presence of virus. Thus, the CD8+ T-cells were depleted from all animals at three weeks after ART was stopped as described in the methods section. The CD8+ and CD4+ T-cells levels were closely monitored throughout the study to assess the levels of CD8+ T-cells depletion and CD4+ T-cells loss, a hallmark of disease progression during HIV infection. The PVL were measured weekly by ddPCR to detect low viral copy number in the experimental animals23,24. The spleen tissues were also collected at necropsy to be used to determine vDNA by ddPCR and vRNA.
Six and 24 hours treatment animals had undetectable to low PVL after ART cessation
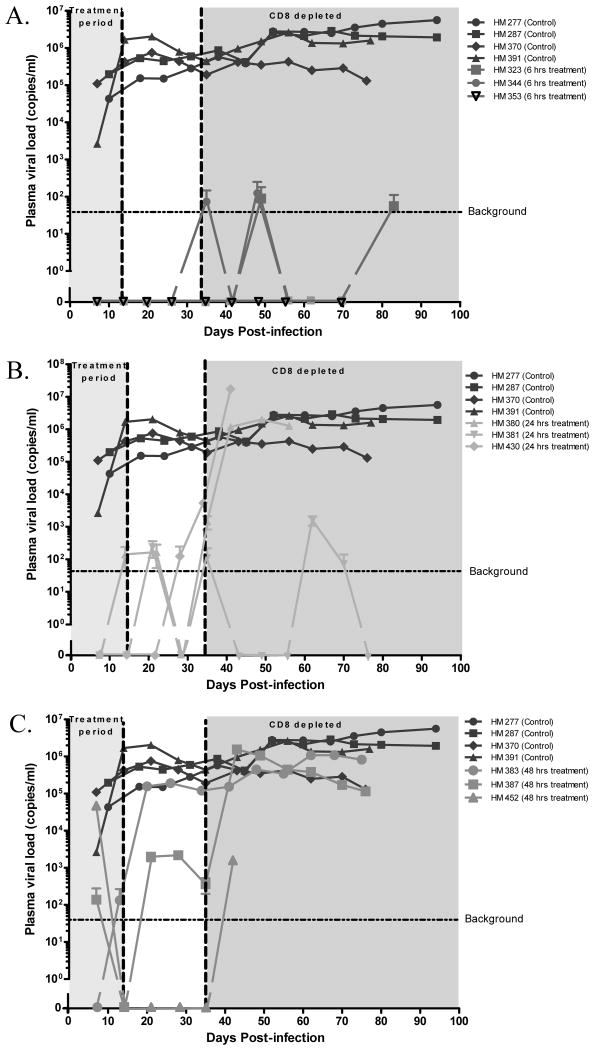
At 1 week p.i., high viremia (2×103 to 1.9×105 copies/ml) was detected in all control animals, which suggests that our virus inoculum and delivery route were able to achieve 100% infection rate (Fig 2A). Importantly, all animals in Rx-6h have undetectable PVL during and after ART were stopped (Fig 2A). In contrast, one animal (HM380) in Rx-24h had low PVL of 1.4×102 copies/ml during ART and all animals in this group developed very low PVL (1×102 to 2×102 copies/ml) after ART was stopped (Fig 2B). For the Rx-48h group, one animal (HM452) had high PVL (4.7×104 copies/ml) during ART but became PVL undetectable after ART was stopped and before CD8+ T-cells depletion; the other two animals (HM383 and 387) in this group developed high PVL (2.1×103 to 1.9×105 copies/ml) after ART was stopped. After CD8 T-cells depletion, all animals became viremic (Fig 2C).
Figure 2. Plasma viral load (PVL).
The identical control animals were included in all graphs for comparison purposes. Background cutoff value of 40 copies/ml was determined with plasma from non-infected hu-BLT mice. The 2 weeks on ART and CD8+ T-cells depletion period are shaded. (A) Rx-6h. (B) Rx-24h. (C) Rx-48h.
Six hours treatment animals had undetectable to low PVL even after CD8+ T-cells depletion
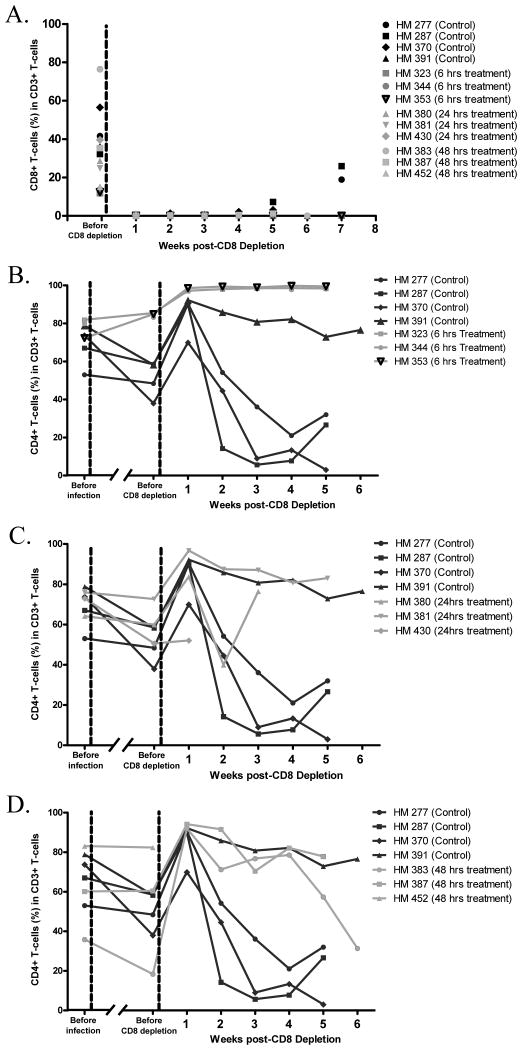
CD8+ T-cells had been shown to suppress viral replication in vivo and led to low viremia25. To determine whether the undetectable to low PVL observed after ART cessation was due to CD8+ T-cells mediated suppression, all the studied animals underwent CD8+ T-cells depletion. Flow cytometric data showed that >99% of CD8+ T-cells in blood were depleted; the depletion was sustained in all animals for up to 4 weeks, and in the majority of animals for up to 7 weeks (Fig 3A).
Figure 3. Flow cytometry.
The identical control animals were included in all graphs for comparison purposes. (A) CD8+ T-cells percentage before and after CD8+ T-cells depletion. (B) CD4+ T-cells percentage of Rx-6h, (C) Rx-24h and (D) Rx-48h treatment groups over the study period.
Surprisingly, CD8+ T-cells depletion did not result in any significant PVL increase in all the Rx-6h animals. Two of these animals (HM323 and 344) had transient low PVL (55 to 1×102 copies/ml) at three time points while the PVL in one animal (HM353) remained undetectable (Fig 2A). In contrast, two animals (HM380 and 430) of the Rx-24h group had PVL increased significantly (2×106 to 1.7×107 copies/ml) after CD8+ T-cells depletion (Fig 2B). One animal (HM381) in Rx-24h developed a high PVL (1.5×103 copies/ml) at only one time point and declined to below detection limit at euthanization (Fig 2B). All animals in the control and Rx-48h developed high PVL after CD8+ T-cells depletion (Fig 2C).
Six hours treatment protected the infected animals from disease progression
CD4+ T-cells count is an important marker for HIV-1 disease progression. To determine if early ART could prevent CD4+ T-cells losses and disease progression, the percentage of CD4+ T-cells were measured before and after infection, as well as after CD8+ T-cells depletion by flow cytometry.
Before CD8+ T-cells depletion, all animals in Rx-6h maintained their CD4+ T-cells levels at pre-infection levels (Fig 3B). Whereas, all the infected animals in the control and in Rx-24h and one of three animals in Rx-48h already showed a decline in CD4+ T-cells relative to their infection baseline (Fig 3C and D).
After CD8+ T-cells depletion, the lack of CD8+ T-cells led to apparent increases in the percentage of CD4+ T-cells across all the experimental groups. More importantly, all animals in Rx-6h maintained a high level of CD4+ T-cells during CD8+ T-cells depletion (Fig 3B). In contrast, all animals in the control, Rx-24h and Rx-48h showed significant CD4+ T-cells losses (Fig 3C and D). These data suggests that early ART at 6 hours p.i. prevented the depletion of CD4+ T-cells in the infected animals.
Six hours treatment did not act as pre-exposure prophylaxis to prevent infection
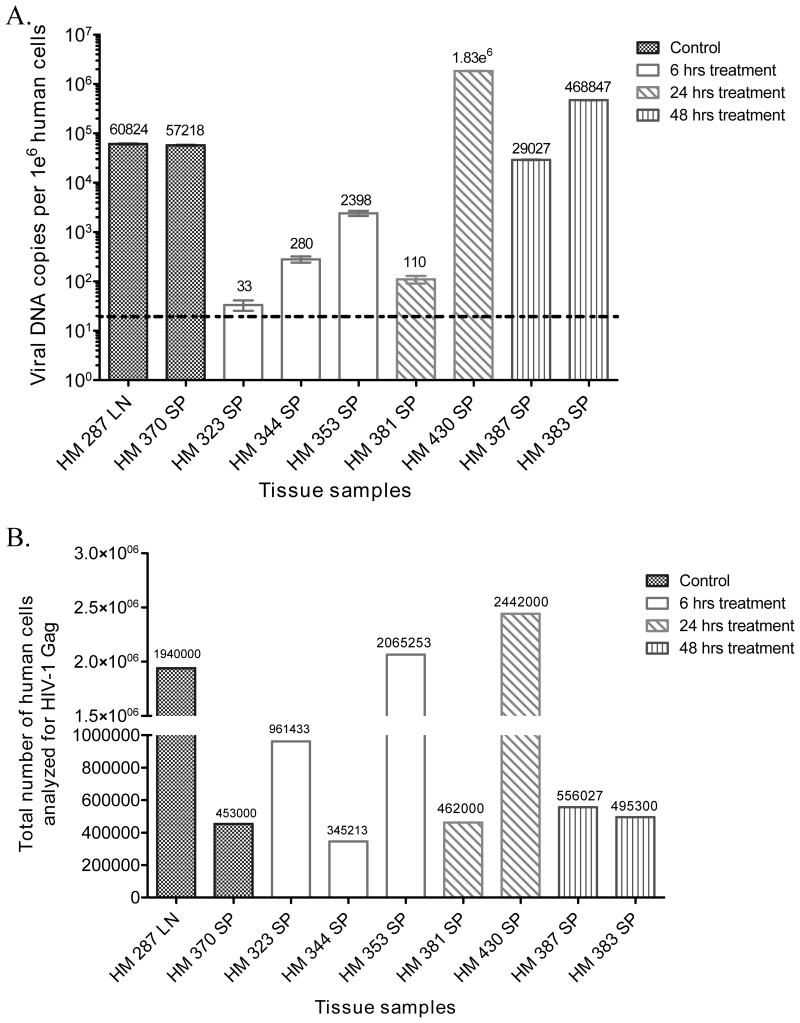
Since ART was administered within hours of infection, there was a concern that it may have functioned as prophylaxis rather than treatment. To address this issue, ddPCR was used to detect the presence of HIV-1 vDNA in the spleen tissues harvested from all animals at necropsy. As expected, the control and Rx-48h animals had high vDNA copy number in spleen tissues (2.9×104 to 4.6×105 vDNA copies/106 human cells) (Fig 4A). Similarly, vDNA was also detected in Rx-24h animals (110 to 1.83×106 vDNA copies/106 human cells) (Fig 4A). Importantly, Rx-6h animals were all found to be vDNA positive (33 to 2.4×103 vDNA copies/106 human cells) (Fig 4A). This strongly suggests that ART initiation at 6 hours p.i. did not act as prophylaxis because it did not prevent the initial infection, the generation of proviral DNA and the establishment of viral reservoirs in the lymphatic tissues where vDNA were detected. The low vDNA copy number in some animals was not due to lower numbers of cells tested, since nearly 1 million human cells were used for the analysis. For example, animal HM323 (Rx-6h) was found to have only 33 vDNA copies/10 human cells (Fig 4B).
Figure 4. HIV-1 DNA copy number in lymphatic tissues.
Viral DNA was detected in the lymph node (LN) or Spleen (SP) of necropsied animals by ddPCR. Background cutoff value of 18 copies/106 human cells was determined with non-infected hu-BLT mice spleen tissue. HM380 (Rx-24h) and 452 (Rx-48h) die before sacrifice, therefore their samples are unavailable. (A) HIV-1 DNA copies/106 human cells for Control, Rx-6h, Rx-24h and Rx-48h group (B) Total number of human cells analyzed for HIV-1 DNA for each animal.
Detection of HIV-1 vRNA in spleen tissues
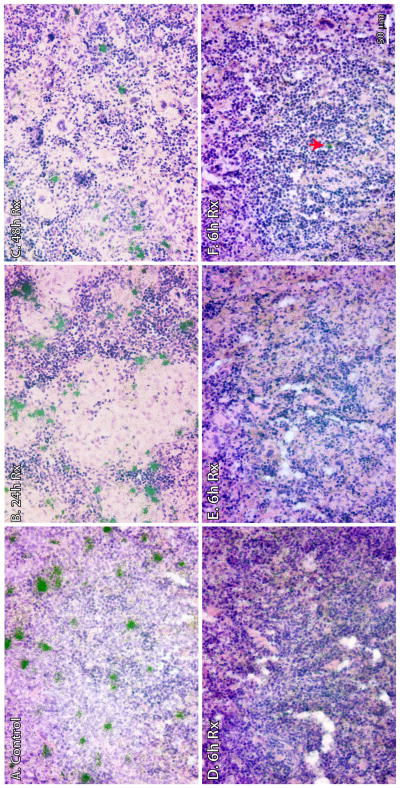
To evaluate whether HIV-1 replication occurred in the lymphatic tissues of the 6 hours treatment animals, ISH was used to detect vRNA+ cells in the spleen tissues, which had undetectable or low PVL. As expected, numerous HIV-1 vRNA+ cells were detected in the control, Rx-24h and Rx-48h animals (Fig 5A, B and C). In contrast, no vRNA+ cells were detected in two Rx-6h animals (HM323 and 353) (Fig 5D and E), and only a few isolated vRNA+ cells (three vRNA+ cells in the whole tissue section analyzed) were detected in the third Rx-6h animal (HM344) (Fig 5F). Therefore, our results confirmed that 6 hours ART treatment p.i. could achieve functional control and suppress viral replication to low or undetectable levels in the lymphatic tissues of treated animals, even upon ART withdrawal and CD8+ T-cells depletion during the follow-up period. Importantly, we observed that HIV-1 infection can be maintained at either latent or non-productive level upon early ART treatment.
Figure 5. HIV-1 vRNA+ cells in the spleens of hu-BLT mice were detected using in situ hybridization.
Spleen tissues were collected after >70 days p.i. and fixed in 4% paraformaldehyde. Clusters of green silver grains overlay HIV-1 vRNA+ cells after radioautography for 7 day exposure. (A) Representative image showed numerous HIV-1 vRNA+ cells in the control animal (HM370). (B) Representative image showed numerous HIV-1 vRNA+ cells in Rx-24h (HM430). (C) Representative image showed numerous HIV-1 vRNA+ cells in Rx-48h (HM383). (D and E) Undetectable vRNA+ cells in two animals of Rx-6h (HM323 and 353, respectively). (F) Isolated vRNA+ cell (arrow) in Rx-6h (HM344).
Discussion
The recently reported “Mississippi pediatric case” and other studies have provided a glimmer of hope that temporary functional control of HIV-1 infection is possible 6,26. However, many questions such as whether this functional control can be sustained over time, what is its underlying mechanism, where are the potential viral reservoirs and whether ART regimen can be further improved remained to be addressed. In this preliminary study, we recapitulated the essential feature of functional control, low or undetectable plasma viral load after withdrawal of ART during the study period, by using the hu-BLT mouse model27. Importantly, our result showed that early initiation of ART at 6 hours, but not at 24 or 48 hours post-infection in this model, achieved functional control in the infected animals and is reminisce of those in the reported clinical cases 6,26.
It was reported that Tenofovir treatment started at 24 hours post-infection achieved a control of virus productive infection in rhesus macaques28. Other animal studies showed that HIV-1 always rebounded after ART was withdrawn in these setting 19,20. However, our study showed that hu-BLT mice which received ART at 6 hours post-infection had undetectable PVL measured by the sensitive ddPCR after ART was stopped. Only after CD8+ T-cells depletion that two out of three animals had transient near-detection limit (40 copies/ml) PVL, and the other animal had undetectable PVL even after CD8+ T-cells depletion for over 7 weeks (Fig 2). Our results suggest that functional control was achieved in the 6 hours post-infection treated animals using this model. We hypothesize that early treatment might limit the size of viral reservoirs in different CD4+ T-cells subsets, allowing the host immune system to eliminate productive reservoirs and select for latent reservoirs that remained non-productive without ART29 Although the time scale difference between hu-BLT mice and human still needs to be determined, the concept of a small window for functional control, even for temporary functional control, remain valid based on our study.
Since ART was initiated early after infection, it is possible that it might had acted as prophylaxis rather than therapeutic. To address this issue, we checked for the presence of HIV-1 vDNA in the lymphatic tissues. Surprisingly, all animals, including the 6 hours treatment group, had detectable vDNA in their spleen tissues at the end of experiment. This suggests that ART initiated at 6 hours post-infection did not prevent establishment of viral reservoirs but acted therapeutically.
Although our detection method cannot determine whether the detected vDNA were in fact integrated viral genomes, evidence from other studies suggested that only integrated viral DNA can be retained upon successful ART30. In addition, our ISH detected a few HIV-1 vRNA+ cells in the spleen of one animal in the 6 hours treatment group, suggesting that some of these vDNA were integrated and functional. The ISH data might explain why near-detection limit PVL can still be observed in the same animal. At this point, we cannot exclude the possibility that ISH might have missed some vRNA+ cells in other tissues or perhaps even in other sections of the same tissue, therefore, further extensive analysis of other treated animals will be needed to explore this possibility. In addition, since some 6 hours treatment animals without detectable PVL had high vDNA copy in their tissues, we cannot exclude the possibility that the detected vDNA are integrated but defective proviral genome in clonally expanded host cells. It is equally possible that these are functional latent proviral genome which can be reactivated for virus production. Nevertheless, our result from the CD8+ T-cells depletion experiment showed that the low or undetected viremia in the 6 hours treatment animals were not mainly due to CD8+ T-cells mediated suppression or lack of target CD4+ T-cells. It strongly suggests that lymphatic tissues such as spleen could be a major viral reservoir despite successful ART and undetectable viremia. More in-depth experiments, such as adoptive transfer of cells and in-vitro stimulation, will be needed to determine the presence of replication competent viral reservoirs.
There are several caveats in this study. First, our study has a small number of animals per treatment group; second, our study may underestimate the functional curable window which may be enlarged by including more ART drugs and increasing the treatment time; third, it is possible that other infection routes may produce different results. Thus, future studies are needed to address these limitations. Regardless, this study serves as a proof of concept that the hu-BLT mouse model can be used for future studies to explore many unanswered questions regarding functional control and its sustainability. Such questions include some of the major challenges that will be prohibitive, if not impossible to study in patients, due to the difficulty in identifying early HIV-1 infection in patients as well as identifying viral genome in different anatomic sites. In addition, further optimization of the timing, various ART combinations and dosages will be needed to improve the potency of early ART for achieving a sustainable functional control of HIV-1 infection. Moreover, this model has the potential to address unanswered questions such as the viral reservoirs and mechanisms of functional control.
Supplementary Material
Acknowledgments
Source of Funding: This work was supported by in part by the NIH COBRE grant P30 GM103509-Nebraska Center for Virology and F43 TW 001429 to CW, and the NIH COBRE pilot project grant from the Nebraska Center for Virology to QL. YL was a Fogarty Fellow.
The authors would like to thank Andrew Demers for his technical support and his contribution to hu-BLT mouse generation, and Ming Sun for his help with sample collections.
Footnotes
Partial data of this study were presented at the CROI conference, March 2014, Boston, Massachusetts.
Conflicts of Interest: None of the authors in this paper have commercial or other association that might pose a conflict of interest.
Author's contributions: QL and CW conceived the conceptual framework; designed the main experiments and oversaw all aspects of this project. FYT generated virus stocks, conducted and analyzed vRNA and vDNA detection using ddPCR. GK managed the mouse experiments, performed ART administration, CD8+ T-cells depletion, animal euthanasia, tissue fixation, embedding and in situ hybridization. GK, WL performed virus inoculation. GK, WL, WF conducted mouse bleeding. YL, WF and ZY performed flow cytometry and data analyses. CJD oversaw the pharmacology aspects of this project and provided manuscript editing. FYT, QL and CW prepared the manuscript.
References
- 1.Piot P, Quinn TC. Response to the AIDS pandemic--a global health model. N Engl J Med. 2013 Jun 6;368(23):2210–2218. doi: 10.1056/NEJMra1201533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phongsamart W, Hansudewechakul R, Bunupuradah T, et al. Long-term outcomes of HIV-infected children in Thailand: the Thailand Pediatric HIV Observational Database. Int J Infect Dis. 2014 Feb 25; doi: 10.1016/j.ijid.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic. 2013 [Google Scholar]
- 5.Hutter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009 Feb 12;360(7):692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 6.Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013 Nov 7;369(19):1828–1835. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.“Mississippi Baby” Now Has Detectable HIV, Researchers Find. NIH News. [ http://www.niaid.nih.gov/news/newsreleases/2014/pages/mississippibabyhiv.aspx.
- 8.Cilio AR, Krishnan A, Mitsuyasu RT, et al. Plasma viremia and cellular HIV-1 DNA persist despite autologous hematopoietic stem cell transplantation for HIV-related lymphoma. J Acquir Immune Defic Syndr. 2013 Aug 1;63(4):438–441. doi: 10.1097/QAI.0b013e31828e6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henrich TJ, Hu Z, Li JZ, et al. Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J Infect Dis. 2013 Jun 1;207(11):1694–1702. doi: 10.1093/infdis/jit086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melkus MW, Estes JD, Padgett-Thomas A, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006 Nov;12(11):1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 11.Sun Z, Denton PW, Estes JD, et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J Exp Med. 2007 Apr 16;204(4):705–714. doi: 10.1084/jem.20062411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denton PW, Estes JD, Sun Z, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 2008 Jan 15;5(1):e16. doi: 10.1371/journal.pmed.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheeler LA, Trifonova R, Vrbanac V, et al. Inhibition of HIV transmission in human cervicovaginal explants and humanized mice using CD4 aptamer-siRNA chimeras. J Clin Invest. 2011 Jun;121(6):2401–2412. doi: 10.1172/JCI45876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brainard DM, Seung E, Frahm N, et al. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J Virol. 2009 Jul;83(14):7305–7321. doi: 10.1128/JVI.02207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudek TE, No DC, Seung E, et al. Rapid evolution of HIV-1 to functional CD8(+) T cell responses in humanized BLT mice. Sci Transl Med. 2012 Jul 18;4(143):143ra198. doi: 10.1126/scitranslmed.3003984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. The American Journal of Hygiene. 1938;27(3):493–497. [Google Scholar]
- 17.Wang LX, Kang G, Kumar P, et al. Humanized-BLT mouse model of Kaposi's sarcoma-associated herpesvirus infection. Proc Natl Acad Sci USA. 2014 Feb 25;111(8):3146–3151. doi: 10.1073/pnas.1318175111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Duan L, Estes JD, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005 Apr 28;434(7037):1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 19.Gianella S, von Wyl V, Fischer M, et al. Effect of early antiretroviral therapy during primary HIV-1 infection on cell-associated HIV-1 DNA and plasma HIV-1 RNA. Antivir Ther. 2011;16(4):535–545. doi: 10.3851/IMP1776. [DOI] [PubMed] [Google Scholar]
- 20.Wyl V, Gianella S, Fischer M, et al. Early antiretroviral therapy during primary HIV-1 infection results in a transient reduction of the viral setpoint upon treatment interruption. PLoS One. 2011;6(11):e27463. doi: 10.1371/journal.pone.0027463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin X, Bauer DE, Tuttleton SE, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999 Mar 15;189(6):991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999 Feb 5;283(5403):857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson S, Graf EH, Dahl V, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013 Feb;9(2):e1003174. doi: 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinheiro LB, Coleman VA, Hindson CM, et al. Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Anal Chem. 2011 Jan 17;84(2):1003–1011. doi: 10.1021/ac202578x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pandrea I, Gaufin T, Gautam R, et al. Functional cure of SIVagm infection in rhesus macaques results in complete recovery of CD4+ T cells and is reverted by CD8+ cell depletion. PLoS Pathog. 2011 Aug;7(8):e1002170. doi: 10.1371/journal.ppat.1002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saez-Cirion A, Bacchus C, Hocqueloux L, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013 Mar;9(3):e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanham G, Buve A, Florence E, Seguin-Devaux C, Saez-Cirion A. What is the significance of posttreatment control of HIV infection vis-a-vis functional cure? AIDS. 2013 Feb 20;28(4):603–605. doi: 10.1097/QAD.0000000000000147. [DOI] [PubMed] [Google Scholar]
- 28.Lifson JD, Rossio JL, Arnaout R, et al. Containment of simian immunodeficiency virus infection: cellular immune responses and protection from rechallenge following transient postinoculation antiretroviral treatment. J Virol. 2000 Mar;74(6):2584–2593. doi: 10.1128/jvi.74.6.2584-2593.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soriano-Sarabia N, Bateson RE, Dahl NP, et al. The quantitation of replication-competent HIV-1 in populations of resting CD4+ T cells. J Virol. 2014 Sep 24; doi: 10.1128/JVI.01900-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koelsch KK, Liu L, Haubrich R, et al. Dynamics of total, linear nonintegrated, and integrated HIV-1 DNA in vivo and in vitro. J Infect Dis. 2008 Feb 1;197(3):411–419. doi: 10.1086/525283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.