Abstract
Background
Globally, as in South Africa, obstetric hemorrhage (OH) remains a leading cause of maternal mortality and morbidity. Although blood transfusion is critical to OH management, the incidence and predictors of transfusion as well as their relation to HIV infection are poorly described.
Study Design and Methods
A cross-sectional study was conducted of all peripartum patients at four major hospitals in South Africa (April to July 2012). Comprehensive clinical data were collected on patients who sustained OH and/or were transfused. Logistic regression was used to model risk factors for OH and transfusion.
Results
A total of 15,725 peripartum women were evaluated, of whom 3,969 (25.2%) were HIV positive. Overall, 387 (2.5%) women sustained OH and 438 (2.8%) were transfused, including 213 (1.4%) women with both OH and transfusion. There was no significant difference in OH incidence between HIV positive (2.8%) and HIV negative (2.3%) patients (adjusted OR = 0.95, 95% CI 0.72–1.25). In contrast, the incidence of blood transfusion was significantly higher in HIV positive (3.7%) than in HIV negative (2.4%) patients (adjusted OR = 1.52, 95% CI 1.14–2.03). Other risk factors for transfusion included OH, low prenatal hemoglobin, the treating hospital, lack of prenatal care and gestational age ≤34 weeks.
Conclusion
In the South African obstetric setting, the incidence of peripartum blood transfusion is ten-fold higher than in the U.S. and other high-income countries while OH incidence is similar. While OH and prenatal anemia are major predictors of transfusion, HIV infection is a common and independent contributing factor.
Keywords: postpartum hemorrhage, blood transfusion, obstetric labor complications, South Africa, epidemiology
Introduction
Obstetric hemorrhage (OH) remains a major international public health challenge and is a leading contributor to both obstetric mortality1–3 and severe acute maternal morbidity4. Frequent, unanticipated OH is directly related to absent or deficient obstetric care5. Lack of early recognition of risk factors for primary or recurrent OH3 as well as failure to provide effective pre- and peripartum care contribute to adverse maternal outcomes2. Consequently, morbidity and mortality due to OH are primarily encountered in resource poor countries; this is the case in South Africa6,7.
Studies suggest that lack of blood for transfusion contributes to a quarter of OH-related deaths and/or “near misses” (severe acute maternal morbidity) in Sub-Saharan Africa1, underscoring the critical role of blood transfusion in obstetric care. Furthermore, the International Confederation of Midwives (ICM) and the International Federation of Gynecology and Obstetrics (FIGO) recommend that there be “blood transfusion facilities in all centers that provide comprehensive health care”2. While the importance of blood transfusion in obstetric practice is widely accepted8, published data on the incidence and clinical use of blood transfusion in obstetric settings are lacking. Pertinent to South Africa, where up to 30% of pregnant women are HIV positive9,10, the transfusion practices in HIV positive OH patients have not been studied. Furthermore, blood transfusion reflects a complicated peripartum course11,12, and an improved understanding of the risk factors for OH and peripartum transfusion may serve to identify deficiencies in care, thus informing corrective interventions.
Therefore, we conducted a cross-sectional study of all peripartum women that presented to four major obstetric hospitals in South Africa to investigate the risk factors for OH and/or peripartum blood transfusion. In addition, we took advantage of the study to gather contemporary data on HIV prevalence and treatment to ascertain compliance with current HIV treatment guidelines in the South Africa obstetric population.
Materials and Methods
Study Design and Subjects
A cross-sectional study was conducted over a four-month period (April to July 2012) on all deliveries at four major hospitals in South Africa: Chris-Hani Baragwanath Hospital (CHB) in Johannesburg, King Edward VIII Hospital (KEH) in Durban, Mowbray Maternity Hospital (MMH) and Groote Schuur Hospital (GSH) in Cape Town. The study received ethical approval from the relevant committees at the participating hospitals in addition to University of California, San Francisco, the South African National Blood Service (SANBS) and RTI International. Written informed consent was elicited from all women presenting at CHB; a waiver of consent for collection of existing data was granted at the other three hospitals.
The obstetric services at the four sites serve low-income, predominantly Black-African and Colored women (Colored in South Africa denotes a specific mixed race population group). The four sites have major second-tier or tertiary obstetric services and the patients reflect a generally urban, high HIV prevalence population in South Africa. We excluded patients who did not consent (CHB only), who were either transferred or discharged before data could be gathered or who left prematurely against medical advice.
Trained research personnel enrolled all peripartum obstetric patients with an index hospitalization at any of the four hospitals during the study period. Limited denominator data from all patients were collected on a ledger-style form. The minority of patients who sustained OH and/or were transfused in the peripartum period were identified through daily review of ward admission logs and maternity, delivery and operating room registers, in combination with direct communication with the blood bank and ward staff. More extensive clinical data were collected on these patients using a newly designed Obstetric Hemorrhage Audit Tool (OHAT). Both machine-readable paper forms [available on request] were scanned for data entry and electronic data were transferred to the data-coordinating center for cleaning and analysis. All data were collected either concurrently with the patients’ admissions or soon after discharge.
Definitions
“OH” was defined as any obstetric-related hemorrhage occurring in the peripartum period of 48 hours pre- or post-delivery. We used the World Health Organization (WHO) definition of peripartum hemorrhage as ≥500 mL blood loss for vaginal delivery or ≥1000 mL blood loss for Caesarean section. We included live births as well as births associated with stillborn fetuses and early neonatal deaths; however, data collection was restricted to women that were at least 26 weeks gestation. “Transfusion” was defined as having received any allogeneic blood product, i.e. red cells, platelets, plasma and/or cryoprecipitate, during the peripartum period. “Booked” refers to patients who had had antenatal care during the index pregnancy; in contrast “Unbooked” refers to patients who had not had antenatal care during the index pregnancy. The prenatal hemoglobin value that was used was the most recently measured hemoglobin prior to delivery: 40% and 58% of prenatal hemoglobin values were within 3 and 30 days of delivery, respectively.
At the time of the study the national policy was to administer three-drug antiretroviral therapy (ART) to all HIV positive pregnant patients with a CD4 count <350 cells per mm3 or with a WHO staging of III or IV. The prevention of mother to child transmission (PMTCT) regimen referred to the use of limited mono therapy during the antenatal period and additional antiretroviral therapy at the time of delivery and was recommended in HIV-positive patients not fulfilling the criteria for ART, or for patients with HIV presenting for the first time in labor.
Statistical Analysis
The descriptive analysis generated counts and percentages for categorical data and distributions for continuous variables. For categorical data, counts and percentages for single variables and combinations of variables were produced, using Chi-squared tests of significance. For the continuous data, distributions were examined individually and stratified by covariates, using T-tests to test differences between means.
Multivariable modeling was conducted using logistic regression. The primary outcome variables were binary while the predictor variables were categorical or ordinal. A larger set of variables was initially considered. The model was refined using backwards elimination at the p=0.05 level to retain variables. Once a set of variables was identified, interactions were investigated. SAS 9.3 (TS1M2) with enhance analytic product SAS/STAT 12.1 (SAS Institute Inc. 2011) was used for the data manipulation and analysis; R version 3.0.1 (2013-05-16) – “Good Sport” (R Core Team 2013) was used for the data visualization. Finally, the models were tested for calibration using the Hosmer-Lemeshow test for goodness of fit.
Results
We included 15,725 women over four months from April to July 2012. A total of 15,670 patients had valid data for hemorrhage and transfusion. These numbers reflect the exclusion of approximately 12% of peripartum women at one hospital (CHB) due to lack of consent mostly due to inability to contact patients; refusals were rare. The majority of women were aged 20 to 29 and of Black race; data on race was not provided on many patients at two hospitals (Table 1). Among all women, 25.2% were HIV positive (prevalence varied by site from 14.8 % at MMH to 37% at KEH) and 95.8% had received at least some antenatal care (booked). Fifty eight percent had vaginal deliveries, 40.5% had Caesarean sections and 1.2% of patients delivered before arrival at the hospital. Mode of delivery differed by HIV status, with Caesarean sections performed in 44% of HIV positive patients as compared to 41% of HIV negative patients (p=0.0008).
Table 1.
Characteristics of the peripartum women included in the study, overall and by hospital, South Africa 2012. Column percentages are shown except for the top row.
| Ledger Variable | All subjects N (%) | CHB N (%) | KEH N (%) | MMH N (%) | GSH N (%) |
|---|---|---|---|---|---|
| All subjects | 15,725 | 7,548 (48.0) | 2,441 (15.5) | 4,336 (27.6) | 1,400 (8.9) |
| Age | |||||
| ≤19 | 2,067 (13.1) | 1,087 (14.4) | 357 (14.6) | 519 (12.0) | 104 (7.4) |
| 20–24 | 4,402 (28.0) | 2,183 (28.9) | 747 (30.6) | 1,238 (28.6) | 234 (16.7) |
| 25–29 | 4,252 (27.0) | 1,898 (25.1) | 693 (28.4) | 1,245 (28.7) | 416 (29.7) |
| 30–34 | 2,834 (18.0) | 1,284 (17.0) | 383 (15.7) | 813 (18.8) | 354 (25.3) |
| 35–39 | 1,624 (10.3) | 812 (10.8) | 210 (8.6) | 393 (9.1) | 209 (14.9) |
| 40+ | 506 (3.2) | 276 (3.7) | 46 (1.9) | 103 (2.4) | 81 (5.8) |
| Missing | 40 (0.3) | 8 (0.1) | 5 (0.2) | 25 (0.6) | 2 (0.1) |
| Race | |||||
| Black | 10,786 (68.6) | 7,470 (99.0) | 2,253 (92.3) | 854 (19.7) | 209 (14.9) |
| Colored | 596 (3.8) | 22 (0.3) | 32 (1.3) | 382 (8.8) | 160 (11.4) |
| Asian | 134 (0.9) | 3 (0.0) | 34 (1.4) | 68 (1.6) | 29 (2.1) |
| White | 45 (0.3) | 1 (0.0) | 31 (1.3) | 10 (0.2) | 3 (0.2) |
| Missing | 4,164 (26.5) | 52 (0.7) | 91 (3.7) | 3,022 (69.7) | 999 (71.4) |
| Prenatal visit | |||||
| Booked | 15,058 (95.8) | 7,291 (96.6) | 2,361 (96.7) | 4,110 (94.8) | 1,296 (92.6) |
| Unbooked | 574 (3.7) | 257 (3.4) | 65 (2.7) | 151 (3.5) | 101 (7.2) |
| Missing | 93 (0.6) | 0 (0.0) | 15 (0.6) | 75 (1.7) | 3 (0.2) |
| Type of delivery | |||||
| Vaginal | 9,117 (58.0) | 4,773 (63.2) | 1,152 (47.2) | 2,567 (59.2) | 625 (44.6) |
| C-section | 6,362 (40.5) | 2,653 (35.1) | 1,220 (50.0) | 1,733 (40.0) | 756 (54.0) |
| BBA | 191 (1.2) | 121 (1.6) | 42 (1.7) | 16 (0.4) | 12 (0.9) |
| Missing | 55 (0.3) | 1 (0.0) | 27 (1.1) | 20 (0.5) | 7 (0.5) |
| Gravidity (includes current pregnancy) | |||||
| 1 | 5,215 (33.2) | 2,647 (35.1) | 717 (29.4) | 1,505 (34.7) | 346 (24.7) |
| 2 | 4,738 (30.1) | 2,285 (30.3) | 766 (31.4) | 1,296 (29.9) | 391 (27.9) |
| 3 | 3,198 (20.3) | 1,495 (19.8) | 563 (23.1) | 834 (19.2) | 306 (21.9) |
| 4+ | 2,524 (16.1) | 1,120 (14.8) | 377 (15.4) | 671 (15.5) | 356 (25.4) |
| Missing | 50 (0.3) | 1 (0.0) | 18 (0.7) | 30 (0.7) | 1 (0.1) |
| Parity (before current delivery) | |||||
| 0 | 5,674 (36.1) | 2,949 (39.1) | 787 (32.2) | 1,533 (35.4) | 405 (28.9) |
| 1 | 5,017 (31.9) | 2,355 (31.2) | 799 (32.7) | 1,406 (32.4) | 457 (32.6) |
| 2 | 3,070 (19.5) | 1,398 (18.5) | 543 (22.2) | 832 (19.2) | 297 (21.2) |
| 3 | 1,239 (7.9) | 563 (7.5) | 192 (7.9) | 341 (7.9) | 143 (10.2) |
| 4+ | 696 (4.4) | 282 (3.7) | 116 (4.8) | 201 (4.6) | 97 (6.9) |
| Missing | 29 (0.2) | 1 (0.0) | 4 (0.2) | 23 (0.5) | 1 (0.1) |
| HIV Status | |||||
| Missing/Unknown | 130 (0.8) | 9 (0.1) | 21 (0.9) | 96 (2.2) | 4 (0.3) |
| Negative | 11,626 (73.9) | 5,362 (71.0) | 1,517 (62.1) | 3,598 (83.0) | 1,149 (82.1) |
| Positive | 3,969 (25.2) | 2,177 (28.8) | 903 (37.0) | 642 (14.8) | 247 (17.6) |
| CD4 ≥ 350 | 1,715 (10.9) | 887 (11.8) | 393 (16.1) | 318 (7.3) | 117 (8.4) |
| CD4 <350 and ≥200 | 1,112 (7.1) | 545 (7.2) | 312 (12.8) | 182 (4.2) | 73 (5.2) |
| CD4 < 200 | 663 (4.2) | 345 (4.6) | 185 (7.6) | 89 (2.1) | 44 (3.1) |
| CD4 missing/Uknown | 479 (3.0) | 400 (5.3) | 13 (0.5) | 53 (1.2) | 13 (0.9) |
Abbreviations
CHB: Chris-Hani Baragwanath Hospital
MMH: Mowbray Maternity Hospital
GSH: Groote Schuur Hospital
KEH: King Edward VIII hospital
BBA: Born before arrival in hospital
C-section: Caesarean section
A total of 387 (2.5%) women sustained OH and 438 (2.8%) were transfused; included in these were 213 (1.4%) women with both OH and transfusion (Table 2). The incidence of OH by hospital was 1.3%, 1.8%, 2.8%, and 4.4% at KEH, MMH, CHB and GSH respectively. In unadjusted analyses, OH was significantly associated with treating hospital, gestational age, gravidity, parity, prenatal care, and birth weight (Table 2).
Table 2.
Characteristics of patients with obstetric hemorrhage (OH) and transfusion compared to those without each condition. Row percentages and Chi-squared p-values are shown. Fifty-five women without valid data on OH and transfusion were excluded from the Table.
| Ledger Variable | No Hemorrhage N (%) | Hemorrhage N (%) | No Transfusion N (%) | Transfusion N (%) |
|---|---|---|---|---|
| Total | ||||
| All Subjects | 15,283 (97.5) | 387 (2.5) | 15,232 (97.2) | 438 (2.8) |
| Age | (p = 0.0535) | (p = 0.4921) | ||
| Missing | 27 (73.0) | 10 (27.0) | 29 (78.4) | 8 (21.6) |
| <=19 | 2,012 (98.0) | 42 (2.0) | 1,993 (97.0) | 61 (3.0) |
| 20–24 | 4,305 (98.1) | 84 (1.9) | 4,286 (97.7) | 103 (2.3) |
| 25–29 | 4,130 (97.4) | 109 (2.6) | 4,122 (97.2) | 117 (2.8) |
| 30–34 | 2,747 (97.2) | 79 (2.8) | 2,739 (96.9) | 87 (3.1) |
| 35–39 | 1,572 (97.1) | 47 (2.9) | 1,572 (97.1) | 47 (2.9) |
| 40+ | 490 (96.8) | 16 (3.2) | 491 (97.0) | 15 (3.0) |
| Race | ||||
| Missing | 4,035 (97.5) | 102 (2.5) | 4,017 (97.1) | 120 (2.9) |
| Asian | 129 (96.3) | 5 (3.7) | 131 (97.8) | 3 (2.2) |
| Black | 10,505 (97.6) | 253 (2.4) | 10,469 (97.3) | 289 (2.7) |
| Colored | 569 (95.5) | 27 (4.5) | 570 (95.6) | 26 (4.4) |
| White | 45 (100.0) | 0 (0.0) | 45 (100.0) | 0 (0.0) |
| Gravidity (incl current preg) | (p < 0.0001) | (p = 0.0017) | ||
| Missing | 48 (100.0) | 0 (0.0) | 48 (100.0) | 0 (0.0) |
| 1 | 5,100 (98.2) | 91 (1.8) | 5,063 (97.5) | 128 (2.5) |
| 2 | 4,609 (97.5) | 120 (2.5) | 4,614 (97.6) | 115 (2.4) |
| 3 | 3,097 (97.1) | 91 (2.9) | 3,089 (96.9) | 99 (3.1) |
| 4+ | 2,429 (96.6) | 85 (3.4) | 2,418 (96.2) | 96 (3.8) |
| Parity (before current delivery) | (p = 0.0008) | (p = 0.0222) | ||
| Missing | 26 (100.0) | 0 (0.0) | 26 (100.0) | 0 (0.0) |
| 0 | 5,546 (98.1) | 105 (1.9) | 5,514 (97.6) | 137 (2.4) |
| 1 | 4,881 (97.5) | 125 (2.5) | 4,875 (97.4) | 131 (2.6) |
| 2 | 2,963 (96.8) | 97 (3.2) | 2,961 (96.8) | 99 (3.2) |
| 3 | 1,197 (97.1) | 36 (2.9) | 1,186 (96.2) | 47 (3.8) |
| 4+ | 670 (96.5) | 24 (3.5) | 670 (96.5) | 24 (3.5) |
| Birth Weight (Grams) | (p = 0.0023) | (p < 0.0001) | ||
| Missing | 11 (91.7) | 1 (8.3) | 9 (75.0) | 3 (25.0) |
| <=2,100 | 1,590 (96.4) | 59 (3.6) | 1,539 (93.3) | 110 (6.7) |
| 2,105–2,525 | 1,505 (97.4) | 40 (2.6) | 1,501 (97.2) | 44 (2.8) |
| 2,530–2,760 | 1,536 (98.1) | 30 (1.9) | 1,522 (97.2) | 44 (2.8) |
| 2,765–2,925 | 1,548 (98.2) | 29 (1.8) | 1,538 (97.5) | 39 (2.5) |
| 2,930–3,060 | 1,517 (98.1) | 29 (1.9) | 1,526 (98.7) | 20 (1.3) |
| 3,065–3,190 | 1,563 (97.9) | 33 (2.1) | 1,565 (98.1) | 31 (1.9) |
| 3,195–3,320 | 1,510 (98.2) | 28 (1.8) | 1,507 (98.0) | 31 (2.0) |
| 3,323–3,480 | 1,532 (97.4) | 41 (2.6) | 1,535 (97.6) | 38 (2.4) |
| 3,485–3,700 | 1,518 (97.0) | 47 (3.0) | 1,525 (97.4) | 40 (2.6) |
| 3,705+ | 1,453 (96.7) | 50 (3.3) | 1,465 (97.5) | 38 (2.5) |
| Gestational Age (Weeks) | (p < 0.0001) | (p < 0.0001) | ||
| Missing | 48 (100.0) | 0 (0.0) | 48 (100.0) | 0 (0.0) |
| <=34 | 1,860 (96.1) | 75 (3.9) | 1,804 (93.2) | 131 (6.8) |
| 35–36 | 1,402 (97.0) | 43 (3.0) | 1,402 (97.0) | 43 (3.0) |
| 37 | 1,498 (97.0) | 47 (3.0) | 1,497 (96.9) | 48 (3.1) |
| 38 | 2,474 (97.4) | 65 (2.6) | 2,471 (97.3) | 68 (2.7) |
| 39 | 2,095 (98.1) | 41 (1.9) | 2,101 (98.4) | 35 (1.6) |
| 40 | 4,237 (98.1) | 80 (1.9) | 4,235 (98.1) | 82 (1.9) |
| 41 | 1,313 (98.1) | 26 (1.9) | 1,317 (98.4) | 22 (1.6) |
| 42+ | 356 (97.3) | 10 (2.7) | 357 (97.5) | 9 (2.5) |
| Hospital | (p < 0.0001) | (p < 0.0001) | ||
| CHB | 7,332 (97.2) | 215 (2.8) | 7,357 (97.5) | 190 (2.5) |
| GSH | 1,332 (95.6) | 61 (4.4) | 1,307 (93.8) | 86 (6.2) |
| KEH | 2,382 (98.7) | 32 (1.3) | 2,334 (96.7) | 80 (3.3) |
| MMH | 4,237 (98.2) | 79 (1.8) | 4,234 (98.1) | 82 (1.9) |
| Delivery | (p = 0.2853) | (p = 0.0074) | ||
| BBA | 186 (97.4) | 5 (2.6) | 179. (93.7) | 12 (6.3) |
| C-section | 6,190 (97.3) | 172 (2.7) | 6,176 (97.1) | 186 (2,9) |
| Vaginal | 8,907 (97.7) | 210 (2.3) | 8,877 (97.4) | 240 (2.6) |
| Prenatal Care | (p < 0.0001) | (p < 0.0001) | ||
| Missing | 85 (100.0) | 0 (0.0) | 83 (97.6) | 2 (2.4) |
| Booked | 14,664 (97.6) | 355 (2.4) | 14,639 (97.5) | 380 (2.5) |
| Unbooked | 534 (94.3) | 32 (5.7) | 510 (90.1) | 56 (9.9) |
| HIV Status | (p = 0.0776) | (p < 0.0001) | ||
| Missing | 368 (97.1) | 11 (2.9) | 363 (95.8) | 16 (4.2) |
| Negative | 11,074 (97.7) | 264 (2.3) | 11,064 (97.6) | 274 (2.4) |
| Postitive | 3,841 (97.2) | 112 (2.8) | 3,805 (96.3) | 148 (3.7) |
| CD4 Category | (p = 0.01350) | (p = 0.6938) | ||
| Missing | 457 (95.8) | 20 (4.2) | 459 (96.2) | 18 (3.8) |
| < 200 | 648 (98.3) | 11 (1.7) | 631 (95.8) | 28 (4.2) |
| 200–349 | 1,072 (96.8) | 36 (3.2) | 1,066 (96.2) | 42 (3.8) |
| 350+ | 1,664 (97.4) | 45 (2.6) | 1,649 (96.5) | 60 (3.5) |
NA-Not applicable: Chi square test was not performed due to high proportion of missing data in the race category
Abbreviations
CHB: Chris-Hani Baragwanath Hospital
MMH: Mowbray Maternity Hospital
GSH: Groote Schuur Hospital
KEH: King Edward VIII hospital
BBA: Born before arrival in hospital
C-section: Caesarean section
The incidence of transfusion was 1.9%, 2.5%, 3.3% and 6.2% at MMH, CHB, KEH and GSH, respectively. Transfusion occurred in more HIV positive (3.7%) than HIV negative (2.4%) patients. In unadjusted analyses, women aged 20–24 years were less likely to be transfused as compared to other age groups and the incidence of transfusion was positively associated with both parity and HIV infection. Women who were transfused received a mean of 2.28 red cell, 0.28 and 0.08 platelet units. No cryoprecipitate was transfused during the study. In those patients who were transfused, the number of RBC units received did not differ significantly by HIV status.
In the multivariable analysis of risk factors for OH (Table 3), HIV status was not associated with OH (OR = 0.95, 95% Wald CI 0.72–1.25). The odds of OH were significantly lower in patients who delivered at KEH and MMH as compared to CHB. Other risk factors for OH included prenatal hemoglobin less than or equal to 9.2 g/dL, receiving no prenatal care, higher birth-weight, low gestational age and high gravidity. Interestingly, patients who were HIV positive with a CD4 count <200 cells per mm3 were less likely to sustain OH as compared to those who were HIV negative (data not shown).
Table 3.
Multivariable logistic regression model of obstetric hemorrhage (OH). Odds ratios with 95% confidence intervals (CI) are shown and the p value for association of each variable with OH is shown in the left-hand column.
| Parameter | OR | 95% CI | ||
|---|---|---|---|---|
| Hospital (<0.0001) | CHB | 1.00 | . | . |
| GSH | 1.19 | 0.84 | 1.67 | |
| KEH | 0.38 | 0.25 | 0.56 | |
| MMH | 0.68 | 0.51 | 0.91 | |
| Age [Years] (0.9620) | <=19 | 1.00 | . | . |
| 20–24 | 0.90 | 0.59 | 1.36 | |
| 25–29 | 1.02 | 0.66 | 1.60 | |
| 30–34 | 0.95 | 0.58 | 1.55 | |
| 35–39 | 0.89 | 0.51 | 1.54 | |
| 40+ | 0.98 | 0.48 | 1.97 | |
| Hemoglobin [g/dL] (<0.0001) | <=9.2 | 3.16 | 2.03 | 4.90 |
| 9.3–10.0 | 1.49 | 0.91 | 2.44 | |
| 10.1–10.5 | 1.39 | 0.83 | 2.34 | |
| 10.6–11.0 | 1.00 | . | . | |
| 11.1–11.4 | 0.96 | 0.55 | 1.69 | |
| 11.5–11.8 | 1.16 | 0.69 | 1.97 | |
| 11.9–12.2 | 1.21 | 0.73 | 2.01 | |
| 12.3–12.6 | 1.06 | 0.61 | 1.84 | |
| 12.7–13.3 | 1.24 | 0.75 | 2.06 | |
| 13.4+ | 0.75 | 0.42 | 1.35 | |
| Prenatal Care (<0.0001) | Booked | 1.00 | . | . |
| Unbooked | 2.56 | 1.59 | 4.10 | |
| Birth Weight [g] (0.0008) | <=2,100 | 1.00 | . | . |
| 2,105–2,525 | 1.19 | 0.73 | 1.94 | |
| 2,530–2,760 | 0.99 | 0.56 | 1.75 | |
| 2,765–2,925 | 1.11 | 0.62 | 1.98 | |
| 2,930–3,060 | 1.03 | 0.56 | 1.90 | |
| 3,065–3,190 | 1.21 | 0.67 | 2.20 | |
| 3,195–3,320 | 1.15 | 0.62 | 2.13 | |
| 3,323–3,480 | 1.83 | 1.04 | 3.23 | |
| 3,485–3,700 | 2.06 | 1.17 | 3.62 | |
| 3,705+ | 2.57 | 1.47 | 4.47 | |
| Gestational Age [Weeks] (0.0030) | <=34 | 2.37 | 1.47 | 3.84 |
| 35–36 | 1.54 | 0.97 | 2.44 | |
| 37 | 1.75 | 1.16 | 2.66 | |
| 38 | 1.25 | 0.86 | 1.82 | |
| 39 | 0.86 | 0.57 | 1.32 | |
| 40 | 1.00 | . | . | |
| 41 | 0.88 | 0.54 | 1.43 | |
| 42+ | 1.31 | 0.64 | 2.68 | |
| HIV Status (0.7233) | No | 1.00 | . | . |
| Yes | 0.95 | 0.72 | 1.25 | |
| Gravidity (0.0493) | 1 | 1.00 | . | . |
| 2 | 1.25 | 0.89 | 1.74 | |
| 3 | 1.41 | 0.96 | 2.07 | |
| 4+ | 1.79 | 1.19 | 2.70 | |
In the multivariable model of risk factors for transfusion, OH was the strongest risk factor for blood transfusion and there was an interaction between OH and prenatal hemoglobin with OH having the most pronounced effect at the lowest levels of antenatal hemoglobin (Table 4 and Figure 2). In contrast to the findings for OH, HIV status was associated with blood transfusion (adjusted OR = 1.52, 95% CI 1.14–2.03). Blood transfusion was also significantly more likely to occur at GSH and KEH as compared to CHB Hospital. Other risk factors significantly associated with blood transfusion included a lack of prenatal care (being unbooked) and low gestational age. Notably, mode of delivery (Caesarean section vs. normal vaginal delivery) was not retained as a significant risk factor in the final logistic regression model. Because of the association between HIV infection and transfusion, we explored models with other HIV variables. A model substituting HIV treatment found an association between transfusion and combination ART (but not short course PMTCT) when compared to no therapy/HIV negative patients.
Table 4.
Multivariable logistic regression model of risk factors for blood transfusion with an interaction between OH and hemoglobin. Odds ratios with 95% confidence intervals (CI) are shown, and the p value for association of each variable with transfusion is shown in the left-hand column.
| Parameter | OR | 95% CI | ||
|---|---|---|---|---|
| Hospital (0.0064) | CHB | 1.00 | . | . |
| GSH | 1.84 | 1.24 | 2.72 | |
| KEH | 1.50 | 1.03 | 2.17 | |
| MMH | 1.03 | 0.73 | 1.45 | |
| Age [Years] (0.8026) | <=19 | 1.00 | . | . |
| 20–24 | 0.74 | 0.49 | 1.12 | |
| 25–29 | 0.79 | 0.52 | 1.20 | |
| 30–34 | 0.88 | 0.56 | 1.37 | |
| 35–39 | 0.79 | 0.47 | 1.33 | |
| 40+ | 0.80 | 0.37 | 1.75 | |
| Gestational Age [Weeks] (<0.0001) | <=34 | 2.90 | 1.98 | 4.25 |
| 35–36 | 1.07 | 0.64 | 1.79 | |
| 37 | 1.53 | 0.95 | 2.45 | |
| 38 | 1.36 | 0.89 | 2.08 | |
| 39 | 0.75 | 0.44 | 1.27 | |
| 40 | 1.00 | . | . | |
| 41 | 1.19 | 0.67 | 2.12 | |
| 42+ | 1.30 | 0.52 | 3.26 | |
| HIV Status (0.0046) | Negative | 1.00 | . | . |
| Positive | 1.52 | 1.14 | 2.03 | |
| Prenatal Care (<0.0001) | Booked | 1.00 | . | . |
| Unbooked | 2.77 | 1.68 | 4.56 | |
| Hemoglobin [g/dL] (p<0.0001) by Hemorrhage (p<0.0001) Interaction (p=0.0198) | ||||
| With OH: Hemoglobin (g/dL) | <=9.2 | 467.0 | 200.1 | >999.9 |
| 9.3–10.0 | 928.2 | 317.1 | >999.9 | |
| 10.1–10.5 | 217.6 | 79.8 | 593.1 | |
| 10.6–11.0 | 264.7 | 95.2 | 736.1 | |
| 11.1–11.4 | 163.3 | 54.0 | 493.8 | |
| 11.5–11.8 | 150.8 | 53.7 | 423.6 | |
| 11.9–12.2 | 287.4 | 106.3 | 777.1 | |
| 12.3–12.6 | 160.9 | 53.9 | 480.1 | |
| 12.7–13.3 | 94.1 | 34.6 | 256.0 | |
| 13.4+ | 158.9 | 50.0 | 505.1 | |
| Without OH: Hemoglobin (g/dL) | <=9.2 | 9.3 | 4.6 | 18.8 |
| 9.3–10.0 | 2.9 | 1.3 | 6.3 | |
| 10.1–10.5 | 2.4 | 1.0 | 5.6 | |
| 10.6–11.0 (ref. cell) | 1.0 | |||
| 11.1–11.4 | 1.6 | 0.6 | 4.0 | |
| 11.5–11.8 | 2.5 | 1.1 | 5.7 | |
| 11.9–12.2 | 1.6 | 0.7 | 3.8 | |
| 12.3–12.6 | 1.4 | 0.5 | 3.7 | |
| 12.7–13.3 | 1.8 | 0.8 | 4.4 | |
| 13.4+ | 1.9 | 0.8 | 4.7 | |
Figure 2.
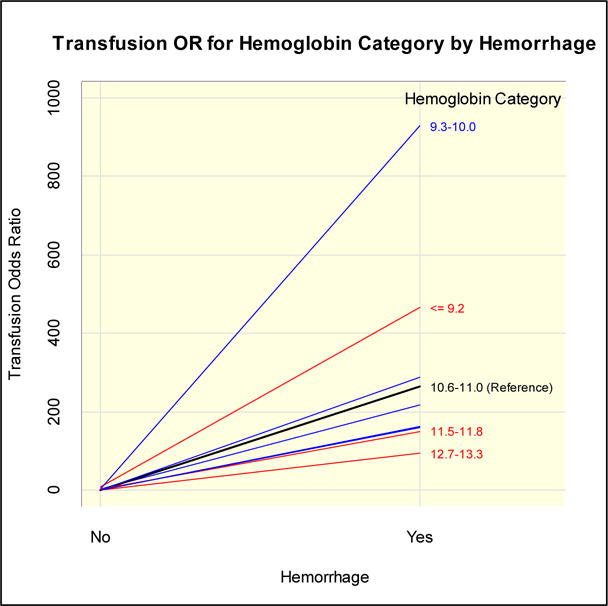
Odds ratio for blood transfusion, according to obstetric hemorrhage (OH) status and prenatal hemoglobin level. OH carries a very strong odds of transfusion, especially in women in the two lowest categories of prenatal hemoglobin.
The mean hemoglobin on hospital admission or at the last prenatal visit was 11.4 g/dL in HIV negative and 11.0 g/dL in HIV positive patients (p< 0.0001; Figure 1). This small difference in means translated to significant differences in the proportion of anemic patients using the cutoff of 11g/dL: 36% for HIV negative and 47% for HIV positive patients (P<0.0001). Mean hemoglobin increased slightly with age and differed by hospital: 10.6, 11.3, 11.4 and 11.5 g/dL at KEH, GSH, MMH and CHB respectively. In contrast, the respective mean pre-transfusion (post-transfusion) hemoglobin values were 7.8 (10.3), 7.5 (9.1), 7.0 (9.0) and 7.7 (8.8) g/dL. The difference in pre- and post-transfusion hemoglobin was associated with hospital (highest at KEH) and age (a linear decrease was observed with advancing age) [data not shown].
Figure 1. Prenatal Hemoglobin by HIV Status.
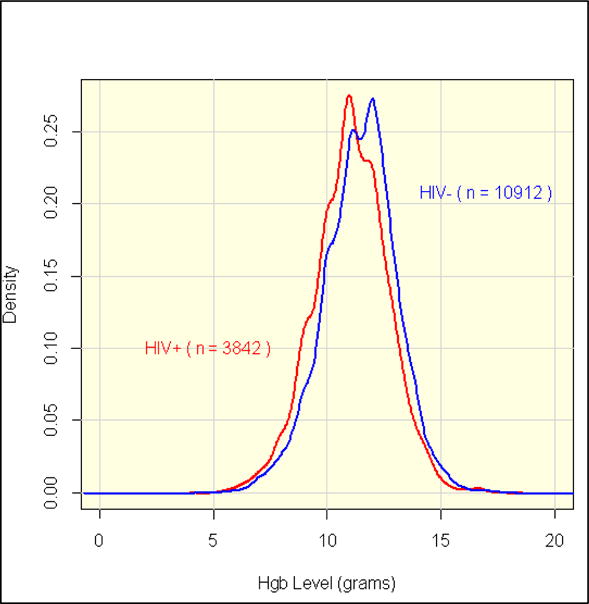
The proportion of each subgroup with each value of hemoglobin is indicated on the Y axis. Mean hemoglobin was 11.4 g/dL among HIV negative and 11.0 g/dL among HIV positive peripartum women.
Among HIV positive patients, 50.9% had a CD4 count <350 cells per mm3. Of these, 81.2% received ART and an additional 14.5% received PMTCT. Among all HIV positive women, ART or PMTCT was administered in 93.1% of cases. Women who failed to access antenatal care were less likely to have received (necessary) ART, although similar rates of PMTCT were observed.
Discussion
These findings show that the incidence of OH in South Africa is similar to that reported in the United States (U.S.) (2.3–2.9%)13 and is not associated with HIV status. In contrast, the incidence of blood transfusion is up to ten-fold higher than that reported in the U.S. (0.24–0.46%)14 and several other high middle income countries11,15. While high rates of transfusion were observed in all patients, transfusion incidence was significantly higher in HIV positive patients after controlling for potential confounders such as age, parity, hospital and mode of delivery. The study also demonstrated good compliance with the current South African obstetric HIV management guidelines whereby the overwhelming majority of HIV positive patients received either therapeutic antiretroviral therapy and/or PMTCT.
In South Africa, OH is the third leading cause of maternal death and mortality rates associated with OH have escalated over the past decade16. From 2008 to 2010, there were 688 deaths attributable to OH in South Africa, representing an increase of 32.4% (18.82 to 24.91/100,000 live births) as compared with 2005 to 2007. The majority of deaths were deemed to have been avoidable and 13.2% were attributed to lack of adequate blood transfusion. This spurred efforts to improve access to blood transfusion in the obstetric setting, such as placement of emergency group O blood in refrigerators at district hospitals where over a third of OH-related deaths reportedly occur16. While this has helped to improve availability of blood, obstetric blood utilization continues to place a significant demand on the South African blood transfusion services.
Mortality ascribed to OH in high resource settings is comparatively low and has been relatively static over recent decades17. For example, OH mortality in Australia during 2003–2005 was 8.4/100,000 live births18. In contrast, morbidity due to OH, which is estimated to be 100-fold more common than OH-associated death13,17, may be increasing12,19,20. However data on non-fatal OH and consequent morbidity are sparse and impeded by variability in the definition of OH and measurement of peripartum blood loss. Reliable international and regional data are needed to inform public health intervention, particularly in Africa21.
In contrast to our a priori expectations, the study found that the incidence of OH in South Africa either approximates or is lower than rates reported from high-income countries19,21,22. For example, the OH incidence in the U.S. was 2.3% in 1994 and 2.9% in 200623, which is similar to the rates observed in our study. In contrast, the rates of blood transfusion in South Africa were found to be five- to ten-fold higher than that reported from the U.S., despite U.S. rates having increased from 2.38 to 4.58 per 1000 deliveries between 1998–1999 and 2004–2005, which was ascribed to a general increase in U.S. blood utilization over a similar time period24. Over half of women with OH in our study were transfused; by comparison, only 11.7% of women with OH in Australia are transfused22..
A major finding of this study was the significantly higher risk of peripartum transfusion in HIV positive patients after controlling for known confounders. We postulate that this association may reflect a high prevalence of unaddressed antenatal anemia as evidenced by significant differences in mean pre-natal hemoglobin and proportion anemic between HIV positive and negative patients. The association between HIV and anemia is well described25–27 and has a multitude of causes, which includes direct effects of the virus itself, infection, neoplasia and therapy (e.g. ART). While severe anemia is an indication for blood transfusion, it also renders patients less likely to tolerate bleeding at time of delivery and may exacerbate bleeding once initiated due to the adverse rheological effects of a low hematocrit on hemostasis28. This hypothesis is supported by the observed interaction between prenatal hemoglobin and OH in relation to transfusion: women with the lowest prenatal hemoglobin showed the greatest impact of OH on their risk of receiving a transfusion. South African maternal mortality reports also show that over a third of OH-related deaths have underlying anemia16. Thus anemia due to HIV disease itself or to ART seems the likely reason for the higher rates of transfusion in HIV positive patients. HIV-related coagulopathy and institutional or physician specific variability in transfusion practice for HIV-positive vs. negative patients are other plausible explanations that warrant future evaluation.
Variability In transfusion practice may be contributing to the high rates of transfusion. While not unique to South Africa, the decision to transfuse is informed both by laboratory parameters (primarily the hemoglobin) as well as the patient’s symptoms and signs (presence of symptomatic anemia). Although South Africa has national transfusion guidelines, they do not establish specific thresholds for transfusion, instead suggesting that “patients with a hemoglobin level below 7g/dl often require a transfusion”. In the context of obstetric hemorrhage, the guidelines recommend that patient’s hemoglobin values be “maintained between 6 and 10g/dl during the resuscitation phase”29. Our study did not evaluate compliance with guidelines directly; however, the observed pre-transfusion hemoglobin values and transfusion delta (post- minus pre-transfusion hemoglobin) do suggest that there is general compliance with those guidelines. Differences in pre- and post-transfusion hemoglobins between hospitals show some variability in transfusion practice that may benefit from targeted blood management interventions such as the use of one vs. two unit blood orders. Importantly, the observed high rates of transfusion in our study are notable for having occurred in a setting where blood inventories are constantly strained: during the study period, SANBS operated on an average blood reserve of 3.88 days (2.2–6.5 days)[personal communication M. Vermeulen, SANBS].
Obstetric transfusion was also associated with the treating hospital, lower gestational age and the absence of prenatal care. The comparatively high rate of transfusion at GSH may be consistent with this hospital’s status as a high-risk obstetric referral unit, as we were unable to fully adjust for comorbidity in our multivariable model. Reasons for higher adjusted odds of transfusion at KEH are less clear and may be attributable to a high prevalence of HIV and anemia in the hospital’s population. Lack of prenatal care is a recognized risk factor for complications during pregnancy and delivery16,30, whereby the absence of prenatal care precludes timely diagnosis and intervention of complicating conditions (e.g. multiple pregnancy, placenta previa, anemia etc.), several of which could require transfusion if neglected.
This study used the opportunity to gather contemporary data on HIV prevalence and treatment in the obstetric setting. HIV/AIDS is currently the leading cause of peripartum mortality in South Africa31. Twenty five percent of patients in our study were HIV positive and the observed differences by hospital (Table 1) mirror regional differences in national HIV surveillances32. Effective management of HIV in pregnancy with three-drug ART significantly reduces the rate of mother to child transmission of HIV to under 2%33. However, South Africa was relatively late in adopting the WHO recommendations on initiation of ART at a CD4 count ≤350/mm3 34 and, as of 2010, there were still an estimated 213,800 HIV Infected mothers still in need of PMTCT34, in addition to 1,584,000 adult infections that require ART. Nevertheless our 2012 data demonstrate good ARV and PMTCT penetrance in the obstetric setting, consistent with recent public health priorities in South Africa32,35. Fully 81% of women with a CD4 count <350 cells/mm3 were on ARV therapy and almost all of those not on routine therapeutic ARV’s received PMTCT.
Despite its contributions, there are several limitations to our study. First, data sources in the hospitals were often incomplete leading to missing data and there was variability as to when prenatal hemoglobins were measured. The pre-natal hemoglobin values were used as a substitute for the pre-transfusion hemoglobin values, when the latter had not been obtained; a proportion of the pre-natal hemoglobin values had been collected over 30 days prior to delivery. Second, while we adhered to a standard definition of OH, we did not assess compliance with the WHO definition and recognize that the estimation of blood loss is often inaccurate and non-reproducible2,36–39. Third, our four large urban hospitals allowed efficient capture of data on a large number of deliveries, but do not represent the full spectrum of obstetric care in South Africa, in particular more rural, under-resourced settings. Our focus on secondary and tertiary level hospitals may also account for the comparatively high rate of cesarean sections; in contrast to our study, the rates of cesarean section in district hospitals is ~18.8%). Finally, we restricted the study to OH in the context of viable pregnancies around time of delivery and did not capture data on maternal hemorrhage that occurs in non-viable pregnancies, e.g. spontaneous abortions, ectopic pregnancies etc.
In conclusion, this study has demonstrated similar rates of OH but disproportionately high rates of transfusion in peripartum patients in South Africa relative to developed countries, and identified HIV as a risk factor for transfusion. While the findings suggest that antenatal anemia may underlie the high risk of transfusion, coagulopathy and variation in transfusion practice in care of HIV-positive patients also warrant investigation. The findings also show good compliance with HIV prescribing guidelines despite earlier delays in implementation. Future directions include the application of this study’s approach to other settings, allowing validation and extension of our findings. If replicated, the study’s findings suggest that systematic treatment of prenatal anemia may be a viable intervention to prevent peripartum blood transfusion.
Acknowledgments
The authors wish to thank the research nurses Ms. Ellen Makhale, Happy Maake, Edna Harmse, Mamsie Msomi, Catherine Thorpe and Elizabeth Mathebula for their invaluable contribution to the study. We are also grateful to the medical and nursing personnel at Chris-Hani Baragwanath Hospital, King Edward VIII Hospital, Mowbray Maternity Hospital and Groote Schuur Hospital for their support.
Funding: National Heart Lung and Blood Institute Recipient Epidemiology and Donor Evaluation Study (REDS-III) research contract HHSN268201100009I.
References
- 1.Bates I, Chapotera GK, McKew S, van den Broek N. Maternal mortality in sub-Saharan Africa: the contribution of ineffective blood transfusion services. BJOG. 2008;115:1331–9. doi: 10.1111/j.1471-0528.2008.01866.x. [DOI] [PubMed] [Google Scholar]
- 2.Lalonde A, Daviss BA, Acosta A, Herschderfer K. Postpartum hemorrhage today: ICM/FIGO initiative 2004–2006. Int J Gynaecol Obstet. 2006;94:243–53. doi: 10.1016/j.ijgo.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Homer C, Clements V, McDonnell N, Peek M, Sullivan E. Maternal mortality: what can we learn from stories of postpartum haemorrhage? Women Birth. 2009;22:97–104. doi: 10.1016/j.wombi.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Kaye DK, Kakaire O, Osinde MO. Systematic review of the magnitude and case fatality ratio for severe maternal morbidity in sub-Saharan Africa between 1995 and 2010. BMC pregnancy and childbirth. 2011;11:65. doi: 10.1186/1471-2393-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geller SE, Adams MG, Kelly PJ, Kodkany BS, Derman RJ. Postpartum hemorrhage in resource-poor settings. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2006;92:202–11. doi: 10.1016/j.ijgo.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi MN, Welz T, Ronsmans C. Severe acute maternal morbidity in rural South Africa. Int J Gynaecol Obstet. 2004;87:180–7. doi: 10.1016/j.ijgo.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Fawcus SR, van Coeverden de Groot HA, Isaacs S. A 50-year audit of maternal mortality in the Peninsula Maternal and Neonatal Service, Cape Town (1953–2002) BJOG. 2005;112:1257–63. doi: 10.1111/j.1471-0528.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 8.Jansen AJ, van Rhenen DJ, Steegers EA, Duvekot JJ. Postpartum hemorrhage and transfusion of blood and blood components. Obstetrical & gynecological survey. 2005;60:663–71. doi: 10.1097/01.ogx.0000180909.31293.cf. [DOI] [PubMed] [Google Scholar]
- 9.Mseleku M, Smith TH, Guidozzi F. HIV seropositive in pregnant South African women who initially refuse routine antenatal HIV screening. BJOG. 2005;112:370–1. doi: 10.1111/j.1471-0528.2004.00424.x. [DOI] [PubMed] [Google Scholar]
- 10.Health Do. National Antenatal Sentinel HIV and Syphilis Prevalence Survey in South Africa, 2009. Pretoria. 2010 [Google Scholar]
- 11.Roberts CL, Ford JB, Thompson JF, Morris JM. Population rates of haemorrhage and transfusions among obstetric patients in NSW: a short communication. The Australian & New Zealand journal of obstetrics & gynaecology. 2009;49:296–8. doi: 10.1111/j.1479-828X.2009.00985.x. [DOI] [PubMed] [Google Scholar]
- 12.Joseph KS, Rouleau J, Kramer MS, Young DC, Liston RM, Baskett TF. Investigation of an increase in postpartum haemorrhage in Canada. BJOG : an international journal of obstetrics and gynaecology. 2007;114:751–9. doi: 10.1111/j.1471-0528.2007.01316.x. [DOI] [PubMed] [Google Scholar]
- 13.Callaghan WM, Creanga AA, Kuklina EV. Severe Maternal Morbidity Among Delivery and Postpartum Hospitalizations in the United States. Obstetrics and gynecology. 2012 doi: 10.1097/aog.0b013e31826d60c5. [DOI] [PubMed] [Google Scholar]
- 14.Kuklina EV, Meikle SF, Jamieson DJ, et al. Severe obstetric morbidity in the United States: 1998–2005. Obstet Gynecol. 2009;113:293–9. doi: 10.1097/AOG.0b013e3181954e5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.New Zealand Ministry of Health. New Zealand Maternity Clinical Indicators. 2011. p. 5. [Google Scholar]
- 16.National Committee for Confidential Enquiry into Maternal Deaths: Department of Health Republic of South Africa. Saving Mothers 2008–2010: Fifth report on the Confidential Enquiries into Maternal Deaths in South Africa. 2012. [Google Scholar]
- 17.Knight M, Callaghan WM, Berg C, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC pregnancy and childbirth. 2009;9:55. doi: 10.1186/1471-2393-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Homer C, Clements V, McDonnell N, Peek M, Sullivan E. Maternal mortality: what can we learn from stories of postpartum haemorrhage? Women and birth : journal of the Australian College of Midwives. 2009;22:97–104. doi: 10.1016/j.wombi.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesthesia and analgesia. 2010;110:1368–73. doi: 10.1213/ANE.0b013e3181d74898. [DOI] [PubMed] [Google Scholar]
- 20.Ford JB, Roberts CL, Simpson JM, Vaughan J, Cameron CA. Increased postpartum hemorrhage rates in Australia. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2007;98:237–43. doi: 10.1016/j.ijgo.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Calvert C, Thomas SL, Ronsmans C, Wagner KS, Adler AJ, Filippi V. Identifying regional variation in the prevalence of postpartum haemorrhage: a systematic review and meta-analysis. PLoS One. 2012;7:e41114. doi: 10.1371/journal.pone.0041114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cameron CA, Roberts CL, Olive EC, Ford JB, Fischer WE. Trends in postpartum haemorrhage. Australian and New Zealand journal of public health. 2006;30:151–6. doi: 10.1111/j.1467-842x.2006.tb00109.x. [DOI] [PubMed] [Google Scholar]
- 23.Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994–2006. Am J Obstet Gynecol. 2010;202:353e1–6. doi: 10.1016/j.ajog.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan MT, Cotten R, Read EJ, Wallace EL. Blood collection and transfusion in the United States in 2001. Transfusion. 2007;47:385–94. doi: 10.1111/j.1537-2995.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- 25.Volberding PA, Levine AM, Dieterich D, et al. Anemia in HIV infection: clinical impact and evidence-based management strategies. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;38:1454–63. doi: 10.1086/383031. [DOI] [PubMed] [Google Scholar]
- 26.Bain BJ. Pathogenesis and pathophysiology of anemia in HIV infection. Curr Opin Hematol. 1999;6:89–93. doi: 10.1097/00062752-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Claster S. Biology of anemia, differential diagnosis, and treatment options in human immunodeficiency virus infection. J Infect Dis. 2002;185(Suppl 2):S105–9. doi: 10.1086/340202. [DOI] [PubMed] [Google Scholar]
- 28.Grabowski EF, Yam K, Gerace M. Evaluation of hemostasis in flowing blood. American Journal of Hematology. 2012;87(Suppl 1):S51–5. doi: 10.1002/ajh.23207. [DOI] [PubMed] [Google Scholar]
- 29.Medical Directors of the South African National Blood Service and the Western Province Blood Transfusion Service. Clinical guidelines for the use of blood products in South Africa. 4. 2008. [Google Scholar]
- 30.Vintzileos AM, Ananth CV, Smulian JC, Scorza WE, Knuppel RA. The impact of prenatal care in the United States on preterm births in the presence and absence of antenatal high-risk conditions. American Journal of Obstetrics and Gynecology. 2002;187:1254–7. doi: 10.1067/mob.2002.127140. [DOI] [PubMed] [Google Scholar]
- 31.Moodley J, Pattinson RC, Baxter C, Sibeko S, Abdool Karim Q. Strengthening HIV services for pregnant women: an opportunity to reduce maternal mortality rates in Southern Africa/sub-Saharan Africa. BJOG : an international journal of obstetrics and gynaecology. 2011;118:219–25. doi: 10.1111/j.1471-0528.2010.02726.x. [DOI] [PubMed] [Google Scholar]
- 32.DOH RoSA. The 2011 National Antenatal Sentinel HIV & Syphilis Prevalence Survey in South Africa. 2012. [Google Scholar]
- 33.Siegfried N, van der Merwe L, Brocklehurst P, Sint TT. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev. 7:CD003510. doi: 10.1002/14651858.CD003510.pub3. [DOI] [PubMed] [Google Scholar]
- 34.National Department of Health SA. CLINICAL GUIDELINES: PMTCT (Prevention of Mother-to-Child Transmission) 2010. [Google Scholar]
- 35.National Department of Health SA. GLOBAL AIDS RESPONSE PROGRESS REPORT 2012. 2012. [Google Scholar]
- 36.Quinones JN, Uxer JB, Gogle J, Scorza WE, Smulian JC. Clinical evaluation during postpartum hemorrhage. Clin Obstet Gynecol. 53:157–64. doi: 10.1097/GRF.0b013e3181cd5d36. [DOI] [PubMed] [Google Scholar]
- 37.Razvi K, Chua S, Arulkumaran S, Ratnam SS. A comparison between visual estimation and laboratory determination of blood loss during the third stage of labour. The Australian & New Zealand journal of obstetrics & gynaecology. 1996;36:152–4. doi: 10.1111/j.1479-828x.1996.tb03273.x. [DOI] [PubMed] [Google Scholar]
- 38.Prasertcharoensuk W, Swadpanich U, Lumbiganon P. Accuracy of the blood loss estimation in the third stage of labor. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2000;71:69–70. doi: 10.1016/s0020-7292(00)00294-0. [DOI] [PubMed] [Google Scholar]
- 39.Stafford I, Dildy GA, Clark SL, Belfort MA. Visually estimated and calculated blood loss in vaginal and cesarean delivery. American Journal of Obstetrics and Gynecology. 2008;199:519e1–7. doi: 10.1016/j.ajog.2008.04.049. [DOI] [PubMed] [Google Scholar]


