Abstract
Background
Telomere shortening and alterations of mitochondrial biogenesis are involved in cellular aging. Childhood adversity is associated with telomere shortening, and several investigations have shown short telomeres in psychiatric disorders. Recent studies have examined whether mitochondria might be involved in neuropsychiatric conditions; findings are limited and no prior work has examined this in relation to stress exposure.
Methods
Two-hundred and ninety healthy adults provided information on childhood parental loss and maltreatment and completed diagnostic interviews. Participants were categorized into four groups based upon the presence or absence of childhood adversity and the presence or absence of lifetime psychopathology (depressive, anxiety, and substance use disorders). Telomere length and mtDNA copy number were measured from leukocyte DNA by qPCR.
Results
Childhood adversity and lifetime psychopathology were each associated with shorter telomeres (p < .01) and higher mtDNA copy numbers (p < .001). Significantly higher mtDNA copy numbers and shorter telomeres were seen in individuals with major depression, depressive disorders, and anxiety disorders, as well as those with parental loss and childhood maltreatment. A history of substance disorders was also associated with significantly higher mtDNA copy numbers.
Conclusion
This study provides the first evidence of an alteration of mitochondrial biogenesis with early life stress and with anxiety and substance use disorders. We replicate prior work on telomere length and psychopathology, and show that this effect is not secondary to medication use or comorbid medical illness. Finally, we show that early life stress and psychopathology are each associated with these markers of cellular aging.
Keywords: mitochondria, telomere, childhood, early life stress, depressive disorder, anxiety disorder
Introduction
Many decades of research have documented robust associations of stress exposure and psychopathology with somatic conditions associated with aging, including cardiovascular disease, inflammatory conditions, and obesity (1–4). This may be due to effects of inflammation, oxidative stress, and glucocorticoid exposure on cellular aging (5, 6). Two key processes in cellular aging are telomere shortening and mitochondrial dysfunction (7–9). New research links telomere biology and mitochondrial biogenesis and function, and there is increasing interest in a role for telomere shortening and mitochondrial dysfunction in psychiatric disorders (10–14).
Mitochondrial Dysfunction, Cellular Aging, and Psychopathology
Mitochondrial respiration provides most of the ATP needed for cellular functions. Mitochondria also play an integral role in cell growth, differentiation, replication, and death. Reactive oxygen species (ROS) are generated during mitochondrial respiration. Aging is characterized by increases in mitochondrial ROS production, declines in mitochondrial function, accumulation of mutations in mitochondrial DNA (mtDNA), and alterations of mitochondrial biogenesis (7, 15, 16). Mitochondrial dysfunction and alterations of mitochondrial biogenesis have been documented in age-related diseases, including cardiovascular disease, diabetes, and various forms of cancer (7, 16, 17).
The possibility that mitochondrial dysfunction might be a pathophysiologic mechanism in psychiatric disorders is intriguing. The brain has high energy demands, and ATP is vital for such neural processes as ion transport, neurotransmitter production and release, and receptor function, (14, 18) as well as neural differentiation, dendritic branching, and synaptic plasticity (14, 19). Several studies support a role for abnormalities of mitochondrial function in psychiatric disorders, in particular bipolar disorder, schizophrenia, and major depressive disorder (MDD) (14, 19). Stress exposure may account for some of this association. Gluococorticoids act on coordinated physiological systems that can induce metabolic stress (20) and exert effects on mitochondria by altering expression of mitochondrial genes and nuclear genes that influence mitochondrial density and function (20, 21). Glucocorticoid receptors bind to mitochondrial membranes to regulate membrane potential (20, 21). Early life stress also enhances glutamate transmission which increases oxidative stress and results in mitochondrial damage (22, 23).
Mitochondrial biogenesis serves the energy demands of the cell and compensates for cell damage (24). A small number of studies have examined mtDNA copy number, an index of mitochondrial biogenesis, in patients with psychiatric disorders. mtDNA copy number has been reported to be high in autism (25), and reduced in a recent study of bipolar disorder (26) but not others (27–31). A few studies have assessed mtDNA copy number in patients with MDD: no difference was found between MDD patients and controls in a small post-mortem study of brain tissue (31) or in a large study in outpatients with MDD and matched controls (32), but there is some evidence of reduced leukocyte mtDNA copy number in association with depression in women over age 60 (33). We are not aware of any investigations of mtDNA copy number in relation to anxiety disorders, alcohol or substance disorder, or psychosocial stress exposure.
Telomere Length, Cellular Aging, and Psychopathology
Telomeres are repetitive nucleotide sequences at the ends of chromosomes that are critical to maintaining chromosomal stability. DNA sequences are lost with each replication cycle, and telomeres serve as a buffer that protects the chromosome from losing critical DNA (34, 35). Telomeres shorten with each cell division, and on average older individuals have shorter telomeres than younger individuals (36). Telomere shortening beyond a critical threshold initiates the DNA damage response pathway and induces replicative senescence or apoptosis (9, 34, 37).
Telomere shortening occurs in several diseases associated with aging, including cardiovascular disease, diabetes, stroke and autoimmune conditions (34, 38, 39). Reduced telomere length has also been documented in MDD (10, 40–44), although some studies have been negative (45, 46). A few investigations also found reduced leukocyte telomere length in anxiety disorders (45, 47), post-traumatic stress disorder (PTSD) (48–51), bipolar II disorder (52), schizophrenia (53), and substance use disorders (54, 55). Chronicity of MDD is linked to leukocyte telomere shortening (43, 47, 56), suggesting that loss of telomere length may be a consequence of other aspects of the disease process.
Psychiatric disorders are associated with high levels of perceived and chronic stress exposure (57, 58), and reduced telomere length is associated with chronic stress (10, 11). Stress-induced activation of glucocorticoids, pro-inflammatory cytokines, and oxidative stress have been implicated in this association (59). Telomeres undergo substantial shortening during early development, and exposure to early adversity is associated with short telomeres in leukocytes and buccal cells in cross-sectional (10, 11), (60–62) and prospective designs (47, 63), however not all studies have shown this effect (64, 65). This suggests that stress exposure may account for the association of psychopathology and telomere shortening. Although prior work has statistically controlled for early life stress exposure in testing for effects of psychiatric conditions (45, 47, 50, 66), we are not aware of any previous studies that have directly compared telomere length in subjects with both early life stress and psychopathology to those with psychopathology or early life stress alone.
Telomere Length and the Regulation of Mitochondrial Biogenesis and Function
Work in animal models and cell culture has recently uncovered shared pathways that regulate telomere length and mitochondrial biogenesis and function. Telomere dysfunction induces expression of p53, which suppresses PGC1α and PGC1β, proteins that regulate mitochondrial biogenesis and function (9, 67). Telomerase reverse transcriptase (TERT) may also regulate mitochondrial function. Under oxidative stress, TERT translocates from the nucleus to the mitochondria, where it appears to modulate respiratory chain function, ROS production, mtDNA damage, and apoptosis (68, 69).
The Present Study
Given this shared regulation of telomere and mitochondrial function and the emerging body of literature implicating these mechanisms of cellular aging in psychiatric disorders, we hypothesized alterations of mtDNA copy number and shorter telomere length in relation to early adversity as well as lifetime depressive, anxiety, and substance use disorders.
Methods and Materials
Subjects
Two hundred ninety participants, 177 women and 113 men, aged 18–61 (31.0 ± 10.7) years, were recruited using separate local, newspaper, and Internet advertisements directed toward healthy adults and individuals with depression, childhood parental loss, or a history of early life stress. Participants were white (n=241), black (n=26), Asian (n=9), Hispanic (n=4) and “other” (n=10). Subjects from our prior study of adversity and telomere length (70) were not included. The study was approved by the Butler Hospital Institutional Review Board. After complete description of the study, voluntary written informed consent was obtained.
Exclusions included prescription medication use other than oral contraceptives, acute or chronic medical illness, pregnancy, and a history of brain injury or seizure disorder. Also excluded were those with night-shift work, current alcohol or substance disorders, and lifetime history of bipolar disorder, psychotic disorder, and obsessive-compulsive disorder.
Demographics
Height and weight were measured without shoes, and body mass index (BMI) (weight (kg)/ht (m)2) calculated. Participants answered a single item about childhood socioeconomic adversity (“My family was generally financially stable when I was growing up, and all of my basic needs (food, shelter, and clothing) were met during my childhood”) and reported their highest level of education.
Diagnoses and Symptoms
Axis I psychiatric diagnoses were assessed using the Structured Clinical Interview for DSM-IV (SCID) (71). The Inventory for Depressive Symptoms, Self Report (IDS-SR) (72), the State-Trait Anxiety Inventory (STAI) (73), the Perceived Stress Scale (PSS) (74), and the Connor-Davidson Resilience Scale (CD-RISC) (75) were also administered.
Childhood Adversity
Parental Loss
Loss of a parent before age 18 (N=76) included death (N=36) and/or prolonged separation/desertion (i.e., parent deserted for at least six months with no attempts at contact or responses to child’s attempts) (N=43).
The Childhood Trauma Questionnaire (CTQ), 28-item version (76)
This measure has high internal consistency, test-retest reliability, and convergent validity (76). Moderate-severe levels of one or more of the five types of maltreatment (physical, sexual, and emotional abuse, physical and emotional neglect) were assessed.
Measures of Cellular Aging
DNA was isolated from whole blood using standard techniques. Three parallel quantitative polymerase chain reactions (qPCRs) were performed to quantitate copy numbers for telomeres, mitochondrial genomes, and the beta-hemoglobin gene as a single-copy standard, as previously described (77). Data were acquired using the ABI Prism HT79000 DNA Sequence Detection System (Applied Biosystems, Grand Island, NY). qPCRs were performed on 384-well plates in a reaction volume of 10 μL containing approximately 25 ng genomic DNA, 300 nM of each primer, and 1x Sybr Select Master Mix (Life Technologies Corporation, Grand Island, NY). For telomere length, the reaction also included 2% DMSO. All reaction plates contained wells with serial dilutions of a cloned amplicon (i.e., cloned telomere amplicon, cloned mitochondrial amplicon, or cloned beta-hemaglobin amplicon) to permit absolute quantitation of telomere, mitochondrial DNA, and beta-hemoglobin copy number. A standard curve was used to directly determine copy number for telomere amplicons, mitochondria, or a single-copy gene in each PCR run. All analyses were performed in triplicate.
Single Copy Gene
The sequences of the forward and reverse primers for the beta-hemoglobin gene were: GCT TCT GAC ACA ACT GTG TTC ACT AGC and CAC CAA CTT CAT CCA CGT. An initial heating step of 95°C for 10 minutes was followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min.
mtDNA Copy Number
The sequences of the forward and reverse mitochondrial primers (directed towards the D-loop region) were: CAT CTG GTT CCT ACT TCA GGG and TGA GTG GTT AAT AGG GTG ATA GA (78). An initial heating step of 95°C for 10 minutes was followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. To obtain an index of mitochondrial number, mtDNA copy number was divided by the copy number for the beta-hemoglobin gene.
Telomere Length
Modified telomere primer sequences were provided by Richard Cawthon (Eccles Institute of Human Genetics, University of Utah): CGG TTT GTT TGG GTT TGG GTT TGG GTT TGG GTT TGG GTT (Tel1b) and GGC TTG CCT TAC CCT TAC CCT TAC CCT TAC CCT TAC CCT (Tel2b). An initial heating step of 95°C for 30 minutes was followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The ratio of telomere copy number over beta-hemoglobin copy number was obtained, multiplied by the length of the telomere amplicon to obtain telomere length per genome, and then divided by the number of chromosome ends.
Statistical Analysis
Telomere length and mtDNA copy number were log transformed to adjust for skewed distributions. Age, gender, education, BMI, and childhood socioeconomic adversity were included as covariates in the statistical models given literature demonstrating their effects on mtDNA copy number and telomere length. As previously reported (79), mtDNA copy number and telomere length were highly correlated (r=.33, p < .001) and were therefore included as covariates. Means and standard deviations shown in figures represent log transformed data and were adjusted for the covariates; unadjusted raw means are provided in the supplementary materials. Subjects were categorized into four groups: no early adversity and no lifetime psychiatric diagnosis (n = 113, No Adversity/No Disorder group); early adversity but no lifetime diagnosis (n = 66, Adversity/No Disorder group); lifetime diagnosis but no history of early adversity (n = 39, No Adversity/Disorder group); and both early adversity and a lifetime diagnosis (n = 72, Adversity/Disorder group). Analyses that involved subjects with substance use disorders included only those with past disorders because those with current substance disorders (meeting criteria in the last month) were excluded from the study. General linear models (GLMs) were used to predict the grouping variable, controlling for covariates; the full models are shown in Supplementary tables S1 and S2. We then examined individual disorder types (MDD, any depressive disorder, PTSD, any anxiety disorder, and past alcohol or substance disorders), parental loss, and childhood maltreatment, in models that controlled for the covariates and used the No Adversity/No Disorder as the comparison group.
Results
Preliminary Analyses
Descriptive statistics are displayed in Table 1. Age and BMI were negatively associated with telomere length (r = −.13, p = .026 and r = −.14, p = .019, respectively), but not with mtDNA copy number. Race was not associated with telomere length or mtDNA copy number. Gender, education, and childhood socioeconomic adversity were also not associated with telomere length or mtDNA copy number. Telomere length and mtDNA copy number were significantly correlated (r =.33, p < .001).
Table 1.
Demographics, disorders, and symptoms.
| Demographics | No Disorder No Adversity (n = 113) |
No Disorder Adversity (n = 66) |
Disorder No Adversity (n = 39) |
Disorder Adversity (n = 72) |
p |
|---|---|---|---|---|---|
| Age, M (SD) | 28.5a (9.2) | 31.3ab (11.1) | 30.7ab (10.4) | 34.8b (12.0) | .00 |
| Sex, N (%) male | 50 (44.2) | 26 (39.4) | 15 (38.5) | 22 (30.6) | .32 |
| Race, N (%) white | 93 (82.3) | 53 (80.3) | 36 (92.3) | 59 (81.9) | .42 |
| College Degree, N (%) | 69 (61.1) | 34 (51.5) | 20 (51.3) | 38 (52.8) | .51 |
| BMI, N (%) | 25.4 (4.5) | 26.3 (5.2) | 26.1 (4.2) | 27.3 (5.8) | .08 |
| Smokers, N (%) | 9 (8.3) | 5 (7.8) | 3 (7.7) | 12 (17.1) | .19 |
|
| |||||
|
Adversity
| |||||
| Emotional abuse, N (%) | -- | 16 (24.2) | -- | 35 (48.6) | .00 |
| Physical abuse, N (%) | -- | 13 (20.0) | -- | 24 (33.3) | .08 |
| Sexual abuse, N (%) | -- | 18 (27.3) | -- | 27 (37.5) | .20 |
| Emotional neglect, N (%) | -- | 16 (24.2) | -- | 36 (50.0) | .00 |
| Physical neglect, N (%) | -- | 11 (16.7) | -- | 21 (29.2) | .08 |
| Parental death, N (%) | -- | 21 (31.9) | -- | 15 (20.8) | .14 |
| Parental desertion, N (%) | -- | 21 (32.8) | -- | 22 (31.0) | .82 |
| Sum adversities, M (SD) | -- | 1.8 (1.2) | -- | 2.5 (1.4) | .00 |
|
| |||||
|
Psychiatric Disorders
| |||||
|
Current Disorders
| |||||
| MDD, N (%) | -- | -- | 6 (15.4) | 7 (9.7) | .38 |
| Depressive, N (%) | -- | -- | 7 (17.9) | 18 (25.0) | .40 |
| PTSD, N (%) | -- | -- | 0 (0.0) | 3 (4.2) | .20 |
| Anxiety, N (%) | -- | -- | 5 (12.8) | 8 (11.1) | .79 |
|
| |||||
|
Past Disorders
| |||||
| MDD, N (%) | -- | -- | 14 (35.9) | 31 (43.1) | .46 |
| Depressive, N (%) | -- | -- | 15 (38.5) | 35 (48.6) | .31 |
| PTSD, N (%) | -- | -- | 2 (5.1) | 8 (11.1) | .29 |
| Anxiety, N (%) | -- | -- | 4 (10.3) | 17 (23.6) | .09 |
| Alcohol/Substance, N (%) | -- | -- | 23 (59.0) | 32 (44.4) | .14 |
|
| |||||
|
Symptoms, Stress and Resilience
| |||||
| Depressive, M (SD) | 6.9 (6.0) | 9.9 (7.3) | 15.2a (11.1) | 17.0a (10.5) | .00 |
| Trait Anxiety, M (SD) | 27.7 (6.5) | 31.2 (9.5) | 36.5a (11.1) | 38.6a (10.6) | .00 |
| State Anxiety, M (SD) | 26.8a (7.5) | 28.4a (7.5) | 33.7b (12.0) | 34.6b (10.0) | .00 |
| Perceived Stress, M (SD) | 16.7 (5.9) | 19.3 (7.2) | 22.5a (8.8) | 23.5a (8.5) | .00 |
| Resilience, M (SD) | 80.4a (10.4) | 76.9ab (13.7) | 72.6bc (11.7) | 71.8c (13.5) | .00 |
Note: In four group analyses, p values indicate ANOVA significance level. In two group analyses, p values indicate t-test or chi-square significance level. For those four group analyses with p values < .05, same superscripts within the same line indicate groups are not significantly different from one another. Alcohol/Substance refers to alcohol abuse or dependence. Depressive disorders include MDD, dysthymia, and depression not otherwise specified. Anxiety disorders include PTSD, generalized anxiety disorder, social phobia, panic disorder, and anxiety disorder not otherwise specified.
mtDNA Copy Number
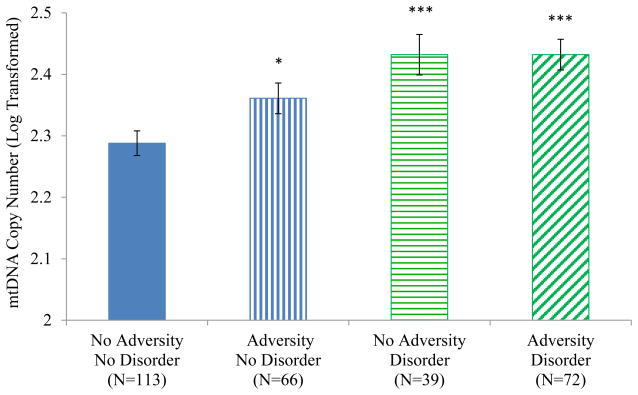
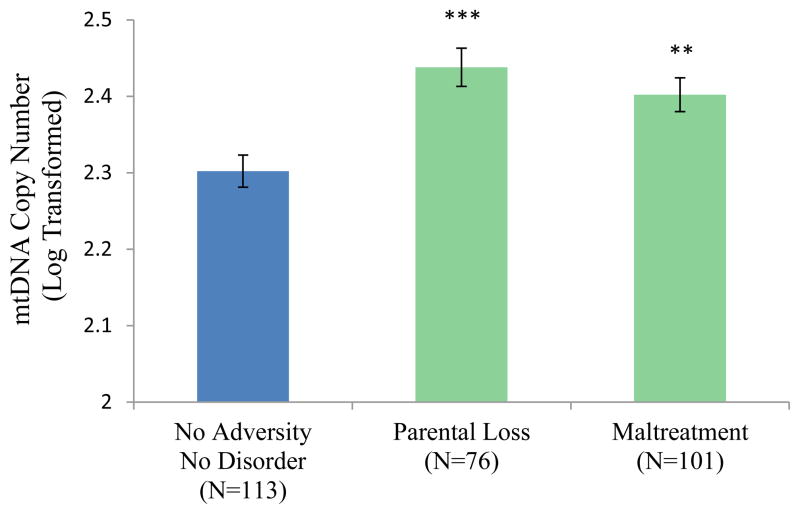
The adversity and diagnosis grouping variable was significantly associated with mtDNA copy number (F(3, 280) = 8.40, p < .001; Figure 1). The No Adversity/No Disorder group had significantly lower mtDNA copy numbers than the three other groups. The Adversity/No Disorder group was also significantly different from the Adversity/ Disorder group (p = .040) and the No Adversity/Disorder group at trend level (p = .089). As shown in Figure 2, in comparison with the No Adversity/No Disorder group, higher mtDNA copy numbers were seen in participants with lifetime MDD (F(1,153) = 12.18, p = .001), any lifetime depressive disorder (including MDD; F(1, 164) = 14.65, p <.001), any lifetime anxiety disorder (including PTSD; F(1, 133) = 14.05, p < .001), and past substance disorders (F(1, 160) = 12.36, p = .001). As shown in Figure 3, elevated mtDNA copy numbers were also seen in participants who experienced parental loss (n = 76; F(1, 181) = 15.85, p < .001) and those who reported any form of maltreatment on the CTQ (n = 101; F(1, 206) = 11.60, p = .001).
Figure 1.
Mitochondrial Copy Number by Adversity/Disorder Group
Note. * p < .05, *** p < .001 in comparison with the No Adversity/No Disorder group.
In addition, the Adversity/Disorder group was significantly different from the Adversity/No Disorder group (p = .040).
Figure 2.
Mitochondrial Copy Number by Disorder Type
Note. ** p < .01, *** p < .001 in comparison with the No Adversity/No Disorder group. Lifetime Depressive Disorder includes major depressive disorder (MDD), dysthymia, and depressive disorder not otherwise specified (NOS). Lifetime anxiety disorder includes post-traumatic stress disorder (PTSD), panic disorder, generalized anxiety disorder, social phobia, and anxiety disorder not otherwise specified (NOS).
Figure 3.
Mitochondrial Copy Number by Adversity Type
Note. ** p < .01, *** p <.001 in comparison with the No Adversity/No Disorder group.
Telomere Length
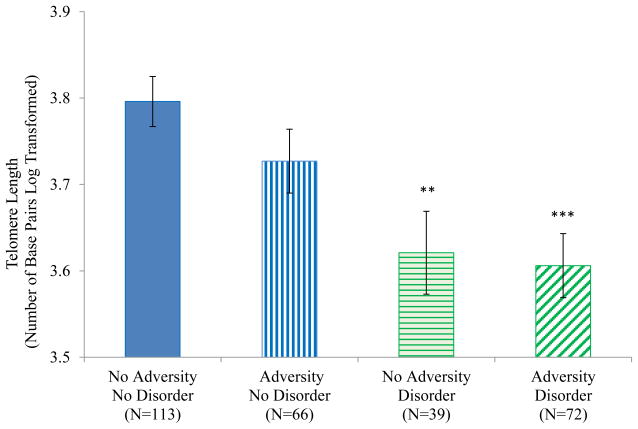
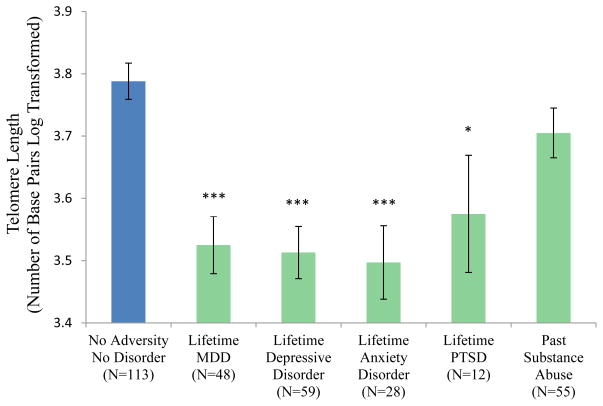
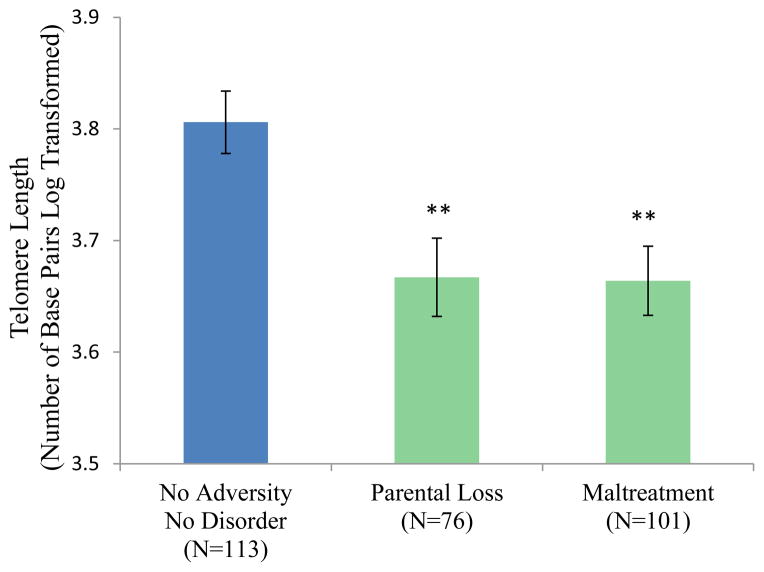
The Adversity/Disorder grouping variable was associated with telomere length (F(3, 280) = 6.39, p < .001; Figure 4). The No Adversity/No Disorder group had significantly longer telomeres on average than the No Adversity/Disorder group (p = .002) and the Adversity/Disorder group (p < .001) but not the Adversity/No Disorder group (p=.153). The Adversity/No Disorder group was significantly different from the Adversity/Disorder group (p = .019) and the No Adversity/Disorder group at trend level (p = .085). Figure 5 shows that shorter telomere lengths were seen in participants with lifetime MDD (F(1,153) = 21.09, p < .001), any lifetime depressive disorder (F(1, 164) = 26.35, p < .001), any lifetime anxiety disorder F(1, 133) = 19.06, p <.001) and lifetime PTSD (F(1, 117 = 4.02, p = .047). Shorter telomere lengths were also seen in participants who experienced parental loss and those who experienced maltreatment (F(1, 181) = 8.84, p = .003 and F(1, 206) = 7.07, p = .008, respectively) (Figure 6).
Figure 4.
Telomere Length by Adversity/Disorder Group
Note. ** p < .01, *** p < .001 in comparison with the No Adversity/No Disorder group.
Figure 5.
Telomere Length by Disorder Type
Note. * p < .05, *** p < .001 in comparison with the No Adversity/No Disorder group. Lifetime Depressive Disorder includes MDD, dysthymia, and depressive disorder NOS. Lifetime anxiety disorder includes PTSD, panic disorder, generalized anxiety disorder, social phobia, and anxiety disorder NOS.
Figure 6.
Telomere Length by Adversity Type
Note. ** p < .01 in comparison with the No Adversity/No Disorder group.
Potential Confounds and Sensitivity Analyses
Race, smoking status, and oral contraceptive use were not associated with mtDNA copy number or telomere length, and the grouping variable remained a significant predictor of telomere length and mtDNA copy number when these variables were included in the statistical models. Additional analyses were conducted that did not control for the significant positive association of telomere length and mtDNA copy number. The grouping variable remained a significant predictor of mtDNA copy number and telomere length. Details of the individual contrasts are provided in the supplementary materials.
As shown in Table 1, the groups differed with respect to depressive symptoms on the IDS-SR, anxiety symptoms on the STAI, perceived stress in the last month on the PSS, and resilience on the CD-RISC; the Adversity/No Disorder group had intermediate scores compared to the two disorder groups and the No Adversity/No Disorder group, and the two disorder groups did not differ. This pattern resembles the differences between groups on telomere length and mtDNA copy number. However, when these variables were entered into the models predicting telomere length and mtDNA number, they did not account for the group effect. Additional analyses were aimed at further elucidating the effects in the two groups that had either childhood adversity or lifetime psychopathology. First, we sought to determine whether the effects of the No Adversity/Disorder group might be explained by an effect of recent perceived stress, low levels of resilience in the face of stress, or the symptom burden in these subjects. Within this group, telomere length was negatively correlated with scores on the PSS (r=−.46, p < .01), IDS-SR (r=−.40, p < .05), STAI-Trait (r=−.34, p < .05), and STAI-State (r=−.37, p < .05) after controlling for the covariates, suggesting that the burden of stress and symptoms may account for effects in this group. There was no effect of the CD-RISC. None of these variables was associated with mtDNA copy number in this group.
Similarly, we examined whether the intermediate scores in the Adversity/No Disorder group might due to an effect of subclinical symptoms, recent perceived stress, or resilience. There were no associations of telomere length or mtDNA number with depressive symptoms, state or trait anxiety, recent perceived stress, or resilience in the Adversity/No Disorder group.
The two other groups (No Adversity/No Disorder and Adversity/Disorder) also did not show any associations of telomere length or mtDNA copy number with any of these three domains.
Discussion
This study provides the first evidence of an association of early stress exposure and lifetime psychopathology with alterations of mtDNA copy number, a proposed measure of cellular aging. These findings are bolstered by our similar results for telomere length, another marker of cellular aging with robust links to stress and psychopathology. Although the specific mechanisms governing mtDNA copy number are poorly understood, mitochondrial replication and degradation can occur as a response to mtDNA dysfunction or other cellular signals (80). Glucocorticoids induce expression of nuclear genes involved in the production of new mitochondria (81, 82), and in skeletal and cardiac muscle, mitochondrial proliferation occurs following mechanical or oxidative stress (83). Consistent with evidence linking the regulation of telomeres and telomerase with mitochondrial function (9), there was a positive association between telomere length and mtDNA copy number in this study. However, early stress and psychopathology were associated with shorter telomeres but higher mtDNA numbers, suggesting that co-regulation of these processes might serve a compensatory role, particularly in healthy adults. Telomere dysfunction leads to activation of the tumor suppressor protein p53. Depending on levels of p53 and the degree of genotoxic stress, p53 can either promote the production of antioxidants and enhance mitochondrial function and biogenesis, or promote the expression of ROS and impair mitochondrial function (9). Glucocorticoids and inflammatory mediators likely also play a role in the associations of stress exposure and psychopathology with telomere length and mitochondrial biogenesis (20, 59).
In a study of peripheral blood mononuclear cells (PBMCs) from depressed patients compared to healthy controls, mitochondrial respiration was impaired and the intracellular density of mitochondria, as measured by citrate synthase activity, was increased. In addition, mitochondrial respiration was negatively correlated with early trauma exposure (84). Another study found impaired mitochondrial respiration in platelets of patients with depression (84, 85). With regard to mtDNA copy number, one study did not show alterations in 17–45 year-old Chinese outpatients with MDD compared with matched healthy controls (32). However, in a study of mtDNA copy number in community-dwelling Korean women over age 60, lower mtDNA copy numbers were seen in a subgroup on antidepressants or with high levels of depressive symptoms (33). In another analysis of this sample, the negative association of depressive symptoms with mtDNA copy number was not significant after controlling for effects of current smoking, hypertension, telomere length and other covariates (79). We speculate that in our study of medically-healthy adults, increased mtDNA copy numbers might be an early compensatory response that could be limited by time and disease burden. We observed a negative association of age and BMI with telomere length, but not with mtDNA copy number. However, when analyzed by group, there were significant negative correlations of mtDNA number with age (r = −.275, p = .018) and BMI (r = −.291, p = .012) only in the Adversity/Diagnosis group. This group had the greatest spread of age and BMI, and the greatest burden of early adversity and psychopathology.
This study is unique in assessing discrete groups of adults with and without childhood adversity and lifetime psychopathology; this allowed examination of whether telomere length and mtDNA copy number might be related to risk or resilience for psychopathology. For both measures, associations with the Adversity/No Disorder group were not as robust as those for the two disorder groups. Within this group, there were no associations of telomere length or mtDNA with subclinical symptoms, perceived stress, or resilience, arguing against the possibility that subgroups of subjects who were at greater risk or more resilient were responsible for the intermediate association. The Adversity/No Disorder group had a lower burden of adversity than the Adversity/Disorder group (Table 1), which could explain why the effects were less robust.
It is of note that lifetime psychopathology without childhood adversity was also linked to telomere length and mtDNA copy number, suggesting that lifetime psychopathology and early adversity share some mechanisms of cellular aging. Within the No Adversity/Disorder group, telomere length was associated with perceived stress and with depressive and anxiety symptoms. Psychological and physiological stress associated with the disorders themselves or other adverse experiences could account for the effect.
It is notable that the Adversity/Disorder group did not differ from the groups with early stress alone or lifetime disorder alone, particularly given that these subjects had a higher burden of illness and stress exposure (Table 1). This could be due to “ceiling” or “floor” effects for mtDNA copy number and telomere length, respectively. There is a critical threshold for telomere shortening beyond which point the cell enters senescence or undergoes apoptosis, and production of excess ROS or other mechanisms might limit compensatory mitochondrial proliferation.
Because we used a sample of mixed leukocytes, it is possible that our findings are influenced by a difference in cell type that might covary with early stress and psychopathology. We excluded individuals with acute and chronic illness and pregnancy, but it remains possible that the groups differed with respect to leukocyte distribution. Few studies have examined these measures in leukocyte subtypes. There is evidence that telomere length is similar in lymphocytes and granulocytes in younger adults, but that lymphocyte telomere length declines at a faster rate with age (86). Given the large differences seen between our groups and the fact that we controlled for age, this would appear unlikely to explain the findings. Moreover, a recent study found that subjects with current depression and those with past episodes both had significantly shorter telomere length than healthy controls in each of three lymphocyte subpopulations (CD4+ helper T cells, CD8+ cytotoxic T cells, and CD20+ B cells) (84). For mtDNA copy number, there is evidence that whole blood is comparable to measurements in isolated lymphocytes (87).
The mechanisms by which mtDNA is elevated in conditions where telomere length is shortened remain to be elucidated. We do not have data on mitochondrial function, oxidative stress or telomerase levels so we cannot assess these factors. Future research should also examine mtDNA mutations; there is some evidence for a role of mtDNA variants, deletions, and mutations in various psychiatric disorders (12, 13) and it has been posited that some mutations might confer a replicative advantage (88). It is also possible that our findings were due to shared heritable influences on psychopathology, early stress, telomere length, and mtDNA copy number, although this is unlikely to fully account for the consistent pattern of findings in these different conditions and distinct markers of cellular aging. Future work could address such influences using multi-generation or twin designs. We did assess for effects of BMI, age, and smoking, but did not investigate quality of sleep, diet, and exercise (19, 89). A major strength of this study is that subjects did not have acute or chronic medical conditions and were not taking psychotropic medications which can alter mitochondrial biogenesis, gene expression, and function (12, 90) and telomere length (91).
In conclusion, we report new evidence of an alteration of mitochondrial biogenesis in association with early life stress and psychopathology. This study replicates prior findings of shortened telomere length in association with depressive and anxiety disorders and shows that this effect is not due to medical comorbidity or medication effects. Early adversity and lifetime psychopathology were uniquely linked to markers of cellular aging, and we suggest that this may be due to shared biological sequelae of these conditions.
Supplementary Material
Acknowledgments
Andrew Huckins-Noss assisted with literature review for an earlier draft of the manuscript. We thank Kelly Colombo for data management. This research was supported by grants R21 MH091508 (ART), and R01 MH068767-08S1 (LLC) from the National Institute of Mental Health (NIMH).
Footnotes
The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIMH.
Financial Disclosures: In the last two years, Drs. Tyrka, Carpenter, Price, and Philip have received support for research from Neuronetics, NeoSync, and Cervel Neurotech. Dr. Carpenter has served as a consultant for Magstim, Naurex, and Taisho, and Dr. Price has served as a consultant for Wiley, Springer, Qatar National Research Fund, and Abbott. Dr. Philip is supported by Department of Veterans Affairs grant 1IK2CX000724. Drs. Kao, Porton, and Parade and Emma Welch declare no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clarke DM, Currie KC. Depression, anxiety and their relationship with chronic diseases: a review of the epidemiology, risk and treatment evidence. Med J Aust. 2009;190:S54–60. doi: 10.5694/j.1326-5377.2009.tb02471.x. [DOI] [PubMed] [Google Scholar]
- 2.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 3.Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009;12:1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1:607–628. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer ME, Jeckel CM, Luz C. The role of stress factors during aging of the immune system. Annals of the New York Academy of Sciences. 2009;1153:139–152. doi: 10.1111/j.1749-6632.2008.03966.x. [DOI] [PubMed] [Google Scholar]
- 6.Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depression and Anxiety. 2010;27:327–338. doi: 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- 7.Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest. 2013;123:951–957. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahin E, DePinho RA. Axis of ageing: telomeres, p53 and mitochondria. Nat Rev Mol Cell Biol. 2012;13:397–404. doi: 10.1038/nrm3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biol Psychiatry. 2013;73:15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, et al. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology. 2013;38:1835–1842. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anglin RE, Mazurek MF, Tarnopolsky MA, Rosebush PI. The mitochondrial genome and psychiatric illness. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:749–759. doi: 10.1002/ajmg.b.32086. [DOI] [PubMed] [Google Scholar]
- 13.Sequeira A, Martin MV, Rollins B, Moon EA, Bunney WE, Macciardi F, et al. Mitochondrial mutations and polymorphisms in psychiatric disorders. Front Genet. 2012;3:103. doi: 10.3389/fgene.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Streck EL, Goncalves CL, Furlanetto CB, Scaini G, Dal-Pizzol F, Quevedo J. Mitochondria and the central nervous system: searching for a pathophysiological basis of psychiatric disorders. Rev Bras Psiquiatr. 2014;36:156–167. doi: 10.1590/1516-4446-2013-1224. [DOI] [PubMed] [Google Scholar]
- 15.Kazachkova N, Ramos A, Santos C, Lima M. Mitochondrial DNA damage patterns and aging: revising the evidences for humans and mice. Aging Dis. 2013;4:337–350. doi: 10.14336/AD.2013.0400337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagouge M, Larsson NG. The role of mitochondrial DNA mutations and free radicals in disease and ageing. J Intern Med. 2013;273:529–543. doi: 10.1111/joim.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu WY, Chang HW, Lin CH, Cho CL. Short telomeres in patients with chronic schizophrenia who show a poor response to treatment. J Psychiatry Neurosci. 2008;33:244–247. [PMC free article] [PubMed] [Google Scholar]
- 18.Marques-Aleixo I, Oliveira PJ, Moreira PI, Magalhaes J, Ascensao A. Physical exercise as a possible strategy for brain protection: evidence from mitochondrial-mediated mechanisms. Prog Neurobiol. 2012;99:149–162. doi: 10.1016/j.pneurobio.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Lopresti AL, Hood SD, Drummond PD. A review of lifestyle factors that contribute to important pathways associated with major depression: diet, sleep and exercise. Journal of Affective Disorders. 2013;148:12–27. doi: 10.1016/j.jad.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Picard M, Juster RP, McEwen BS. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nature Reviews Endocrinology. 2014;10:303–310. doi: 10.1038/nrendo.2014.22. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Zhou R, Li X, Ursano RJ, Li H. Stress-induced change of mitochondria membrane potential regulated by genomic and non-genomic GR signaling: a possible mechanism for hippocampus atrophy in PTSD. Medical Hypotheses. 2006;66:1205–1208. doi: 10.1016/j.mehy.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 22.Atlante A, Calissano P, Bobba A, Giannattasio S, Marra E, Passarella S. Glutamate neurotoxicity, oxidative stress and mitochondria. FEBS Letters. 2001;497:1–5. doi: 10.1016/s0014-5793(01)02437-1. [DOI] [PubMed] [Google Scholar]
- 23.Toya S, Takatsuru Y, Kokubo M, Amano I, Shimokawa N, Koibuchi N. Early-life-stress affects the homeostasis of glutamatergic synapses. The European Journal of Neuroscience. 2014 doi: 10.1111/ejn.12728. [DOI] [PubMed] [Google Scholar]
- 24.Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17:491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giulivi C, Zhang YF, Omanska-Klusek A, Ross-Inta C, Wong S, Hertz-Picciotto I, et al. Mitochondrial dysfunction in autism. JAMA. 2010;304:2389–2396. doi: 10.1001/jama.2010.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang CC, Jou SH, Lin TT, Liu CS. Mitochondrial DNA variation and increased oxidative damage in euthymic patients with bipolar disorder. Psychiatry Clin Neurosci. 2014;68:551–557. doi: 10.1111/pcn.12163. [DOI] [PubMed] [Google Scholar]
- 27.de Sousa RT, Uno M, Zanetti MV, Shinjo SM, Busatto GF, Gattaz WF, et al. Leukocyte mitochondrial DNA copy number in bipolar disorder. Progress in Neuro-psychopharmacology & Biological Psychiatry. 2014;48:32–35. doi: 10.1016/j.pnpbp.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Kakiuchi C, Ishiwata M, Kametani M, Nelson C, Iwamoto K, Kato T. Quantitative analysis of mitochondrial DNA deletions in the brains of patients with bipolar disorder and schizophrenia. Int J Neuropsychopharmacol. 2005;8:515–522. doi: 10.1017/S1461145705005213. [DOI] [PubMed] [Google Scholar]
- 29.Sabunciyan S, Kirches E, Krause G, Bogerts B, Mawrin C, Llenos IC, et al. Quantification of total mitochondrial DNA and mitochondrial common deletion in the frontal cortex of patients with schizophrenia and bipolar disorder. J Neural Transm. 2007;114:665–674. doi: 10.1007/s00702-006-0581-8. [DOI] [PubMed] [Google Scholar]
- 30.Torrell H, Montana E, Abasolo N, Roig B, Gaviria AM, Vilella E, et al. Mitochondrial DNA (mtDNA) in brain samples from patients with major psychiatric disorders: gene expression profiles, mtDNA content and presence of the mtDNA common deletion. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:213–223. doi: 10.1002/ajmg.b.32134. [DOI] [PubMed] [Google Scholar]
- 31.Vawter MP, Tomita H, Meng F, Bolstad B, Li J, Evans S, et al. Mitochondrial-related gene expression changes are sensitive to agonal-pH state: implications for brain disorders. Mol Psychiatry. 2006;11:663–679. doi: 10.1038/sj.mp.4001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He Y, Tang J, Li Z, Li H, Liao Y, Tang Y, et al. Leukocyte mitochondrial DNA copy number in blood is not associated with major depressive disorder in young adults. PloS One. 2014;9:e96869. doi: 10.1371/journal.pone.0096869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim MY, Lee JW, Kang HC, Kim E, Lee DC. Leukocyte mitochondrial DNA (mtDNA) content is associated with depression in old women. Arch Gerontol Geriatr. 2011;53:e218–221. doi: 10.1016/j.archger.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 34.Mason PJ, Perdigones N. Telomere biology and translational research. Transl Res. 2013;162:333–342. doi: 10.1016/j.trsl.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silvestre DC, Londono-Vallejo A. Telomere dynamics in mammals. Genome Dynamics. 2012;7:29–45. doi: 10.1159/000337128. [DOI] [PubMed] [Google Scholar]
- 36.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88:557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 37.Lin P, Mobasher ME, Alawi F. Acute dyskerin depletion triggers cellular senescence and renders osteosarcoma cells resistant to genotoxic stress-induced apoptosis. Biochem Biophys Res Commun. 2014;446:1268–1275. doi: 10.1016/j.bbrc.2014.03.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bojesen SE. Telomeres and human health. J Intern Med. 2013;274:399–413. doi: 10.1111/joim.12083. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson PM, Tufvesson H, Leosdottir M, Melander O. Telomeres and cardiovascular disease risk: an update 2013. Transl Res. 2013;162:371–380. doi: 10.1016/j.trsl.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Rizo C, Fernandez-Egea E, Miller BJ, Oliveira C, Justicia A, Griffith JK, et al. Abnormal glucose tolerance, white blood cell count, and telomere length in newly diagnosed, antidepressant-naive patients with depression. Brain, Behavior, and Immunity. 2013;28:49–53. doi: 10.1016/j.bbi.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartmann N, Boehner M, Groenen F, Kalb R. Telomere length of patients with major depression is shortened but independent from therapy and severity of the disease. Depression and Anxiety. 2010;27:1111–1116. doi: 10.1002/da.20749. [DOI] [PubMed] [Google Scholar]
- 42.Hoen PW, de Jonge P, Na BY, Farzaneh-Far R, Epel E, Lin J, et al. Depression and leukocyte telomere length in patients with coronary heart disease: data from the heart and soul study. Psychosom Med. 2011;73:541–547. doi: 10.1097/PSY.0b013e31821b1f6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verhoeven JE, Revesz D, Epel ES, Lin J, Wolkowitz OM, Penninx BW. Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol Psychiatry. 2013;19:895–901. doi: 10.1038/mp.2013.151. [DOI] [PubMed] [Google Scholar]
- 44.Wikgren M, Maripuu M, Karlsson T, Nordfjall K, Bergdahl J, Hultdin J, et al. Short Telomeres in Depression and the General Population Are Associated with a Hypocortisolemic State. Biol Psychiatry. 2011;71:294–300. doi: 10.1016/j.biopsych.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 45.Hoen PW, Rosmalen JG, Schoevers RA, Huzen J, vander Harst P, de Jonge P. Association between anxiety but not depressive disorders and leukocyte telomere length after 2 years of follow-up in a population-based sample. Psychological Medicine. 2013;43:689–697. doi: 10.1017/S0033291712001766. [DOI] [PubMed] [Google Scholar]
- 46.Shaffer JA, Epel E, Kang MS, Ye S, Schwartz JE, Davidson KW, et al. Depressive symptoms are not associated with leukocyte telomere length: findings from the Nova Scotia Health Survey (NSHS95), a population-based study. PloS One. 2012;7:e48318. doi: 10.1371/journal.pone.0048318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shalev I, Moffitt TE, Braithwaite AW, Danese A, Fleming NI, Goldman-Mellor S, et al. Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Mol Psychiatry. 2014;19:1163–1170. doi: 10.1038/mp.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ladwig KH, Brockhaus AC, Baumert J, Lukaschek K, Emeny RT, Kruse J, et al. Posttraumatic stress disorder and not depression is associated with shorter leukocyte telomere length: findings from 3,000 participants in the population-based KORA F4 study. PloS One. 2013;8:e64762. doi: 10.1371/journal.pone.0064762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malan S, Hemmings S, Kidd M, Martin L, Seedat S. Investigation of telomere length and psychological stress in rape victims. Depression and Anxiety. 2011;28:1081–1085. doi: 10.1002/da.20903. [DOI] [PubMed] [Google Scholar]
- 50.O’Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70:465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L, Hu XZ, Benedek DM, Fullerton CS, Forsten RD, Naifeh JA, et al. The interaction between stressful life events and leukocyte telomere length is associated with PTSD. Mol Psychiatry. 2013;19:855–856. doi: 10.1038/mp.2013.141. [DOI] [PubMed] [Google Scholar]
- 52.Elvsashagen T, Vera E, Boen E, Bratlie J, Andreassen OA, Josefsen D, et al. The load of short telomeres is increased and associated with lifetime number of depressive episodes in bipolar II disorder. Journal of Affective Disorders. 2011;135:43–50. doi: 10.1016/j.jad.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Kao HT, Cawthon RM, Delisi LE, Bertisch HC, Ji F, Gordon D, et al. Rapid telomere erosion in schizophrenia. Mol Psychiatry. 2008;13:118–119. doi: 10.1038/sj.mp.4002105. [DOI] [PubMed] [Google Scholar]
- 54.Pavanello S, Hoxha M, Dioni L, Bertazzi PA, Snenghi R, Nalesso A, et al. Shortened telomeres in individuals with abuse in alcohol consumption. Int J Cancer. 2011;129:983–992. doi: 10.1002/ijc.25999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Z, Ye J, Li C, Zhou D, Shen Q, Wu J, et al. Drug addiction is associated with leukocyte telomere length. Sci Rep. 2013;3:1542. doi: 10.1038/srep01542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su Y, et al. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress--preliminary findings. PloS One. 2011;6:e17837. doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- 58.Liu RT, Alloy LB. Stress generation in depression: A systematic review of the empirical literature and recommendations for future study. Clin Psychol Rev. 2010;30:582–593. doi: 10.1016/j.cpr.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verhoeven JE, Revesz D, Wolkowitz OM, Penninx BW. Cellular aging in depression: Permanent imprint or reversible process?: An overview of the current evidence, mechanistic pathways, and targets for interventions. BioEssays : News and Reviews in Molecular, Cellular and Developmental Biology. 2014;36:968–978. doi: 10.1002/bies.201400068. [DOI] [PubMed] [Google Scholar]
- 60.Asok A, Bernard K, Roth TL, Rosen JB, Dozier M. Parental responsiveness moderates the association between early-life stress and reduced telomere length. Dev Psychopathol. 2013;25:577–585. doi: 10.1017/S0954579413000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Savolainen K, Eriksson JG, Kananen L, Kajantie E, Pesonen AK, Heinonen K, et al. Associations between early life stress, self-reported traumatic experiences across the lifespan and leukocyte telomere length in elderly adults. Biol Psychol. 2014;97:35–42. doi: 10.1016/j.biopsycho.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Theall KP, Brett ZH, Shirtcliff EA, Dunn EC, Drury SS. Neighborhood disorder and telomeres: connecting children’s exposure to community level stress and cellular response. Soc Sci Med. 2013;85:50–58. doi: 10.1016/j.socscimed.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, et al. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry. 2013;18:576–581. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glass D, Parts L, Knowles D, Aviv A, Spector TD. No correlation between childhood maltreatment and telomere length. Biol Psychiatry. 2010;68:e21–22. doi: 10.1016/j.biopsych.2010.02.026. author reply e23–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jodczyk S, Fergusson DM, Horwood LJ, Pearson JF, Kennedy MA. No Association between Mean Telomere Length and Life Stress Observed in a 30 Year Birth Cohort. PloS One. 2014;9:e97102. doi: 10.1371/journal.pone.0097102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ale-Agha N, Dyballa-Rukes N, Jakob S, Altschmied J, Haendeler J. Cellular functions of the dual-targeted catalytic subunit of telomerase, telomerase reverse transcriptase - Potential role in senescence and aging. Experimental Gerontology. 2014;56:189–193. doi: 10.1016/j.exger.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 69.Jaiswal RK, Kumar P, Yadava PK. Telomerase and its extracurricular activities. Cell Mol Biol Lett. 2013;18:538–554. doi: 10.2478/s11658-013-0105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biol Psychiatry. 2009;67:531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.First MB, Gibbon M, Spitzer RL, Williams JB, Benjamin L. Clinical Version. 1997. Structured Clinical Interview for DSM-IV Axis I (SCID-I) [Google Scholar]
- 72.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychological Medicine. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 73.Spielberger CD. State-Trait Anxiety Inventory: A Comprehensive Bibliography. Palo Alto, CA: Consulting Psychologists Press; 1989. [Google Scholar]
- 74.Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 75.Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC) Depression and Anxiety. 2003;18:76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- 76.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. The American Journal of Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 77.O’Callaghan NJ, Fenech M. A quantitative PCR method for measuring absolute telomere length. Biol Proced Online. 2011;13:3–13. doi: 10.1186/1480-9222-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bai RK, Wong LJ. Simultaneous detection and quantification of mitochondrial DNA deletion(s), depletion, and over-replication in patients with mitochondrial disease. J Mol Diagn. 2005;7:613–622. doi: 10.1016/S1525-1578(10)60595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim JH, Kim HK, Ko JH, Bang H, Lee DC. The relationship between leukocyte mitochondrial DNA copy number and telomere length in community-dwelling elderly women. PloS One. 2013;8:e67227. doi: 10.1371/journal.pone.0067227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clay Montier LL, Deng JJ, Bai Y. Number matters: control of mammalian mitochondrial DNA copy number. Journal of Genetics and Genomics. 2009;36:125–131. doi: 10.1016/S1673-8527(08)60099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Psarra AM, Sekeris CE. Glucocorticoid receptors and other nuclear transcription factors in mitochondria and possible functions. Biochimica et Biophysica Acta. 2009;1787:431–436. doi: 10.1016/j.bbabio.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 82.Psarra AM, Sekeris CE. Glucocorticoids induce mitochondrial gene transcription in HepG2 cells: role of the mitochondrial glucocorticoid receptor. Biochimica et Biophysica Acta. 2011;1813:1814–1821. doi: 10.1016/j.bbamcr.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 83.Xiao J, Chen L, Wang X, Liu M, Xiao Y. eNOS Correlates with Mitochondrial Biogenesis in Hearts of Congenital Heart Disease with Cyanosis. Arquivos Brasileiros de Cardiologia. 2012;99:780–788. doi: 10.1590/s0066-782x2012005000072. [DOI] [PubMed] [Google Scholar]
- 84.Karabatsiakis A, Kolassa IT, Kolassa S, Rudolph KL, Dietrich DE. Telomere shortening in leukocyte subpopulations in depression. BMC Psychiatry. 2014;14:192–198. doi: 10.1186/1471-244X-14-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hroudova J, Fisar Z, Kitzlerova E, Zverova M, Raboch J. Mitochondrial respiration in blood platelets of depressive patients. Mitochondrion. 2013;13:795–800. doi: 10.1016/j.mito.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 86.Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, et al. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med. 1999;190:157–167. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chan SW, Chevalier S, Aprikian A, Chen JZ. Simultaneous quantification of mitochondrial DNA damage and copy number in circulating blood: a sensitive approach to systemic oxidative stress. Biomed Res Int. 2013;2013:157547. doi: 10.1155/2013/157547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holt IJ, Reyes A. Human mitochondrial DNA replication. Cold Spring Harb Perspect Biol. 2012;4:1–15. doi: 10.1101/cshperspect.a012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin J, Epel E, Blackburn E. Telomeres and lifestyle factors: roles in cellular aging. Mutat Res. 2012;730:85–89. doi: 10.1016/j.mrfmmm.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 90.Neustadt J, Pieczenik SR. Medication-induced mitochondrial damage and disease. Mol Nutr Food Res. 2008;52:780–788. doi: 10.1002/mnfr.200700075. [DOI] [PubMed] [Google Scholar]
- 91.Savolainen K, Raikkonen K, Kananen L, Kajantie E, Hovatta I, Lahti M, et al. History of mental disorders and leukocyte telomere length in late adulthood: the Helsinki Birth Cohort Study (HBCS) J Psychiatr Res. 2012;46:1346–1353. doi: 10.1016/j.jpsychires.2012.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.