Abstract
Objective
To identify approximately 500 cases of incident cognitive impairment (ICI) in a large, national sample adapting an existing cognitive test-based case definition and to examine relationships of vascular risk factors with ICI.
Method
Participants were from the REGARDS study, a national sample of 30,239 African-American and white Americans. Participants included in this analysis had normal cognitive screening and no history of stroke at baseline, and at least one follow-up cognitive assessment with a three test battery (TTB). Regression-based norms were applied to TTB scores to identify cases of ICI. Logistic regression was used to model associations with baseline vascular risk factors.
Results
We identified 495 participants with ICI out of 17,630 eligible participants. In multivariable modeling, income (OR 1.83 CI 1.27,2.62), stroke belt residence (OR 1.45 CI 1.18,1.78), history of transient ischemic attack (OR 1.90 CI 1.29,2.81), coronary artery disease(OR 1.32 CI 1.02,1.70), diabetes (OR 1.48 CI 1.17,1.87), obesity (OR 1.40 CI 1.05,1.86), and incident stroke (OR 2.73 CI 1.52,4.90) were associated with ICI.
Conclusions
We adapted a previously validated cognitive test-based case definition to identify cases of ICI. Many previously identified risk factors were associated with ICI, supporting the criterion-related validity of our definition.
Keywords: epidemiology, risk factors, methods, cognitive disorders, mild cognitive impairment, cognitive aging, stroke
Introduction
5.4 million people in the United States have clinically significant cognitive impairment and about 12% of those progress to dementia annually (Plassman et al., 2008). Development of treatments that delay progression of cognitive impairment is of enormous public health significance, as such interventions would delay the onset of dementia and its attendant functional and financial losses (IOM, 2009; Sloane et al., 2002). Identification of risk factors and biomarkers associated with development of cognitive impairment could assist in diagnosis and may also be helpful in developing strategies for prevention and treatment (Revkin, Shear, Pouleur, Ryder, & Orloff, 2007; Vasan, 2006). Large-scale epidemiological studies that have thousands of subjects provide the most diverse samples and most robust risk factor and biomarker information available; however, these projects are typically lacking a clinical diagnosis component due to the high costs associated with this procedure.
In this paper, we demonstrate how a large epidemiological study may efficiently use cognitive data to identify clinically relevant incident cognitive impairment (ICI) and relate risk factors and a representative biomarker to this endpoint. Our goal was to identify approximately 500 cases of ICI in the REasons for Geographic and Racial Differences in Stroke (REGARDS) study, a national cohort of 30,239 African-American and white Americans, to be used in future nested case-control studies investigating biomarkers and ICI. We describe our methodology for identifying ICI in REGARDS using telephone administered cognitive tests during follow up as a basis for classification. To our knowledge, this is the first application of this method of identifying clinically meaningful cognitive impairment in such a large cohort using telephone-administered tests. Based on previously reported risk factors for cognitive decline and dementia, we expected ICI identified with this case-selection method to be associated with vascular risk factors and additional socio-demographic factors, supporting the validity of our definition. We also report on the association of C-reactive protein (CRP) with ICI, as it is an inflammation biomarker currently used in clinical settings to assess cardiovascular risk, and is also associated with risk of dementia(Ravaglia et al., 2007; Schmidt et al., 2002), and cognitive dysfunction (Mooijaart et al., 2011; Tilvis et al., 2004). Study of CRP also provides an example of how we can investigate baseline biomarker levels in relation to ICI.
Neuropsychological tests are useful for characterizing cognitive ability and have utility in clinical classification and diagnosis (Howieson DB & MD, 2007; Jacova, Kertesz, Blair, Fisk, & Feldman, 2007; Petersen, Stevens, et al., 2001). In particular, an approach to classification of individuals based solely on cognitive test scores called the absolute score method has high concordance to consensus diagnoses panels using multiple sources of information including history, interview, physical and neurological examination, laboratory tests, and neuroimaging (Ivnik et al., 2000). The absolute score method utilizes a single score (i.e., a cut-score) on each test that defines normalcy vs. impairment. Ivnik et al. (Ivnik et al., 2000) found that this approach was superior to other solely cognitive score-based approaches (e.g., difference scores, change scores, and profile variability) in identifying clinically diagnosed patients. This absolute scores approach to case definition offers a means to define cases in the absence of a clinical diagnostic process. In this paper, we adapt and apply the Mayo absolute score method of case definition to the telephone-based cognitive battery used in REGARDS. We then use the adapted case definition to examine relationships of ICI with known vascular risk factors.
Method
Participants
Participants in this study were drawn from the REGARDS Study (Howard et al., 2005), which aimed to identify factors underlying excess stroke mortality in the Southeastern U.S. (North Carolina, South Carolina, Georgia, Tennessee, Mississippi, Alabama, Louisiana, and Arkansas, known as the “Stroke Belt”) and among African-Americans. Higher stroke mortality has been observed in the stroke belt since the 1940s (Lanska, 1993) and has been the subject of much investigation in the stroke research community(Perry & Roccella, 1998). REGARDS participants were recruited by random selection of telephone numbers from a commercially available nationwide list (Genesys Inc., Daly City, CA). One person from each household was identified and eligibility was assessed via computer assisted telephone interview (CATI) by a centrally trained staff at the Survey Research Unit (SRU) at the University of Alabama at Birmingham, which provides telephone interviews for the Behavioral Risk Factor Surveillance System, and other national phone surveys. Exclusion criteria were 1) race other than African-American or white, 2) Hispanic ethnicity, 3) active treatment for cancer, 4) medical conditions that would prevent long-term participation, 5) difficulties with hearing, articulation, comprehension of English language, or general confusion that interfered with assessment 6) residence in or inclusion on a waiting list for a nursing home, or 7) inability to communicate in English. Interviewers using standardized, computer-programmed scripts and real-time data entry were trained and certified by the SRU. Cognitive assessment interviews were monitored routinely by a supervisor for ongoing quality control.
Once eligibility was established, verbal consent was obtained. Study procedures and methods of data collection are outlined in Table 1. Following consent, medical history, including risk factor evaluation, was collected by CATI, which provided a high level of quality control and standardization (Table 1). About one month following the telephone interview, participants underwent an in-home examination conducted by Examination Management Services, Inc. (EMSI), which included written consent, height, weight, and blood pressure measurements, resting electrocardiogram (ECG), medication inventory, and fasting blood and urine samples. EMSI examiners were trained at their local offices by centrally-trained supervisors on REGARDS-specific procedures. Samples were processed in the field by EMSI and shipped to the Central Laboratory at the University of Vermont where they were processed further and frozen. Lab work was performed by technicians blinded to the identity, risk factor and outcome status of the participants. Results from specimens collected in this manner were validated using a paired samples technique (Gillett et al., 2014). ECG tracings were mailed to a core laboratory at Wake Forest University School of Medicine where they were visually inspected for technical errors and were given a quality grade. ECGs were then coded using the Standard Minnesota ECG Classification by trained physician electrocardiographers, and all abnormalities were re-read by a second physician electrocardiographer. Methods were approved by the institutional review boards of all participating institutions.
Table 1.
REGARDS procedures and methods of data collection
| Component | Baseline telephone Interview | In-home Exam | Self-administered and mailed | Every 6 months telephone call | Every 2 years telephone call |
|---|---|---|---|---|---|
| Verbal Consent | X | ||||
| Medical history | X | ||||
| Demographics | X | ||||
| Stroke free status | X | X | |||
| Physical activity | X | ||||
| Depression | X | ||||
| Cognitive screening* | X* | ||||
| Six Item Screener** | X** | X** | |||
| Three-test battery† | X | ||||
| Perceived health/quality of life | X | ||||
| Social support | X | ||||
| Potential caregiver status | X | ||||
| Written consent | X | ||||
| Blood collection | X | ||||
| Urine collection | X | ||||
| Height, weight, waist circumference | X | ||||
| Blood pressure, pulse | X | ||||
| Electrocardiography | X | ||||
| Medications used in the past 2 weeks | X | ||||
| Residential history | X | ||||
| Dietary intake | X | ||||
| Family history | X |
cognitive status was judged by the computer assisted telephone interview (CATI) interviewer. Exclusion was for low ability to communicate (express and comprehend) in English as rated by the study staff based on the telephone conversation to that point of the screening. It included difficulties with hearing, articulation, accent, and general confusion that interfered with the assessment.
The six item screener was added to the baseline CATI 11 months after enrollment began (December 2003) and then administered yearly via CATI
The three-test battery consists of the word list learning (WLL), word list recall (WLR), and animal fluency (AF) tests. They were added to the follow-up CATI calls in 2006 and administered every 2 years. WLL/WLR and AF were initially administered on different calls to reduce participant burden, but were shifted to the same call in 2008 such that the cognitive battery was administered as a unit.
From January 2003 to October 2007, the study enrolled 30,239 Americans ≥ 45 years of age. Race distribution in the sample was 58% white and 42% African-American. Gender distribution was 45% male and 55% female. Regional distribution was 56% residing in the Stroke Belt and 44% residing in the other 40 contiguous United States. Participants were considered lost to follow up if they could not be reached at 4 consecutive scheduled calls. Contact of listed proxies, Lexis-Nexis searches, and 2 formal letters to the participant’s last known address were used to attempt contact when a participant misses a call. Attrition rates are low in REGARDS. As of April 2011, 3785(12%) of 30,239 participants were considered lost to follow up.
Cognitive Assessment
Approximately 100 telephone interviewers were carefully trained by the SRU to administer a telephone-based computer-assisted assessment of cognitive function. Interviewers’ performance was continually monitored by SRU supervisors and group meetings were held periodically with REGARDS operations Center personnel to discuss questions and unusual circumstances. REGARDS includes a two-level assessment (Table 1): 1) the Six-Item Screener(C. M. Callahan, F. W. Unverzagt, S. L. Hui, A. J. Perkins, & H. C. Hendrie, 2002) (SIS) added to the baseline assessment in December 2003 and then performed annually; and 2) a three test battery (TTB) introduced in 2006 and performed at 2 year intervals, which includes Word List Learning (WLL), Word List Recall (WLR) (Morris et al., 1989) and Animal Fluency (AF) (Rosen, 1980). WLL and WLR were administered on the same call, whereas AF originally was administered on a different call to reduce the burden of testing on each call. The SIS is a brief screening measure with 3 temporal orientation items and delayed recall of 3 objects. Scores range from 0–6 with a score of 4 or fewer correct indicative of cognitive impairment. The SIS has been validated against clinical diagnoses of dementia and mild cognitive impairment (74% sensitivity and 80% specificity for both groups combined versus cognitively normal elders) (C. M. Callahan, F. W. Unverzagt, S. L. Hui, A. Perkins, & H. C. Hendrie, 2002) and has been used to document cognitive impairment in older patients seen in emergency departments (Wilber, Lofgren, Mager, Blanda, & Gerson, 2005) and older depressed patients in a large randomized controlled trial (Steffens et al., 2006). The WLL is the total number of words recalled on a 10-item, three-trial word list learning task (range 0–30); WLR is the number of words recalled after a filled delay (range 0–10). Both immediate and delayed recall are decreased in Alzheimer and frontotemporal dementia(Wicklund, Johnson, Rademaker, Weitner, & Weintraub, 2006). AF measures ability to name as many animals as possible in 60 seconds; such semantic fluency is decreased in mild cognitive impairment (MCI) (Nutter-Upham et al., 2008) and may help screening accuracy of the Mini Mental State exam for MCI and non-Alzheimer disease dementia (Kim et al., 2014). These measures are widely used in observational and interventional studies of cognitive aging and dementia. Scoring of the WLL and WLR was completed by calculating summaries of valid responses captured in real time by interviewers during the telephone-based assessments. AF responses were digitally recorded for later playback and scoring by trained technicians who were certified by a PhD level expert scorer who conducted a second scoring for each scorer’s first 50 files. Agreement of .88 or higher was required for certification. Ongoing quality control was conducted on 10% of each scorer’s files. Exact agreement between each scorer and the expert scorer consistently exceeded 95%.
Because the SIS was added 11 months after enrollment began, there were 3218 participants (18% of the cohort) who did not have a SIS score at baseline for whom we considered the first administered SIS the baseline SIS. Median time between enrollment and first SIS for these participants was 2.0 years with 90% completing the first SIS by 2.2 years after enrollment.
Measurement of Covariates
Baseline demographic and health behavior variables were self-reported, collected via CATI and included age, race (African-American or white), gender (male or female), education (categorized as <HS, high school, some college, college and above), yearly income (categorized as <$20,000, $20,000–34,999, $35,000–74,999, ≥ $75,000 or refused), region (categorized as stroke belt or non-stroke belt), cigarette smoking (categorized as never, past, or current), alcohol intake (categorized as none, moderate [≤14 drinks per week for men, ≤7 drinks/week for women] or heavy [>14 drinks/week for men, >7 drinks/week for women]), and physical activity level (categorized as any weekly exercise vs. none). Presence of vascular disease or vascular risk factors was collected at baseline via CATI or during the in-home physical examination (see Table 1) and included: self-reported history of transient ischemic attack (TIA); history of coronary artery disease (CAD), including myocardial infarction by self-report or electrocardiogram (ECG), or self-reported history of coronary revascularization (stenting, coronary artery bypass surgery, or percutaneous transluminal coronary angioplasty); atrial fibrillation (self-reported or by ECG); left ventricular hypertrophy (LVH) by ECG; body mass index (BMI) calculated from height and weight measurements; hypertension (systolic blood pressure>140 mmHg or diastolic blood pressure >90 mmHg on average of 2 measurements, or self-reported use of hypertension medication); dyslipidemia (total cholesterol ≥ 240 mg/dL, LDL ≥ 160, HDL ≤ 40 or self-reported lipid-lowering drug use); and diabetes (fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, or self-reported use of diabetes medications). C-reactive protein (CRP) was measured in plasma samples collected at baseline using a validated, high-sensitivity, particle enhanced immunonephelometric assay (N High Sensitivity CRP, Dade Behring Inc., Deerfield, IL; interassay CVs 2.1–5.7%). The definitions and procedures used for stroke adjudication have been previously published (Howard et al., 2011). In brief summary, reports of possible stroke during follow-up generated a request for retrieval of medical records that were centrally adjudicated by physicians. Stroke events were defined following the World Health Organization (WHO) definition (“Stroke--1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders,” 1989) and classified as hemorrhagic or ischemic. Events not meeting the WHO definition but with symptoms lasting <24 hours and neuroimaging consistent with acute ischemia were classified as clinical strokes.
Case Definition of Incident Cognitive Impairment (ICI)
We developed a case definition of ICI that aimed to identify approximately 500 cases of ICI to be used in a subsequent nested case control study of biomarkers and risk of ICI in REGARDS. The sample size was set a priori at N=500 to allow appropriate statistical power, cost efficiency, and to include a wide range of age, race, gender, and education levels in the case group. Ivnik et al. (Ivnik et al., 2000) previously demonstrated that an absolute score approach, in which a cut-score for impairment was identified for each cognitive test, produced the highest rates of correspondence to a clinical diagnosis of cognitive impairment compared to other solely test-based approaches including difference scores, profile variability, and change scores. Participants’ most recent TTB assessment that had been obtained, scored, and included in the REGARDS dataset as of April 2011 was used to define impairment. To determine the cut-scores for each of the tests in the TTB, age, education, gender, and race were regressed onto each TTB score. The resultant beta weights were used to generate predicted scores for each participant. Participants scoring more than 1.5 SD units below his or her predicted score on at least 2 of 3 of the TTB measures at the most recent assessment were identified as having ICI (n = 577). We selected a threshold of 1.57 SD units, which is slightly more restrictive than the typical 1.50 threshold, to achieve the desired sample size. Importantly, the threshold we used selected subjects with clinically significant cognitive performance below expectation, producing a case group that is more clearly below expectation than the traditional 1.50 SD cut-off.
Statistical Analysis
The analytic sample consisted of 17,630 participants free of stroke and cognitive impairment at the time of first SIS assessment and who had at least one assessment with the TTB during follow-up (Figure 1). Exclusions from the analytic sample were as follows: 1) prevalent stroke (N=1958), 2) missing baseline cognitive assessment with the SIS (N=409), 3) prevalent cognitive impairment on the SIS (N=2319), 4) insufficient cognitive testing during follow up to determine cognitive status (N=7786), and 5) anomalous data (N=56). As TTB measures originally were not all completed in the same phone call, participants may have only completed part of the TTB at the time of the data lock for the present analyses. Exclusions based on insufficient testing were as follows: 1) no TTB testing (N=5098); 2) score on only 1 TTB measure (N=2348); or 3) score on 2 TTB measures but scores were discordant (i.e., one score below threshold and one score above threshold, N=340). A statistical algorithm was used to define incident cognitive impairment status based solely on TTB score without regard to risk factor status. Analysis of risk factor associations with ICI status was performed only after the case group was identified.
Figure 1.
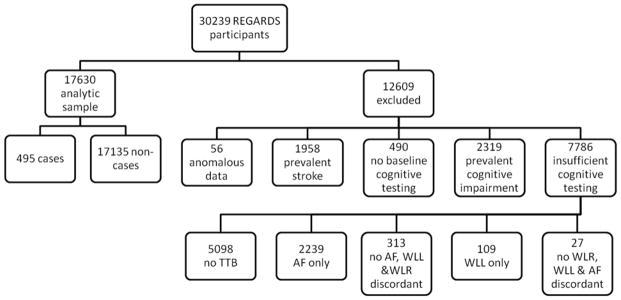
REGARDS analytic sample, exclusion criteria, and number excluded.
SIS= Six-item Screener; TTB= Three Test Battery, which consists of: WLL= Word List Learning, WLR = Word List Recall, AF = Animal Fluency.
Anomalous data refers to participants with questionable identity at baseline for whom all baseline data is unavailable. Prevalent stroke includes participants who reported stroke at the time of enrollment (N=1930) and those who had a stroke between the time of enrollment and the first administered SIS (N=28). No baseline cognitive testing refers to participants who never had a SIS. Prevalent cognitive impairment is defined as those who scored ≤4 on the first SIS. Insufficient cognitive testing to determine incident cognitive impairment status includes participants who never had any of the three tests in the TTB, participants who only had one of the three tests in the TTB, and participants who had 2 tests in the TTB but were impaired on one of those and not the other (discordant).
After cases were selected, we compared baseline characteristics of participants with ICI to the remainder of the analytic sample. Chi square tests were used for dichotomous or nominal variables, and t-tests for continuous variables. A logistic regression model was used to calculate odds ratios (OR) and 95% confidence intervals (CI) of ICI for vascular and demographic risk factors, first in a univariable fashion and then in multivariable models. Model 1 included income, region, smoking status, alcohol use, exercise, diabetes, hypertension, dyslipidemia, history of TIA, atrial fibrillation, LVH, CAD, BMI category and CRP ≥90th percentile. Model 2 added incident stroke during follow up prior to the most recent cognitive assessment. Incident stroke was not censored, as it may be in the causal pathway between vascular risk factors and ICI. A sensitivity analysis was performed where participants with incident stroke were excluded from the analysis. Age, race, gender, and education were not included in the multivariable models as these factors were accounted for in the regression-based approach to case definition. In multivariable models, participants with missing data were excluded from analysis. In order to reflect traditional definitions of ICI based on mild cognitive impairment, we re-analyzed the data using a more liberal cut-point of −1.50 SD from predicted score and there was no material difference in any results (data not shown).
Results
A total of 495 participants were identified as having ICI including 412 with scores below the cut-off on WLL and WLR; 27 with scores below cut-off on WLL and AF; 13 with scores below cut-off on WLR and AF; and 43 with scores below cut-off on WLL, WLR, and AF (see Figure 2). All participants included in the analytic sample had a SIS of 5 or 6 at baseline; mean (SD) SIS score was 5.78 (0.41). Mean scores on the most recently administered TTB and number of participants for whom it was the first, second, or third administration are listed in Table 2.
Figure 2.
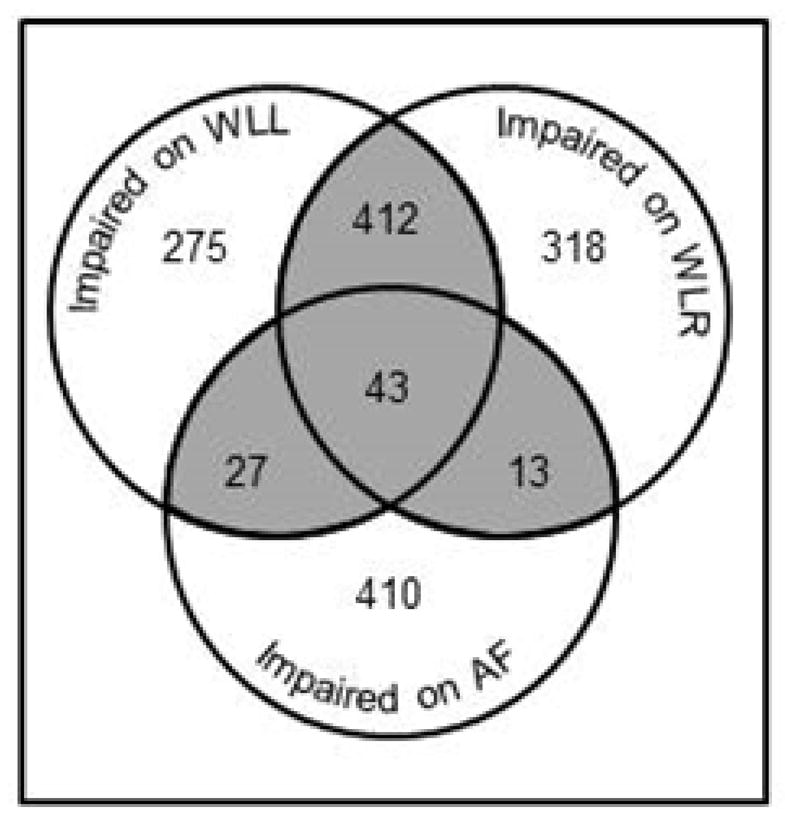
Number of participants with scores at or below demographically-adjusted cut-off on the Three Test Battery.
WLL = Word List Learning, WLR = Word List Recall, AF= Animal Fluency. Numbers are people who scored >1.57 SD below age-, race-, gender-, and education-adjusted predicted scores at the most recent occasion of measurement. Shaded area represents those identified with ICI.
Table 2.
Number of participants with multiple administrations of cognitive tests and most recent mean (SD) score
| Test/Number of administrations at the time of the data lock | 0 | 1 | 2 | 3 | Most recent mean (SD) |
|---|---|---|---|---|---|
| Word List Learning | 0 | 9246 | 6419 | 1965 | 17.43 (5.16) |
| Word List Recall | 133 | 9439 | 6148 | 1910 | 6.55 (2.12) |
| Animal Fluency | 5105 | 9903 | 2530 | 92 | 17.38 (5.77) |
Baseline characteristics of participants with and without ICI are listed in Table 3. Participants with ICI tended to have lower income, residence in the stroke belt, reported current smoking, no alcohol consumption, no weekly exercise, and were obese. Participants with ICI also had higher prevalence of diabetes, hypertension, LVH, and history of TIA or CAD. They also had higher systolic blood pressure, BMI, and CRP. As expected based on the use of demographically-adjusted cognitive scores for case definition, there were no group differences in age, race, gender, or education.
Table 3.
Baseline characteristics of participants with and without incident cognitive impairment
| Variable* | Level | Incident Cognitive Impairment (N= 495) | No Incident Cognitive Impairment (N=17135) | p-value** |
|---|---|---|---|---|
| Age, Mean (SD) | Years | 64.6 (10.1) | 64.0 (8.75) | 0.15 |
|
| ||||
| Race | White | 331 (67) | 10978 (64) | 0.20 |
| Black | 164 (33) | 6157 (36) | ||
|
| ||||
| Sex | Female | 293 (59) | 9829 (57) | |
| Male | 202 (41) | 7306 (43) | 0.42 | |
|
| ||||
| Education | < High school | 40 (8) | 1529 (9) | 0.79 |
| High school | 125 (25) | 4319 (25) | ||
| Some college | 129 (26) | 4655 (27) | ||
| ≥College graduate | 201 (41) | 6632 (39) | ||
|
| ||||
| Income | < $20k | 122 (25) | 2406 (14) | |
| $20k–$34k | 99 (20) | 3949 (23) | ||
| $35k–$74k | 145 (29) | 5686 (33) | <0.001 | |
| $75k and above | 57 (12) | 3166 (18) | ||
| Refused | 72 (15) | 1928 (11) | ||
|
| ||||
| Region | Belt | 324 (66) | 9388 (21) | |
| Nonbelt | 171 (34) | 7747 (45) | <0.001 | |
|
| ||||
| Smoking Status | Current | 86 (17) | 2167 (13) | |
| Past | 184 (37) | 6790 (40) | ||
| Never | 224 (45) | 8104 (47) | 0.01 | |
| Missing | 1 (<1) | 74 (<1) | ||
|
| ||||
| Alcohol Use | Heavy | 12 (2) | 713 (4) | |
| Moderate | 137 (28) | 6060 (35) | ||
| None | 335 (68) | 10062 (59) | <0.001 | |
| Missing | 11 (2) | 300 (2) | ||
|
| ||||
| Exercise | Any weekly | 305 (61) | 11552 (67) | |
| None | 184 (37) | 5405 (32) | 0.01 | |
| Missing | 6 (1) | 209 (1) | ||
|
| ||||
| Diabetes | No | 336 (68) | 13548 (79) | |
| Yes | 140 (28) | 3009 (18) | <0.001 | |
| Missing | 19 (4) | 578 (3) | ||
|
| ||||
| Systolic blood pressure, mean (SD) | mmHg | 128 (18) | 127 (16) | 0.02 |
|
| ||||
| Hypertension | No | 192 (39) | 7559 (44) | |
| Yes | 303 (61) | 9540 (56) | 0.02 | |
| Missing | 0 (0) | 36 (<1) | ||
|
| ||||
| Dyslipidemia | No | 188 (38) | 6982 (41) | |
| Yes | 289 (58) | 9570 (56) | 0.23 | |
| Missing | 18 (4) | 583 (3) | ||
|
| ||||
| History of Transient Ischemic Attack | No | 460 (93) | 16459 (96) | |
| Yes | 31 (6) | 587 (3) | 0.001 | |
| Missing | 4 (1) | 89 (1) | ||
|
| ||||
| Atrial Fibrillation | No | 432 (87) | 15545 (91) | |
| Yes | 47 (9) | 1268 (7) | 0.07 | |
| Missing | 16 (3) | 322 (2) | ||
|
| ||||
| Left Ventricular Hypertrophy | No | 430 (87) | 15455 (90) | |
| Yes | 56 (11) | 1453 (8) | 0.03 | |
| Missing | 9 (2) | 227 (1) | ||
|
| ||||
| History of Coronary Artery Disease | No | 381 (77) | 14395 (84) | |
| Yes | 99 (20) | 2482 (15) | <0.001 | |
| Missing | 15 (3) | 258 (2) | ||
|
| ||||
| Body Mass Index, mean (SD) | kg/m2 | 30.0 (6.1) | 29.3 (6.1) | 0.01 |
|
| ||||
| BMI Category | <18.5 underweight | 6 (1) | 140 (1) | |
| >18.5 <25 normal | 89 (18) | 4076 (24) | ||
| >=25<30 overweight | 169 (34) | 6390 (37) | ||
| >=30 obese | 230 (46) | 6425 (38) | <0.001 | |
| Missing | 1 (<1) | 104 (1) | ||
|
| ||||
| C-Reactive Protein | ≥90th percentile, mg/L | 65 (13) | 1601 (9) | 0.004 |
| Missing | 30 (6) | 994 (6) | ||
N (%) unless otherwise noted
p-values according to pooled t-test for continuous variables, and chi-square test for categorical variables.
Results from the univariable and multivariable modeling are shown in Table 4. In univariable analyses, low income, residence in the stroke belt, current smoking, alcohol abstinence, no weekly exercise, diabetes, hypertension, LVH, history of TIA, CAD, obesity, CRP ≥90th percentile and incident stroke were all associated with higher odds of ICI. In multivariable model 1, those in the lowest income group (<$20,000/year) had 84% higher odds of ICI compared to those in the highest group (>$75,000/year). Residence in the stroke belt region was associated with 45% higher odds of ICI. Diabetes was associated with 49% higher odds of ICI, and those with a self-reported history of TIA at baseline were almost twice as likely to have ICI as those who did not report TIA. CAD was associated with 33% higher odds of ICI. Obese participants had 39% higher odds of ICI than normal weight participants.
Table 4.
Odds ratios of incident cognitive impairment in univariable and multivariable models.
| Variable | Level | OR (CI) unadjusted | OR (CI) Model 1* | OR (CI) Model 2** |
|---|---|---|---|---|
| Age | Per decade | 1.08 (0.97, 1.19) | n/a | n/a |
|
| ||||
| Race | African-American (white is reference) | 0.88 (0.73, 1.07) | n/a | n/a |
|
| ||||
| Gender | Male (female is reference) | 0.93 (.077, 1.11) | n/a | n/a |
|
| ||||
| Education | ≥ College graduate | reference | n/a | n/a |
| Some college | 0.91 (0.73, 1.14) | |||
| High school graduate | 0.95 (0.76, 1.20) | |||
| < High school | 0.86 (0.61, 1.22) | |||
|
| ||||
| Income | $75k and above | reference | Reference | reference |
| $35k–$74k | 1.42 (1.04, 1.93) | 1.13 (0.81, 1.57) | 1.13 (0.81, 1.56) | |
| $20k–$34k | 1.39 (1.00, 1.94) | 1.07 (0.74, 1.51) | 1.06 (0.74, 1.50) | |
| < $20k | 2.82 (2.05, 3.87) | 1.84 (1.28, 2.62) | 1.83 (1.27, 2.62) | |
| Refused | 2.07 (1.45, 2.95) | 1.62 (1.11, 2.39) | 1.62 (1.10, 2.38) | |
|
| ||||
| Region | Stroke Belt (non-belt is reference) | 1.56 (1.30, 1.89) | 1.45 (1.18, 1.77) | 1.45 (1.18, 1.78) |
|
| ||||
| Smoking Status | Never | reference | Reference | Reference |
| Past | 0.98 (.80, 1.19) | 1.01 (0.81, 1.25) | 1.01 (0.81, 1.25) | |
| Current | 1.43 (1.11, 1.85) | 1.24 (0.92, 1.66) | 1.22 (0.91, 1.64) | |
|
| ||||
| Alcohol Use | None | reference | Reference | reference |
| Moderate | 0.68 (0.56, 0.83) | 0.84 (0.67, 1.06) | 0.84 (0.67, 1.06) | |
| Heavy | 0.51 (0.28, 0.90) | 0.54 (0.28, 1.07) | 0.55 (0.28, 1.08) | |
|
| ||||
| Exercise | None (any weekly is reference) | 1.29 (1.07, 1.55) | 1.11 (0.91, 1.37) | 1.12 (0.91,1.37) |
|
| ||||
| Diabetes | Yes | 2.42 (2.24, 2.62) | 1.49 (1.18, 1.89) | 1.48 (1.17, 1.87) |
|
| ||||
| Systolic Blood Pressure | Per 10 mmHg | 1.07 (1.01, 1.12) | n/a | n/a |
|
| ||||
| Hypertension | Yes | 1.25 (1.04, 1.50) | 0.97 (0.78, 1.20) | 0.96 (0.78, 1.19) |
|
| ||||
| Dyslipidemia | Yes | 1.12 (0.93, 1.35) | 0.98 (0.80, 1.20) | 0.98 (0.80, 1.20) |
|
| ||||
| History of Transient Ischemic Attack | Yes | 1.89 (1.30, 2.74) | 1.91 (1.29, 2.82) | 1.90 (1.29,2.81) |
|
| ||||
| Atrial Fibrillation | Yes | 1.33 (0.98, 1.80) | 1.07 (0.76, 1.52) | 1.08 (0.76, 1.52) |
|
| ||||
| Left Ventricular Hypertrophy | Yes | 1.39 (1.04, 1.84) | 1.16 (0.85, 1.60) | 1.15 (0.84, 1.58) |
|
| ||||
| History of Coronary Artery Disease | Yes | 1.51 (1.20, 1.89) | 1.33 (1.03, 1.71) | 1.32 (1.02, 1.70) |
|
| ||||
| Body Mass Index (BMI) | Per 1 kg/m2 | 1.02 (1.01, 1.03) | n/a | n/a |
|
| ||||
| BMI Category | <18.5 underweight | 1.96 (0.84, 4.56) | 1.66 (0.65, 4.20) | 1.70 (0.67, 4.30) |
| ≥18.5 <25 normal | reference | Reference | Reference | |
| ≥25 <30 overweight | 1.21 (0.93, 1.57) | 1.17 (0.88, 1.56) | 1.17 (0.88, 1.56) | |
| ≥30 obese | 1.64 (1.28, 2.10) | 1.39 (1.04, 1.85) | 1.40 (1.05, 1.86) | |
|
| ||||
| C-reactive Protein | ≥90th percentile (<90th percentile is reference) | 1.48 (1.13, 1.93) | 1.17 (0.88, 1.57) | 1.17 (0.87, 1.56) |
|
| ||||
| Incident Stroke | Yes | 2.58, (1.46, 4.57) | n/a | 2.73 (1.52, 4.90) |
Notes: Bold font denotes confidence interval does not include 1, p < 0.05. OR = odds ratio, CI= 95% confidence interval.
Model 1 is adjusted for income, region, smoking status, alcohol use, exercise, diabetes, hypertension, dyslipidemia, history of TIA, atrial fibrillation, left ventricular hypertrophy, history of coronary artery disease, BMI category, and CRP >90th percentile. Age, race, sex, and education were not included in the multivariable model as they were accounted for in the definition of ICI. Continuous BMI and SBP were not included in multivariable models as they were incorporated in our definition of hypertension and BMI category.
Model 2 is adjusted for all of the factors in Model 1 plus incident stroke occurring before the most recent cognitive assessment.
In the time between the first SIS and the most recent TTB assessment, 190 participants survived an incident stroke; 177 of these did not have ICI and 13 had ICI. Those surviving an incident stroke had a 2.6-fold increased risk of ICI (OR 2.58, CI 1.46, 4.57) in an unadjusted model and about 2.7-fold increased risk in a multivariable model (Table 4). The addition of incident stroke to the multivariable model did not attenuate the associations of income, region, CAD, diabetes, or TIA with ICI (Table 4). When we excluded participants with incident stroke, the direction and magnitude of observed associations did not change (data not shown).
To estimate confounding effects of age, race, gender, and education given their association with other covariates, we conducted sensitivity analyses in which these variables were added to model 2. Results were largely unchanged: income, region, diabetes, obesity, and history of TIA remained independent predictors of ICI, the association of CAD became borderline (OR 1.28, CI 0.99, 1.66), and alcohol abstinence emerged as an independent predictor of ICI (moderate alcohol consumption compared to abstainers OR 0.77, CI 0.61, 0.97).
Compared with those participants who were excluded due to missing data, participants in the analytic sample were more likely to be white, female, have higher income, have more education, be nonsmokers, and have lower BMI. They were also more likely to reside outside the stroke belt, report alcohol use and weekly exercise, and less likely to have diabetes, history of TIA, hypertension, dyslipidemia, atrial fibrillation, LVH, or CAD, and had lower CRP (Supplemental Table 1).
Discussion
In this study we adapted a previously validated absolute scores case identification methodology (Ivnik et al., 2000) to identify 495 individuals with clinically important ICI over 3.4 years follow up within the REGARDS national cohort study. We used a demographically-adjusted, regression-based norms modification of the absolute scores approach. Our study has important epidemiologic implications as it shows how a test-based method can be adapted to define cognitive impairment in the absence of a physician-lead diagnostic clinical assessment. The extension of this method to a brief telephone-based test allowed us to assess thousands of geographically diverse participants. Among participants with intact global cognitive status at enrollment, subsequently attaining absolute scores greater than 1.5 SD below one’s demographically matched peers on two or three out of three more detailed cognitive tests is clinically meaningful since this threshold is consistent with conventions used to define mild cognitive impairment (Petersen, 2004; Petersen et al., 2010; Petersen, Doody, et al., 2001; Petersen et al., 1995; Petersen et al., 1999; Winblad et al., 2004), and meets criteria for both severity (level) and pervasiveness (number of tests) of impairment. Taking the definition we developed using a more stringent cutpoint of scores >1.57 SD from predicted norms based on age, sex, race and education, we identified associations of previously identified risk factors for cognitive impairment to support of the validity of our method. While these findings do not indicate immediate use of this type of cognitive testing in practice, we have identified a clinically relevant level of ICI that was related to baseline risk factors, an important demonstration of the validity of the case definition. Our findings also add to the building evidence that vascular health plays a role in cognitive function and suggest that some risk factors for cognitive impairment may be modifiable and targeted with interventions. Patients with these risk factors warrant increased clinical suspicion, and risk of cognitive impairment may be another motivating factor for patients to improve their vascular health.
Univariable analyses showed an association of several risk factors with development of ICI: lower income, stroke belt residence, current smoking, alcohol abstinence, absent exercise, diabetes, high systolic blood pressure, and history of hypertension, TIA, LVH, and CAD, obesity, incident stroke, and high levels CRP. Multivariable analyses revealed independent relationships of lower income, stroke belt residence, obesity, incident stroke, and history of diabetes, TIA, and CAD with risk of ICI. These associations did not change when incident stroke was excluded as a covariate, suggesting that clinically recognized stroke is not the sole mechanism underlying the association of these risk factors with ICI and that cases of ICI are not limited to those with post-stroke cognitive dysfunction.
This work builds on previous research on cognitive impairment in REGARDS. When ICI was defined using SIS scores, age, race, gender, education, and stroke belt residence were all independent predictors of incident impairment (Wadley et al., 2011) as were elements of the Framingham Stroke Risk Score including systolic blood pressure, antihypertensive medication, diabetes, LVH, atrial fibrillation, heart disease, and smoking(Unverzagt et al., 2011). The consistency in pattern and magnitude of associations across these REGARDS studies is notable given the differences in how ICI was defined and use of demographic adjustment of cognitive scores in the present investigation.
Low income was strongly associated with ICI in our study. Socioeconomic status is generally understood as a surrogate for many unmeasured individual characteristics and has been associated with a wide variety of health outcomes (Pickett & Pearl, 2001). Others have shown that low income is associated with increased risk of Alzheimer disease (Evans et al., 1997). One prospective study of the relationship of socioeconomic factors to cognitive function found that higher income was associated with less cognitive decline over 4 years even after adjustment for vascular and health comorbidities suggesting that pathways outside general physical health contribute to cognitive outcomes (Koster et al., 2005; Lee, Buring, Cook, & Grodstein, 2006).
Our finding that diabetes is related to cognitive decline has been corroborated by many (Alonso et al., 2009; Carmelli et al., 1998; Comijs et al., 2009; Debette et al., 2011; Wessels et al., 2011; Yaffe et al., 2011), but not all (Cherbuin et al., 2009; Knopman, Mosley, Catellier, & Coker, 2009; Yaffe et al., 2009) studies. The underlying mechanism may be through buildup of glycation end products in the brain (Yaffe et al., 2011) leading to subcortical and cortical ischemia and brain dysfunction.
Our finding of a protective effect of moderate alcohol consumption has been reported in other studies of cognitive impairment and dementia (Anstey, Mack, & Cherbuin, 2009; Ganguli, Vander Bilt, Saxton, Shen, & Dodge, 2005; Mukamal et al., 2003; A. Ruitenberg et al., 2002; Stampfer, Kang, Chen, Cherry, & Grodstein, 2005) (Hendrie, Gao, Hall, Hui, & Unverzagt, 1996). Likewise TIA (Pendlebury, Wadling, Silver, Mehta, & Rothwell, 2011) and obesity (Debette et al., 2011; Kivipelto et al., 2005; Whitmer, Gunderson, Barrett-Connor, Quesenberry, & Yaffe, 2005; Xu et al., 2011) have been linked to cognitive decline and dementia.
We found that higher BMI was associated with increased risk of cognitive impairment. Other investigators noted an inverse relationship where higher BMI was protective against dementia (Luchsinger, Patel, Tang, Schupf, & Mayeux, 2007; Annemieke Ruitenberg et al., 2002) (Gao et al., 2011). This difference may be due to the fact that the dementia endpoint occurs in much older age compared to the ICI endpoint in our study. Factors like frailty, sarcopenia, and terminal drop are much more prevalent in the oldest-old and overlap with weight loss, dementia onset, and death.
Hypertension has been consistently associated with cognitive impairment and dementia (Alonso et al., 2009; Freitag et al., 2006; Kivipelto et al., 2005; Skoog et al., 1996; Tsivgoulis et al., 2009; Tzourio, Dufouil, Ducimetiere, & Alperovitch, 1999; Verdelho et al., 2007; Whitmer, Sidney, Selby, Johnston, & Yaffe, 2005) (Liu et al., 2013), and the interaction between blood pressure and obesity as components of metabolic syndrome has been implicated in cognitive decline (Wolf et al., 2007; Yaffe, 2007a, 2007b). Hypertension was not an independent predictor of ICI in this analysis. One explanation for this may be that hypertension does not exert an effect in the presence of more powerful markers of advanced vascular disease, as has been shown for hypertension in relation to Left ventricular hypertrophy (Unverzagt et al., 2011).
Studies of the association of smoking with incident dementia and cognitive decline report inconsistent results. Some studies report no association of smoking with cognitive decline (Doll, Peto, Boreham, & Sutherland, 2000; Whittington & Huppert, 1997) while others reported smokers had higher odds of dementia (Juan et al., 2004; Sabia S & et al., 2012). Survivor bias, as smokers are more likely to die from other causes before suffering from dementia, may underestimate the risk(Hernán, Alonso, & Logroscino, 2008). A meta-analysis concluded that current smoking at baseline was a risk factor for incident AD and cognitive decline (Anstey, von Sanden, Salim, & O’Kearney, 2007).
CRP ≥90th percentile was associated with increased risk of ICI in unadjusted analyses but not in the fully adjusted model Although many longitudinal studies (Engelhart et al., 2004; Gimeno, Marmot, & Singh-Manoux, 2008; Hoth et al., 2008; Laurin, David Curb, Masaki, White, & Launer, 2009; Marioni et al., 2009; Yaffe et al., 2003) reported an association of CRP with cognitive decline, some reported no association (Alley, Crimmins, Karlamangla, Hu, & Seeman, 2008; Dik et al., 2005; Haan, Aiello, West, & Jagust, 2008). Our findings suggest that the relationship of CRP with ICI as defined here was confounded by other ICI risk factors. CRP is increased in other conditions also related to vascular risk factors such as coronary disease, peripheral artery disease, and stroke. Thus, although inflammation may have direct neurotoxic effects, its association with ICI may also be due to indirect effects through other vascular disorders.
Our study had strengths and weaknesses. As strengths, REGARDS is a large national sample that is over-represented with African Americans and has excellent characterization of measured vascular risk factors. In addition, we used age-, education-, gender-, and race-adjusted regression-based norms to define ICI thus providing a clearer focus on modifiable and biological risk factors. On the other hand, the large sample (over 30,000 participants) made it impossible to use a gold standard definition of clinical cognitive disorder (e.g., MCI, AD dementia) based on standard clinical diagnostic assessment (i.e., physician examination, laboratory studies, cognitive testing, imaging, and consensus diagnostic panel) due to prohibitively high costs. The large sample size also dictated our use of a telephone-based administration format and a brief assessment. While telephone-based cognitive assessment returns scores that are equivalent to those obtained during in-person assessments (Unverzagt et al., 2007), a more extensive battery of cognitive function would likely have improved sensitivity to ICI, and the current study may have misclassified participants as either impaired or non-impaired. This non-differential misclassification would be expected to underestimate associations of risk factors with ICI (Copeland, Checkoway, McMichael, & Holbrook, 1977).
A limitation of this study is selective attrition of those in poorer health and a non-random pattern of missing follow-up data. This type of selection bias is common in longitudinal studies of cognitive outcomes (Euser, Schram, Hofman, Westendorp, & Breteler, 2008). To the degree that the described biases are present, our odds ratios likely are underestimates of the actual relationships. To help reduce this bias, we used the “most recent” assessment with the TTB to identify ICI, allowing us to include participants with only 1 assessment as well as those with 2 or 3 assessments after baseline. Additionally, we determined impairment by comparing participants to their peers in age, race, gender, and education, which were factors associated with exclusion from the analysis (Supplemental Table 1). Despite these weaknesses, the large sample size (over 17,000 participants) and good representation of risk categories allowed us to observe significant relationships in predicted directions. The potential for practice effect was evaluated for those with 2 or 3 test visits, and the analyses did not find any significant systematic practice effects (data not shown).
In summary, we adapted a previously validated cognitive test-based approach to using regression-based norms to define ICI in a large national cohort. A number of demographic and vascular risk factors were associated with ICI, some of which are potentially modifiable and should be a focus of future prevention efforts.
Supplementary Material
Acknowledgments
The authors thank Aleena Mosher for assistance with data analysis. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org. This study was supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke (NINDS) and R01 HL080477 and T32 HL07594-24 from the National Heart, Lung and Blood Institute (NHLBI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS, the NHLBI or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data.
References
- Alley DE, Crimmins EM, Karlamangla A, Hu P, Seeman TE. Inflammation and rate of cognitive change in high-functioning older adults. J Gerontol A Biol Sci Med Sci. 2008;63(1):50–55. doi: 10.1093/gerona/63.1.50. 63/1/50 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, Mosley TH, Jr, Gottesman RF, Catellier D, Sharrett AR, Coresh J. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. Journal of Neurology, Neurosurgery & Psychiatry. 2009;80(11):1194–1201. doi: 10.1136/jnnp.2009.176818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey KJ, Mack HA, Cherbuin N. Alcohol Consumption as a Risk Factor for Dementia and Cognitive Decline: Meta-Analysis of Prospective Studies. American Journal of Geriatric Psych. 2009;17(7):542–555. doi: 10.1097/JGP.1090b1013e3181a1092fd1007. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, von Sanden C, Salim A, O’Kearney R. Smoking as a Risk Factor for Dementia and Cognitive Decline: A Meta-Analysis of Prospective Studies. American Journal of Epidemiology. 2007;166(4):367–378. doi: 10.1093/aje/kwm116. [DOI] [PubMed] [Google Scholar]
- Callahan CM, Unverzagt FW, Hui SL, Perkins A, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Medical Care. 2002;40(9):771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Reed T, Miller B, Wolf PA, Jarvik GP, Schellenberg GD. Midlife cardiovascular risk factors, ApoE, and cognitive decline in elderly male twins. Neurology. 1998;50(6):1580–1585. doi: 10.1212/wnl.50.6.1580. [DOI] [PubMed] [Google Scholar]
- Cherbuin N, Reglade-Meslin C, Kumar R, Jacomb P, Easteal S, Christensen H, Anstey KJ. Risk factors of transition from normal cognition to mild cognitive disorder: the PATH through Life Study. Dementia and Geriatric Cognitive Disorders. 2009;28(1):47–55. doi: 10.1159/000229025. [DOI] [PubMed] [Google Scholar]
- Comijs HC, Kriegsman DM, Dik MG, Deeg DJ, Jonker C, Stalman WA. Somatic chronic diseases and 6-year change in cognitive functioning among older persons. Archives of Gerontology and Geriatrics. 2009;48(2):191–196. doi: 10.1016/j.archger.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Copeland KT, Checkoway H, McMichael AJ, Holbrook RH. Bias due to misclassification in the estimation of relative risk. American Journal of Epidemiology. 1977;105(5):488–495. doi: 10.1093/oxfordjournals.aje.a112408. [DOI] [PubMed] [Google Scholar]
- Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64(8):1371–1377. doi: 10.1212/01.WNL.0000158281.08946.68. 64/8/1371 [pii] [DOI] [PubMed] [Google Scholar]
- Doll R, Peto R, Boreham J, Sutherland I. Smoking and dementia in male British doctors: prospective study. British Medical Journal. 2000;320(7242):1097–1102. doi: 10.1136/bmj.320.7242.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Breteler MM. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Archives of Neurology. 2004;61(5):668–672. doi: 10.1001/archneur.61.5.668. 61/5/668 [pii] [DOI] [PubMed] [Google Scholar]
- Evans DA, Hebert LE, Beckett LA, Scherr PA, Albert MS, Chown MJ, Taylor JO. Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Archives of Neurology. 1997;54(11):1399–1405. doi: 10.1001/archneur.1997.00550230066019. [DOI] [PubMed] [Google Scholar]
- Euser SM, Schram MT, Hofman A, Westendorp RG, Breteler MM. Measuring cognitive function with age: the influence of selection by health and survival. Epidemiology. 2008;19(3):440–447. doi: 10.1097/EDE.0b013e31816a1d31. [DOI] [PubMed] [Google Scholar]
- Freitag MH, Peila R, Masaki K, Petrovitch H, Ross GW, White LR, Launer LJ. Midlife pulse pressure and incidence of dementia: the Honolulu-Asia Aging Study. Stroke. 2006;37(1):33–37. doi: 10.1161/01.STR.0000196941.58869.2d. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Vander Bilt J, Saxton JA, Shen C, Dodge HH. Alcohol consumption and cognitive function in late life: a longitudinal community study. Neurology. 2005;65(8):1210–1217. doi: 10.1212/01.wnl.0000180520.35181.24. [DOI] [PubMed] [Google Scholar]
- Gao SJ, Nguyen JT, Hendrie HC, Unverzagt FW, Hake A, Smith-Gamble V, Hall K. Accelerated Weight Loss and Incident Dementia in an Elderly African-American Cohort. Journal of the American Geriatrics Society. 2011;59(1):18–25. doi: 10.1111/j.1532-5415.2010.03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillett SR, Boyle RH, Zakai NA, McClure LA, Jenny NS, Cushman M. Validating laboratory results in a national observational cohort study without field centers: The Reasons for Geographic and Racial Differences in Stroke cohort. Clinical Biochemistry. 2014 doi: 10.1016/j.clinbiochem.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno D, Marmot MG, Singh-Manoux A. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology. 2008;33(10):1322–1334. doi: 10.1016/j.psyneuen.2008.07.006. S0306-4530(08)00184-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan MN, Aiello AE, West NA, Jagust WJ. C-reactive protein and rate of dementia in carriers and non carriers of Apolipoprotein APOE4 genotype. Neurobiology of Aging. 2008;29(12):1774–1782. doi: 10.1016/j.neurobiolaging.2007.04.020. S0197-4580(07)00195-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrie HC, Gao SJ, Hall KS, Hui SL, Unverzagt FW. The relationship between alcohol consumption, cognitive performance, and daily functioning in an urban sample of older black Americans. Journal of the American Geriatrics Society. 1996;44(10):1158–1165. doi: 10.1111/j.1532-5415.1996.tb01364.x. [DOI] [PubMed] [Google Scholar]
- Hendrie HC, Ogunniyi A, Hall KS, Baiyewu O, Unverzagt FW, Gureje O, Hui SL. Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. Journal of the American Mecial Association. 2001;285(6):739–747. doi: 10.1001/jama.285.6.739. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Alonso A, Logroscino G. Cigarette smoking and dementia: potential selection bias in the elderly. Epidemiology. 2008;19(3):448. doi: 10.1097/EDE.0b013e31816bbe14. [DOI] [PubMed] [Google Scholar]
- Hoth KF, Haley AP, Gunstad J, Paul RH, Poppas A, Jefferson AL, Cohen RA. Elevated C-reactive protein is related to cognitive decline in older adults with cardiovascular disease. Journal of the American Geriatrics Society. 2008;56(10):1898–1903. doi: 10.1111/j.1532-5415.2008.01930.x. JGS1930 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Howard G. Disparities in stroke incidence contributing to disparities in stroke mortality. Annals of Neurology. 2011;69(4):619–627. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howieson DB, MDL . The Neuropsychological Evaluation. In: Yudofsky SC, HRE, editors. The American Psychiatric Publishing Textbook of Neuropsychiatry and Behavioral Neurosciences. 5. Washington, DC: American Psychiatric Press; 2007. pp. 215–243. [Google Scholar]
- IOM. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: The National Academies Press; 2009. [Google Scholar]
- Ivnik RJ, Smith GE, Petersen RC, Boeve BF, Kokmen E, Tangalos EG. Diagnostic accuracy of four approaches to interpreting neuropsychological test data. Neuropsychology. 2000;14(2):163–177. doi: 10.1037//0894-4105.14.2.163. [DOI] [PubMed] [Google Scholar]
- Jacova C, Kertesz A, Blair M, Fisk JD, Feldman HH. Neuropsychological testing and assessment for dementia. Alzheimer’s & Dementia. 2007;3(4):299–317. doi: 10.1016/j.jalz.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Juan D, Zhou DHD, Li J, Wang JYJ, Gao C, Chen M. A 2-year follow-up study of cigarette smoking and risk of dementia. European Journal of Neurology. 2004;11(4):277–282. doi: 10.1046/j.1468-1331.2003.00779.x. [DOI] [PubMed] [Google Scholar]
- Kim JW, Lee DY, Seo EH, Sohn BK, Choe YM, Kim SG, Woo JI. Improvement of Screening Accuracy of Mini-Mental State Examination for Mild Cognitive Impairment and Non-Alzheimer’s Disease Dementia by Supplementation of Verbal Fluency Performance. Psychiatry Investigations. 2014;11(1):44–51. doi: 10.4306/pi.2014.11.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Archives of Neurology. 2005;62(10):1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Mosley TH, Catellier DJ, Coker LH. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimer’s & Dementia. 2009;5(3):207–214. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- Koster A, Penninx BWJH, Bosma H, Kempen GIJM, Newman AB, Rubin SM, Rosano C. Socioeconomic differences in cognitive decline and the role of biomedical factors. Annals of Epidemiology. 2005;15(8):564–571. doi: 10.1016/j.annepidem.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Lanska DJ. Geographic distribution of stroke mortality in the United States: 1939–1941 to 1979–1981. Neurology. 1993;43(9):1839–1851. doi: 10.1212/wnl.43.9.1839. [DOI] [PubMed] [Google Scholar]
- Laurin D, David Curb J, Masaki KH, White LR, Launer LJ. Midlife C-reactive protein and risk of cognitive decline: a 31-year follow-up. Neurobiology of Aging. 2009;30(11):1724–1727. doi: 10.1016/j.neurobiolaging.2008.01.008. S0197-4580(08)00030-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Buring JE, Cook NR, Grodstein F. The relation of education and income to cognitive function among professional women. Neuroepidemiology. 2006;26(2):93–101. doi: 10.1159/000090254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Gao SJ, Hall KS, Unverzagt FW, Lane KA, Callahan CM, Hendrie HC. Optimal Blood Pressure for Cognitive Function: Findings from an Elderly African-American Cohort Study. Journal of the American Geriatrics Society. 2013;61(6):875–881. doi: 10.1111/jgs.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Measures of adiposity and dementia risk in elderly persons. Archives of Neurology. 2007;64(3):392–398. doi: 10.1001/archneur.64.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Annals of Neurology. 2008;63(4):494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni RE, Stewart MC, Murray GD, Deary IJ, Fowkes FG, Lowe GD, Price JF. Peripheral levels of fibrinogen, C-reactive protein, and plasma viscosity predict future cognitive decline in individuals without dementia. Psychosomatic Medicine. 2009;71(8):901–906. doi: 10.1097/PSY.0b013e3181b1e538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschia JF, Brott TG, Chukwudelunzu FE, Hardy J, Brown RD, Jr, Meissner I, O’Brien PC. Verifying the stroke-free phenotype by structured telephone interview. Stroke. 2000;31(5):1076–1080. doi: 10.1161/01.str.31.5.1076. [DOI] [PubMed] [Google Scholar]
- Mooijaart SP, Sattar N, Trompet S, Polisecki E, de Craen AJ, Schaefer EJ, Westendorp RG. C-Reactive Protein and Genetic Variants and Cognitive Decline in Old Age: The PROSPER Study. PLoS One. 2011;6(9):e23890. doi: 10.1371/journal.pone.0023890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT, Jr, Mittleman MA, Siscovick DS. Prospective study of alcohol consumption and risk of dementia in older adults. Journal of the American Medical Association. 2003;289(11):1405–1413. doi: 10.1001/jama.289.11.1405. [DOI] [PubMed] [Google Scholar]
- Nutter-Upham KE, Saykin AJ, Rabin LA, Roth RM, Wishart HA, Pare N, Flashman LA. Verbal fluency performance in amnestic MCI and older adults with cognitive complaints. Archives of Clinical Neuropsychology. 2008;23(3):229–241. doi: 10.1016/j.acn.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendlebury ST, Wadling S, Silver LE, Mehta Z, Rothwell PM. Transient cognitive impairment in TIA and minor stroke. Stroke. 2011;42(11):3116–3121. doi: 10.1161/strokeaha.111.621490. [DOI] [PubMed] [Google Scholar]
- Perry HM, Roccella EJ. Conference report on stroke mortality in the southeastern United States. Hypertension. 1998;31(6):1206–1215. doi: 10.1161/01.hyp.31.6.1206. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Weiner MW. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Winblad B. Current concepts in mild cognitive impairment. Archives of Neurology. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Ivnik RJ, Tangalos EG, Schaid DJ, Thibodeau SN, Kurland LT. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. Journal of the American Medcical Association. 1995;273(16):1274–1278. [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: Early detection of dementia: Mild cognitive impairment (an evidence-based review) - Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. Journal of Epidemiology and Community Health. 2001;55(2):111–122. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Wallace RB. Prevalence of cognitive impairment without dementia in the United States. Annals of Internal Medicine. 2008;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Chiappelli M, Montesi F, Tumini E, Patterson C. Blood inflammatory markers and risk of dementia: The Conselice Study of Brain Aging. Neurobiology of Aging. 2007;28(12):1810–1820. doi: 10.1016/j.neurobiolaging.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Revkin JH, Shear CL, Pouleur HG, Ryder SW, Orloff DG. Biomarkers in the prevention and treatment of atherosclerosis: need, validation, and future. Pharmacological Reviews. 2007;59(1):40–53. doi: 10.1124/pr.59.1.1. [DOI] [PubMed] [Google Scholar]
- Rosen WG. Verbal fluency in aging and dementia. Journal of Clinical Neuropsychology. 1980;2(2):135–146. [Google Scholar]
- Ruitenberg A, van Swieten JC, Witteman JC, Mehta KM, van Duijn CM, Hofman A, Breteler MM. Alcohol consumption and risk of dementia: the Rotterdam Study. The Lancet. 2002;359(9303):281–286. doi: 10.1016/s0140-6736(02)07493-7. [DOI] [PubMed] [Google Scholar]
- Ruitenberg A, van Swieten JC, Witteman JCM, Mehta KM, van Duijn CM, Hofman A, Breteler MMB. Alcohol consumption and risk of dementia: the Rotterdam Study. The Lancet. 2002;359(9303):281–286. doi: 10.1016/s0140-6736(02)07493-7. [DOI] [PubMed] [Google Scholar]
- Sabia SEADA, et al. Impact of smoking on cognitive decline in early old age: The whitehall ii cohort study. Archives of General Psychiatry. 2012;69(6):627–635. doi: 10.1001/archgenpsychiatry.2011.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Annals of Neurology. 2002;52(2):168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, Svanborg A. 15-year longitudinal study of blood pressure and dementia. The Lancet. 1996;347(9009):1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- Sloane PD, Zimmerman S, Suchindran C, Reed P, Wang L, Boustani M, Sudha S. The public health impact of Alzheimer’s disease 2000–2050: potential implication of treatment advances. Annual Review of Public Health. 2002;23:213–231. doi: 10.1146/annurev.publhealth.23.100901.140525. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Kang JH, Chen J, Cherry R, Grodstein F. Effects of Moderate Alcohol Consumption on Cognitive Function in Women. New England Journal of Medicine. 2005;352(3):245–253. doi: 10.1056/NEJMoa041152. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Snowden M, Fan MY, Hendrie H, Katon WJ, Unutzer J. Cognitive impairment and depression outcomes in the IMPACT study. American Journal of Geriatric Psychiatry. 2006;14(5):401–409. doi: 10.1097/01.JGP.0000194646.65031.3f. [DOI] [PubMed] [Google Scholar]
- Stroke. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. (1989) Stroke. 1989;20(10):1407–1431. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- Tilvis RS, Kahonen-Vare MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. Journal of Gerontology A Biological Science Medical Science. 2004;59(3):268–274. doi: 10.1093/gerona/59.3.m268. [DOI] [PubMed] [Google Scholar]
- Tsivgoulis G, Alexandrov AV, Wadley VG, Unverzagt FW, Go RC, Moy CS, Howard G. Association of higher diastolic blood pressure levels with cognitive impairment. Neurology. 2009;73(8):589–595. doi: 10.1212/WNL.0b013e3181b38969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio C, Dufouil C, Ducimetiere P, Alperovitch A. Cognitive decline in individuals with high blood pressure: a longitudinal study in the elderly. EVA Study Group. Epidemiology of Vascular Aging. Neurology. 1999;53(9):1948–1952. doi: 10.1212/wnl.53.9.1948. [DOI] [PubMed] [Google Scholar]
- Unverzagt FW, McClure LA, Wadley VG, Jenny NS, Go RC, Cushman M, Howard G. Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology. 2011;77:1729–1736. doi: 10.1212/WNL.0b013e318236ef23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unverzagt FW, Monahan PO, Moser LR, Zhao Q, Carpenter JS, Sledge GW, Champion VL. The Indiana university telephone-based assessment of neuropsychological status: a new method for large scale neuropsychological assessment. Journal of the International Neuropsychological Society. 2007;13(5):799–806. doi: 10.1017/S1355617707071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113(19):2335–2362. doi: 10.1161/circulationaha.104.482570. [DOI] [PubMed] [Google Scholar]
- Verdelho A, Madureira S, Ferro JM, Basile AM, Chabriat H, Erkinjuntti T, Inzitari D. Differential impact of cerebral white matter changes, diabetes, hypertension and stroke on cognitive performance among non-disabled elderly. The LADIS study. Journal of Neurology, Neurosurgery & Psychiatry. 2007;78(12):1325–1330. doi: 10.1136/jnnp.2006.110361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadley VG, Unverzagt FW, McGuire LC, Moy CS, Go R, Kissela B, Howard G. Incident cognitive impairment is elevated in the stroke belt: The REGARDS Study. Annals of Neurology. 2011;70(2):229–236. doi: 10.1002/ana.22432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels AM, Lane KA, Gao S, Hall KS, Unverzagt FW, Hendrie HC. Diabetes and cognitive decline in elderly African Americans: a 15-year follow-up study. Alzheimer’s & Dementia. 2011;7(4):418–424. doi: 10.1016/j.jalz.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. British Medical Journal. 2005;330(7504):1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–281. doi: 10.1212/01.wnl.0000149519.47454.f2. [DOI] [PubMed] [Google Scholar]
- Whittington JE, Huppert FA. Smoking and cognitive decline. Human Psychopharmacology: Clinical and Experimental. 1997;12(5):467–480. [Google Scholar]
- Wicklund AH, Johnson N, Rademaker A, Weitner BB, Weintraub S. Word list versus story memory in Alzheimer disease and frontotemporal dementia. Alzheimer Disease and Associated Disorders. 2006;20(2):86–92. doi: 10.1097/01.wad.0000213811.97305.49. [DOI] [PubMed] [Google Scholar]
- Wilber ST, Lofgren SD, Mager TG, Blanda M, Gerson LW. An evaluation of two screening tools for cognitive impairment in older emergency department patients. Academic Emergency Medicine. 2005;12(7):612–616. doi: 10.1197/j.aem.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Current Alzheimer Research. 2007;4(2):111–116. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. 2011;76(18):1568–1574. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K. Metabolic syndrome and cognitive decline. Current Alzheimer Research. 2007a;4(2):123–126. doi: 10.2174/156720507780362191. [DOI] [PubMed] [Google Scholar]
- Yaffe K. Metabolic syndrome and cognitive disorders: is the sum greater than its parts? Alzheimer Disesae and Associated Disorders. 2007b;21(2):167–171. doi: 10.1097/WAD.0b013e318065bfd6. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Fiocco AJ, Lindquist K, Vittinghoff E, Simonsick EM, Newman AB, Harris TB. Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology. 2009;72(23):2029–2035. doi: 10.1212/WNL.0b013e3181a92c36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Harris T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61(1):76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Schwartz AV, Vitartas C, Vittinghoff E, Satterfield S, Harris T. Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology. 2011;77(14):1351–1356. doi: 10.1212/WNL.0b013e3182315a56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


