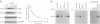Abstract
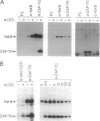
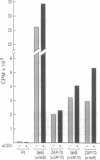
One of the earliest responses of T and B lymphocytes to stimulation through their antigen receptors is the activation of protein tyrosine kinases and the tyrosine phosphorylation of multiple cellular substrates. Here we describe a tyrosine kinase substrate, fakB, a putative homologue of the focal adhesion kinase pp125FAK. Tyrosine phosphorylation of fakB was rapidly augmented in human T and B cells following antigen receptor cross-linking with antibody, while pp125FAK was nonresponsive. Costimulation of the T-cell antigen receptor (TCR/CD3) with either the CD2 or CD4 costimulatory receptors induced synergistic fakB tyrosine phosphorylation in normal human T cells. Engagement of TCR/CD3 induced the stable association of fakB with ZAP-70, the TCR/CD3 sigma-chain-associated tyrosine kinase involved in antigen receptor-induced T-cell activation. In addition, preformed complexes of fakB and ZAP-70 were observed in T-cell leukemia lines. Phosphorylation of fakB on serine, threonine, and tyrosine residues was observed both in vivo and in vitro, where a functional increase of in vitro kinase activity was observed following TCR/CD3 stimulation. fakB is thus a focal adhesion kinase-related tyrosine kinase substrate that is differentially regulated from that of pp125FAK and likely plays a role in antigen-induced lymphocyte signaling.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André E., Becker-André M. Expression of an N-terminally truncated form of human focal adhesion kinase in brain. Biochem Biophys Res Commun. 1993 Jan 15;190(1):140–147. doi: 10.1006/bbrc.1993.1022. [DOI] [PubMed] [Google Scholar]
- Burridge K., Turner C. E., Romer L. H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992 Nov;119(4):893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. C., Iwashima M., Turck C. W., Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992 Nov 13;71(4):649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992 Aug 20;358(6388):690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Trevithick J. E., Hynes R. O. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991 Nov;2(11):951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkind J. S., Robbins K. C. Activation of transforming G protein-coupled receptors induces rapid tyrosine phosphorylation of cellular proteins, including p125FAK and the p130 v-src substrate. Biochem Biophys Res Commun. 1992 Oct 15;188(1):155–161. doi: 10.1016/0006-291x(92)92363-3. [DOI] [PubMed] [Google Scholar]
- Hamawy M. M., Mergenhagen S. E., Siraganian R. P. Tyrosine phosphorylation of pp125FAK by the aggregation of high affinity immunoglobulin E receptors requires cell adherence. J Biol Chem. 1993 Apr 5;268(10):6851–6854. [PubMed] [Google Scholar]
- Hanks S. K., Calalb M. B., Harper M. C., Patel S. K. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L., Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993 Feb;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner S. B., Damle N. K., Blake J., Aruffo A., Ledbetter J. A. CD2/LFA-3 ligation induces phospholipase-C gamma 1 tyrosine phosphorylation and regulates CD3 signaling. J Immunol. 1992 Apr 1;148(7):2023–2029. [PubMed] [Google Scholar]
- Kanner S. B., Reynolds A. B., Parsons J. T. Immunoaffinity purification of tyrosine-phosphorylated cellular proteins. J Immunol Methods. 1989 Jun 2;120(1):115–124. doi: 10.1016/0022-1759(89)90296-2. [DOI] [PubMed] [Google Scholar]
- Kanner S. B., Reynolds A. B., Vines R. R., Parsons J. T. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci U S A. 1990 May;87(9):3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Samelson L. E. T cell antigen receptor activation pathways: the tyrosine kinase connection. Cell. 1991 Mar 8;64(5):875–878. doi: 10.1016/0092-8674(91)90310-u. [DOI] [PubMed] [Google Scholar]
- Kornberg L. J., Earp H. S., Turner C. E., Prockop C., Juliano R. L. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg L., Earp H. S., Parsons J. T., Schaller M., Juliano R. L. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992 Nov 25;267(33):23439–23442. [PubMed] [Google Scholar]
- Kupfer A., Singer S. J. The specific interaction of helper T cells and antigen-presenting B cells. IV. Membrane and cytoskeletal reorganizations in the bound T cell as a function of antigen dose. J Exp Med. 1989 Nov 1;170(5):1697–1713. doi: 10.1084/jem.170.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., June C. H., Grosmaire L. S., Rabinovitch P. S. Crosslinking of surface antigens causes mobilization of intracellular ionized calcium in T lymphocytes. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1384–1388. doi: 10.1073/pnas.84.5.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipfert L., Haimovich B., Schaller M. D., Cobb B. S., Parsons J. T., Brugge J. S. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol. 1992 Nov;119(4):905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D. B., Weiss A. The CD3 chains of the T cell antigen receptor associate with the ZAP-70 tyrosine kinase and are tyrosine phosphorylated after receptor stimulation. J Exp Med. 1993 Nov 1;178(5):1523–1530. doi: 10.1084/jem.178.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wange R. L., Malek S. N., Desiderio S., Samelson L. E. Tandem SH2 domains of ZAP-70 bind to T cell antigen receptor zeta and CD3 epsilon from activated Jurkat T cells. J Biol Chem. 1993 Sep 15;268(26):19797–19801. [PubMed] [Google Scholar]
- Weiss A. T cell antigen receptor signal transduction: a tale of tails and cytoplasmic protein-tyrosine kinases. Cell. 1993 Apr 23;73(2):209–212. doi: 10.1016/0092-8674(93)90221-b. [DOI] [PubMed] [Google Scholar]
- Whitney G. S., Chan P. Y., Blake J., Cosand W. L., Neubauer M. G., Aruffo A., Kanner S. B. Human T and B lymphocytes express a structurally conserved focal adhesion kinase, pp125FAK. DNA Cell Biol. 1993 Nov;12(9):823–830. doi: 10.1089/dna.1993.12.823. [DOI] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. Focal adhesion kinase (p125FAK): a point of convergence in the action of neuropeptides, integrins, and oncogenes. Cell. 1992 Dec 11;71(6):891–894. doi: 10.1016/0092-8674(92)90385-p. [DOI] [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J., Rozengurt E. Bombesin, vasopressin, and endothelin stimulation of tyrosine phosphorylation in Swiss 3T3 cells. Identification of a novel tyrosine kinase as a major substrate. J Biol Chem. 1992 Sep 25;267(27):19031–19034. [PubMed] [Google Scholar]