Abstract
Daily intermittent access to sugar solutions results in intense bouts of sugar intake (i.e. bingeing) in rats. Bingeing on sucrose, a disaccharide of glucose and fructose, has been associated with a “primed” mesolimbic dopamine (DA) pathway. Recent studies suggest glucose and fructose engage brain reward and energy sensing mechanisms in opposing ways and may drive sucrose intake through unique neuronal circuits. Here, we examined in male Sprague-Dawley rats whether or not (1) intermittent access to isocaloric solutions of sucrose, glucose or fructose results in distinctive sugar bingeing profiles and (2) previous sugar bingeing alters cocaine locomotor activation and/or reward, as determined by conditioned place preference (CPP). To encourage bingeing, rats were given 24-h access to water and 12 h-intermittent access to chow plus an intermittent bottle that contained water (control) or 8% solutions of sucrose, glucose or fructose for 9 days, followed by ad libitum chow diet and a 10 day cocaine (15 mg/kg; i.p.) CPP paradigm. By day 4 of the sugar bingeing diet, sugar bingeing in the fructose group surpassed the glucose group, with the sucrose group being intermediate. All three sugar groups had similar chow and water intake throughout the diet. In contrast, controls exhibited chow bingeing by day 5 without altering water intake. Similar magnitudes of cocaine CPP were observed in rats with a history of sucrose, fructose or chow (control) bingeing. Notably, the glucosebingeing rats did not demonstrate a significant cocaine CPP despite showing similar cocaine-induced locomotor activity as the other diet groups. Overall, these results show that fructose and glucose, the monosaccharide components of sucrose, produce divergent degrees of bingeing and cocaine reward.
Keywords: Sugar bingeing, cocaine conditioned place preference, sucrose, glucose, fructose
1. Introduction
In the United States sugar consumption exceeds the dietary guidelines more than any other macronutrient, with added sugar intake comprising over 15% of daily calories (USDA, 2011). Added sugar calories are commonly derived from sucrose, a glucose-fructose disaccharide, and high fructose corn syrup, a mixture of free sugars, most often containing 55% fructose, 42% glucose and 3% polycose, a glucose polymer. Although glucose and fructose are commonly consumed together, it is now appreciated that glucose and fructose utilize different mechanisms for absorption, cellular transport and metabolism and that they stimulate opposing endocrine and hypothalamic responses (Teff et al., 2004; Cha et al., 2008; Stanhope et al., 2008; Tappy and Le, 2010; Page et al., 2013). A human imaging study found ingestion of glucose, but not fructose, increases the functional connectivity between the hypothalamus and the striatum, areas critical for energy-sensing and reward processing, respectively (Page et al., 2013). In a follow up study, drinking a fructose-sweetened drink, as compared to a glucose-sweetened drink, was linked with greater hunger ratings and willingness to give up monetary reward in exchange for palatable food (Luo et al., 2015). In combination, these studies suggest that glucose and fructose may contribute to sucrose intake and reward through unique mechanisms and these diverging processes ultimately affect feeding behavior. Understanding the rewarding properties produced by these monosaccharides, as well as the individual mechanisms underlying these properties, may help to identify therapies to curb excessive consumption of complex sugars (i.e. sucrose and high fructose corn syrup).
Previous work has shown that rats given repeated intermittent access to highly palatable food (foods high in sugar, fat or both) develop bingeing behavior and behavioral and neurochemical signs of dysfunction in their stress and reward circuitry (Bello et al., 2002; Avena and Hoebel, 2003; Bello et al., 2003; Gosnell, 2005; Rada et al., 2005; Avena et al., 2006b; Wojnicki et al., 2007; Cottone et al., 2008; Wojnicki et al., 2008; Corwin and Wojnicki, 2009; Hoebel et al., 2009; Johnson and Kenny, 2010; Lê et al., 2011; Cifani et al., 2012; Iemolo et al., 2012; Micioni Di Bonaventura et al., 2014). The various bingeing models differ in the macronutrient composition, whether chow is offered concurrently and in onset and duration of palatable food access; but all models robustly increase palatable food intake at the onset of food access, which is termed a “binge”. Here we used a sugar bingeing model developed by Drs. Hoebel, Avena and colleagues that fosters bingeing behavior by cycling rats between 12 h of food deprivation and 12 h of sugar and chow access, coupled with delaying food access until 4 h into the dark cycle (Reviewed here Hoebel et al., 2009). Within several days of this diet, rats shift to consuming a large sugar meal within the 1st h of food presentation, i.e. a sugar binge, while water and chow intake remains unchanged (Rada et al., 2005; Avena et al., 2006b; Rorabaugh et al., 2014). The majority of sugar bingeing papers has used a 10% sucrose solution, although 25% glucose and 8–12% fructose solutions also produce bingeing behavior (Colantuoni et al., 2001; Avena and Hoebel, 2003; Gosnell, 2005; Rada et al., 2005; Avena et al., 2006a; Wojnicki et al., 2007; Rorabaugh et al., 2014). However, the wide range of sugar concentrations with varying caloric densities used in the different bingeing studies confound any direct comparisons between these three sugars.
Previous studies have found sucrose bingeing enhances the locomotor responses to cocaine and amphetamine (Avena and Hoebel, 2003; Gosnell, 2005). This cross-sensitization is thought to reflect hypersensitivity in dopamine (DA) systems, also known as “priming” (Reviewed here Robinson and Berridge, 2008). Likewise, sucrose-bingeing rats show some of the neurochemical signs of DA hypersensitivity including elevated extracellular DA levels in response to sucrose intake, decreased DA D2 receptor (D2R) levels and increased DA transporter levels within the nucleus accumbens (NAc) (Bello et al., 2002; 2003; Rada et al., 2005; Avena et al., 2006b). Glucose-bingeing, but not fructose-bingeing, rats also display reduced D2R levels within the NAc (Colantuoni et al., 2001; Rorabaugh et al., 2014). A history of sucrose bingeing enhances locomotor responses to cocaine; however, whether bingeing on sucrose, or its components glucose and fructose, similarly alters the rewarding properties of cocaine has not been investigated. Here, we used the sugar bingeing model to assess whether isocaloric 8% sucrose, glucose and fructose solutions result in similar or distinct bingeing profiles and whether previous sugar bingeing alters cocaine-induced locomotion and reward, as determined by the development of cocaine conditioned place preference (CPP).
2. Experimental procedures
2.1 Sugar bingeing model
The University of Colorado Denver IACUC approved all animal procedures. This research program operates in accordance with the National Institutes of Health’s and National Research Council’s guidelines (Guide for Care and Use of Animals, 8th Edition, 2011). A total of 40, outbred male Sprague-Dawley rats (Charles Rivers Laboratories, Wilmington, MA), weighing 200 – 220 g on arrival, were used. A 12-h light-dark cycle was used throughout testing (lights on 0300–1500). Rats were singly housed with food (Teklad 2020X chow: 3.1 kcal/g, 24% protein, 16% fat, 60% carbohydrate; Harlan Laboratories, Denver, CO) and water available ad libitum for 5 days prior to commencing experiments. Rats were subsequently tested using the sugar bingeing model, as previously described (Avena et al., 2006a; Rorabaugh et al., 2014). At the onset of the experiment, rats continued to have 24-h access to an ad libitum water bottle but were cycled between 12 h of food deprivation and 12 h of access to chow and a second intermittent bottle that contained water (control; n=10), 8% sucrose, 8% glucose, or 8% fructose solution (0.29 kcal/mL; n=10/group). Food access was shifted 4 h into the dark cycle (1900–0700). Sugar, chow and water intake was recorded daily following 1 and 12 h of food access for each rat. Rats were also weighed daily. The sugar bingeing diet was maintained for 9 days; this diet length corresponds to the period during which we observed maximal 8% fructose bingeing in previous cohorts (3 published, 1 unpublished) (Rorabaugh et al., 2014). An 8% sugar concentration was chosen because (1) it is in the range of sugar concentrations that produce fructose (8%–12%) and sucrose (10%) bingeing, (2) it is the most preferred sucrose concentration in a 2-bottle choice test and (3) it is a similar concentration as in most sodas and fruit juices (Smith and Sclafani, 2002; Rada et al., 2005; Avena et al., 2006a; Rorabaugh et al., 2014). All sugars were purchased from Fisher Scientific (Waltham, MA). Consistent with the model, all results are expressed in raw intake values (mL or kcal) (Colantuoni et al., 2001; Rada et al., 2005; Avena et al., 2006a; Rorabaugh et al., 2014).
2.2 Cocaine conditioned place preference paradigm
After 9 days of the intermittent sugar diet, rats were switched to an ad libitum chow diet without any sugar for the remainder of the study. Rats were given a day to adjust to ad libitum feeding prior to CPP conditioning/testing, which occurred during the animals’ light cycle between 0700 and 1300. The CPP boxes (Med Associates Inc., St. Albans, VT) were housed in sound attenuating cabinets and had three distinct chambers equipped with photobeams: two larger conditioning chambers (10.5″ X 8″ X 8″) connected by a smaller neutral chamber (4.5″ X 8″ X 8″). The chambers were separated by doors and had distinct visual, tactile and bedding odor cues. On day 1 of the CPP procedure, rats were placed in the neutral chamber and allowed free access to all three chambers for 15 min to measure any preconditioning chamber preferences. Over the next 8 days, animals underwent a single, daily 30-min conditioning session in which rats were injected on alternate days with either saline (1 mL/kg; i.p.) or cocaine (15 mg/kg; i.p.) and then confined to the respective saline- or cocaine-paired chamber. If baseline chamber preferences were observed, cocaine was paired to the less preferred chamber; otherwise, the cocaine injection was randomly assigned to a chamber. The control diet rats (n=10) were split into saline- and cocaine-conditioned groups (n=5/group). The sucrose, glucose and fructose groups (n=10/group) were all cocaine-conditioned. After 8 days of conditioning, a second 15-min preference test, identical to the preconditioning test, was performed; and the time spent in the cocaine-paired and saline-paired chambers was recorded.
2.3 Data analysis and statistics
Group data are expressed as mean values ± standard error of the mean (SEM). Statistical significance was set at p < 0.05. All statistical tests were performed using either Prism (GraphPad Software, La Jolla, CA) or SPSS (IBM, Armonk, NY) software. Bingeing behavior was defined as a statistically significant increase in sugar, water or chow intake within the 1st h of food presentation by comparing day 1 and subsequent diet days using a 1-way repeated measures analysis of variance (RMANOVA) followed by Dunnett’s post hoc analysis. CPP chamber preference scores were calculated as the ratio of time spent in the cocaine-paired over saline-paired chambers or, in the case of the saline conditioned controls, the initially preferred over less preferred chamber. Cocaine CPP was defined as an increase in the ratio of time spent in the cocaine-paired versus saline-paired chambers after cocaine conditioning compared to before cocaine conditioning, as determined by 2-way RMANOVA. Comparisons among feeding groups during the sugar bingeing model and cocaine CPP procedures were made using a 2-way RMANOVA. Unless otherwise stated, all significant ANOVAs were followed by Tukey’s post hoc analysis.
3. Results
3.1 Intermittent access to fructose produced larger binges than glucose
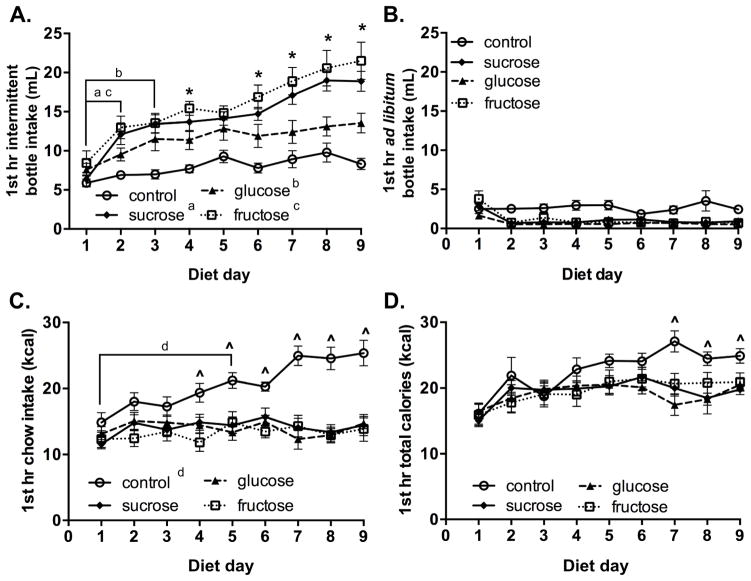
In order to determine if sucrose, glucose and fructose produce distinct bingeing profiles, rats were given daily intermittent 12-h access to chow and an intermittent bottle that contained water (controls), or an 8% solution of the aforementioned sugars (n=10/group). To ensure sugar intake was not driven by thirst, all rats also had 24-h ad libitum access to water. Bingeing was defined as a significant increase in sugar, water or chow intake during the 1st h of food access on subsequent diet days, as compared to day 1. Starting on day 1, all sugar groups drank similar amounts of sugar solution within the 1st h of food access (Fig. 1A). By day 2 of the diet, the sucrose [F(8,72)= 22.36, p < 0.0001] and fructose [F(8,72)= 14.44, p < 0.0001] groups exhibited robust sugar bingeing, as determined by a within group 1-way RMANOVA followed by Dunnett’s post hoc analysis (Fig. 1A). Likewise, the glucose group showed sugar bingeing on day 3 [F(8,72)= 8.59, p < 0.0001], whereas the controls [F(8,72)=, p = 0.070] did not significantly alter water intake from the intermittent or ad libitum water bottles (Figs. 1A and 1B). Overall, a 2-way RMANOVA of 1st h sugar intake (or water intake from the combined intermittent and ad libitum bottles in controls) showed a diet x day interaction [F(24,288)=3.21, p < 0.0001]. Tukey’s post hoc analysis revealed that after day 5, the fructose group had consistently larger binges than the glucose group, while sucrose bingeing was not statistically different from fructose or glucose intake (Fig. 1A). In addition, once bingeing was established, each of the three sugar groups drank more sugar solution within the 1st h of food access than the control group drank water (Figs. 1A and 1B). In contrast to the 1st h bingeing results, however, over the entire 12 h of sugar and chow access no differences in sugar intake were observed among the sucrose, glucose and fructose groups over the course of the 9-day diet (Table 1).
Figure 1. 8% fructose produced larger binges than 8% glucose.
Consumption during the 1st h of food presentation during the 9-day sugar bingeing diet. Intake was measured from the intermittent bottle containing a sugar solution (sucrose, glucose or fructose) or water (control) (A); from the ad libitum water bottle (B); of chow (C); and of calories (D). All sugar solutions were 8% (w/v) with a caloric density of 0.29 kcal/mL. Bingeing was defined as a significant increase in sugar, water or chow intake from day 1. Mean values ± SEM for n=10/group. p< 0.05, 1st day of bingeing in the sucrose (a), glucose (b), fructose (c) and control (d) groups; within group 1-way RMANOVA and Dunnett’s post hoc analysis. p < 0.05, between group comparisons across days: glucose vs. fructose (*), control vs. sucrose, glucose or fructose groups (^); 2-way RMANOVA and Tukey’s post hoc analysis.
Table 1.
Daily (12 h) food intake and rat weight throughout sugar bingeing diet
| Control | Sucrose | Glucose | Fructose | |
|---|---|---|---|---|
| Day 1 | ||||
| Sugar (mL) | 14.2 ± 3.2 | 42.3 ± 4.0 * | 47.7 ± 7.1 * | 49.4 ± 3.9 * |
| Water (mL) | 10.4 ± 2.4 | 4.8 ± 0.8 | 3.5 ± 1.2 | 7.1 ± 1.9 |
| Chow (kcal) | 68.5 ± 2.9 | 64.7 ± 2.3 | 64.8 ± 2.4 | 71.1 ± 3.0 |
| Total calories (kcal) | 68.5 ± 2.9 | 52.5 ± 3.1 | 50.8 ± 1.4 | 55.7 ±3.1 |
| Rat weight (kg) | 0.26 ± 0.01 | 0.28 ± 0.01 | 0.27 ± 0.01 | 0.26 ± 0.01 |
| Day 9 | ||||
| Sugar (mL) | 17.1 ± 1.8 | 98.7 ± 8.6 * | 78.1 ± 10.8 * | 98.7 ± 12.0 * |
| Water (mL) | 11.6 ± 2.4 | 2.2 ± 3.3 | 1.8 ± 0.4 | 1.7 ± 0.3 |
| Chow (kcal) | 79.0 ± 2.1 | 68.7 ± 2.6 | 68.4 ± 3.1 | 66.9 ± 2.0 |
| Total calories (kcal) | 79.0 ± 2.1 | 98.0 ± 3.1 * | 89.9 ± 4.5 | 96.7 ± 4.8 * |
| Rat weight (kg) | 0.34 ± 0.01 | 0.34 ± 0.01 | 0.34 ± 0.01 | 0.33 ± 0.01 |
Mean ± SEM,
p < 0.05 vs. control; 2-way RMANOVA
3.2 Intermittent sugar access did not increase overall consummatory behaviors
Similar to previous studies, the sugar bingeing model did not increase general consummatory behaviors (i.e. water and chow intake) throughout the 9-day diet. Once bingeing behavior was established, the sugar groups drank very little water (< 1 mL) from the ad libitum bottle in the 1st h of food presentation compared to the sugar solution (10–20 mL) from the intermittent bottle (Figs. 1A and 1B). Furthermore, the three sugar groups consumed similar amounts of chow during the 1st h and entire 12 h of food presentation over the 9-day diet (Fig. 1C and Table 1). Similar to our previous studies, the control group [F(8,72)= 3.56, p < 0.01] binged on chow by day 5 of the diet (Fig. 1C) (Rorabaugh et al., 2014). A 2-way RMANOVA revealed a diet x day interaction for chow intake in the 1st h of food access [F(24,288)=3.46, p < 0.001], and 1st h caloric intake showed a trend for an interaction [F(24,288)=1.5, p=0.065]. After day 6, the control group consumed more chow and calories during the 1st h of food presentation than the sugar groups (Figs. 1C and 1D). From the start of the diet, the control group consumed more daily (12 h) chow than the sugar groups (Table 1). A 2-way RMANOVA of daily chow intake revealed main effects of diet [F(3,36)=7.86, p < 0.0001] and day [F(8,288)= 30.75, p < 0.0001], without a diet x day interaction (Table 1). Similar to previous fructose bingeing studies, the 2-way RMANOVA of daily caloric intake showed a diet x day interaction [F(24,288)=29.0, p < 0.0001], and after day 3, the fructose and sucrose groups consumed more daily calories than controls (Table 1) (Rorabaugh et al., 2014). Nevertheless, the body weights of the rats did not differ among the groups over the course of the experiment [F(3,36)= 0.67, p= 0.58; Table 1]. As such, normalizing chow or sugar intake to body weight produces similar results as reported above (normalized data not shown).
3.3 Previous glucose bingeing blunted cocaine CPP
Next, we examined whether previous sugar bingeing altered the locomotor and/or rewarding properties (as determined by CPP) of cocaine (15 mg/kg; i.p.). In order to limit potential devaluation of cocaine by concurrent sugar access, all diet groups were placed on an ad libitum chow diet during conditioning and testing procedures. Throughout conditioning, all diet groups consumed similar amounts of chow and water (data not shown). The former control diet group (n=10) was subdivided into cocaine- and saline-conditioned groups (n=5/group). The three previous sugar groups were all cocaine-conditioned (n=10/group).
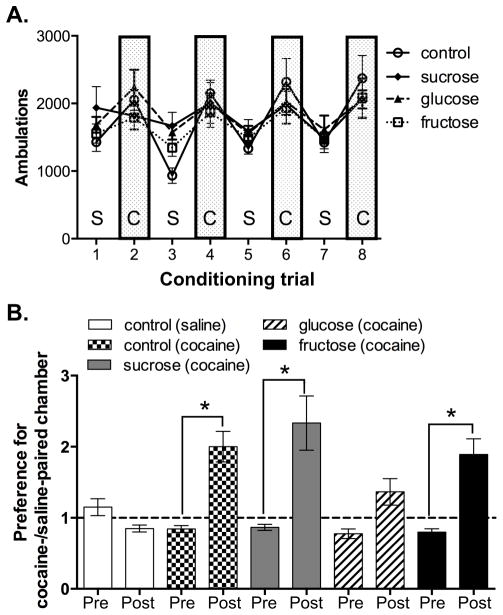
Across the 8 conditioning trials, all diet groups showed similar locomotor responses to cocaine and saline pretreatments within the CPP apparatus (Fig. 2A). A 2-way RMANOVA showed a significant effect of drug (cocaine vs. saline) [F(7,252)=13.68, p < 0.001] but not diet history. In contrast, during the post-conditioning preference test, the cocaine-conditioned control group (p < 0.01), sucrose group (p < 0.01) and fructose group (p < 0.01), but not glucose group (p > 0.05), developed a robust cocaine CPP, as determined by a significant two-fold increase in the ratio of time spent in the cocaine-paired over the saline-paired chambers compared to preconditioning (Fig. 2B). A 2-way RMANOVA of cocaine CPP scores revealed an interaction between CPP score x diet history [F(4,45)=8.61; p < 0.0001]. Furthermore, the CPP exhibited by the cocaine-conditioned control group (p < 0.01), sucrose group (p < 0.01) and fructose group (p < 0.01) was greater than the saline-conditioned control group, which spent similar amounts of time in both of the saline-paired chambers pre- and post-conditioning.
Figure 2. Previous glucose bingeing blunted cocaine conditioned place preference (CPP) but not locomotor activity.
Cocaine CPP was assessed in rats with a history of sugar or chow (controls) bingeing (see Figure 1). Locomotor activity was monitored during once daily saline (S) and cocaine (C; 15 mg/kg;i.p.) conditioning trials (A). Pre- and post-conditioning preferences (ratio of time spent in the cocaine-paired versus saline-paired chambers) were measured in rats that had previously binged on chow (controls), sucrose (gray columns), glucose (striped columns) or fructose (black columns; n=10/group) (B). The control group (n=10/group) was subdivided into saline-conditioned (white, n=5) and cocaine-conditioned (checkered, n=5) groups. Mean ± SEM. *p< 0.05, pre- vs. post-conditioning 2-way RMANOVA.
4. Discussion
4.1 Intermittent access to sucrose and its monosaccharide components produced different levels of sugar bingeing
Here we examined whether intermittent access to 8% sucrose, glucose or fructose stimulated distinctive bingeing responses and if previous sugar bingeing altered cocaine locomotor and/or reward activities. In all three sugar groups, bingeing occurred within 2–3 days exposure to the intermittent diet. Within 4–6 days, maximal fructose bingeing surpassed glucose bingeing. Sucrose bingeing was intermediate in comparison to its monosaccharide components. On day 1 of the diet, the three sugar groups showed similar 1st h sugar intake, indicating bingeing differences were not due to initial disparities in sugar sampling. Likewise, 2-bottle choice studies show no initial preference for 8% glucose or 8% fructose solutions (Ackroff and Sclafani, 1991; 1997). In addition, all three sugar groups consumed similar amounts of daily (12 h) sugar, suggesting bingeing differences in the 1st h of food access were not due to differences in long-term sugar-induced satiety. Similarly, intragastric infusions of isocaloric sucrose, glucose and fructose solutions equally reduce subsequent sucrose feeding both 10 min and 4 h after infusion (Warwick and Weingarten, 1994). With these facts in mind, one interpretation of our sugar bingeing results is that less 8% glucose may have been consumed because it has lower hedonic value than 8% fructose. This observation agrees with previous reports that studied bingeing behavior of only one sugar but used a higher concentration of glucose (25%) than of sucrose (10%) or fructose (8%) to stimulate bingeing behavior (Colantuoni et al., 2001; Rada et al., 2005; Rorabaugh et al., 2014).
An alternate interpretation of our results is that 8% glucose bingeing is more rewarding or produces less tolerance than 8% fructose bingeing. Thus, less glucose than fructose would be needed to produce reward, resulting in smaller binges. This idea may seem counterintuitive; however, less rewarding foods are often consumed in greater amounts over time than more rewarding foods (Stice et al., 2008a; 2008b). For example, lower levels of reward pathway activation, as determined by BOLD-fMRI, during palatable food consumption have been linked with overeating and weight gain in adolescent girls (Stice et al., 2008b). The existence of sugar tolerance has not been thoroughly examined, but behavior consistent with tolerance (i.e. a steady increase in binge size after the initial plateau) has been observed during longer 21- or 28-day sugar bingeing studies (Colantuoni et al., 2001; Rada et al., 2005; Rorabaugh et al., 2014). Our results are consistent with either of these potential interpretations, and future bingeing studies are needed to determine the relative potencies of glucose and fructose reward and the possibility of sugar tolerance.
While we found that fructose produces greater bingeing than glucose, previous 2-bottle choice studies show the following 8% sugar preference ranking: sucrose > glucose > fructose (Sclafani and Mann, 1987; Ackroff and Sclafani, 1991; 1997). It is important to note that we did not assess preference between sugars in individual animals; in our study, rats were given access to only one sugar and comparisons were made across groups. The differing results may also be due to variations in experimental procedures (i.e. length of sugar access, flavor pairing and concurrent chow availability) between these two behavioral paradigms. Sugar bingeing studies using concurrent access to fructose and glucose would be required to directly address this issue.
4.2 A history of sucrose and fructose bingeing did not alter cocaine CPP
To examine whether a history of sucrose, glucose or fructose bingeing could alter the rewarding effects of cocaine, rats underwent cocaine CPP training and testing following the 9 days of the intermittent sugar diet. The rats with a history of sucrose and fructose bingeing showed robust levels of cocaine CPP, similar to the chow bingeing controls. Surprisingly, the rats with a history of glucose bingeing failed to express significant cocaine CPP, despite showing similar cocaine-induced locomotor responses as the other diet groups during cocaine conditioning. Locomotor sensitization was not observed in any of the diet groups during the conditioning trials. This is not completely unexpected as locomotor sensitization in CPP apparatus decreases with the number of distinct cues within the respective chambers (Shimosato and Ohkuma, 2000). While we did not observe enhanced cocaine CPP in the sugar bingeing rats, this does not rule out a potential priming of psychostimulant reward. For example, sucrose bingeing rats may be more sensitive to cocaine CPP. In support of this idea, rats given ad libitum access to sucrose show a leftward shift in the dose-response curve for amphetamine CPP, as compared to chow-fed control rats; yet, both groups exhibit similar absolute magnitudes of CPP at high doses of amphetamine (Vitale et al., 2003). Likewise, a similar leftward shift in cocaine response in the glucose bingeing group may have increased the aversive aspects of cocaine and be responsible for the lack of cocaine CPP observed in these rats. Furthermore glucose bingeing, but not fructose bingeing, rats have increased D1Rs within the NAc, which are necessary for the formation and expression of cocaine CPP (Colantuoni et al., 2001; Nazarian et al., 2004; Rorabaugh et al., 2014). This may provide a molecular mechanism through which the glucose bingeing rats may be more sensitive to cocaine reward than sucrose and fructose bingeing rats. Furthermore, withdrawal from intermittent glucose access is associated with elevated anxiety-like responses, which may predispose these rats to experience the aversive properties of cocaine more than the other sugar groups (Colantuoni et al., 2002). Stress responses and corticotrophin-releasing factor signaling are critical in mediating sweet-fat bingeing behavior in rats (Cottone et al., 2009; Iemolo et al., 2013; Lê et al., 2011; Nair et al., 2010). Although there is evidence that different macronutrients (i.e. sugars versus fats) engage different neurocircuitry, it is likely that stress circuits play a role in the bingeing and cocaine responses observed here (Avena et al. 2009; Corwin et al, 2009; Parylak et al., 2012; Wong et al., 2009).
An alternative interpretation is that glucose bingeing produces a rightward shift in the cocaine dose response curve and these rats are less sensitive to the rewarding effects of cocaine. While elevated D1R levels are observed in glucose bingeing rats, it is not known whether they remain elevated after removal from the sugar bingeing diet. Chronic cocaine injections increase D1Rs, while withdrawal from cocaine causes a rapid, long lasting reduction in D1Rs (Macedo et al., 2004; Ben-Shahar et al., 2007). It is possible that glucose bingeing produces a similar increase in D1Rs during intermittent glucose access followed by reduced D1R levels upon termination of the glucose diet, thus attenuating cocaine CPP. Additional dose-response experiments are necessary to determine whether a history of sugar bingeing produces leftward or rightward shifts in cocaine CPP.
4.3 Distinct behavioral responses following intermittent access to glucose versus fructose
We found that repeated, intermittent glucose or fructose access produced different degrees of bingeing and cocaine CPP. While not directly tested here, these behavioral phenotypes might reflect differences in the neuronal circuits engaged by these monosaccharides. In humans, intake of glucose, but not fructose, reduces hunger ratings and desire for immediately available palatable food. Glucose also enhances striatal activation, as determined by BOLD-fMRI, indicating that glucose consumption may more effectively engage and alter DA circuitry than fructose (Page et al., 2013; Luo et al., 2015). Likewise, glucose, but not fructose, bingeing rats have lower D2R levels within the NAc and striatum, a neurochemical marker of chronic DA signaling (Colantuoni et al., 2001; Rorabaugh et al., 2014). Similarly, we previously reported evidence that fructose bingeing is partially mediated via a lateral hypothalamic orexin circuit, rather than a DA circuit (Rorabaugh et al., 2014). In addition, compared to glucose, fructose intake fails to effectively elevate the anorexic hormones insulin, glucagon-like peptide 1 and leptin, or to reduce the orexigenic hormone ghrelin (Teff et al., 2004; Page et al., 2013). These hormones also influence DA neuron firing, and as such may contribute to the differential behavioral responses to glucose and fructose observed here (Morton et al., 2009; Mebel et al., 2012; Mietlicki-Baase et al., 2013; Cone et al., 2014). Overall, our results support the idea that glucose and fructose intake are driven through separate neuronal circuits, resulting in differential behavioral responses to these sugars.
5. Conclusions
We found that at an 8% concentration, intermittent access to sucrose and its monosaccharide components, glucose and fructose, stimulated different magnitudes of sugar bingeing and differentially affected cocaine CPP in rats. Notably, glucose produced low levels of bingeing and abolished cocaine CPP, whereas fructose produced high bingeing behavior and preserved cocaine CPP. These results support the idea that individual components of mixed sugars (i.e. sucrose and high fructose corn syrup) may differentially engage and change reward circuitry within the brain. Further analysis of the neuronal circuits stimulated by each sugar should provide important insights into the brain regions contributing to sugar reward. Gaining a greater understanding of how the individual components influence sugar intake and reward may increase our understanding about ways to limit sugar, and potentially palatable food, overconsumption.
Highlights.
Intermittent access to an 8% sugar solution produces fructose bingeing ≈ sucrose bingeing > glucose bingeing
Overall daily (12 h) sugar and chow intake was comparable between sucrose, glucose and fructose groups
Previous glucose, but not sucrose or fructose, bingeing blocked cocaine CPP but not cocaine-induced locomotor activity
Acknowledgments
We thank Dr. Thomas E. Finger for his generous advice and comments, Mr. Dayton Goodell and Dr. Laura Saba for their valuable help with statistical analysis, and Dr. Bruce H. Mandt, Dr. David Weinshenker and Ms. Kelsey Barcomb for their critical reading of our manuscript. This research was supported by NIH grants R01 DA004216 (NRZ), T32 GM007635 (JMR), F32 DC012025 (JMS), and K05 DA015050 (NRZ), as well as by the Rocky Mountain Taste and Smell Center (P30 DC04657).
Abbreviations
- ANOVA
analysis of variance
- CPP
conditioned place preference
- DA
dopamine
- i.p
intraperitoneal
- NAc
nucleus accumbens
- R
receptor
- RMANOVA
repeated measures analysis of variance
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackroff K, Sclafani A. Flavor preferences conditioned by sugars: Rats learn to prefer glucose over fructose. Physiol Behav. 1991;50:815–824. doi: 10.1016/0031-9384(91)90023-h. [DOI] [PubMed] [Google Scholar]
- Ackroff K, Sclafani A. Diabetic rats prefer glucose-paired flavors over fructose-paired flavors. Appetite. 1997;28:73–83. doi: 10.1006/appe.1996.0058. [DOI] [PubMed] [Google Scholar]
- Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience. 2003;122:17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Sugar bingeing in rats. Curr Protoc Neurosci. 2006a;Chapter 9 doi: 10.1002/0471142301.ns0923cs36. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience. 2006b;139:813–820. doi: 10.1016/j.neuroscience.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009;139:623–628. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bello NT, Lucas LR, Hajnal A. Repeated sucrose access influences dopamine D2 receptor density in the striatum. Neuroreport. 2002;13:1575–1578. doi: 10.1097/00001756-200208270-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello NT, Sweigart KL, Lakoski JM, Norgren R, Hajnal A. Restricted feeding with scheduled sucrose access results in an upregulation of the rat dopamine transporter. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1260–R1268. doi: 10.1152/ajpregu.00716.2002. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Keeley P, Cook M, Brake W, Joyce M, Nyffeler M, Heston R, Ettenberg A. Changes in levels of D1, D2, or NMDA receptors during withdrawal from brief or extended daily access to IV cocaine. Brain Research. 2007;1131:220–228. doi: 10.1016/j.brainres.2006.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha SH, Wolfgang M, Tokutake Y, Chohnan S, Lane MD. Differential effects of central fructose and glucose on hypothalamic malonyl-CoA and food intake. Proc Natl Acad Sci USA. 2008;105:16871–16875. doi: 10.1073/pnas.0809255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifani C, Koya E, Navarre BM, Calu DJ, Baumann MH, Marchant NJ, Liu Q-R, Khuc T, Pickel J, Lupica CR, Shaham Y, Hope BT. Medial prefrontal cortex neuronal activation and synaptic alterations after stress-induced reinstatement of palatable food seeking: A study using c-fos-GFP transgenic female rats. J Neurosci. 2012;32:8480–8490. doi: 10.1523/JNEUROSCI.5895-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–3552. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–489. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Cone JJ, McCutcheon JE, Roitman MF. Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci. 2014;34:4905–4913. doi: 10.1523/JNEUROSCI.4404-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FH. Baclofen, raclopride, and naltrexone differentially affect intake of fat and sucrose under limited access conditions. Behav Pharmacol. 2009;20:537–548. doi: 10.1097/FBP.0b013e3283313168. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1066–R1076. doi: 10.1152/ajpregu.90309.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, Fekete EM, Steardo L, Rice KC, Grigoriadis DE, Conti B, Koob GF, Zorrilla EP. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci USA. 2009;106:20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosnell BA. Sucrose intake enhances behavioral sensitization produced by cocaine. Brain Research. 2005;1031:194–201. doi: 10.1016/j.brainres.2004.10.037. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Avena NM, Bocarsly ME, Rada P. Natural Addiction. J Addict Med. 2009;3:33–41. doi: 10.1097/ADM.0b013e31819aa621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemolo A, Valenza M, Tozier L, Knapp CM, Kornetsky C, Steardo L, Sabino V, Cottone P. Withdrawal from chronic, intermittent access to a highly palatable food induces depressive-like behavior in compulsive eating rats. Behav Pharmacol. 2012;23:593–602. doi: 10.1097/FBP.0b013e328357697f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemolo A, Blasio A, St Cyr SA, Jiang F, Rice KC, Sabino V, Cottone P. Receptor system in the central and basolateral nuclei of the amygdala differentially mediates excessive eating of palatable food. Neuropyschopharmacology. 2013;38:2456–2466. doi: 10.1038/npp.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology (Berl) 2011;218:89–99. doi: 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Monterosso JR, Sarpelleh K, Page KA. Differential effects of fructose versus glucose on brain and appetitive responses to food cues and decisions for food rewards. Proc Natl Acad Sci USA; 2015; 2015. p. 03358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo DS, Correia EE, Vasconcelos S, Aguiar L, Viana G, Sousa F. Cocaine treatment causes early and long-lasting changes in muscarinic and dopaminergic receptors. Cell Mole Neurobio. 2004;24:129–136. doi: 10.1023/B:CEMN.0000012718.08443.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebel DM, Wong JCY, Dong YJ, Borgland SL. Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake. Eur J Neurosci. 2012;36:2336–2346. doi: 10.1111/j.1460-9568.2012.08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micioni Di Bonaventura MV, Ciccocioppo R, Romano A, Bossert JM, Rice KC, Ubaldi M, St Laurent R, Gaetani S, Massi M, Shaham Y, Cifani C. Role of bed nucleus of the stria terminalis corticotrophin-releasing factor receptors in frustration stress-induced binge-like palatable food consumption in female rats with a history of food restriction. J Neurosci. 2014;34:11316–11324. doi: 10.1523/JNEUROSCI.1854-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, Hayes MR. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am J Physiol Endocrinol Metab. 2013;305:E1367–E1374. doi: 10.1152/ajpendo.00413.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Blevins JE, Kim F, Matsen M, Figlewicz DP. The action of leptin in the ventral tegmental area to decrease food intake is dependent on Jak-2 signaling. Am J Physiol Endocrinol Metab. 2009;297:E202–E210. doi: 10.1152/ajpendo.90865.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Navarre BM, Cifani C, Pickens CL, Bossert JM, Shaham Y. Role of dorsal medial prefrontal cortex dopamine D1-family receptors in relapse to high-fat food seeking induced by the anxiogenic drug yohimbine. Neuropyschopharmacology. 2010;36:497–510. doi: 10.1038/npp.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian A, Russo SJ, Festa ED, Kraish M, Quinones-Jenab V. The role of D1 and D2 receptors in the cocaine conditioned place preference of male and female rats. Brain Res Bull. 2004;63:295–299. doi: 10.1016/j.brainresbull.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Page KA, Chan O, Arora J, Roehmholdt B, Cline GW, Naik S, Sinha R, Constable RT, Sherwin RS. Effects of fructose vs glucose on regional cerebal blood flow in brain regions involved with appetite and reward pathways. JAMA. 2013;309:63–71. doi: 10.1001/jama.2012.116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parylak SL, Cottone P, Sabino V, Rice KC, Zorrilla EP. Effects of CB1 and CRF1 receptor antagonists on binge-like eating in rats with limited access to a sweet fat diet: Lack of withdrawal-like responses. Physiol Behav. 2012;107:231–242. doi: 10.1016/j.physbeh.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Avena N, Hoebel B. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B: Bio Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorabaugh JM, Stratford JM, Zahniser NR. A relationship between reduced nucleus accumbens shell and enhanced lateral hypothalamic orexin neuronal activation in long-term fructose bingeing behavior. PLoS ONE. 2014;9:e95019–e95030. doi: 10.1371/journal.pone.0095019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Mann S. Carbohydrate taste preferences in rats: Glucose, sucrose, maltose, fructose and polycose compared. Physiol Behav. 1987;40:563–568. doi: 10.1016/0031-9384(87)90097-7. [DOI] [PubMed] [Google Scholar]
- Shimosato K, Ohkuma S. Simultaneous monitoring of conditioned place preference and locomotor sensitization following repeated administration of cocaine and methamphetamine. Pharmacol Biochem Behav. 2000;66:285–292. doi: 10.1016/s0091-3057(00)00185-4. [DOI] [PubMed] [Google Scholar]
- Smith JC, Sclafani A. Saccharin as a sugar surrogate revisited. Appetite. 2002;38:155–160. doi: 10.1006/appe.2001.0467. [DOI] [PubMed] [Google Scholar]
- Stanhope KL, Griffen SC, Bair BR, MSM, Keim NL, Havel PJ. Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr. 2008;87:1194–1204. doi: 10.1093/ajcn/87.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008a;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: A functional magnetic resonance imaging study. J Abnom Psych. 2008b;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- Teff KL, Elliott SS, Tschöp M, Kieffer TJ, Rader D, Heiman M, Townsend RR, Keim NL, D’Alessio D, Havel PJ. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–2972. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- USDA. Dietary Guidelines for Americans. 2011:1–112. [Google Scholar]
- Vitale MA, Chen D, Kanarek RB. Chronic access to a sucrose solution enhances the development of conditioned place preferences for fentanyl and amphetamine in male Long–Evans rats. Pharmacol Biochem Behav. 2003;74:529–539. doi: 10.1016/s0091-3057(02)01034-1. [DOI] [PubMed] [Google Scholar]
- Warwick ZS, Weingarten HP. Dynamics of intake suppression after a preload: role of calories, volume, and macronutrients. Am J Physiol Regul Integr Comp Physiol. 1994;266:R1314–R1318. doi: 10.1152/ajpregu.1994.266.4.R1314. [DOI] [PubMed] [Google Scholar]
- Wojnicki FHE, Johnson DS, Corwin RLW. Access conditions affect binge-type shortening consumption in rats. Physiol Behav. 2008;95:1–9. doi: 10.1016/j.physbeh.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnicki FHE, Stine JG, Corwin RLW. Liquid sucrose bingeing in rats depends on the access schedule, concentration and delivery system. Physiol Behav. 2007;92:566–574. doi: 10.1016/j.physbeh.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Wong KJ, Wojnicki FHE, Corwin RLW. Baclofen, raclopride, and naltrexone differentially affect intake of fat/sucrose mixtures under limited access conditions. Pharm Biochem Behav. 2009;92:528–536. doi: 10.1016/j.pbb.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]