Abstract
Brain ischemia and reperfusion (I/R) injury occurs in various pathological conditions, but there is no effective treatment currently available in clinical practice. Methylene blue (MB) is a century old drug with a newly discovered protective function in the ischemic stroke model. In the current investigation we studied the MB-induced neuroprotective mechanism focusing on stabilization and activation of hypoxia inducible factor-1α (HIF-1α) in an in vitro oxygen and glucose deprivation (OGD)-reoxygenation model.
Methods
HT22 cells were exposed to OGD (0.1% O2, 6h) and reoxygenation (21% O2, 24h). Cell viability was determined with the calcein AM assay. The dynamic change of intracellular O2 concentration was monitored by fluorescence lifetime imaging microscopy (FLTIM). Glucose uptake was quantified using the 2-[N-(7-Nitrobenz-2-Oxa- 1,3-Diazol-4-yl)Amino]- 2-Deoxy-D-Glucose (2-NBDG) assay. ATP concentration and glycolytic enzyme activity were examined by spectrophotometry. Protein content changes were measured by immunoblot: HIF-1α, prolyl hydroxylase 2(PHD2), erythropoietin (EPO), Akt, mTOR, and PIP5K. The contribution of HIF-1α activation in the MB-induced neuroprotective mechanism was confirmed by blocking HIF-1α activation with 2-methoxyestradiol-2 (2-MeOE2) and by transiently transfecting constitutively active HIF-1α.
Results
MB increases cell viability by about 50% vs. OGD control. Compared to the corresponding control, MB increases intracellular O2 concentration and glucose uptake as well as the activities of hexokinase and G-6-PDH, and ATP concentration. MB activates the EPO signaling pathway with a corresponding increase in HIF-1α. Phosphorylation of Akt was significantly increased with MB treatment followed by activation of the mTOR pathway. Importantly, we observed, MB increased nuclear translocation of HIF-1α vs. control (about 3 folds), which was shown by a ratio of nuclear:cytoplasmic HIF-1α protein content.
Conclusion
We conclude that MB protects the hippocampus derived neuronal cells against OGD-reoxygenation injury by enhancing energy metabolism and increasing HIF-1α protein content accompanied by an activation of the EPO signaling pathway.
Introduction
Ischemic stroke is the leading cause of severe adult disability and the 4th leading cause of death in the U.S. While the incidence of stroke in the Medicare population 65 years of age has dropped about 40% in the last two decades, possibly due to the preventive interventions such as anti-hypertension and diet control1, there is no currently available treatment for ischemic stroke. Indeed, recombinant tissue plasminogen activator (rtPA) remains the only FDA approved treatment for ischemic stroke2. Unfortunately, the therapeutic window of rtPA is less than 4.5 hours, thus, less than 4 % of stroke patients have received rtPA thrombolytic therapy3,4. Development of an alternative or combined therapy for ischemic stroke is urgently needed.
Hypoxia inducible factors (HIF) are the most pertinent transcription factors in the maintenance of cellular homeostasis under ischemic/hypoxic conditions. Three isoforms of HIF have been identified: HIF-1, HIF-2, and HIF-35. Although physiological functions and regulatory mechanisms of HIF-1 and HIF-2 are relatively well documented, the role of HIF-3 is less known, except as a negative regulator of HIF-1 and HIF-26. HIF-1 plays critical roles as a transcriptional activator regulating various proteins in energy metabolism and in maintenance of cellular homeostasis in low O2 conditions5,7. The importance of HIF can be underscored with its primary functions in angiogenesis, hematopoiesis, energy metabolism, and anti-/pro-apoptosis5,8-10. All of the primary functions of HIF-1α directly or indirectly contribute to the increase the O2 content in ischemic organs. HIF has two subunits: α and β (also known as aryl hydrocarbon nuclear translocator (ARNT)). Those proteins belong to the basic helix-loop-helix-per-ARNT (bHLH-PAS) protein family11. Although both subunits are continuously synthetized, the O2-regulated HIF-α subunit is recognized as a critical regulatory subunit because of short half-life (< 3 minutes) under normoxia. In the presence of O2, iron, and ascorbate, the HIF-α subunit is rapidly degraded via proline hydroxylation in the O2-dependent degradation domain, ubiqutination by von Hippel-Lindau protein (VHL), and the proteasomal degradation pathway. Interaction between HIF-1α and PHD2, which is a key enzyme catalyzing proline hydroxylation, occurs in both nucleus and cytoplasm, but recent reports state that a significant portion of HIF-1α proline hydroxylation occurs in the nucleus12. Many studies focusing on pharmacological inhibition of PHD2 to study the role of HIF-1α have been conducted13,14,15.
A series of metabolic and pharmacological HIF-1α stabilizers under normoxia are proposed, such as pyruvate16,17, moderate level of reactive oxygen species (ROS)18,19, and PHD inhibitor15. Stabilization of HIF protects O2-sensitive organs, such as brain and heart, by mitigating inflammatory20 and apoptotic21 responses, but a HIF-1-induced neuronal protective mechanism is not clear. Empirically, outcomes of HIF-1α stabilization and HIF-1 activation seem varied depending on the way HIF-1α is stabilized or activated.
Methylene blue, the first synthetic drug, has been used in clinics for various diseases for more than a century 22,23,24. Recently MB has been proposed as a potential treatment for cancer 25, hepatopulmonary syndrome 26, and septic shock 27,28. Furthermore, the neuroprotective function of MB, which is able to traverse the BBB, has been reported 29. Our previous studies have demonstrated that MB increases ATP synthesis and minimizes ROS generation30,31. The MB-induced antioxidant function is unique compared to the traditional ROS scavengers. MB is not capable of detoxifying the glucose oxidase-generated H2O2 30, rather it reduces newly formed ROS by minimizing electron leakage from the mitochondrial electron transport chain (ETC) ,shuttling electrons from complex I to cytochrome c bypassing complex II and complex III, a primary source for superoxide generation30. Since MB increases byproducts of glucose metabolism and reduces ROS generation, it could be reasonably speculated that MB stabilizes HIF-1α under a normoxic environment.
Therefore, we hypothesized, based on previous reports including ours, that MB-induced neuroprotection is mediated by stabilizing HIF-1α and MB activates HIF-1 by increasing glucose metabolism along with activating the EPO-mTOR pathway and enhancing nuclear translocation.
Materials and Methods
OGD and reoxygenation stress model
Murine hippocampal cell line, HT22 (< 20 passages), was maintained in DMEM supplemented with 10% fetal bovine serum (FBS) and penicillin (10,000 units/ml)-streptomycin (10,000 μg/ml). At 18 h prior to OGD and reoxygenation, HT22 cells (5,000/well, 96-well plate) were seeded in high glucose DMEM supplemented with 10% FBS and 1% penicillin-streptomycin cocktail. OGD stress was introduced by replacing media with DMEM without FBS, glucose or pyruvate (Gibco, NY, USA) in which O2 concentration is maintained at 0.1% with auto-controlled N2 gas injection. After 6h OGD, reoxygenation was initiated by transferring the cells to normoxic 5% CO2 cell culture incubator. At the beginning of reoxygenation, Dextrose (11mM) and pyruvate (1mM) were restored to simulate in vivo reperfusion.
Primary Neuron preparation
Primary neurons were generated as described previously with modifications. Briefly, cortex tissue from postnatal day 0 C57BL/6 pups was dissociated by incubating in TrypLE Express (Invitrogen) at 37 °C for 20 minutes. Single cell suspension was made by passing tissue through fire-polished glass pipettes and a cell strainer. Cells were re-suspended in neuron culture medium (neurobasal medium with 2% B27 and 1% Glutamax) and seeded on poly-L-lysine coated coverslips. After incubation in cell culture incubator at 37 °C for 3–6 h, old medium was removed and fresh warm neuron culture medium was added. Two days after plating, cytosine arabinoside (araC; 1-b-D-arabinofuranosylcytosine) was added to the medium to a final concentration of 5 μM to inhibit the proliferation of glia cells. After 24 hours, old medium containing araC was replaced with fresh warm neuron culture medium. Cells were cultured for ~10 days before experiment.
Cell viability
After 24 h reoxygenation, cell viability was tested with the calcein AM assay. Cells were washed with PBS (pH 7.0) and incubated with calcein AM (1μM; Anaspec, Fremont, CA, USA) in PBS for 15 min at 37 °C. Fluorescence was measured using a Tecan Infinite F200 plate reader (Maennedorf, Switzerland) with 485/530 nm excitation/emission. Percent viability was calculated by comparing to the corresponding control. Treatment groups consisted of four independent trials, each in sextuplicate. After spectrophotometric analysis, green fluorescent images were obtained using a Zeiss Observer Z1 fluorescence microscope.
Glucose uptake, glycolytic enzyme activity, pyruvate concentration and ATP assay
The effect of MB on glucose uptake was determined by using the 2-[N-(7- Nitrobenz-2-Oxa- 1,3-Diazol-4-yl)Amino]- 2-Deoxy-D-Glucose (2-NBDG) assay as previously described 32. HT22 cells were seeded in 96-well plates 24 h prior to the experiment, and subjected to the treatments. HT22 cells were incubated in glucose-free Krebs Ringer HEPES (KRH) buffer (NaCl (129 mM), NaHCO3 (5 mM), KCl (4.8 mM), KH2PO4 (1.2 mM), CaCl2 (1 mM), MgCl2 (1.2 mM), HEPES (10 mM); pH 7.4) for 30 min, and in glucose free KRH buffer containing 2-NBDG (100 μM) and different concentrations of MB (1 and 10 μM) for 5 min. Glucose uptake was determined and photographed by spectrophotometry at 465/540 nm of excitation/emission and Zeiss Observer Z1 microscope, separately. Activity of glucose-6-phosphate dehydrogenase (G-6-PDH) and hexokinase (HK), and pyruvate concentration were examined with spectrophotometry. Enzyme activities were measured with Flexstation (Molecular devices) at 37 °C as described earlier with minor modification 33. Enzyme activities were expressed as units per milligram protein. ATP concentration was measured with commercial assay kit (Invitrogen, Eugene, OR, USA) 34. ATP concentrations were normalized to the protein concentration of corresponding samples measured with a protein assay kit (Thermo Scientific, Rockford, IL, USA) at 660 nm, and presented as percentage of the mean ATP concentration of normoxic control (% normoxic control) experiments.
Intracellular O2 concentration
HT22 cells were seeded at a density of 20,000 cells/mL and cultured on cell culture dishes (35mm) with a cover glass in high glucose DMEM supplemented with pyruvate (1mM), L-glutamine (4 mM), and 10% FBS. For the fluorescence life time imaging microscopy (FLTIM), cells were incubated in tris (2, 2′-bipyridyl) dichlororuthenium (II) hexahydrate (120 μM), an oxygen sensing dye, for 2 h. Washed Cells were incubated in Dextrose (10 mM)-supplemented sterile Dulbecco's phosphate buffered saline. MB (10 μM) and glucose oxidase (GO), as a positive control, were added during microscopy. Time resolved images were obtained on a confocal MicroTime 200 system (PicoQuant GmbH, Berlin). Excitation was provided from 470 nm pulsed diode laser operating at a 320 kHz repetition rate and it was reflected off of a 490 nm dichroic plate into an Olympus IX71 inverted microscope. The light passed through an Olympus 60X 1.2 NA objective, and the collected fluorescence was filtered by a 488 nm long wave pass, interference filter before passing through a 50μm confocal pinhole. The signal from the detector was routed into time correlated single photon counting module (PicoHarp 300). Fluorescence decay curves were analyzed by the software SymPhoTime, v. 5.3.2.
Western blot analysis
A series of protein contents were analyzed with immunoblotting (n=5) of whole cell and nuclear lysates; α subunit of HIF-1α, Erythropoietin (EPO), Akt and phosphorylated Akt, mTOR and phosphorylated mTOR, phosphorylated P70S6K, and prolyl hydroxylase 2 (PHD2). Whole cell protein was extracted as following; PBS-washed HT22 cells were lysed in the cell lysis buffer, containing Tri (20mM), NaCl (100mM), and EDTA (1mM), mixed with inhibitors of protease and phosphatase for 15 min at the 4 °C on shaker and followed by centrifugation at 110,000g for 20 min at 4 °C. Supernatant containing whole cell extract was saved at −80 °C for later use. Nuclear protein was extracted with commercial kit (FIVEphoton Biochemicals, San Diego, CA). Cells were extracted with cytoplasmic isolation solution mixed with inhibitors of protease cocktail and phospholipase. Cells and cytoplasmic isolation solution mixture was centrifuged at 588g for 3 min at 4 °C to separate cytoplasmic fraction. The nuclear fraction was isolated with nuclear isolation solution plus protease inhibitor cocktail and phospholipase inhibitor. Equal nuclear protein loading was confirmed with histone deacetylase 1 (HDAC1). Protein concentration of lysates was measured with a protein assay kit (Thermo Scientific, Rockford, IL, USA) at 660 nm to ensure equal loading. Protein (15μg/lane) was separated with SDS-PAGE electrophoresis and transferred to nitrocellulose membrane. The following primary antibodies were used; HIF-1α, EPO, and PHD2 (Novus Biologicals, Littleton, CO); Akt and p-Akt (Santa Cruz, Dallas, TX); and mTOR signaling pathway (Cell signaling technology, Danvers, MA, USA). Goat anti-mouse and rabbit secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA) were used at 1:4500 dilutions for 1 hour at the room temperature. Protein contents were quantified with protein densitometry (Ultraviolet Products, Upland, CA, USA) and normalized to actin band density.
Nuclear Translocation of HIF-1α
Constitutively active HIF-1α plasmids (gift from Dr. JA Garcia, UT Southwestern Medical Center35) encoding constitutively active human HIF-1α (by alanine substitution of the conserved Pro or Asp residues normally modified in an oxygen-dependent manner), tagged with GFP, and cloned into pIRES-hrGFP (Stratagene, La Jolla, CA) was transiently transfected to the HT22 cells. DNA-liposome complex was formed with P1P2N HIF-1α plasmid (200ng) 36 and transfection agent (1μL) (Lipofectamine 2000; Invitrogen, Carlsbad, CA). DNA-liposome complex was added to the 50% confluent HT22 cells in 48 well plates, incubated 48 h in normoxic CO2 incubator at 37 °C. After transfection, before and after MB treatment, live cell images of HIF-1α translocation was photographed with a temperature and time controlled (37°C) Zeiss Observer Z1 fluorescence microscope. Additionally, nuclear HIF-1α protein content/Cytoplasmic HIF-1α content ratio was obtained from the immunoblot analysis.
Inhibition of HIF-1
2-MeOE2 (2-methoxyestradiol) inhibits transcriptional expression of target genes regulated by HIF-1α without affecting HIF-1α transcription37. 2-MeOE2 inactivates HIF-1α by blocking nuclear translocation of HIF-1α. HT22 cells were treated with 2-MeOE2 (1μM) during OGD and reoxygenation. The involvement of HIF-1 activation in the MB-induced neuronal protection was confirmed with calcein AM assay with 2-MeOE2 treatment.
Inhibition of mTOR
in order to inhibit mTOR, KU-63794 (1 μM, EMD Milipore) was pretreated 3 h prior to the OGD. KU-63794 is known to inhibit the phosphorylation of mTOR at the Ser2448/2481.38,39 Inhibition of mTOR phosphorylation was confirmed with immunoblot and effects of mTOR inhibition on MB-induced protection against OGD-reoxygenation was monitored with Calcein AM assay.
Statistical analysis
Data was expressed as mean ± SEM. Multiple comparisons between OGD-reoxygenation exposed groups and normoxia groups was accomplished by one way analysis of variance combined with Turkey multiple comparison test to identify statistically meaningful differences. Probability values < 0.05 were taken to indicate statistically significant effects.
Results
MB protects HT22 cells from OGD and reoxygenation protection
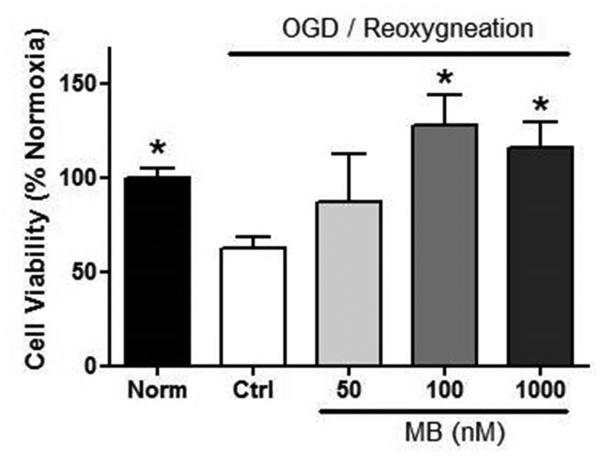
MB-induced neuroprotection was studied in hippocampus derived HT22 cells subjected to OGD/reoxygenation. Six hours of OGD and 24 h of reoxygenation killed about 45% of HT22 cells in the control group (Figure 1). MB (100 nM) treatment at the time of reoxygenation was able to significantly rescue the cells from OGD/reoxygenation injury.
Figure 1. MB protects HT22 cells against OGD-reoxygenation stress.
MB treated at the reoxygenation protects HT22 cells in a dose dependent manner. † p < 0.05 vs. Norm, * p < 0.05 vs. Ctr
MB enhances glucose utilization
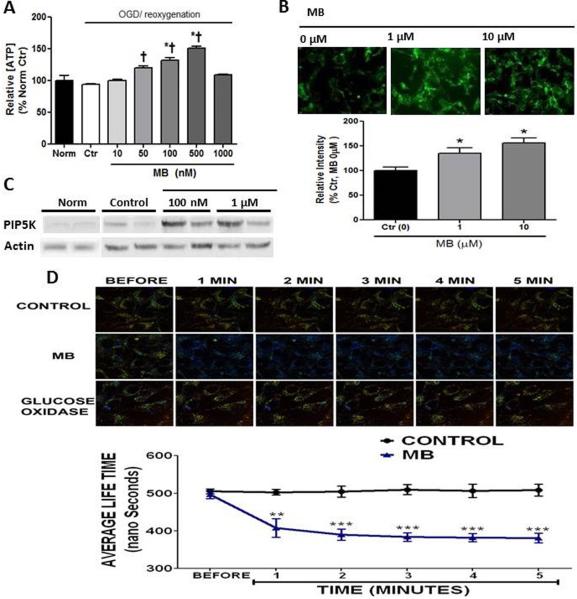
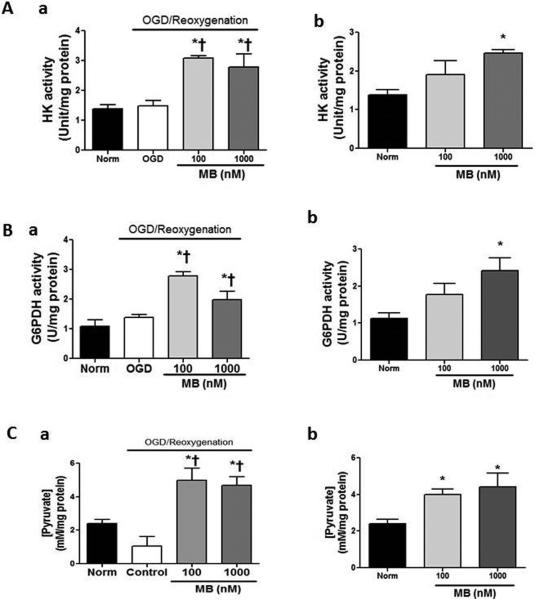
MB increases ATP after 24h reoxygenation in a dose dependent manner (Figure 2A). Increased ATP concentration with MB treatment is associated with increased glucose uptake. MB (100 nM and 1 μM) increases glucose uptake in a dose dependent manner (25% and 50%, respectively) which was manifested with 2-deoxy-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl) amino]-D-glucose (2NBDG) (Figure 2B). Immunoblot assay shows that MB increased PIP5K which is known to function in glucose uptake by stimulating endocytosis of glucose (Figure 2C). The role of PIP5K in anti-apoptosis was also reported40. MB increases intracellular O2 concentration, which provides a favorable condition for aerobic glucose metabolism. (Figure 2D) MB-enhanced ATP concentration was consistent with increased activities of hexokinase (Figure 3A-a,b) and glucose-6-phosphodehydrogenase (G6PDH). (Figure 3B-a,b) This increased enzyme activity resulted in an increased endogenous pyruvate concentration. (Figure 3C-a,b)
Figure 2. MB increases energy production.
A) ATP concentration was significantly increases in a dose dependent manner, but MB (1μM) increased toxicity. ATP value in control group: 9.89 ± 0.88 μmol/g protein (Mean ± SE) B) MB increases glucose uptake, tested with 2-[N-(7-Nitrobenz-2-Oxa- 1,3-Diazol-4-yl)Amino]-2-Deoxy-D-Glucose (2-NBDG) assay, because of experiment limitation, relatively high doses of MB (1μM and 10μM) were used. C) Phosphatidylinositol 4-phosphate 5-kinase (PIP5K) protein content was increased with MB vs. normoxic and OGD controls D) Fluorescence life time imaging microscopy (FLTIM) showed that MB enhanced intracellular O2 concentration vs. Control. Glucose oxidase was used as a positive control. † p < 0.05 vs. Norm, * p < 0.05 vs. Ctr
Figure 3. MB enhances glycolytic enzyme activity and increases endogenous pyruvate.
Activities of hexokinase (A) and glucose-6-phosphate dehydrogenase (B) are significantly increased in both normoxia (a), and OGD (b) vs. Norm, OGD controls. C) Increased pyruvate concentration with MB treatment in both normoxia (a) and OGD (b) was consistent with enzyme activities. † p < 0.05 vs. Norm, * p < 0.05 vs. Ctr
MB-enhanced stability of HIF-1α contributes to the MB-induced neuroprotection
The role of HIF in cell survival from I/R injury has been widely reported14,15,21,41. MB significantly increases HIF-1α protein content compared to both normoxic and OGD control groups (Figure 4A). EPO is a protective cytokine that is mainly regulated by activation of HIF-1. EPO was significantly increased with MB treatment (Figure 4A,B). The binding of EPO to its membrane receptor triggers the Jak-STAT signaling pathway, and, in turn, activates anti-apoptotic protein kinase, such as Akt. Akt and mTOR pathways were activated by MB treatment (Figure 4C). In the current setting, the involvement of HIF activation in the MB-induced neuroprotective mechanism was examined by blocking HIF-1α translocation to the nucleus with 2-methoxyestradiol (2-MeOE2) (Figure 4D). 2-MeOE2 treatment significantly abolished MB's neuroprotection against OGD-reoxygenation (Figure 4D). Activated mTOR is newly proposed as an HIF-1 regulator at the messenger level42-45. The involvement of mTOR activation in MB-induced protection was tested by treatment with KU-63794, which inhibits phosphorylation of mTOR. KU-63794 dampened MB-induced protection against OGD-reoxygenation stress. (Figure 4E)
Figure 4. MB-induced neuroprotection mediated via activation of HIF-1.
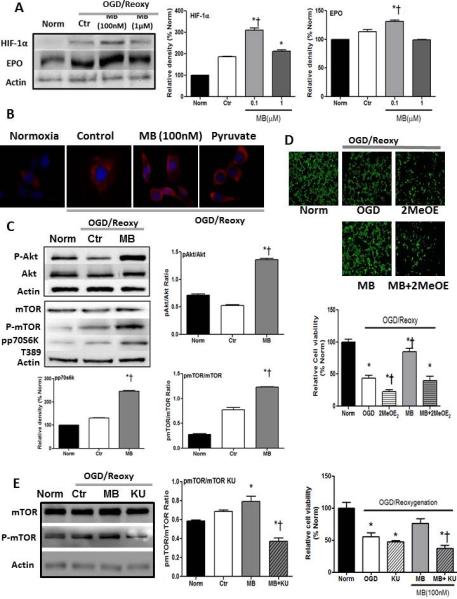
(A) HIF-1α and EPO (A,B) protein contents are significantly increased in MB treated group. C) Akt -mTOR in EPO signaling pathway are activated with MB treatment. D) Inhibition of HIF-1 activation with 2MeOE2 dampened MB-induced protection against OGD-reoxygenation stress. E) Inhibition of mTOR with KU-63794 blocks protective effects of MB. n=5, * p < 0.05 vs. Norm, † p < 0.05 vs. OGD
MB enhances nuclear translocation of HIF-1α
To examine the role of MB on HIF-1α nuclear translocation, HT22 cells were transiently transfected with constitutively active P1P2N HIF-1α or empty vector. GFP-tagged HIF-1α enables us to track the HIF-1α translocation. Additionally, primary neuron cultures were treated with CoCl (100μM) to stabilize HIF-1α under normoxia. HIF-1α accumulation was observed in the nucleus of both HT22 cells (Figure 5A) and primary neurons (Figure 5D) within 10 minutes of treatment with 1μM and 10μM MB, respectively. Additionally, the nuclear/cytoplasmic HIF-1α ratio was significantly increased in the MB treated group vs. both normoxic and OGD/reoxygenation controls. (Figure 5B) It has been reported that PHD2 interacts with HIF-1α located in the nucleus as well as with cytoplasmic HIF-1α, thus, even after HIF-1α enter the nucleus, HIF-1α degradation continuously occurs. MB lowered PHD2 content in the whole cell extract compared to the both normoxic and OGD control group. (Figure 5C)
Figure 5. MB stimulates nuclear translocation of HIF-1α.
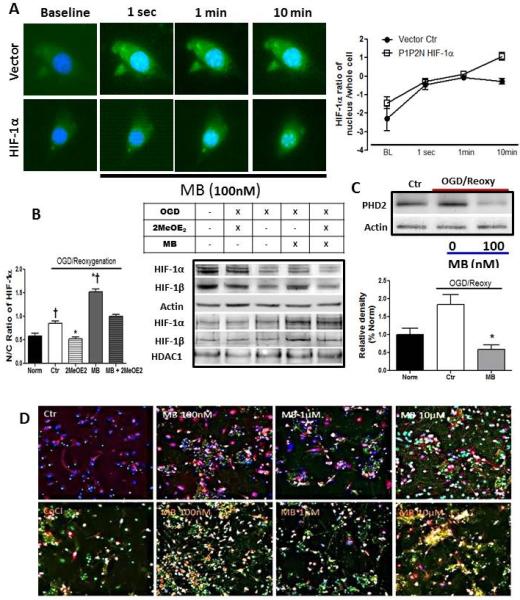
A) GFP-tagged HIF-1α reveals the concentrated HIF-1α expression in the nucleus with MB treatment (green, P1P2N HIF-1α, blue: DAPI). B) The ratio of nuclear and cytosolic HIF-1α significantly increased in MB group vs. Norm and OGD groups. 2MeOE2 inhibits nuclear translocation of HIF-1α. (n=4) C) MB significantly lowers prolyl hydroxylase 2 (PHD2) content vs. OGD control D) MB enhances nuclear HIF-1α expression in primary neurons which express constant HIF-1α expression with CoCl (100μM treatment. Red: Neurofilament, Green HIF-1α, Blue: DAPI. * p < 0.05 vs. Ctr, † p < 0.05 vs. Norm
Discussion
Although MB's neuroprotective function failed to be proven in some cases46, several laboratories have reported the neuroprotective effects of MB in I/R models30,47. The role of ROS in I/R injury in various pathological cases, such as ischemic stroke48, myocardial infarction48, traumatic brain injury49, and organ transplantation50 is well documented. Previously, we reported that MB minimizes electron leakage from the electron transport chain of the mitochondria, significantly reducing ROS generation30,31. Herein, we are proposing a possible mechanism for this MB protection from in vitro OGD-reoxygenation stress in neurons with special emphasis on the effect of MB on HIF-1. We demonstrated that MB-induced neuronal protection is mediated via activation of HIF-1, and proposed that MB stabilizes and increases HIF-1 , a regulatory subunit of HIF-1 by 1) increasing glucose metabolism, culminating in increased endogenous pyruvate, which is consistent with increased ATP concentration, 2) activating EPO and mTOR signaling pathways, and 3) stimulating nuclear localization of HIF-1α.
HIF-1 is an important transcription factor for cellular adaptation to intense hypoxic conditions. Activation of HIF-1 is involved in the regulation of numerous genes in glucose metabolism, anti- and pro-apoptosis, cellular proliferation, pH regulation, angiogenesis, erythropoiesis, matrix metabolism, and metal transport51,52 in the ischemic condition. HIF-1α is a well characterized oxygen-dependent transcription factor 53, but recently also proposed as a mTOR-dependent transcription factor 54,55. The α-subunit of HIF-1 is continuously produced, but readily degraded by the activation of prolyl hydroxylases in the presence of oxygen and cofactors, such as Fe2+ and ascorbate56. Although, HIF-1α is rapidly degraded under normoxic conditions, in the presence of normoxic HIF-1α stabilizers, HIF-1α could become stable in a normoxic or even a hyperoxic environment16. Normoxic HIF-1α stabilizers fall into the following categories: metabolites from glucose metabolism, growth factors, antioxidants, and inflammatory cytokines. For example, insulin increases HIF-1α in both the messenger and the protein levels and non-pathological concentrations of ROS also stabilize HIF-1α by inhibiting PHD57. The current study showed that MB significantly increased HIF-1α even 24 h after 6 h OGD. This MB-induced stabilization of HIF-1α is correlated with an increased effect of MB on glucose metabolism. In the in vitro OGD-reoxygenation model and also in the rodent Parkinson model, MB significantly increased ATP concentration, and reduced H2O2 toxicity31,32. Conceivably, the increased ATP concentration with MB treatment is closely related to the increased glucose uptake, intracellular O2 concentration, and content of phosphatidylinositol 4-phosphate 5-kinase (PIP5K) protein in the MB group. PIP5K generates phosphatidylinositol 4,5-bisphosphate (PIP2), which plays a key role in increased translocation of GLUT4 58. In accordance with increased ATP concentration, the activities of HK and G-6-PDH were significantly increased with MB treatment. This culminated in an increased concentration of endogenous pyruvate, an intermediary metabolite and an efficient energy yielding fuel, entering into the TCA cycle. Previously, we reported that exogenous pyruvate perfused during reperfusion in rodent transient middle cerebral artery occlusion (MCAO) model significantly reduced brain lesion volume and DNA fragmentation17. Pyruvate-induced cerebral protection against I/R injury is mediated by activation of HIF-1α and the endogenous EPO signaling pathway17. Indeed, knock down of HIF-1α with small interfering RNA transfection and inhibition of the EPO signaling pathway with soluble EPO receptor abrogated pyruvate-induced cerebral protection17. Similarly, Inhibition of HIF-1α nuclear translocation eliminated the favorable effect of MB on neuroprotection against OGD-reoxygenation. Therefore, MB-induced neuroprotection is mediated via HIF-1α stabilization and a possible mechanism of action for this MB stabilization of HIF-1α is the increase of glucose metabolism, so that increased endogenous pyruvate could contribute to the mitigation of HIF-1α degradation.
HIF-1 was originally characterized as an O2-dependent transcription factor, and involvement of mTOR in HIF-1 activation was recently reported 44,59. In addition to MB's function in the maintenance of HIF-1α stability under normoxia by increasing glucose metabolism, MB could also increase HIF-1α protein content by stimulating transcription of HIF-1α by activating the mTOR pathway which could be a second mechanism of MB-induced HIF-1α regulation. MB activates the EPO signaling pathway including the activation of anti-apoptotic kinase, Akt, followed by activation of the mTOR pathway. Indeed, combined treatment of MB with mTOR inhibitor decreased HIF-1α protein content and MB's neuroprotective effect was significantly moderated. This suggests that activation of mTOR is involved in the sustainably elevated HIF-1α protein content with MB treatment. Therefore, transiently activating HIF-1α by MB treatment might lead to a second phase elevation of HIF-1α and possibly to long-term recovery.
Because of the short half-life of HIF-1α in normoxia, its stabilization is a critical step. Nuclear translocation of HIF-1α is also an essential step for HIF activation. In HIF-1α degradation, the role of PHD is important. Of special significance is the reported HIF-1α degradation by direct interaction between PHD2 and HIF-1α in the nucleus12. MB significantly decreased PHD2 protein content and increased nuclear HIF-1α protein content in this study. Thus, the rate of HIF-1 activation should be associated with both stability of HIF-1α and inhibition of interaction between PHD2 and HIF-1α in the nucleus, which results in increased expression of HIF-1α in the nucleus.
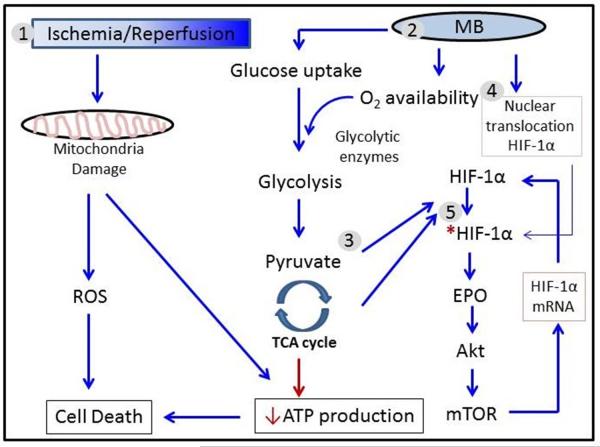
In conclusion, MB protects hippocampus-derived neuronal HT22 cells by enhancing glucose metabolism and activating the HIF-1/EPO signaling pathway. MB also stabilizes the α subunit of HIF-1 by increasing endogenous pyruvate, a known normoxic HIF-1α stabilizer (Figure 6).
Figure 6. Proposed mechanism of MB-induced neuronal protection against ischemia and reperfusion injury.
1) Ischemia and/or reperfusion damages cells and tissues by increased ROS and energy depletion which mediated by mitochondrial damage. 2) MB preserves mitochondria function, and increases intracellular O2 concentration and glucose uptake which culminate in restoration of ATP. 3) Increased energy production increases endogenous pyruvate and other glycolytic metabolites, which stabilize HIF-1α . Additionally, 4) MB enhances nuclear translocation of HIF-1α followed by activation of HIF-1. 5) HIF-1 activation stimulates several protein synthesis, including EPO. Activated EPO in turn activate mTOR signaling pathway. Activated mTOR transcriptionally increases HIF-1α. Therefore, MB could make HIF-1 being involved with protection of HT22 cells against ischemia-reperfusion injury. HIF-1α: Hypoxia inducible factor-1α;EPO: Erythroppietin; mTOR: mammalian target of Raoanycin; ROS: reactive oxygen species; ↓ Positive effect; ↓ Negative effect; * Activation.
Limitation
This study provided convincing data to support the proposed hypothesis that MB protects HT22 cells from OGD and reoxygenation stress by stabilizing HIF-1α and activating HIF-1. Due to possible aberrant physiological characteristics of transformed cells, i.e. aberrant energy metabolism, confirmation of the current findings with primary neurons have been begun to demonstrate neuron-specific HIF-1 activation in OGD-reoxygenation. Furthermore, it is highly possible that in the in vivo environment various endogenous factors are interconnected and might create other outcomes. Therefore, it is necessary for the findings presented here to be confirmed in in vivo models.
Highlights of submitted manuscript entitled “Methylene blue-induced Neuronal Protective Mechanism against Hypoxia-Reoxygenation Stress”.
■ MB stabilizes hypoxia inducible factor-1α.
■ MB increased ATP production after OGD-reoxygenation stress.
■ MB-induced HIF-1α stabilization is associated with increased glucose metabolism.
■ MB produces favorable intracellular environment for the aerobic glycolysis.
Acknowledgements
This work was partly supported by National Institutes of Health grants R01NS054651 (SY), R01NS088596 (SY), Institute of Aging and Alzheimer' s Disease Research grant RI6148 (MGR) American Heart Association grant SDG16960084 (RL), and National Natural Science Foundation of China Grant 81228009 (SY, FY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Fang MC, Perraillon MC, Ghosh K, Cutler DM, Rosen AB. Trends in Stroke@ Rates, Risk, and Outcomes in the United States, 1988-2008. Am J Med. 2014 doi: 10.1016/j.amjmed.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Collins VE, et al. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 3.Hacke W, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 4.Green AR. Pharmacological approaches to acute ischaemic stroke: reperfusion certainly, neuroprotection possibly. British journal of pharmacology. 2008;153(Suppl 1):S325–338. doi: 10.1038/sj.bjp.0707594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang P, et al. Hypoxia-inducible factor 3 is an oxygen-dependent transcription activator and regulates a distinct transcriptional response to hypoxia. Cell reports. 2014;6:1110–1121. doi: 10.1016/j.celrep.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Lu H, et al. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. The Journal of biological chemistry. 2005;280:41928–41939. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- 8.Forsythe JA, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marti HH. Erythropoietin and the hypoxic brain. J Exp Biol. 2004;207:3233–3242. doi: 10.1242/jeb.01049. [DOI] [PubMed] [Google Scholar]
- 10.Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Science's STKE : signal transduction knowledge environment. 2007;2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- 11.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pientka FK, et al. Oxygen sensing by the prolyl-4-hydroxylase PHD2 within the nuclear compartment and the influence of compartmentalisation on HIF-1 signalling. Journal of cell science. 2012;125:5168–5176. doi: 10.1242/jcs.109041. [DOI] [PubMed] [Google Scholar]
- 13.Demidenko ZN, Blagosklonny MV. The purpose of the HIF-1/PHD feedback loop: to limit mTOR-induced HIF-1alpha. Cell cycle (Georgetown, Tex.) 2011;10:1557–1562. doi: 10.4161/cc.10.10.15789. [DOI] [PubMed] [Google Scholar]
- 14.Ong SG, et al. HIF-1 reduces ischaemia-reperfusion injury in the heart by targeting the mitochondrial permeability transition pore. Cardiovascular research. 2014;104:24–36. doi: 10.1093/cvr/cvu172. [DOI] [PubMed] [Google Scholar]
- 15.Chen RL, et al. HIF prolyl hydroxylase inhibition prior to transient focal cerebral ischaemia is neuroprotective in mice. Journal of neurochemistry. 2014 doi: 10.1111/jnc.12804. [DOI] [PubMed] [Google Scholar]
- 16.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. The Journal of biological chemistry. 2002;277:23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 17.Ryou MG, et al. Pyruvate protects the brain against ischemia-reperfusion injury by activating the erythropoietin signaling pathway. Stroke; a journal of cerebral circulation. 2012;43:1101–1107. doi: 10.1161/STROKEAHA.111.620088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzy RD, et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell metabolism. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Mansfield KD, et al. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell metabolism. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtis VF, et al. Stabilization of HIF through inhibition of Cullin-2 neddylation is protective in mucosal inflammatory responses. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014 doi: 10.1096/fj.14-259663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trollmann R, Richter M, Jung S, Walkinshaw G, Brackmann F. Pharmacologic stabilization of hypoxia-inducible transcription factors protects developing mouse brain from hypoxia-induced apoptotic cell death. Neuroscience. 2014;278:327–342. doi: 10.1016/j.neuroscience.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Meissner PE, et al. Methylene blue for malaria in Africa: results from a dose-finding study in combination with chloroquine. Malar J. 2006;5:84. doi: 10.1186/1475-2875-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schirmer RH, et al. Methylene blue as an antimalarial agent. Redox Rep. 2003;8:272–275. doi: 10.1179/135100003225002899. [DOI] [PubMed] [Google Scholar]
- 24.Boylston M, Beer D. Methemoglobinemia: a case study. Crit Care Nurse. 2002;22:50–55. [PubMed] [Google Scholar]
- 25.Wondrak GT. NQO1-activated phenothiazinium redox cyclers for the targeted bioreductive induction of cancer cell apoptosis. Free radical biology & medicine. 2007;43:178–190. doi: 10.1016/j.freeradbiomed.2007.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schenk P, Madl C, Rezaie-Majd S, Lehr S, Muller C. Methylene blue improves the hepatopulmonary syndrome. Ann Intern Med. 2000;133:701–706. doi: 10.7326/0003-4819-133-9-200011070-00012. [DOI] [PubMed] [Google Scholar]
- 27.Preiser JC, et al. Methylene blue administration in septic shock: a clinical trial. Critical care medicine. 1995;23:259–264. doi: 10.1097/00003246-199502000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Kwok ES, Howes D. Use of methylene blue in sepsis: a systematic review. J Intensive Care Med. 2006;21:359–363. doi: 10.1177/0885066606290671. [DOI] [PubMed] [Google Scholar]
- 29.O'Leary JL, Petty J, Harris AB, Inukai J. Supravital staining of mammalian brain with intra-arterial methylene blue followed by pressurized oxygen. Stain Technol. 1968;43:197–201. doi: 10.3109/10520296809115068. [DOI] [PubMed] [Google Scholar]
- 30.Poteet E, et al. Neuroprotective actions of methylene blue and its derivatives. PloS one. 2012;7:e48279. doi: 10.1371/journal.pone.0048279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen Y, et al. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. The Journal of biological chemistry. 2011;286:16504–16515. doi: 10.1074/jbc.M110.208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin AL, et al. Methylene blue as a cerebral metabolic and hemodynamic enhancer. PloS one. 2012;7:e46585. doi: 10.1371/journal.pone.0046585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergmeyer H-U. Methods of Enzymatic Analysis. Academic Press; New York: 1983. [Google Scholar]
- 34.Ryou MG, et al. Pyruvate minimizes rtPA toxicity from in vitro oxygen-glucose deprivation and reoxygenation. Brain research. 2013;1530:66–75. doi: 10.1016/j.brainres.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dioum EM, et al. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 36.Dioum EM, Clarke SL, Ding K, Repa JJ, Garcia JA. HIF-2alpha-haploinsufficient mice have blunted retinal neovascularization due to impaired expression of a proangiogenic gene battery. Investigative ophthalmology & visual science. 2008;49:2714–2720. doi: 10.1167/iovs.07-1469. [DOI] [PubMed] [Google Scholar]
- 37.Mabjeesh NJ, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 38.Olsen JM, et al. Glucose uptake in brown fat cells is dependent on mTOR complex 2-promoted GLUT1 translocation. The Journal of cell biology. 2014;207:365–374. doi: 10.1083/jcb.201403080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Martinez JM, et al. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR). The Biochemical journal. 2009;421:29–42. doi: 10.1042/BJ20090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halstead JR, et al. A role for PtdIns(4,5)P2 and PIP5Kalpha in regulating stress- induced apoptosis. Current biology : CB. 2006;16:1850–1856. doi: 10.1016/j.cub.2006.07.066. [DOI] [PubMed] [Google Scholar]
- 41.Ryou MG, et al. Pyruvate-fortified cardioplegia evokes myocardial erythropoietin signaling in swine undergoing cardiopulmonary bypass. American journal of physiology. Heart and circulatory physiology. 2009;297:H1914–1922. doi: 10.1152/ajpheart.01213.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hudson CC, et al. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Molecular and cellular biology. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mi C, et al. 4′,6-dihydroxy-4-methoxyisoaurone inhibits the HIF-1alpha pathway through inhibition of Akt/mTOR/p70S6K/4E-BP1 phosphorylation. Journal of pharmacological sciences. 2014;125:193–201. doi: 10.1254/jphs.13273fp. [DOI] [PubMed] [Google Scholar]
- 44.Park SH, et al. GABARBP down-regulates HIF-1alpha expression through the VEGFR-2 and PI3K/mTOR/4E-BP1 pathways. Cell Signal. 2014;26:1506–1513. doi: 10.1016/j.cellsig.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 45.Harada H, et al. The Akt/mTOR pathway assures the synthesis of HIF-1alpha protein in a glucose- and reoxygenation-dependent manner in irradiated tumors. The Journal of biological chemistry. 2009;284:5332–5342. doi: 10.1074/jbc.M806653200. [DOI] [PubMed] [Google Scholar]
- 46.Audet JN, Soucy G, Julien JP. Methylene blue administration fails to confer neuroprotection in two amyotrophic lateral sclerosis mouse models. Neuroscience. 2012;209:136–143. doi: 10.1016/j.neuroscience.2011.12.047. [DOI] [PubMed] [Google Scholar]
- 47.Auchter A, Williams J, Barksdale B, Monfils MH, Gonzalez-Lima F. Therapeutic Benefits of Methylene Blue on Cognitive Impairment during Chronic Cerebral Hypoperfusion. Journal of Alzheimer's disease : JAD. 2014;42:S525–535. doi: 10.3233/JAD-141527. [DOI] [PubMed] [Google Scholar]
- 48.Kleikers PW, et al. NADPH oxidases as a source of oxidative stress and molecular target in ischemia/reperfusion injury. Journal of molecular medicine (Berlin, Germany) 2012;90:1391–1406. doi: 10.1007/s00109-012-0963-3. [DOI] [PubMed] [Google Scholar]
- 49.Cheng G, Kong RH, Zhang LM, Zhang JN. Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies. British journal of pharmacology. 2012;167:699–719. doi: 10.1111/j.1476-5381.2012.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elias-Miro M, Jimenez-Castro MB, Rodes J, Peralta C. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free radical research. 2013;47:555–568. doi: 10.3109/10715762.2013.811721. [DOI] [PubMed] [Google Scholar]
- 51.Maxwell PH, Ratcliffe PJ. Oxygen sensors and angiogenesis. Semin Cell Dev Biol. 2002;13:29–37. doi: 10.1006/scdb.2001.0287. [DOI] [PubMed] [Google Scholar]
- 52.Semenza GL, Shimoda LA, Prabhakar NR. Regulation of gene expression by HIF-1. Novartis Found Symp. 2006;272:2–8. discussion 8-14, 33-16. [PubMed] [Google Scholar]
- 53.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Molecular and cellular biology. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong H, et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer research. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 55.Leontieva OV, Blagosklonny MV. M(o)TOR of pseudo-hypoxic state in aging: rapamycin to the rescue. Cell cycle (Georgetown, Tex.) 2014;13:509–515. doi: 10.4161/cc.27973. [DOI] [PubMed] [Google Scholar]
- 56.Schofield CJ, Zhang Z. Structural and mechanistic studies on 2-oxoglutarate- dependent oxygenases and related enzymes. Current opinion in structural biology. 1999;9:722–731. doi: 10.1016/s0959-440x(99)00036-6. [DOI] [PubMed] [Google Scholar]
- 57.Niecknig H, et al. Role of reactive oxygen species in the regulation of HIF-1 by prolyl hydroxylase 2 under mild hypoxia. Free radical research. 2012;46:705–717. doi: 10.3109/10715762.2012.669041. [DOI] [PubMed] [Google Scholar]
- 58.Funaki M, DiFransico L, Janmey PA. PI 4,5-P2 stimulates glucose transport activity of GLUT4 in the plasma membrane of 3T3-L1 adipocytes. Biochimica et biophysica acta. 2006;1763:889–899. doi: 10.1016/j.bbamcr.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang J, Ma WY. Nerve growth factor regulates the expression of vascular endothelial growth factor in human HaCaT keratinocytes via PI3K/mTOR pathway. Genet Mol Res. 2014;13 doi: 10.4238/2014.January.24.14. [DOI] [PubMed] [Google Scholar]