Abstract
The existing evidence shows great promise for plasma as the first resuscitation fluid in both civilian and military trauma. We embarked on the Control of Major Bleeding After Trauma (COMBAT) trial with the support of the Department of Defense, in order to determine if plasma-first resuscitation yields hemostatic and survival benefits. The methodology of the COMBAT study represents not only three years of development work, but the integration of nearly two-decades of technical experience with the design and implementation of other clinical trials and studies. Herein, we describe the key features of the study design, critical personnel and infrastructural elements, and key innovations. We will also briefly outline the systems engineering challenges entailed by this study. COMBAT is a randomized, placebo controlled, semi-blinded prospective Phase IIB clinical trial, conducted in a ground ambulance fleet based at a Level I trauma center, and part of a multicenter collaboration. The primary objective of COMBAT is to determine the efficacy of field resuscitation with plasma first, compared to standard of care (normal saline). To date we have enrolled 30 subjects in the COMBAT study. The ability to achieve intervention with a hemostatic resuscitation agent in the closest possible temporal proximity to injury is critical and represents an opportunity to forestall the evolution of the “bloody vicious cycle”. Thus, the COMBAT model for deploying plasma in first response units should serve as a model for RCTs of other hemostatic resuscitative agents.
Keywords: trauma induced coagulopathy, plasma first resuscitation, hemostatic resuscitation, COMBAT, randomized clinical trial, trauma, hemorrhage, hemorrhagic shock, plasma, FFP, FP24
Introduction
Trauma induced coagulopathy (TIC) is present in up to one-third of severely injured civilian trauma patients and is associated with a four-fold increase in risk of mortality when present.(1-7) Today, “hemostatic resuscitation” and “damage control” are watchwords for the trauma community, and the history of hemostatic resuscitation dates back at least as far as the use of plasma as a resuscitation fluid in World War II. Nevertheless, our understanding of how to intervene effectively with the massively bleeding trauma patient remains incomplete.(8, 9) This understanding has taken numerous turns over the intervening decades since the 1940s. A key milestone was the recognition of the “bloody vicious cycle” of hemorrhagic shock, hypothermia, acidosis and coagulopathy.(10, 11) This realization spawned the recommendation for empiric resuscitation with fixed ratios of plasma to PRBCs as early as 1981.(12) Efforts since then have focused on early intervention with the intent to restore hemostasis, chiefly with empiric use of various ratios of blood components.
The military experience with empiric resuscitation has strongly suggested that the administration of a 1:1 ratio of plasma to PRBCs yields a survival benefit.(13-16) However, data from civilian trauma centers in the United States suggest that a ratio of 1:2 may be more beneficial in this setting, and indeed highlight the potential dangers of excessive plasma use.(17-23) Complicating the interpretation of the existing military and civilian studies of empiric plasma resuscitation strategies, are the patients' demographic dissimilarities, the differences in available interventions in terms of both blood products and transport time, and the necessary differences in study designs between urban trauma centers and battlefield environments.(15, 16, 23, 24) Therefore, according the PICO (patients, interventions, comparators and outcomes) format for applying the evidence of a study to care of one's own patients, we remain uncertain has to how to interpret the existing studies of early plasma resuscitation.(25, 26)
Additionally, most existing studies are complicated by survivor bias, as most are retrospective.(18, 27, 28) As Snyder et. al. note of their own 2009 study of blood product ratios: “The non-survivors in our study population did not die because they got a lower plasma: PRBC ratio; they got a lower ratio because they died”.(29, 30) Moreover, as most of the resuscitative efforts with severely injured patients have either succeeded or failed by 3-6 hour after injury, most studies lack the detailed information about the early events in the patient's resuscitation to draw reliable conclusions about causality. Our recent study of goal-directed resuscitation using thrombelastography (TEG), highlights this difficulty: patients in this study were enrolled based on the criteria of initiation of a massive transfusion protocol and by this time, valuable information about their physiologic and hematologic state from the time of injury onward was lost.
These confounders of existing studies not withstanding, in aggregate the existing evidence shows great promise for plasma as the first resuscitation fluid in both civilian and military trauma. We embarked on the Control of Major Bleeding After Trauma (COMBAT) trial with the support of the Department of Defense, in order to determine definitively if plasma-first resuscitation yields survival and hemostatic benefits. The considerations enumerated above prompted us to radically alter the approach to this clinical trial compared to previous ones we have engaged in. Our experience in the Polyheme trial gave us the necessary background to conduct a trial of intervention with blood products in the field, utilizing our ground ambulance fleet.(31) This ability enables us to deliver plasma at the earliest possible time after injury, thus maximizing the potential benefit. Development of our Multiple Organ Failure database project over the past 22 years, has given us experience in collecting and processing enormous amounts of patient data, such as the COMBAT trial will generate.(32) Lastly our recent experience with our trial of TEG-guided resuscitation has built a team of personnel adept at the collection and handling of patient samples and data at numerous, tightly clustered time points. While COMBAT goes beyond all of these studies in terms of the level of effort and detail required, they laid the essential groundwork.
Materials and Methods
The methodology of the COMBAT study represents not only three years of development work, but also the integration of nearly two-decades of technical experience with the design and implementation of other clinical trials and studies. The detailed methods and procedures of the COMBAT trial comprise several hundred pages of standard operating procedures. Herein, we will attempt only to describe the key features of the study design, critical personnel and infrastructural elements, and key innovations. We will also briefly outline the systems engineering challenges entailed by this study.
Study Design
COMBAT is a randomized, placebo controlled, semi-blinded, prospective, Phase IIB clinical trial, based at Denver Health Medical Center's (DHMC) Level I trauma center, with enrolment and interventions conducted in the ground ambulance fleet based at this center. The COMBAT study in Denver is one component of a larger DoD-funded multicenter trial of plasma-first resuscitation, including the University of Pittsburgh (principal investigator: Jason L. Sperry, MD) and Virginia Commonwealth University (principal investigator: Bruce D. Spiess, MD). Notably, the COMBAT study is the only center utilizing a frozen plasma product, thawed at the scene of injury. Data from the multicenter study is also utilized by the Transagency Collaboration for Trauma Induced Coagulopathy (TACTIC) consortium (principal investigator: Kenneth G. Mann, PhD), a large NIH-supported collaborative effort with aims focused on elucidating the basic mechanisms driving TIC.
The primary objective of COMBAT is to determine the efficacy of field resuscitation with plasma first, compared to standard of care (normal saline). The primary end-point is 28-day mortality, for which the study is powered to detect large differences (17-19%) between arms. Secondary endpoints include improvement in immediately post-injury coagulopathy and clot strength, markers of physiologic exhaustion (e.g. acidosis) and reduction in multiple organ failure (MOF) and 24 hour mortality. Projected enrolment is 150 individuals (75 per arm) at a rate of 50 per year.
Eligibility is determined at the scene of injury, by the responding paramedics of the ground ambulance crew. Inclusion criteria are all traumatically injured adults with either a systolic blood pressure (SBP) ≤ 70 mmHg or 71-90 mmHg with an accompanying heart rate ≥108. Prisoners and visibly pregnant patients are excluded, as are those with isolated gunshot wounds to the head or requiring CPR in the field prior to enrolment. Patients may be enrolled at any time during their ambulance transport, but infusion of >800 mL of crystalloid prior to meeting criteria (for example in an initially normotensive patient who then decompensates and meets criteria) results in exclusion. The criteria are based upon those developed by the Resuscitation Outcomes Consortium (ROC) for their similarly structured trial of hypertonic saline as an initial resuscitation fluid.(33) Based on pilot studies, the ROC group determined that these vital sign criteria captured the patient population most likely to be in hemorrhagic shock and thus with the greatest likelihood to benefit from the experimental intervention. Liberalizing the criteria to all patients with an SBP ≤90 would only have increased enrollment by 25% while exposing many more minimally injured patients to the risks of the study intervention.(34, 35) In the case of the COMBAT trial, the need for conservative enrollment criteria is even greater, as AB plasma is a scarce medical resource and the potential hazards of exposure to this therapy are greater than hypertonic saline.
Patients are enrolled under a waiver of informed consent, as permitted under US Federal Regulation 21 CFR 50.24. In order to comply with this regulation, the study was conducted after a process of community consent, wherein the local community has been informed via multiple media outlets and has been provided with the option to opt out by wearing a “No COMBAT” bracelet or dog tags. Additionally, the waiver of consent element of the study design required that the study be conducted under FDA regulation, as an Investigational New Drug study (IND no. 15216). The ethical and practical considerations involved in implementing a waiver of consent in the COMBAT trial are discussed in detail elsewhere. (36)
The field blood sample is drawn immediately upon enrolment, prior to the administration of any therapy. Patients are then randomized by the act of opening a preloaded and sealed cooler which contains either two units of frozen plasma (FP24, approximately 250 mL each), which is immediately thawed and administered; or a dummy load, which prompts the paramedics to administer normal saline per the current standard of care. Plasma and dummy payloads are block randomized in lots of 20. These payloads are delivered to paramedic division staff in sealed aluminum cassettes by study personnel uninvolved in enrolment and data analysis, thus maintaining allocation concealment.
Thawing and administration of the first unit of plasma generally takes a total 6 to 7 minutes, by which time the second unit is thawed and hanging. Generally, this means that patients in the plasma arm arrive at the ED with the second unit running and having received less than 250 mL (frequently zero) of crystalloid in the field. Patients in the control arm generally receive a comparable volume of normal saline, which, given our short transport times, is usually less than 800 mL, thus maintaining equipoise with respect to total prehospital volume of resuscitation. Blinding is partial, with the paramedics blinded to therapy (i.e. cooler contents) until after enrolment. While we were unable to blind the admitting physician completely by using a placebo that resembles plasma (colorants in intravenous solutions are forbidden by the FDA), we have generally found that the “controlled chaos” of a trauma activation is sufficient to obscure the nature of the field therapy, as the paramedics do not report this information to the receiving staff. Upon arrival at the hospital, all study interventions are complete and the patient's ongoing care proceeds with all hospital staff blind to the field intervention (plasma or saline).
A key design feature of the COMBAT study is tightly clustered early time points for data and sample collection, again with the aims of removing survivor bias and detecting the most proximal effects of the study intervention before the effect is diluted by subsequent stochastic factors. Immediately upon hospital arrival collection of the next set of blood samples occurs, followed by samples at 2, 4, 6, 12, 24, 48, and 72 hours, and days 5 and 7 post injury (see figure 1). Patients are followed for outcomes for 28 days or until discharged. This early granularity, coupled with long term follow up allows us to obtain a complete picture of the patients' clinical course as impacted by early intervention. Moreover, since most of the expected deaths and other measurable study objectives are expected to occur within the first 6 hours, highly detailed analysis of the patients' physiologic and coagulation status within this period is critical to achieving its scientific aims.
Figure 1. COMBAT Biological Sampling Timeline.
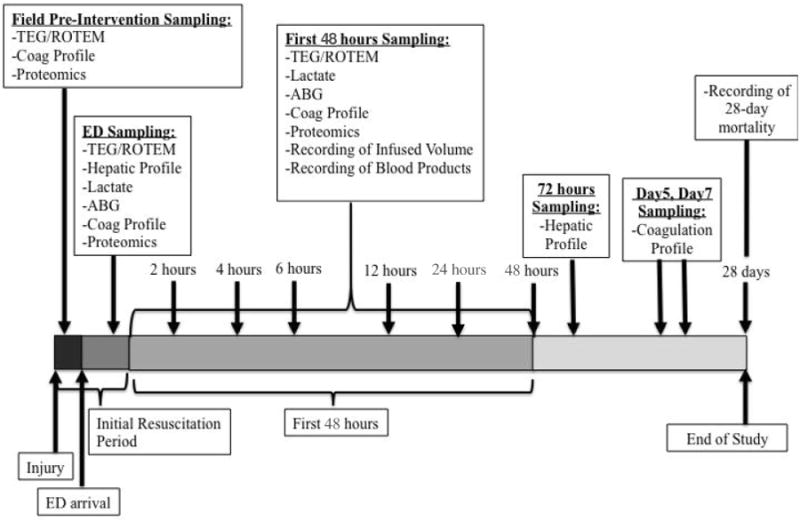
Samples are drawn at 11 time points, starting in the field, immediately before randomization and the administration of the initial resuscitative fluids (FP24 or normal saline). Subsequent samples are drawn at ED arrival and hours 2, 4, 6, 12, 24, 48 and days 5 and 7 after injury. The samples comprise a comprehensive battery of coagulation and other biochemical assays detailed in figure 2, and are typical for each time point with the exception of the field sample which lacks a lactate measurement (due to sample handling constraints) and the addition of a hepatic function panel at the 72 hour time point. At 28 days post-injury, mortality, complications, and other outcomes data are finalized.
Study Metrics
In addition to the primary and secondary endpoints described above, the COMBAT study is designed to capture detailed biochemical and coagulation data at each of the 11 time points, with the aim of describing the molecular natural history of TIC and the mechanism of response to plasma-first resuscitation therapy. At each time point, whole blood undergoes a battery of 12 channels of viscoelastic hemostatic assays on the TEG® and ROTEM® platforms, including TEG® Platelet Mapping™. In addition, whole blood impedance aggregometry is performed with ADP and TRAP agonists on the ROTEM® platelet platform. The remaining blood is immediately chilled, centrifuged to yield platelet free plasma and flash frozen in liquid nitrogen. These plasma samples are used for coagulation factor level tests (II, V, VII, X, and XIII), cytokine and chemokine panels, proteomics, metabolomics and ELISAs for proteins of specific interest relevant to TIC, such as tPA, PAI-1, syndecan and α-enolase, among others. An additional citrated blood tube and EDTA tube go to the DHMC central clinical laboratory for a CBC and conventional coagulation tests (PT, PTT, von Clauss fibrinogen level and D-dimer level). A blood tube containing protease inhibitors is also collected and immediately chilled and centrifuged to yield plasma which, along with aliquots from the other tubes, are contributed to the Trans Agency Research Consortium for Trauma-Induced Coagulopathy (TACTIC) collaboration. The detailed sample processing procedure is described in schematic in figure 2.
Figure 2. COMBAT Biological Sample Processing Chart.
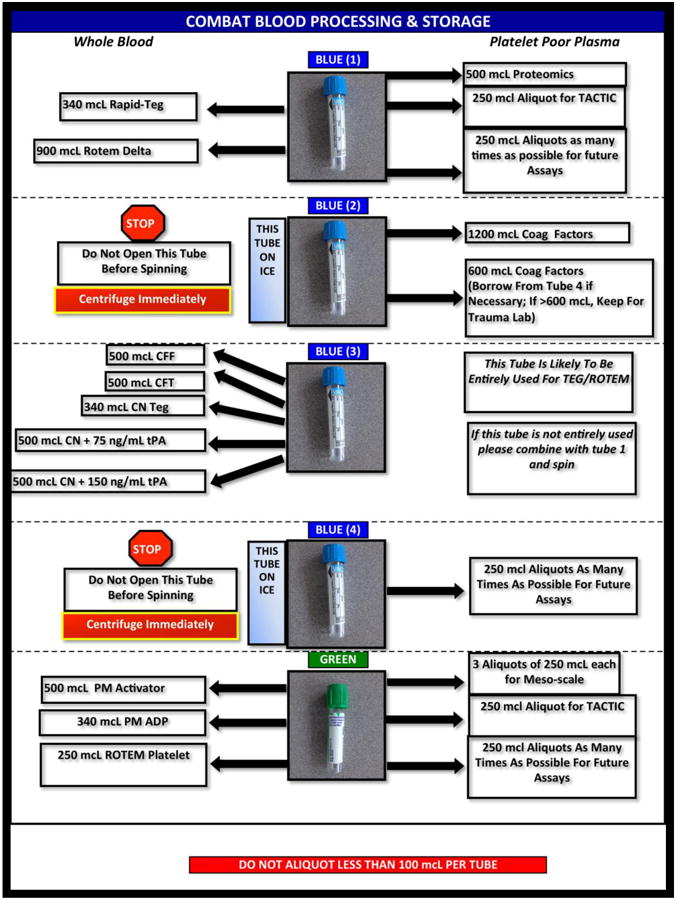
This guide is carried by the COMBAT technical staff to aid in proper sample handling and allocation. Five sodium citrate (blue top), one lithium heparin (green), one EDTA, and one protease inhibitor vacuum tube are collected at each time point. One citrate and the EDTA tube are sent to the DHMC central clinical laboratory for a CBC and conventional coagulation tests. The protease inhibitor tube is immediately chilled and centrifuged to yield plasma. The remaining tubes are processed according to this chart. Whole blood undergoes a battery of 12 channels of viscoelastic hemostatic assays on the TEG® and ROTEM® platforms, including TEG® Platelet Mapping™ as well as ROTEM® platelet aggregometry. The remaining blood is immediately chilled, centrifuged to yield platelet free plasma and flash frozen in liquid nitrogen. These plasma samples are used for coagulation factor level tests (II, V, VII, X, and XIII), cytokine and chemokine panels, proteomics, metabolomics, and ELISAs for proteins of specific interest relevant to trauma induced coagulopathy, including tPA, PAI-1, syndecan and α-enolase. Aliquots are also contributed to the Trans Agency Research Consortium for Trauma-Induced Coagulopathy (TACTIC) collaboration.
Statistical Methods
COMBAT is a Phase IIB trial and is powered to detect large (17 to 19 %) differences in mortality or other binary outcomes (e.g. massive transfusion) between the two groups, using Fisher's exact test. Power analyses for the primary and secondary endpoints were performed with Pass 11 (PASS 11. NCSS, LLC. Kaysville, UT, USA).The study is adequately powered to detect small differences between groups with regard to continuous biological data relevant to coagulation. For example, sample sizes of 60 and 60 achieve 80% power to detect a difference of 1.18 dynes/cm2 in viscoelastic clot strength (TEG G parameter) between the two groups (which is smaller than a clinically relevant difference) at a significance level (alpha) of 0.05 using a two-sided test. All power analyses account for two interim analyses for a total of three sequential, equally-spaced analyses, using the O'Brien-Fleming method to calculate two-sided boundaries, assuming a 95% confidence, 80% power and attrition rates of 0%, 10%, and 20%. The first interim analysis will be conducted when n=50, the second at n=100, and the final at end of the 3-year period of accrual.
An “intent-to-treat” approach will be used for all primary and secondary outcome analyses. All analyses will be conducted using SAS for Windows vs. 9.3 (SAS Institute Inc., Cary NC, USA) on de-identified data. Outcome and effect variables that are not normally distributed will be either categorized or analyzed using non-parametric methods. Missing data will be managed during analysis using the method proposed by Sauaia et al.(37)
Effectiveness of randomization will be examined by comparing the two groups regarding demographic variables (age, gender), injury severity (Injury Severity Score, blunt versus penetrating mechanism), degree of shock (field SBP, field heart rate, field hematocrit), and a field coagulation measure (field INR). We will adjust all analyses of endpoints for the covariates showing different distribution (either statistically significant difference at p<0.05 or clinically significant difference as determined by the DSMB).
Mobile Blood Banking
Delivery of freshly thawed, frozen AB (universal donor) plasma to trauma victims at the scene of injury was the greatest technical challenge faced during the design of this study. Early in the development of the study, we elected to use FP24, which is our locally available frozen plasma product, and is functionally equivalent to fresh frozen plasma (FFP), but conforming to a slightly different set of manufacturing standards.FP24 is plasma frozen with 24 hours of collection, whereas FFP is required to be frozen within 8 hours of collection. While FP24 has slightly lower concentrations of coagulation factors than FFP, the difference is thought to be of minimal clinical impact, except with regard to factor VIII (which can be significantly lower in FP24) and the two products are generally used interchangeably, except for specific indications for factor VIII replacement.(38) Moreover, in our specific case, the local AB FP24 product from Bonfils blood center is collected at the processing facility and packaged immediately and is thus functionally equivalent to FFP, although labeled as FP24.
As noted above, the Denver COMBAT study is the only participant in the DoD's multicenter plasma-first study to utilize a frozen plasma product, thawed on demand at the scene of injury; whereas, the other centers use pre-thawed plasma. While pre-thawed plasma presents some logistical advantages, there are significant disadvantages to its use as well. Pre-thawed previously frozen plasma has a nominal shelf life of 5 days, but has been shown to degrade rapidly in terms of both its hemostatic and anti-inflammatory potential. (38-40) Moreover, as the targeted patient population presents infrequently (roughly 50 cases per year at our center), equipping our entire fleet of 32 ambulances with thawed plasma at all times would result in the waste of nearly 7000 units of AB plasma per year, lost to expiration. This would cost over $5 million in the course of a three-year study and deplete our region of a precious medical resource, as AB donors comprise less than 3% of the donor pool. Lyophilized plasma and other room temperature stable products are not available in the United States.
Therefore, a system to store, transport, and rapidly thaw plasma in our ambulance fleet had to be devised. The only existing methodology for rapid plasma thawing was a microwave based system. This system proved unacceptable on several counts. First, its thaw times were unpredictable ranging from 4 to 8 minutes and occasionally far longer depending on the shape and volume of the plasma unit. The microwave was difficult to load and use, and mishandling during the stress of a trauma response could result in burned plasma, partially frozen plasma or punctured bags. The microwaves were also bulky and fragile and drew enormous amounts of electrical power, all of which rendered them unsuitable for installation in a vehicle. In short, the microwave system was neither sufficiently robust nor fool proof to be used in a mobile/first response environment, and, above all, the system did not fail safe.
Thus, we were compelled to invent an entirely new methodology for rapid plasma thawing, which in aggregate we refer to as the Field Plasma System (FPS) a schematic of which is shown in figure 3. A dry (i.e. contained circulation) warm water bath (the Plasmatherm™, Barkey GmbH & Co., Leopoldshoehe Germany) had recently been approved for the US market, and this system proved very simple to use and reliable, and was easily adapted for vehicular use. However, it thawed FP24 units no more rapidly that any other conventional warm water bath: approximately 20 minutes. This exceeded our usual ground ambulance transport time, but also meant that a patient in shock would require normal saline administration before we could thaw the first unit of plasma, undermining the central purpose of the study: “plasma first” resuscitation. This necessitated a complete redesign of our plasma packaging to increase the surface area to volume ratio. We worked with our local blood donation center (Bonfils Blood Center, Denver, CO) to collect donated 250 mL plasma units directly into oversized 2 liter bags. These were then frozen under compression to yield plasma units with a thin, flat form factor, which readily thawed in under 3 minutes in the Plasmatherm™. These units are, however, extremely fragile and must be transported in a padded, rigid case within an outer insulated cooler.
Figure 3. Field Plasma System Schematic.
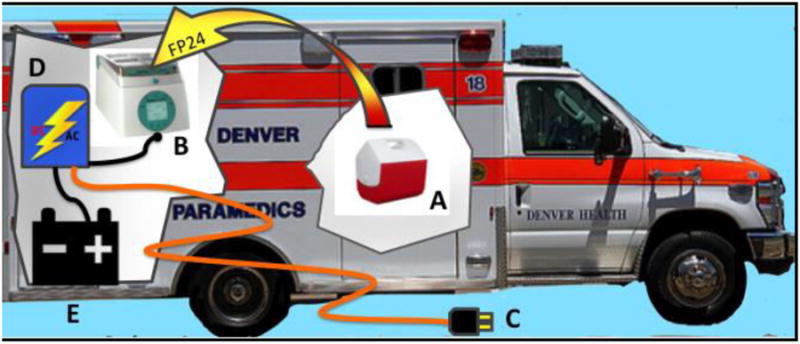
FP24 is stored frozen at less than -18 °C in a high performance cooler (A) passively chilled with a phase change material. This cooler is unpacked at the time of randomization and if it contains FP24 (instead of a dummy payload for the control arm) these units are transferred to the Plasmatherm™ dry warm water bath system (B) where they are thawed in approximately 3 minutes. The Plasmatherm™ is powered by a self-contained electrical system that is charged by connection to conventional 110 VAC mains power via a shore power coupling (C) when the ambulance is parked. A power inverter/battery charger (D) both charges a lithium-ion storage battery (E), which supplies power to the system while the ambulance is in the field, and converts the battery's DC power output to AC to run the Plasmatherm™.
Storing and transporting these plasma units also presented challenges. The FDA mandates that FP24 and FFP be stored at or below -18° C. We had to deliver reliably frozen plasma to up to 44 ambulance crew shifts per day, and this plasma might stay in the field for up to 16 hours. To supply this demand, three -30°C blood bank freezers were installed in the equipment room of our ambulance garage. These freezers are stocked with sufficient units of FP24 to supply three consecutive fleet shifts of the ambulance service, as well as bottles of an aqueous salt solution which changes phase at -22° C. Half of the FP24 packages actually contain a dummy load for the control arm of the study. Both the FP24 units and the dummy loads are concealed within a protective aluminum cassette to maintain blinding during loading and unloading of the coolers. Two FP24 units (or a dummy load) and 4 one-liter bottles of frozen phase change material are packed into specially designed coolers for each ambulance shift. These coolers consist of a5 cm layer of pentane-blown rigid polyurethane foam insulation sandwiched between an inner shock absorbing cross-linked polyethylene foam liner and a highly durable steel outer shell constructed from a repurposed 30 mm ammunition can. These coolers are capable of storing the FP24 units below the mandated temperature of -18° C for at least 28 hours even during Summer weather conditions and can protect their payload from up to a 6 foot drop onto concrete. Integral with the physical elements of this system is the creation of an electronic (barcode-based) tracking system for the issuance and recovery of all fielded and consumable elements of the system. This system was built upon the existing system for tracking the issuance of drugs and equipment to the ambulance crews. Additional record keeping and quality assurance duties (e.g. maintenance of freezer temperature logs), as would be associated with any blood bank, had to be implemented by the team of vehicle service technicians (VSTs) under the DHMC Paramedic Command, as well as by the professional research assistants (PRAs) of the DHMC/University of Colorado study team. A just-in-time (JIT) supply system from Bonfils Blood Center to replace expended units within 24 hours also had to be put in place, as we stock no working reserve, in order to retain the integrity of our block randomization.
Lastly, we had to provide reliable power to the Plasmatherm™ warm water bath. After field tests where the enormous electrical draw of the microwave units damaged the ambulances' charging system, it was decided that the electrical power system for the study equipment should be entirely self-contained. Even after changing to the lower current water bath system, this approach was maintained as an important safety feature, such that no possible failure of the FPS could render the ambulance inoperable. Wide safety margins were engineered into the FPS electrical components. The FPS is built around a 300 ampere-hour (AH) 12-volt lithium-ion battery (Smart Battery, model SB300,Tampa, FL, USA), which can supply power to the water bath for up to 36 hour of idle operation plus the thawing of 4 units of FP24. A 2000 watt, 200 ampere power inverter/charger (Magnum Energy, model MS2012-20B, Everett, WA, USA) converts the battery's DC power to AC to run the Plasmatherm™ (which draws up to 1600 watts) and charges the battery in less than 4 hours even if completely drained and while running the Plasmatherm™. Lastly the ambulances and garage were fitted for connection to shore power (i.e. AC mains) at every parking spot, with a 20 amp, 110 volt AC service delivered to a GFCI protected, all-weather receptacle mounted at the end of a retractable cord reel, and coupled to an automatic plug ejection system on the ambulance to prevent accidental damage to the system by driving off with the shore line still connected. Ambulance crews and VSTs were all extensively trained on a “preflight” checklist for the FPS to insure operability and safety during their shift in the field.
Systems and Subsystems
Once the scientific aims were determined, COMBAT fundamentally became a systems engineering project. The FPS and plasma storage systems detailed are the most innovative components of the study, but represent only a small fraction of the systems, subsystems and integration work which comprise the whole of the COMBAT apparatus. In terms of sheer complexity, COMBAT (in comparison to our previous trials at DHMC) is analogous to the moon landing of the Apollo program, compared to the low Earth orbit of the Mercury program. Both human and materiel resources had to be organized into systems that interlocked and overlapped. Individual personnel frequently were made responsible for the use and maintenance of numerous systems, from the FPS to the ambulance itself, to the patients' blood samples. In terms of physical equipment alone, ranging from freezers to batteries to thromboelastographs and cryogenic storage equipment, over 75 subsystems are specified in our procedures, each subdivided into numerous components in their own right. Figure 4 shows a highly abbreviated systems tree for the COMBAT study. In this view, only the “Plasma Thawing and Administration” subsystem is broken out into third and fourth order detail as an example. The impact of this fact is two-fold: first, studies of this kind necessitate the inclusion of managerial personnel with systems engineering experience from their inception and second, enormous amounts of manpower and extensive training of these personnel on all elements of the study are required for seamless and safe operation, as well as assuring maximization of enrolment.
Figure 4. Systems and Subsystems of the COMBAT Study.
This highly abbreviated systems tree for the COMBAT study is excerpted from the organizational documents used during development of the study. In this view, only the “Plasma Thawing and Administration” subsystem is broken out into third and fourth order detail as an example. If all the nested systems, subsystems and component descriptions were unpacked, the document would run to several hundred pages. Such organizational trees are essential to define and organize operational elements throughout the development of a study of this complexity, and also aid in the process of quality assurance and improvement.
Results
The authors remain blinded to the treatment arm of each patient until our first interim analysis. Therefore, the results presented herein are restricted to the descriptive characteristics of the total enrolled population. To date we have enrolled 30 subjects in the COMBAT study. The median patient was 32 years old (IQR 24-48) and 27 (90%) were male. Ten (33%) had penetrating wounds. The median time from injury to arrival at the ED was 29 minutes (IQR 22-32).The median NISS was 17 (IQR 9-36); the median SBP in the field was 70 mmHg (IQR 60-78). Seventeen patients displayed clinical signs of coagulopathy and15 patients required immediate transfusion of PRBCs, 5 of them massive (≥10 units in 6 hours).Four patients required resuscitative thoracotomy in the ED. Three patients (10%) died, one of massive hemorrhage from a transecting thoracic aortic injury, one of unsurvivable brain injury and one of multiple organ failure. Sixteen patients were randomized to and received FP24 in the field and 14 were randomized to and received standard of care treatment with normal saline. Five eligible subjects were not enrolled during this time period. Two of these failures to enroll were attributable to response to the scene from the single older-model ambulance in our fleet, which was not able to be equipped with the FPS due to space constraints unique to its construction. One failure was due to response from an ambulance whose Plasmatherm had been removed for maintenance immediately before the ambulance was put into service, with no spare equipment available at that time. One was not enrolled because of the proximity of the injury scene to the hospital, such that transport time was insufficient for the randomization procedure, and the last was not enrolled owing to the simultaneous transport of several patients from a multiple casualty event. Notably, during the study period to date, eight additional patients who did not meet COMBAT enrollment criteria suffered massive hemorrhage due to trauma, four of whom required resuscitative thoracotomy, and only three of whom survived.
Discussion
A prospective, placebo-controlled RCT for the use of plasma as an initial resuscitation fluid in trauma has been urgently needed to determine whether the civilian trauma population can indeed benefit from a plasma-first resuscitation strategy. The COMBAT trial was designed to answer this question. Through the methodology of this trial, we are giving the highest quality plasma product available in the United States (frozen) as close to the time of injury as is theoretically possible; faster, in fact, than if we were to use lyophilized plasma, which takes longer to reconstitute than our specially packaged FP24 units take to thaw. Indeed, the only two patients we were forced to exclude owing to timing issues were a pedestrian struck by a car in front of the hospital who had a transport time of under a minute, and another patient whose prolonged extrication required that they receive more crystalloid in the field than allowed by study criteria.
This ability to achieve intervention with a hemostatic resuscitation agent in the closest possible temporal proximity to injury is critical to avoiding the survivor bias than has confounded previous similar studies. Moreover, it is intuitively evident in terms of achieving hemostasis in trauma, an ounce of prevention is worth more than a pound of cure, as to intervene early with a hemostatic agent represents an opportunity to forestall the evolution of the “bloody vicious cycle” of acidosis, hypothermia and worsening coagulopathy and hemorrhagic shock. Thus, the COMBAT model for deploying plasma in first response units should serve as a model for RCTS of other hemostatic resuscitative agents, both extant and on the horizon such as cryoprecipitate, fibrinogen concentrates, novel platelet formulations and platelet-derived agents, PCCs and even antifibrinolytics. If these agents are indeed of benefit in preventing or forestalling TIC, then the best chance of proving their value is by utilizing them in the mode of COMBAT: as early as possible, at or en route from the scene of injury in a rigorously controlled RCT.
Several opportunities for improvement of the COMBAT model are, however, evident. The major limitation of the COMBAT model is that it is prone to Type II error. This is chiefly due to the fact that the response and transport times of our ground ambulance fleet in the Denver metropolitan area are so short (usually less than 30 minutes from injury to ED arrival), that there is less difference in time to first plasma between field and hospital administration than may be present in most parts of the country. This disparity in first response times is particularly evident in rural areas or urban trauma centers without a centralized, professional ambulance system based out of their center. Our fortunate circumstances with regard to the organization and efficiency of our paramedic command and its ground ambulance service make the COMBAT study a logistical possibility, but the associated short transport times are a significant confounder. Now that we have established a safe and efficient system for delivery of frozen plasma by ground ambulance, it would be a logical next step to expand the study to encompass localities with far longer transport times, where the beneficial impact of early plasma may be more evident. This could potential include other austere environments such as are seen in military applications and developing countries, although significant infrastructural hurdles would have to be overcome. One promising development that may aid in the generalizability of plasma-first resuscitation is recent work demonstrating that group A plasma is a safe alternative to AB for unmatched transfusion, thus expanding the available donor pool for emergency use plasma products by roughly 10-fold.(41) The increasing availability of improved lyophilized plasma products both abroad and in the United States, provides the best hope for future generalizability of the results of COMBAT.
While the COMBAT study is powered to detect changes in continuous variables such asclot strength, time to first PRBC requirement, and levels of specific coagulation factors, the infrequent nature of the very severely injured patients meeting COMBAT enrolment criteria mean that the study is not adequately powered to detect small benefits with regard to discrete variables such as mortality. Both from an ethical and scientific standpoint, it was an important and appropriate design consideration of this study to target only the highest acuity trauma patients with the highest likelihood of death. These are the patients most likely to receive benefit from empiric plasma therapy and least likely to be put at increased risk by an intervention given under a waiver of consent.
Necessarily, however, the rigorous inclusion criteria, demanded by this design feature of the study, exclude many badly injured patients who could conceivably benefit from early hemostatic resuscitation. As noted above, during this initial period, wherein 30 patients were enrolled in COMBAT, 8 additional patients with hemorrhagic shock, but who did not quite meet COMBAT criteria, were also admitted to our center from the catchment area of our ground ambulance fleet. These patients were, however, enrolled in our observational Trauma Activation Protocol (TAP) study, which, among other aims, will allow us to identify more accurate inclusion and exclusion criteria to target the enrolment of patients with significant traumatic hemorrhage (and specifically TIC) for future studies similar to COMBAT. Such criteria may include better scoring systems for our first responders to apply, the inclusion of qualitative criteria, or possibly even the application of point-of-care laboratory testing such as viscoelastic hemostatic assays (e.g. TEG or ROTEM) in the field. To this end, we have embarked upon an observational study, which runs in parallel to COMBAT. The Trauma Activation Protocol (TAP) study targets all high-level trauma activation patients who fail to meet COMBAT criteria. Blood is collected in the field on these patients in an identical manner to COMBAT, but no interventions are performed. The patients receive standard care throughout their hospital course. It is hoped that analysis of the outcomes, injury patterns and coagulation lab results from the field blood samples of these patients will yield insights as to how to structure future RCTs of hemostatic resuscitation in the field, in order to capture the patient population most likely to benefit from these interventions.
Acknowledgments
The authors wish to thank our clinical research assistants Sarah Ammons, Andrea Emard, Courtney Fleming and Raymond Shepherd-Singh for their invaluable efforts in obtaining samples for this study. Research reported in this publication was supported by the US Army Medical Research Acquisition Act of the Department of Defense under Contract Award Number W81XWH1220028 and the National Institute of General Medical Sciences and National Heart, Lung, and Blood Institutes of the National Institutes of Health under Award Numbers P50GM049222, T32GM008315 and UMHL120877. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health and the Department of Defense.
Disclosure Statement: This study is supported by the US Army Medical Research Acquisition Act of the Department of Defense under Contract Award Number W81XWH1220028 and the National Institutes of Health (P50 GM049222, T32 GM008315, and UMHL120877). We receive research support from Haemonetics Corporation and TEM GmbH. This study is listed on Clinical Trials.Gov as “A Prospective Randomized Study of Fresh Frozen Plasma Versus Crystalloid as Initial Prehospital Fluid Resuscitation” (NCT01838863, April 22, 2013) and is regulated by the Food and Drug Administration (IND no. 15216) and the Colorado Multiple Institutional Review Board (COMIRB protocol #: 12-1349).
Contributor Information
Michael P. Chapman, Email: Michael.chapman@ucdenver.edu.
Ernest E. Moore, Email: ernest.moore@dhha.org.
Theresa L Chin, Email: Theresa.chin@ucdenver.edu.
Arsen Ghasabyan, Email: arsen.ghasabyan@dhha.org.
James Chandler, Email: james.chandler@dhha.org.
John Stringham, Email: john.stringham@ucdenver.edu.
Eduardo Gonzalez, Email: Eduardo.gonzalez@ucdenver.edu.
Hunter B. Moore, Email: hunter.moore@ucdenver.edu.
Anirban Banerjee, Email: anirban.banerjee@ucdenver.edu.
Christopher C Silliman, Email: Christopher.silliman@ucdenver.edu.
Angela Sauaia, Email: angela.sauia@ucdenver.edu.
References
- 1.Blackbourne LH, Baer DG, Cestero RF, Inaba K, Rasmussen TE. Exsanguination shock: the next frontier in prevention of battlefield mortality. J Trauma. 2011;71(1 Suppl):S1–3. doi: 10.1097/TA.0b013e3182211286. [DOI] [PubMed] [Google Scholar]
- 2.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245(5):812–8. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gruen RL, Brohi K, Schreiber M, Balogh ZJ, Pitt V, Narayan M, Maier RV. Haemorrhage control in severely injured patients. Lancet. 2012;380(9847):1099–108. doi: 10.1016/S0140-6736(12)61224-0. [DOI] [PubMed] [Google Scholar]
- 4.Frith D, Goslings JC, Gaarder C, Maegele M, Cohen MJ, Allard S, Johansson PI, Stanworth S, Thiemermann C, Brohi K. Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J Thromb Haemost. 2010;8(9):1919–25. doi: 10.1111/j.1538-7836.2010.03945.x. [DOI] [PubMed] [Google Scholar]
- 5.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, Pittet JF. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64(5):211–7. doi: 10.1097/TA.0b013e318169cd3c. discussion 7. [DOI] [PubMed] [Google Scholar]
- 6.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care. 2007;13(6):680–5. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 7.Santry HP, Alam HB. Fluid resuscitation: past, present, and the future. Shock. 2010;33(3):229–41. doi: 10.1097/SHK.0b013e3181c30f0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MJ. Towards hemostatic resuscitation: the changing understanding of acute traumatic biology, massive bleeding, and damage-control resuscitation. Surg Clin North Am. 2012;92(4):877–91. viii. doi: 10.1016/j.suc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Counts RB, Haisch C, Simon TL, Maxwell NG, Heimbach DM, Carrico CJ. Hemostasis in massively transfused trauma patients. Ann Surg. 1979;190(1):91–9. doi: 10.1097/00000658-197907000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn EL, Moore EE, Breslich DJ, Galloway WB. Acidosis-induced coagulopathy. Surg Forum. 1979;30:471–3. [PubMed] [Google Scholar]
- 11.Elerding SC, Aragon GE, Moore EE. Fatal hepatic hemorrhage after trauma. Am J Surg. 1979;138(6):883–8. doi: 10.1016/0002-9610(79)90316-7. [DOI] [PubMed] [Google Scholar]
- 12.Kashuk JL, Moore EE, Millikan JS, Moore JB. Major abdominal vascular trauma--a unified approach. J Trauma. 1982;22(8):672–9. doi: 10.1097/00005373-198208000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Armand R, Hess JR. Treating coagulopathy in trauma patients. Transfus Med Rev. 2003;17(3):223–31. doi: 10.1016/s0887-7963(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 14.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–13. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 15.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, Flaherty SF, Grathwohl KW, Spinella PC, Perkins JG, Beekley AC, McMullin NR, Park MS, Gonzalez EA, Wade CE, Dubick MA, Schwab CW, Moore FA, Champion HR, Hoyt DB, Hess JR. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62(2):307–10. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 16.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, Williams KL, Park MS. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–58. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, Cocanour CS, Balldin BC, McKinley BA. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62(1):112–9. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 18.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL, Banerjee A, Sauaia A. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65(2):261–70. doi: 10.1097/TA.0b013e31817de3e1. discussion 70-1. [DOI] [PubMed] [Google Scholar]
- 19.Lucas CE, Ledgerwood AM. Fresh frozen plasma/red blood cell resuscitation regimen that restores procoagulants without causing adult respiratory distress syndrome. J Trauma Acute Care Surg. 2012;72(4):821–7. doi: 10.1097/TA.0b013e3182484111. [DOI] [PubMed] [Google Scholar]
- 20.Lucas CE, Ledgerwood AM, Saxe JM, Dombi G, Lucas WF. Plasma supplementation is beneficial for coagulation during severe hemorrhagic shock. Am J Surg. 1996;171(4):399–404. doi: 10.1016/S0002-9610(97)89618-3. [DOI] [PubMed] [Google Scholar]
- 21.Martin DJ, Lucas CE, Ledgerwood AM, Hoschner J, McGonigal MD, Grabow D. Fresh frozen plasma supplement to massive red blood cell transfusion. Ann Surg. 1985;202(4):505–11. doi: 10.1097/00000658-198510000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davenport R, Curry N, Manson J, De'Ath H, Coates A, Rourke C, Pearse R, Stanworth S, Brohi K. Hemostatic effects of fresh frozen plasma may be maximal at red cell ratios of 1:2. J Trauma. 2011;70(1):90–5. doi: 10.1097/TA.0b013e318202e486. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- 23.Watson GA, Sperry JL, Rosengart MR, Minei JP, Harbrecht BG, Moore EE, Cuschieri J, Maier RV, Billiar TR, Peitzman AB Inflammation, Host Response to Injury I. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67(2):221–7. doi: 10.1097/TA.0b013e3181ad5957. discussion 8-30. [DOI] [PubMed] [Google Scholar]
- 24.Miller RD. Massive blood transfusions: the impact of Vietnam military data on modern civilian transfusion medicine. Anesthesiology. 2009;110(6):1412–6. doi: 10.1097/ALN.0b013e3181a1fd54. [DOI] [PubMed] [Google Scholar]
- 25.Sauaia A, Moore EE, Crebs JL, Maier RV, Hoyt DB, Shackford SR. The anatomy of an article: title, abstract, and introduction. J Trauma Acute Care Surg. 2014;76(5):1322–7. doi: 10.1097/TA.0000000000000210. [DOI] [PubMed] [Google Scholar]
- 26.Sauaia A, Moore EE, Crebs J, Maier R, Hoyt DB, Shackford SR. The anatomy of an article: the discussion section: “how does the article I read today change what I will recommend to my patients tomorrow?”. J Trauma Acute Care Surg. 2013;74(6):1599–602. doi: 10.1097/TA.0b013e318292cb49. [DOI] [PubMed] [Google Scholar]
- 27.Brown JB, Cohen MJ, Minei JP, Maier RV, West MA, Billiar TR, Peitzman AB, Moore EE, Cushieri J, Sperry JL Inflammation, Host Response to Injury I. Debunking the survival bias myth: characterization of mortality during the initial 24 hours for patients requiring massive transfusion. J Trauma Acute Care Surg. 2012;73(2):358–64. doi: 10.1097/TA.0b013e31825889ba. discussion 64. [DOI] [PubMed] [Google Scholar]
- 28.Cosgriff N, Moore EE, Sauaia A, Kenny-Moynihan M, Burch JM, Galloway B. Predicting life-threatening coagulopathy in the massively transfused trauma patient: hypothermia and acidoses revisited. J Trauma. 1997;42(5):857–61. doi: 10.1097/00005373-199705000-00016. discussion 61-2. [DOI] [PubMed] [Google Scholar]
- 29.Snyder CW, Weinberg JA, McGwin G, Jr, Melton SM, George RL, Reiff DA, Cross JM, Hubbard-Brown J, Rue LW, 3rd, Kerby JD. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma. 2009;66(2):358–62. doi: 10.1097/TA.0b013e318196c3ac. discussion 62-4. [DOI] [PubMed] [Google Scholar]
- 30.Sperry JL, Ochoa JB, Gunn SR, Alarcon LH, Minei JP, Cuschieri J, Rosengart MR, Maier RV, Billiar TR, Peitzman AB, Moore EE Inflammation the Host Response to Injury I. An FFP:PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65(5):986–93. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 31.Moore EE, Johnson JL, Moore FA, Moore HB. The USA Multicenter Prehosptial Hemoglobin-based Oxygen Carrier Resuscitation Trial: scientific rationale, study design, and results. Crit Care Clin. 2009;25(2):325–56. doi: 10.1016/j.ccc.2009.01.002. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson JL, Moore EE, Kashuk JL, Banerjee A, Cothren CC, Biffl WL, Sauaia A. Effect of blood products transfusion on the development of postinjury multiple organ failure. Arch Surg. 2010;145(10):973–7. doi: 10.1001/archsurg.2010.216. [DOI] [PubMed] [Google Scholar]
- 33.Bulger EM, May S, Kerby JD, Emerson S, Stiell IG, Schreiber MA, Brasel KJ, Tisherman SA, Coimbra R, Rizoli S, Minei JP, Hata JS, Sopko G, Evans DC, Hoyt DB investigators ROC. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Ann Surg. 2011;253(3):431–41. doi: 10.1097/SLA.0b013e3181fcdb22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bulger EM, Jurkovich GJ, Nathens AB, Copass MK, Hanson S, Cooper C, Liu PY, Neff M, Awan AB, Warner K, Maier RV. Hypertonic resuscitation of hypovolemic shock after blunt trauma: a randomized controlled trial. Arch Surg. 2008;143(2):139–48. doi: 10.1001/archsurg.2007.41. discussion 49. [DOI] [PubMed] [Google Scholar]
- 35.Brasel KJ, Bulger E, Cook AJ, Morrison LJ, Newgard CD, Tisherman SA, Kerby JD, Coimbra R, Hata JS, Hoyt DB Resuscitation Outcomes Consortium I. Hypertonic resuscitation: design and implementation of a prehospital intervention trial. J Am Coll Surg. 2008;206(2):220–32. doi: 10.1016/j.jamcollsurg.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Chin TL, Moore EE, Coors ME, Chandler JG, Ghasabyan A, Harr JN, Stringham JR, Ramos CR, Ammons S, Banerjee A, Sauaia A. Exploring ethical conflicts in emergency trauma research: The COMBAT (Control of Major Bleeding after Trauma) study experience. Surgery. 2015;157(1):10–9. doi: 10.1016/j.surg.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38(2):185–93. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Benjamin RJ, McLaughlin LS. Plasma components: properties, differences, and uses. Transfusion. 2012;52(Suppl 1):9S–19S. doi: 10.1111/j.1537-2995.2012.03622.x. [DOI] [PubMed] [Google Scholar]
- 39.Matijevic N, Wang YW, Cotton BA, Hartwell E, Barbeau JM, Wade CE, Holcomb JB. Better hemostatic profiles of never-frozen liquid plasma compared with thawed fresh frozen plasma. J Trauma Acute Care Surg. 2013;74(1):84–90. doi: 10.1097/TA.0b013e3182788e32. discussion -1. [DOI] [PubMed] [Google Scholar]
- 40.Cao Y, Dua A, Matijevic N, Wang YW, Pati S, Wade CE, Ko TC, Holcomb JB. Never-frozen liquid plasma blocks endothelial permeability as effectively as thawed fresh frozen plasma. J Trauma Acute Care Surg. 2014;77(1):28–33. doi: 10.1097/TA.0000000000000276. discussion. [DOI] [PubMed] [Google Scholar]
- 41.Chhibber V, Greene M, Vauthrin M, Bailey J, Weinstein R. Is group A thawed plasma suitable as the first option for emergency release transfusion? (CME) Transfusion. 2014;54(7):1751–5. doi: 10.1111/trf.12537. quiz 0. [DOI] [PubMed] [Google Scholar]



