Abstract
Colorectal cancer, a leading cause of cancer death, has been linked to inflammation and obesity. Berberine, an isoquinoline alkaloid, possesses anti-inflammatory, anti-diabetes and anti-tumor properties. In the azoxymethane initiated and dextran sulfate sodium (AOM/DSS) promoted colorectal carcinogenesis mouse model, berberine treated mice showed a 60% reduction in tumor number (P=0.009), a 48% reduction in tumors <2 mm, (P=0.05); 94% reduction in tumors 2-4 mm, (P=0.001) and 100% reduction in tumors >4 mm (P=0.02) compared to vehicle treated mice. Berberine also decreased AOM/DSS induced Ki-67 and COX-2 expression. In vitro analysis showed that in addition to its anti-proliferation activity, berberine also induced apoptosis in colorectal cancer cell lines. Berberine activated AMP-activated protein kinase (AMPK), a major regulator of metabolic pathways, and inhibited mammalian target of rapamycin (mTOR), a downstream target of AMPK. Furthermore, 4E-binding protein-1 and p70 ribosomal S6 kinases, downstream targets of mTOR, were down regulated by berberine treatment. Berberine did not affect Liver kinase B1 (LKB1) activity or the mitogen-activated protein kinase pathway. Berberine inhibited Nuclear Factor kappa-B (NF-κB) activity, reduced the expression of cyclin D1 and survivin, induced phosphorylation of p53 and increased caspase-3 cleavage in vitro. Berberine inhibition of mTOR activity and p53 phosphorylation was found to be AMPK dependent, while inhibition NF-κB was AMPK independent. In vivo, berberine also activated AMPK, inhibited mTOR and p65 phosphorylation and activated caspase-3 cleavage. Our data suggests that berberine suppresses colon epithelial proliferation and tumorigenesis via AMPK dependent inhibition of mTOR activity and AMPK independent inhibition of NF-κB.
Keywords: Berberine, Colorectal cancer treatment, AMP-activated protein kinase, Mammalian target of rapamycin, Proliferation, Prevention
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer-related mortality in the U.S [1]. Inflammatory bowel diseases including ulcerative colitis (UC) and Crohn's disease (CD), correlate with a high risk of developing colorectal cancer [2]. Current conventional chemotherapeutic methods for CRC treatment can have strong side effects affecting the quality of life of patients prompting the need to find new effective therapeutics with fewer side effects. Herbal derived medicines, such as, berberine, may serve as promising therapies for the treatment of CRC.
AMP-activated protein kinase (AMPK) has emerged as a promising cancer related target. AMPK is a key regulator of metabolism and can negatively regulate tumor proliferation. Activation of AMPK is thought to require phosphorylation of AMPK at Thr172 [3]. AMPK can regulate glucose, lipid and protein metabolism in response to changes in fuel availability, oxidative stress, heat shock and hormones [4]. The AMPK pathway can regulate tumor growth and proliferation through its negative regulation of the mammalian target of rapamycin (mTOR) pathway [5]. mTOR is frequently activated in cancer and its activation results in the phosphorylation of the serine/threonine kinase p70S6k and the translational repressor eukaryotic initiation factor 4E binding protein (4EBP1), both of which are important regulators of cell growth, proliferation and protein synthesis [6].
Berberine, a natural compound extracted from the herbal plant Rizoma Coptidis, has been used in Chinese medicine for many years. Berberine has anti-diarrheic, anti-inflammation, anti-microbial [7], and anti-tumor properties [7-10]. Berberine has shown efficacy in treating diabetic patients [11]. In rodent models berberine activates AMPK and improves insulin sensitivity [12]. The anti-diabetic drug metformin, which also activates AMPK, is currently being assessed as a therapeutic agent for cancer prevention, including colorectal cancer [13]. The anti-tumor roles of metformin are thought to be mainly due to its activation of the AMPK pathway [14,15]. While it has been shown that berberine can suppress colon tumor growth in a mouse xenograft model [16], the effects of berberine on tumor promotion and progression of CRC have not been determined and the mechanisms of berberine's anti-tumor activity are unclear. We hypothesized that berberine would have chemopreventive and tumor suppressing effects on colorectal carcinogenesis by activating AMPK.
We show both in vitro and in a mouse model of colon carcinogenesis that berberine activates AMPK during the early stages of tumorigenesis inhibiting late stage mTOR and inhibits Nuclear Factor kappaB (NF-κB) activation, leading to inhibition of colon tumorigenesis.
Materials and Methods
Reagents
Berberine (>90% purity by high-performance liquid chromatography, with chemical structure as shown in Supplementary Figure1), was obtained from National Institutes of Food and Drug Control, China. RPMI-1640, Dulbecco's Modified Eagle's Medium (DMEM) and 0.25% Trypsin-EDTA were purchased from Gibco (Life Technologies, Grand Island, NY, USA). Fetal Bovine Serum (FBS) was obtained from Atlantic Biological. TACS MTT Cell Proliferation Assay kits were ordered from Trevigen (Helgerman, Gaithersburg, MD, USA). Azoxymethane was obtained from Sigma Aldrich (St. Louis, MO, USA). Dextran Sodium Sulfate, 36 000 to 50 000 kDa, was obtained from MP Biomedicals LL, (Solon, OH, USA). NE-PER Nuclear and Cytoplasmic Extraction Kit was ordered from Thermo Scientific (Rockford, IL, USA). Annexin V-FITC Apoptosis Detection Kit Ⅰ was ordered from BD Biosciences (San Diego, CA, USA).
Antibodies
Anti-phospho-AMPK (Thr172), anti-AMPK, anti-phospho-Acetyl-CoA Carboxylase (Ser79), anti-Acetyl-CoA Carboxylase, anti-phospho-mTOR, anti-mTOR, anti-phosphop70s6K, anti-p70s6K, anti-phospho-4E-BP1, anti-4E-BP1, anti-COX-2, anti-cleaved-caspase-3, anti-cleaved-PARP, anti-phospho-p53 antibodies, anti NF-κB kit, anti-phospho-LKB1, anti-LKB1, anti-phospho-AKT, anti-AKT, anti-phospho-ERK1/2, anti-ERK1/2, anti-rabbit and anti-mouse antibodies were from Cell Signaling Technology (Danvers, MA, USA); anti-β-actin was from Sigma-Aldrich (St. Louis, MO, USA); anti-Ki-67 and anti-CD-45 were from Abcam (San Francisco, CA, USA).
Animal Studies (AOM/DSS Induced Carcinogenesis)
All animal studies were conducted in accordance with Frederick National Laboratory of Cancer Research (FNLCR) guidelines for care and use of laboratory animals. Female FVB mice (6-week-old) were purchased from Charles River (Frederick, MD). Animal Diets were from Teklad Lab. After one week accustomed to the new environment and controlled diet (AIN93G), all the mice were randomly divided into 4 groups; the AOM/DSS treated groups, treated with PBS (n=15) or berberine (n=15) and the control groups (no AOM/DSS) treated with PBS (n=5) or berberine (n=5). For AOM/DSS treatment (Supplementary Figure 2), mice were given a single intraperitoneal injection of 10 mg/kg Azoxymethane. One week later the mice were given 2% Dextran Sodium Sulfate (m.w. 36–50 kDa; MP Biomedicals) in drinking water for 7 days. Berberine treatment began 1 day after DSS treatment ended and mice were returned to normal drinking water. Mice were treated with berberine (40 mg/kg in PBS) or PBS by oral gavage, 5 times a week for two continuous weeks (days 15-19 and 23-27), followed by 3 times a week (days 30-70) until to the end of the study. On day 28 after AOM injection, 5 mice of each group were euthanized and the colon tissues were collected for H&E and immunohistochemistry analysis. Liver samples were also collected for Western blotting analysis. The remaining mice of each group (n=10) were euthanized 10 weeks post AOM injection. Colon tissues were collected and the tumor numbers and size were determined. Colon tissue was evaluated by H&E and immunohistochemistry analysis. For the control (no AOM/DSS), mice were administered berberine (40 mg/kg) or PBS 5 times a week for two weeks. After two weeks (day 28) the mice were euthanized and the liver samples were collected for Western blotting analysis and the colon tissue was collected for H&E and immunohistochemistry analysis.
Histopathology
Colon tissue was harvested from mice and fixed in 4% formaldehyde overnight and stored in 70% ethanol. Fixed sections of colonic tissues were embedded in paraffin, cut into 6-μm sections and put onto microscopic slides by Histoserv Inc. (Germantown, MD, USA). Slides were stained with hematoxylin–eosin (H&E) for histological analysis. Histology of the colon was evaluated by a board certified pathologist (MSM). Colon tumor formation was diagnosed applying the criteria of a consensus report on murine colon tumors [17] (none, mild, modest, severe). Erosion was only diagnosed if an underlying inflammation was present. Histopathology diagonsis criteria and grading scheme as shown in Saud et al. [18].
Immunohistochemistry
Colonic tissue was harvested from mice, fixed in 4% formaldehyde overnight and stored in 70% ethanol; 5-μm-thick paraffin embedded sections on microscopic slides were obtained from Histoserv Inc. (Germantown, MD, USA). Slides were deparaffinized in xylene and rehydrated in graded ethanol. The sections were subject to Citrate (pH 6) antigen retrieval in pressure chamber. The sections were pre-incubated in 3% H2O2 for 20 minutes and incubated in normal goat serum for 1 hour. Sections were incubated overnight in primary antibody solution diluted as follows: Ki-67, CD45, 1:100; p-mTOR, p-AMPK, and COX-2, 1:500; Cleaved caspase-3, 1: 200; p-p65, 1:50. Slides were incubated in a biotin-conjugated secondary antibody, 1:200 dilutions. The sections were treated with ABC reagent (Vectorstain ABC kit-PK-4000, Vector labs, CA, USA), detected with Diaminobenzidine (Sigma, St. Louis, Missouri, USA) and counterstained in Hematoxylin. Analysis of cells positive for Ki-67 was performed on digitized images using image analysis software ImageProPlus 4.5 (Diagnostic Instruments, USA). Representative images were captured from 5 independently samples.
Cell Culture
HCT116, SW480 and LOVO colon cancer cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and Division of Cancer Treatment and Diagnosis (DCTD) Tumor Repository (National Cancer Institute, USA). HCT116 and LOVO cells were cultured in RPMI1640 supplemented with 10% FBS, 1% penicillin streptomycin and 1% L-Glutamine. SW480 cells were cultured in DMEM supplemented with 10% FBS, 1% penicillin streptomycin and 1% L-Glutamine. Cultures were maintained in a humidified incubator at 37°C in 5% CO2. A 100 mmol/L stock solution of berberine was prepared in DMSO and diluted as needed into cell culture medium, with a final DMSO concentration ≤ 0.1%.
Cell Proliferation Assay
Cells were seeded at a density of 2×104 cells per well in a flat-bottomed 96-well plate and cultured for 24 hours. Cells were treated with berberine (0–100 μmol/L) or 0.1% DMSO for 24, 48 and 72 hours. Subsequently, 10 μl MTT reagent was added into each well and incubated for another 4 hours until purple dye is visible. 100 μl detergent reagent was added and the plate was incubated at RT in the dark for 2-4 hours. Absorbance was measured at 570 nm with BIO-RAD Model 680 Micro plate reader. The growth of the berberine treated cells was compared with DMSO-treated control cells. The experiments were independently performed and repeated three times.
Cell Apoptosis Assay
HCT116 and SW480 cells were seeded in 100 mm dishes at a density of 2×106 cells per dish in growth medium and grown overnight at 37°C in a humidified incubator with 5% CO2. Cells were treated with berberine (0-60 μmol/L) or DMSO (0.1%) for 24 hours. Apoptosis was determined with the Annexin V-FITC Apoptosis Detection Kit Ⅰ (BD Biosciences,San Diego, CA, USA). The experiments were independently performed and repeated three times.
Western Blot Analysis
Colon cancer cells were seeded on 100 mm plates and treated with either berberine (0 μmol/L-60 μmol/L) or DMSO (0.1%) for 24 or 48 hours. The media was removed and cells were washed with cold PBS on ice. Whole-cell extracts were prepared in RIPA lysis buffer (Thermo scientific, Rockford, IL, USA) supplement with proteinase inhibitors (Roche, Mannheim, Germany). Protein concentration was determined by Micro BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA). 30 μg total protein was denatured, heated for 5 minutes and fractionated on a 10% SDS-PAGE. Proteins were transferred to Nitrocellulose membrane (Bio-Rad) for 2 hours at 300 mA, blocked and incubated with a primary specific antibody in 5% of non-fat milk for 1 hour at room temperature or overnight at 4°C. After washing, blots were incubated with secondary horse radish peroxidase (HRP)-conjugated anti-mouse (1:5000) or anti-rabbit (1:5000) at RT for 1 hour. Antigens were detected with Super Signal West Femto Maximum Sensitivity Substrate (Thermo Scientific, Rockford, IL, USA) exposure on X-ray film. The band intensities were quantified by optical densitometry with Image J software. The experiments were independently performed and repeated three times.
Soft Agar Assay
HCT116 cells were trypsinized and resuspended at 6000 cells in 2 × RPMI media. Cells were treated with DMSO (0.1%) vehicle, berberine (30 μmol/L), Compound C (10 μmol/L), or berberine plus Compound C. The bottom layer consisted of 2 ml of 1.2% agarose. The cell suspensions were mixed 1:1 with 0.5% agarose (2 ml/well for a 6 well plate) and layered on top. The cells were maintained in an incubator for 10 days after which the colonies were stained with Nitrotetrazolium Blue chloride (NBT), scanned and counted with GelCount (Oxford Optronix Ltd, Oxford, United Kingdom). The experiments were independently performed and repeated three times.
Statistical Analyses
All data is presented as the means ± standard deviation. The significance of the difference between groups was evaluated by one-way repeated-measures analysis of variance (ANOVA) and multiple comparisons with Prism 5.0 software. Student's t-test for paired observation was used where appropriate. P<0.05 were considered to be statistically significant.
Results
Berberine Decreases Tumorigenesis in AOM/DSS Induced Colon Carcinogenesis
In clinical trials, berberine at 300 to 500 mg/kg has been shown to have similar hypoglycemic effects as the anti-diabetic drug metformin [19] and metformin has been shown to suppressed colorectal aberrant crypt foci [20]. To determine the efficacy of berberine on inhibiting colorectal cancer, we treated mice with AOM to initiate tumorigenesis followed by DSS to promote tumorigenesis [21,22]. One day after exposure to AOM/DSS mice were treated for 10 weeks with PBS or berberine (40 mg/kg, a dose previously shown to prevent inflammation [23]) (Supplementary Figure 2). Berberine treated mice showed a 60% reduction (P=0.009) in tumor number compared to the PBS treated group. Furthermore, the tumors that developed in the berberine treated mice were significantly smaller compared to the tumors in the control group (Table 1). Histopathological analysis of the tumors revealed that mice in both groups developed multifocal tubular colon adenomas with focal high grade dysplasia. Unlike previously described studies [21,22], tumors in this study were premalignant and did not develop into adenocarcinomas. In addition, the length of colons after AOM/DSS treatment, a marker for evaluating colitis associated loss of elasticity, showed that the colons from the berberine treated mice were significantly longer than the colons from PBS treated mice (Table 1).
Table 1.
Effect of berberine on tumor multiplicity (Mean ± SEM)
| Mice N | Tumor Number N/mouse | Tumor size† |
Colon Length (cm) | |||
|---|---|---|---|---|---|---|
| <2mm | 2-4mm | >4mm | ||||
| Control (PBS) | 10 | 13.6±2.4 | 9.6±1.7 | 3.3±0.7 | 3.3±0.7 | 5.4±0.2 |
| Berberine | 10 | 5.4±1.3 | 5.0±1.1 | 0.2±0.2 | 0.0±0.0 | 6.3±0.4 |
| P Group Differences* | 0.0009 | 0.05 | 0.001 | 0.02 | 0.03 | |
FVB mice were treated with 40mg/kg berberine or PBS vehicle for 10 weeks, then collected the colon tissue and observed tumor numbers and measured tumor size under microscopy. Data was presented are mean ± SEM.
P-values are compared to PBS vehicle.
Average tumor numbers per mouse classified by tumor size. 95% Confidence Interval for different tumor size range: tumor size <2mm, 2.46-13.46; tumor size 2-4mm, –0.25-4.99; tumor size>4mm, 0.00-1.29.
Berberine Inhibits AOM/DSS-induced Inflammation and Proliferation
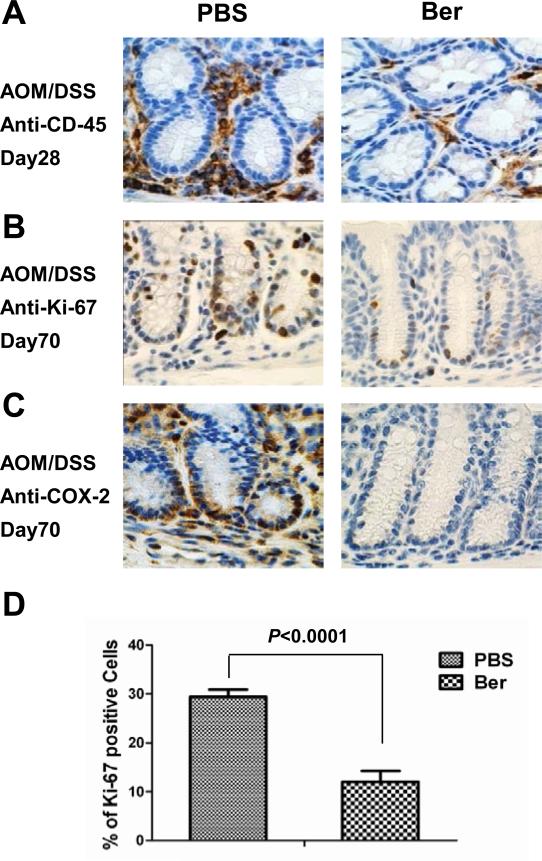
One cycle of DSS exposure is sufficient to produce an inflammatory response in FVB mice [22]. Early onset of DSS-induced inflammation was inhibited by berberine as shown by CD-45 expression, a receptor-linked protein tyrosine phosphatase expressed on leukocytes (Figure 1A). COX-2 is up-regulated in a variety of malignancies and has been linked to the process of tumorigenesis, mainly due to its role in the production of pro-inflammatory prostanoids during the late stages of tumorigenesis [24,25]. We found COX-2 expression to be significantly decreased in berberine treated animals on day 70 (Figure 1C).
Figure 1. Berberine treatment decreases colitis associated inflammation and aberrant proliferation.
Immunohistochemistry shows berberine reduced (A) CD-45+ leucocyte infiltration, at day 28 and (B) Ki-67+ aberrant proliferation and (C) COX-2 expression at day 70 post-AOM/DSS. Photomicrographs depict a representative section of colon with 5 mice in each group used for analysis. (D) Ki-67 expression quantification from immune-stain at day 70. P value <0.05 was considered statistically significant.
Tumorigenesis induced by AOM/DSS results in aberrant proliferation in colon crypts [26]. On day 70 the AOM/DSS treated mice showed increased proliferation in the crypts as detected by Ki-67 immunohistochemistry (IHC) staining (Figure 1B). Berberine suppressed expression of epithelial proliferation marker Ki-67 after AOM/DSS exposure by 60% compared to the PBS control (Figure 1D). In the berberine treated mice, Ki-67 expression was primarily in the basal portion of the colon crypt that contains the multipotent stem cells. Interestingly, berberine had no effect on AOM/DSS induced activation of β-catenin (data not shown), suggesting that berberine inhibition of aberrant proliferation in the crypts occurs downstream of activated β-catenin. Taken together, berberine inhibits AOM/DSS-induced inflammation and proliferation, preventing tumorigenesis.
Berberine Inhibits Growth and Induces Apoptosis of CRC Cells In Vitro
In order to elicit possible chemopreventive mechanisms of berberine, we treated three colon cancer cell lines with varied doses of berberine (0-100 μmol/L) for 24, 48 and 72 hours. Berberine treatment caused a concentration and time-dependent inhibition of cell growth in HCT116, SW480 and LOVO colorectal carcinoma cells (Supplementary Figure 3A, B and C). Berberine did not inhibit proliferation in the non-tumor cell line HEK293 (data not shown). These results suggest that berberine has strong effect on colon cancer cell proliferation.
To determine the role of programmed cell death on the growth inhibitory effects of berberine, HCT116 and SW480 cells were treated with berberine and followed by staining with FITC Annexin Ⅴ staining kit. We found that berberine treatment resulted in a dose dependent increase the number of apoptotic HCT116 and SW480 cells (Supplementary Figure 3D, E). These results suggest that the inhibition of CRC cell number by berberine is at least in part due to an increase in programmed cell death.
Berberine Activates AMPK and Acetyl-CoA Carboxylase (ACC) in CRC Cells
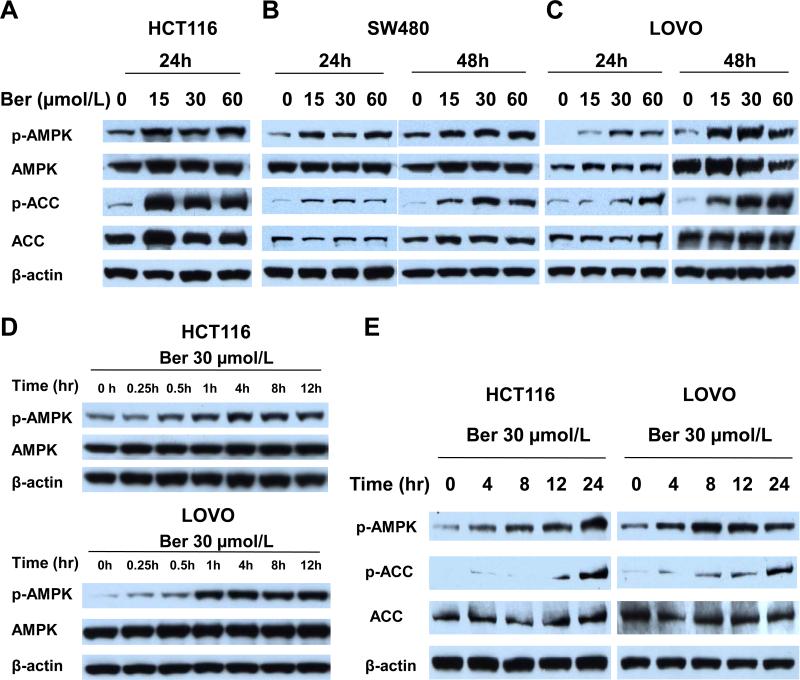
Studies have shown that berberine has beneficial effects in the treatment of diabetes and obesity and that these beneficial effects are at least in part mediated by AMPK activity [27]. To determine if berberine inhibition of colon carcinogenesis is linked to the metabolic signal pathway of AMPK, we measured the effects of berberine (0, 15, 30 and 60 μmol/L) on AMPK activation in 3 CRC cell lines. Berberine increased phosphorylation of AMPK at Thr172, which reflects AMPK activation, at 24 hours in HCT116 and at 24 and 48 hours in SW480 and LOVO cells (Figure 2A, B and C). HCT116 cells treated with berberine for 48 hours showed significant loss of attachment to the dish. The 15 μmol/L treatment was able to activate AMPK in all three lines, with LOVO cells showing the least activity. Phosphorylation of acetyl-CoA carboxylase (ACC), a well-established AMPK substrate and indicator of AMPK enzyme activity, was increased in berberine treated cells at similar doses (Figure 2A, B and C).
Figure 2. Berberine activates AMPK in three CRC cell lines.
HCT116, SW480 and LOVO cells were seeded as 2×104 in 100 mm plates and after 24 hours were treated with 0.1% DMSO, 15, 30, or 60 μmol/L berberine. After 24 and 48 hours of treatment, extracts were analyzed by Western blotting analysis. (A-C) Total and phosphorylated AMPK and ACC. Representative blots of three colon cancer cell lines HCT116 (A) SW480 (B) and LOVO cell (C). Blots are representative of three independent experiments. (D, E) Activation of AMPK by berberine begins as early as 1 hour post berberine treatment. Time course activation of AMPK (D) and ACC (E) in HCT1116 and LOVO cells treated with 30 μmol/L berberine from 0 hour to 24 hours and analyzed by Western blot. Phospho-AMPK (p-AMPK) and phospho-ACC (p-ACC). Blots are representative of three independent experiments.
In a time-dependence experiment HCT116 and LOVO cells were treated with 30 μmol/L berberine from 0 to 24 hours. AMPK phosphorylation was detected as early as 30 min after berberine treatment (Figure 2D, E) and continued to increase up to 24 hours. Consistent with activation of AMPK, ACC phosphorylation increased over time, but lagged behind AMPK phosphorylation (Figure 2E). The immediate activation of AMPK by berberine suggests direct interaction with the AMPK signaling pathway.
Activation of AMPK by Berberine is Independent of LKB1 in CRC Cells
Liver Kinase B1 (LKB1) is the primary upstream kinase that phosphorylates Thr172 on AMPK [28-30]. To determine if berberine activation of AMPK was dependent on LKB1, we measured LKB1 activity (phosphorylation) in HCT116, SW480, and LOVO cells. Berberine treatment had no apparent effect on phosphorylation of LKB1 (Supplementary Figure 4A). We also determined that berberine had no apparent effect on phosphorylation of calcium/calmodulin-dependent protein kinase (CaMKK) (data not shown). These findings indicate that berberine activation of AMPK in CRC cell lines is independent of LKB1 and CaMKK.
Berberine Inhibits mTOR Signaling in CRC Cells
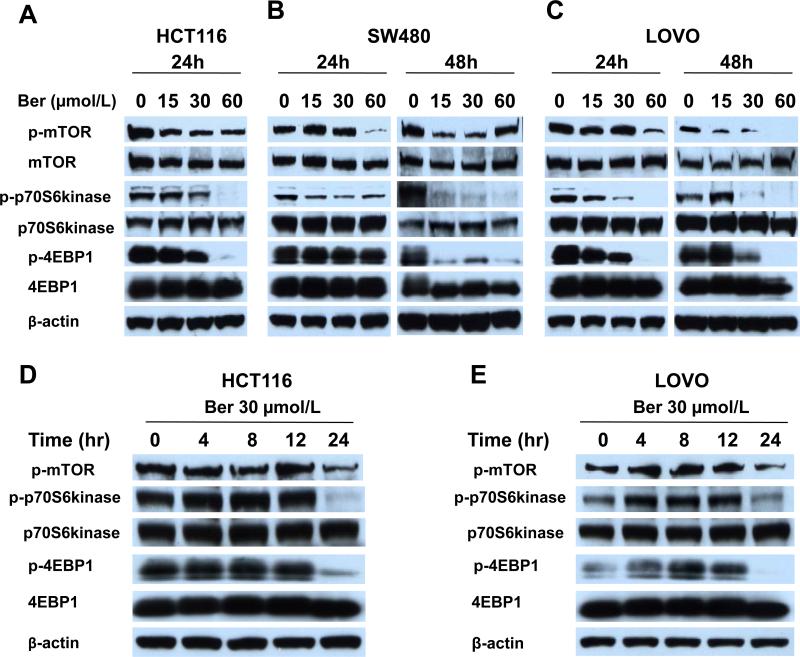
mTOR signaling controls cell survival and regulates metabolism [31]. As AMPK activation not only reprograms metabolism, it also enforces a metabolic checkpoint on the cell growth through its effects on p53 and mTOR signaling, suggesting AMPK-activating drugs might be useful for cancer therapeutics. We explored effects of berberine on the mTOR signal pathway. Berberine inhibited mTOR activity all 3 CRC lines (Figure 3A, B and C). In HCT116, 15 μmol/L berberine was sufficient to inhibit mTOR activation within 24 hours. Consistent with the decreased mTOR activity, phosphorylation of p70S6kinase and of 4EBP1 decreased in a dose-dependent manner (Figure 3A, B and C). Treating HCT116 and LOVO cells with 30 μmol/L berberine from 0 to 24 hours showed that the inhibition of mTOR phosphorylation and its downstream targets lagged behind activation of AMPK with the greatest inhibition of activity seen at 24 hours after berberine exposure (Figure 3D, E). These results suggest that prolonged activation of AMPK leads to inhibition of mTOR activity.
Figure 3. Berberine inhibits mTOR activity for three CRC cell lines.
HCT1116 (A) SW480 (B) LOVO (C) cells were treated with 0.1 % DMSO, 15, 30 or 60 μmol/L berberine for 24 and 48 hours. (D, E) HCT116 and LOVO cells were treated with 30 μmol/L berberine for 0 to 24 hours. Cells were lysed and analyzed by Western blotting for total and phosphorylated mTOR, p70S6kinase and 4EBP1. Representative blots from three independently performed experiments are shown. Phospho-mTOR (pmTOR), phospho-p70S6kinase (p-p70S6kinase) and phospho-4EBP1 (p-4EBP1).
Berberine does not Inhibit AKT and ERK Activity in CRC Cells
Misregulation of AKT and ERK signaling pathways can affect mTOR activity, cell growth and tumorigenesis. Berberine had no apparent inhibitory effect on AKT or ERK activity in all three CRC cell lines (Supplementary Figure 4B). In fact there appeared to be a slight increase in AKT and ERK phosphorylation, which maybe the results of inhibition of the mTOR feedback regulation of AKT activity [32]. Similar results were shown for AICAR, which is also an activator of AMPK [30].
Activation of AMPK by Berberine Inhibits COX-2 Activity and Increases p53 Phosphorylation In Vitro
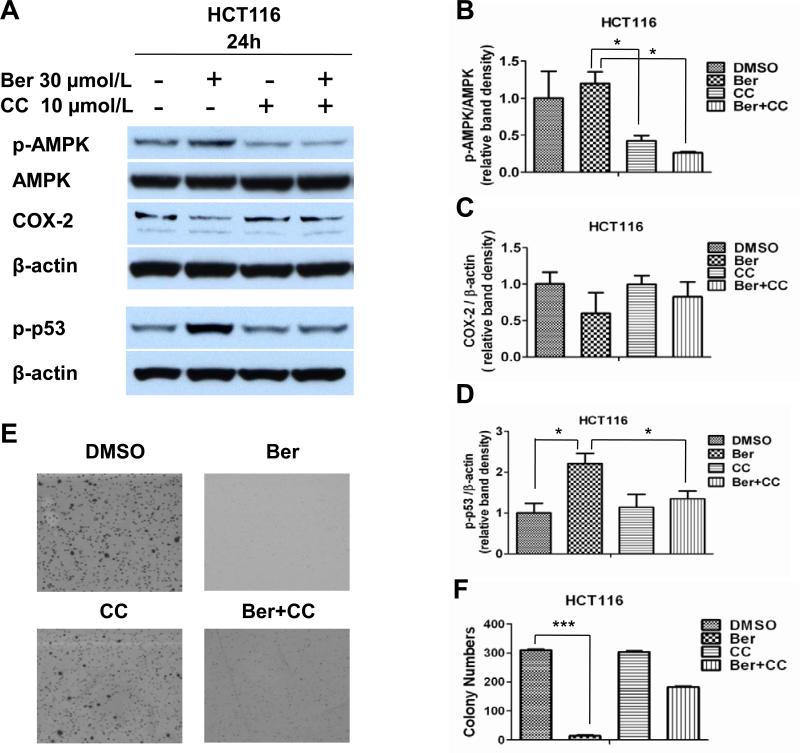
COX-2 plays a causal role in colorectal cancer development in the AOM/DSS tumor induction model [33]. As previously shown by Ishikawa and Herschman [33], COX-2 expression was detected primarily in the stroma (Figure 1C). Berberine inhibited COX-2 expression in AOM/DSS treated mice (Figure 1C). Although COX-2-expression in the AOM/DSS model in mainly seen in macrophages, fibroblasts and endothelial cells, other models of CRC and in human CRC, COX-2 expression is detected in epitheal tumors [33]. To determine if COX-2 expression is dependent on AMPK activation, we treated HCT116 cells with berberine and Compound C, an AMPK inhibitor. Berberine induced AMPK activation and inhibited COX-2 expression (Figure 4A, B and C). Compound C decreased AMPK phosphorylation in untreated and berberine treated cells and reversed the inhibition of COX-2 expression in the berberine treated cells, indicating that COX-2 expression in CRC cells is regulated by AMPK.
Figure 4. Berberine inhibition of COX-2 expression and anchorage independent growth, and induction of p53 phosphorylation are dependent on AMPK activation.
(A-C) HCT116 cells were treated with vehicle, berberine, Compound C or berberine plus Compound C for 24 hours. Cells were lysed and analyzed for total and phosphorylated AMPK and p53, and for COX-2 expression (A) Densitometry analyses of Western blots were determined by Image J to quantify the relative protein levels of (B) p-AMPK versus AMPK and (C) COX-2 and (D) p-p53 verses β-actin. Three independent experiments were analysis. *p<0.05 versus berberine treated cells. (E, F) HCT116 cells were treated with vehicle, berberine, Compound C, or berberine plus Compound C for 10 days in soft agar and the average colony numbers were counted. Column, mean of triplicate samples, bars, standard deviation, ***p<0.001 versus DMSO control cells. Vehicle (DMSO 0.1%), berberine (Ber), Compound C (CC) and combination berberine and Compound C (Ber+CC).
Previous studies have shown that AMPK activation can induce phosphorylation of p53 [34,35]. We tested the ability of berberine to phosphorylate p53 by treating HCT116 colorectal cancer cells with 0, 15, 30 and 60 μmol/L of berberine. We found that 15 μmol/L berberine was sufficient to increase phosphorylation of p53 without increasing p53 expression (data not shown). To determine if berberine-induced p53 phosphorylation was AMPK dependent, we treated HCT116 cells with 30 μmol/L berberine or a combination or berberine and compound C. Compound C reversed the berberine induced phosphorylation of p53 (Figure 4A, D), suggesting that in HCT116 CRC cells, berberine induced phosphorylation of p53 is AMPK dependent.
Activation of AMPK by Berberine Inhibits oncogenic phenotypes of CRC Cells
Expression of phosphorylated AMPK is associated with increased colorectal cancer-specific survival [35] and AMPK activators, metformin and AICAR, have been shown to reduce tumor growth in mouse xenografts [36]. To determine if the oncogenetic phenotype of CRC cells can be inhibited by activation of AMPK, we measured the effects of berberine on the migration and invasion capabilities of HCT116 cells. In a migration (scratch) assay and Matrigel invasion assay, treating CRC cells with different doses of berberine had little to no effect on migration or invasion (data not shown). In anchorage independent growth assays, however, berberine inhibited colony formation of HCT116 by 90% (P<0.001) compared with DMSO control group (Figure 4E and F). Inhibition of basal AMPK activity with Compound C had little effect on colony formation, suggesting the small amount of activated AMPK in untreated cells has little effect on regulating tumorigenesis. Interestingly, inhibition of berberine dependent activation of AMPK only partially restored growth in agar, suggesting that berberine inhibition of anchorage independent growth of HCT116 cells is only partially dependent on AMPK activation.
Berberine Inhibits NF-κB Translocation and Activates Cleaved-caspase-3
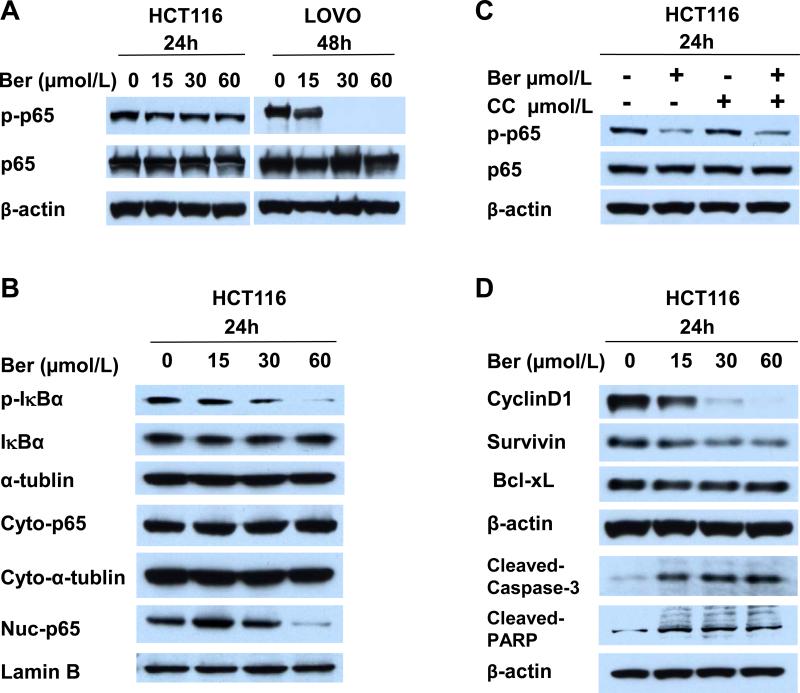
We found that inhibition of anchorage independent growth by berberine treatment was only partially reversed by AMPK inhibitor Compound C, suggesting that activating AMPK is not the only mechanism for anti-cancer activity of berberine. NF-κB activation is associated with the increased survival of cancer cells and resistance to chemotherapy, and berberine has been shown to suppress NF-κB activity [23,37]. Figure 5 A shows that berberine inhibited phosphorylation of NF-κB p65 in HCT116 and LOVO cells. Phosphorylation of IκBα, which releases p65 from sequestration in the cytoplasm, and nuclear translocation of p65 were also inhibited by berberine (Figure 5B). Berberine inhibition of p65 phosphorylation was not reversed by Compound C (Figure 5C) indicating that inhibition of NF-κB activity is independent of AMPK activation in HCT116 cells. Berberine also inhibited expression of cyclin D1 and survivin (Figure 5D), both of which are associated with increased tumorigenesis.
Figure 5. Berberine inhibits NF-κB activity and activates cleaved-caspase-3.
(A-B) Berberine inhibits phosphorylation of p65. HCT116 and LOVO cells were treated with 0 to 60 μmol/L berberine for 24 and 48 hours. (A) Total p65 and phosphorylated p65 (pp65) analyzed by Western blot. (B) Berberine inhibits phosphorylation of IκBα and nuclear localization of p65. HCT116 cells were treated with 0 to 60 μmol/L berberine for 24 hours and the cytoplasmic extracts were analyzed for total and phosphorylated IκBα levels. Cytoplasmic and nuclear extracts were analyzed for cytoplasmic (Cyto-p65) and nuclear (Nuc-p65) localization of p65. α-tubulin and Laminin B were used as cytoplasmic and nuclear markers respectively. Blots are representatives of three independent experiments. (C) Activation of NF-κB is AMPK independent. HCT116 cells were treated with DMSO, berberine, Compound C, and berberine plus Compound C for 24 hours. Total and phosphorylated p65 was analyzed Western blot. Blots are representatives of three independent experiments. (D) Expression of candidate NF-κB targets Cyclin D1 and Survivin were inhibited by berberine. HCT116 cells were treated with 0 to 60 μmol/L berberine for 24 hours. Cyclin D1, Survivin and Bcl-xL expression was analyzed by Western blot. Berberine activated cleavage of caspase-3 and PARP. HCT116 cells were treated with 0 to 60 μmol/L berberine for 24 hours. Cleaved caspase-3 and PARP were analyzed by Western blot. Blots are representatives of three independent experiments.
Berberine's anti-cancer activity in human epidermoid carcinoma is mediated through activation of caspase-3-dependent apoptosis [38]. Cleaved-caspase-3 and cleaved poly (ADP-ribose) polymerase (PARP) were activated by berberine treatment in HCT116 (Figure 5D). These data suggest that berberine has multiple anti-carcinogenic activities.
Berberine Activates AMPK and Inhibits mTOR In Vivo
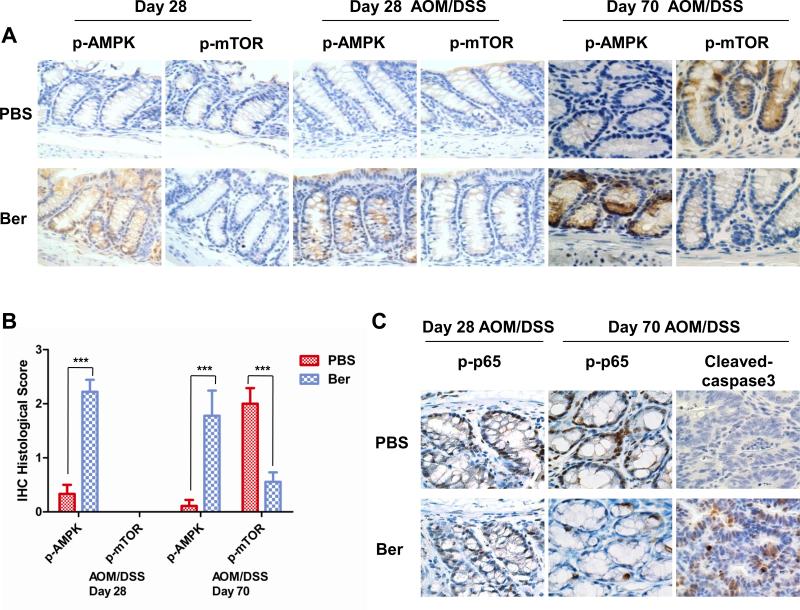
To determine if the berberine induced activation of AMPK and inhibition of mTOR signaling seen in vitro can be translated to our mouse model of colon cancer, we measured AMPK and mTOR activity in AOM/DSS treated mice and quantified the immunohistochemistry slides. We found that berberine activated AMPK in both control and AOM/DSS treated mice after 14 days of berberine treatment (Day 28 after AOM) (Figure 6A, B). Interestingly, we detected little to no phosphorylation of mTOR at this time point after AOM/DSS exposure (Figure 6A, B). By day 70, however, mTOR phosphorylation was readily detected in the epithelial tissue of the control mice treated with AOM/DSS (Figure 6A, B), suggesting that mTOR is not activated in the acute inflammatory stage shortly after DSS but is activated by chronic inflammatory seen at later times after DSS exposure. More importantly, in the berberine treated mice where AMPK was strongly activated, mTOR phosphorylation was inhibited (Figure 6A, B). Berberine also induced a slight increase (not significant) in phospho-AMPK in liver, a major site for metabolism and AMPK activity (Supplementary Figure 4C), however, there was little to no effect on mTOR activity in the liver (data not shown). These findings suggest that berberine activates AMPK at an earlier time and continues to regulate AMPK activity and inhibit mTOR at later times in colon tissue of AOM/DSS induced colorectal carcinogenesis.
Figure 6. Berberine activates AMPK signal pathway and inhibits NF-κB in vivo.
(A) AMPK was activated by berberine in AOM/DSS untreated and treated mice. Immunohistochemistry staining with phospho-AMPK (p-AMPK), phospho-mTOR (pmTOR) of colon tissue on Day 28 showed berberine induced activation of AMPK. Day 70 showed sustained activation of AMPK and inhibition of AOM/DSS induced mTOR activity in berberine treated mice. Representative pictures (60×) of each group are shown. Colon tissues from 5 mice in each group were analyzed. (B) Immunohistochemical phenotypes were assessed by a semi-quantitative scoring method classified into four categories: negative=0, poorly positive=1, positive =2 and strongly positive=3 according to the intensity of staining and specificity. Data are shown as the mean score for 5 mice with standard deviation, ***p<0.001 versus PBS treatment group. (C) Immunohistochemistry staining with phospho-p65 (p-p65) showed inhibition of p-p65 by berberine 70 days after AOM/DSS treatment. Berberine induced cleavage of caspase-3 was also detected by immunohistochemistry at day 70. Representative pictures (60×) are shown. Colon tissues from 5 mice in each group were used for analysis.
Berberine Inhibits NF-κB Activity and Induces Caspase-3 cleavage In Vivo
In CRC cells, berberine inhibited NF-κB p65 phosphorylation and activated caspase-3 cleavage (Figure 5A, D). In AOM/DSS treated mice, phosphorylation of p65 was detected as early as day 28 in both PBS and berberine treated mice (Figure 6C). At later times, when activation of NF-κB remained high in the PBS treated mice, berberine treatment produced a decrease in phosphorylation of p65 (Figure 6C). Finally, berberine induced cleavage of caspase-3 in AOM/DSS treated mice (Figure 6C). These findings indicate that berberine inhibits colitis-associated colorectal carcinogenesis through multiple mechanisms.
Discussion
Berberine, one of the main alkaloids of Rhizoma coptidis, offers a rich source of potential chemopreventive and therapeutic properties [39,40]. In a mouse model of colorectal cancer, we evaluated the efficacy of berberine for preventing colorectal cancer. In this model, berberine reduced leucocytes infiltration, epithelial proliferation and tumor burden in mice treated with AOM/DSS. COX-2 expression and activation of mTOR and NF-κB, induced by AOM/DSS, was also inhibited by berberine in vivo (Figure 7). Furthermore, berberine induced activation of AMPK both in vivo and in vitro. In CRC cells, berberine inhibition of mTOR activity, COX-2 expression and activation of p53 phosphorylation were found to be dependent on AMPK activation. Berberine did not reduce ERK or AKT activity and activation of AMPK by berberine was independent of LKB1 in CRC cell lines. Berberine also activated cleavage of caspase-3 in vitro and in colon tissue from AOM/DSS treated mice. These results indicate that the anti-inflammation and anti-cancer activities of berberine are, at least in part, due to activation of AMPK, which inhibits mTOR activity, COX-2 expression and activates phosphorylation of p53. The anti-tumor and chemopreventive effects of berberine appear to be mediated by activating AMPK and inhibiting NF-κB.
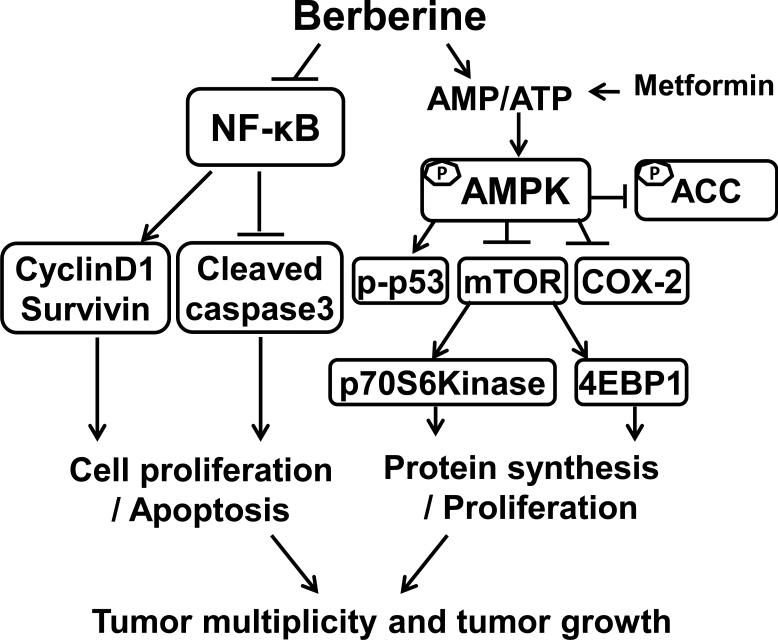
Figure 7. Mechanisms of berberine in suppressing colon cancer.
Berberine activated AMPK negatively regulates the mTOR signal pathway, resulting in inhibition of colon cancer proliferation and growth. Activated AMPK negatively regulates COX-2 in colon cancer. Activation of AMPK also induces phosphorylation of p53 which is a tumor suppressor. Inhibition of tumorigenesis by berberine is partially dependent on inhibition of the NF-κB signaling pathway. This NF-κB suppression results in inhibiting transcription of NF-κB targets CyclinD1 and Survivin and stimulating the cleavage of caspase-3, modulating cell proliferation and apoptosis. Interactions leading to activation of molecular targets are indicated by arrows; those that inhibited are indicated by a bar. Berberine modulation of both pathways leads to inhibition of tumor multiplicity and tumor growth.
AMPK Functions as a Tumor Suppressor in Colorectal Carcinogenesis
AMPK is an energy sensor, which can be activated by many factors, such as low glucose levels, fasting, and exercise [41-43]. In various cancer cells a loss of AMPK signaling has been demonstrated and AMPK activation has been proposed as a therapeutic approach to reduce cancer cell growth [4]. Activation of AMPK in response to energy deficiencies leads to the inhibition of pathways of energy utilization and stimulation of pathways of energy production [44]. AMPK also regulates inflammation and activation of AMPK can reduce inflammation [34]. AMPK activation by anti-inflammatory drugs can suppress tumor carcinogenesis and inflammation [42]. Activation of AMPK has been linked to increased CRC survival [13], while the loss of AMPK activation in cancer cells has been demonstrated in numerous studies [4]. Berberine has beneficial effects in the treatment of diabetes and obesity, and these effects have been linked to AMPK activity [25]. LKB1 is a tumor suppressor and a major mediator of the cellular response to energy stress [41]. LKB1 activation of AMPK regulates a wide range of cellular functions that include metabolism, proliferation and cell shape [45]. Berberine activated AMPK and inhibited colon carcinogenesis in AOM/DSS induced CRC by an LKB independent mechanism.
AMPK Functions as a Tumor Suppressor by Negatively Regulating mTOR
Aberrant mTOR signaling has been long implicated in CRC [46]. mTOR signaling regulates cell growth and proliferation by regulating the energy status of the cell [45]. AMPK negatively regulates mTOR via TSC1/2-dependent and independent mechanisms, and by regulating energy levels, which serves as a checkpoint to allow cellular proliferation only when energy levels are sufficient [47]. Inactivation of mTOR has previously been demonstrated to be important for the ability of AMPK to enforce a metabolic checkpoint [48,49]. NSAIDs and known AMPK activators such as metformin, have been shown to inhibit mTOR and decreased CRC growth in vitro and in CRC animal models [41]. We showed that, similar to metformin, berberine inhibited mTOR activation both in vitro and in vivo. Interestingly, we found that while AMPK as activated at early times, little or no mTOR activity was detected at this time. Activated mTOR was detected at later times in AOM/DSS treated mice. These data suggest that AMPK will inhibit mTOR activation and prevent progression of progression of colitis associated cancer.
Berberine has Anti-inflammatory and Anti-cancer Properties
This study provides further evidence for the efficacy of berberine in preventing CRC. Berberine is a potent anti-inflammatory agent and may prevent colorectal cancer by modulating inflammation. Previous studies have shown that berberine reduced DSS-induced colitis, preventing DSS-induced weight loss, myeloperoxidase activity and acute epithelial damage [50]. We have extended these results to show that, in addition to inhibition of inflammation, berberine inhibits tumorigenesis in AOM/DSS treated mice. We suspect the protective effect may involve targeting NF-κB or AMPK. We show that berberine decreased NF-κB activity at earlier and later times, suggesting that targeting NF-κB is responsible for inflammation protection. NF-κB is a pro-inflammatory transcription factor that is activate in colitis associated tumorigenesis [51]. Berberine suppresses IκBα phosphorylation, NF-κB p65 phosphorylation and nuclear localization, and to inhibit expression of NF-κB target genes in lung adenocarcinoma, leukemia and multiple myeloma cell lines [23]. These authors concluded that the effects of berberine may be mediated in part through the suppression of the NF-κB activation. We showed that berberine inhibited NF-κB activity in colitis associated CRC in vivo and in CRC cells in vitro and that inhibition of NF-κB in CRC cells was independent of AMPK activity.
In addition to its anti-inflammation activity, berberine has anti-cancer properties. Previous study shown that berberine suppressed colon tumor growth in the HT-29 cell xenograft mouse model through inhibiting epidermal growth factor receptor activity and proliferation [16]. In human epidermoid carcinoma berberine induced caspase-3-dependent apoptosis [38]. We used the AOM/DSS induced colorectal carcinoma mouse model to evaluate the effects of berberine on tumor promotion and progression of CRC. We found that berberine decreased tumor numbers and suppressed tumor growth by decreasing proliferation (Figure 1) and increasing apoptosis (Figure 6C).
Summary
Activation of AMPK plays key role on cancer treatment and cancer prevention. AMPK can be activated by many different factors, including phosphorylation by LKB1 or calcium/calmodulin-dependent protein kinase (CaMKK), by a high AMP/ATP ratios or low glucose levels, and by small molecules or natural products. While AMPK is activated by hormones, cytokines, pharmacological agents and natural products, the mechanisms by which these agents activate AMPK are not fully understood [52]. It was reported that berberine, like metformin, activates AMPK by inhibition the respiratory chain complex 1 in the mitochondria, decreasing ATP level [53,54]. While we detected a small but not significant decrease in ATP with berberine (data not shown), the mechanism for berberine induce activation of AMPK in CRC is unclear.
Berberine inhibits colon carcinogenesis by AMPK dependent and AMPK independent mechanisms (Figure 7). The AMPK dependent mechanisms includes inhibition of mTOR signaling and inducing phosphorylation of p53, while the AMPK independent mechanisms include the inhibition of NF-κB activity, and its downstream targets cyclin D1 and survivin. Thus, berberine has multiple targets and may serve as an alternative therapy to prevent and/or treat colorectal cancer, alone or in combination with conventional therapies.
Supplementary Material
Acknowledgement
The authors thank Lisa Dodge and Craig Driver from the Laboratory Animal Sciences Program, Leidos Biomedical Research, Inc. for animal handling and sample collection. We thank Dr. Jeffrey D. White, Office of Cancer Complementary and Alternative Medicine, National Cancer Institute for support of this study. We thank Dr. Gerd Bobe, Linus Pauling Institute, for critical review. This work was supported by National Cancer Institute, National Institutes of Health intramural funding (ZIA BC 011159), National Natural Science Foundation of China (No.81273718 and 81102587), China Postdoctoral Science Foundation (No.20110490559 and 2012T50199).
Abbreviations
- AMPK
AMP-activated protein kinase
- AOM
azoxymethane
- DSS
dextran sulfate sodium
- CRC
Colorectal cancer
- UC
Ulcerative colitis
- CD
Crohn's Disease
- mTOR
mammalian target of rapamycin
- 4EBP1
eukaryotic translation initiation factor 4E binding protein 1
- ACC
acetyl-CoA carboxylase
- LKB1
Liver Kinase B1
- COX-2
Cyclooxygenase 2
- NF-κB
nuclear factor kappaB
Footnotes
Conflict of Interest: There are no conflicts of interest.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. American journal of physiology Gastrointestinal and liver physiology. 2004;287(1):G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 3.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. The Biochemical journal. 2007;403(1):139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fogarty S, Hardie DG. Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochimica et biophysica acta. 2010;1804(3):581–591. doi: 10.1016/j.bbapap.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Fay JR, Steele V, Crowell JA. Energy homeostasis and cancer prevention: the AMP-activated protein kinase. Cancer prevention research. 2009;2(4):301–309. doi: 10.1158/1940-6207.CAPR-08-0166. [DOI] [PubMed] [Google Scholar]
- 6.Brunn GJ, Hudson CC, Sekulic A, et al. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277(5322):99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- 7.Xie Q, Johnson BR, Wenckus CS, Fayad MI, Wu CD. Efficacy of berberine, an antimicrobial plant alkaloid, as an endodontic irrigant against a mixed-culture biofilm in an in vitro tooth model. Journal of endodontics. 2012;38(8):1114–1117. doi: 10.1016/j.joen.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Choi JH, Kim JB, et al. Berberine suppresses TNF-alpha-induced MMP-9 and cell invasion through inhibition of AP-1 activity in MDA-MB-231 human breast cancer cells. Molecules. 2008;13(12):2975–2985. doi: 10.3390/molecules13122975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Cao B, Liu X, et al. Berberine suppresses androgen receptor signaling in prostate cancer. Molecular cancer therapeutics. 2011;10(8):1346–1356. doi: 10.1158/1535-7163.MCT-10-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou H, Mineshita S. The effect of berberine chloride on experimental colitis in rats in vivo and in vitro. The Journal of pharmacology and experimental therapeutics. 2000;294(3):822–829. [PubMed] [Google Scholar]
- 11.Zhang H, Wei J, Xue R, et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism: clinical and experimental. 2010;59(2):285–292. doi: 10.1016/j.metabol.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 12.Turner N, Li JY, Gosby A, et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57(5):1414–1418. doi: 10.2337/db07-1552. [DOI] [PubMed] [Google Scholar]
- 13.Garrett CR, Hassabo HM, Bhadkamkar NA, et al. Survival advantage observed with the use of metformin in patients with type II diabetes and colorectal cancer. British journal of cancer. 2012;106(8):1374–1378. doi: 10.1038/bjc.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocha GZ, Dias MM, Ropelle ER, et al. Metformin amplifies chemotherapy-induced AMPK activation and antitumoral growth. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(12):3993–4005. doi: 10.1158/1078-0432.CCR-10-2243. [DOI] [PubMed] [Google Scholar]
- 15.Zakikhani M, Dowling RJ, Sonenberg N, Pollak MN. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer prevention research. 2008;1(5):369–375. doi: 10.1158/1940-6207.CAPR-08-0081. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Cao H, Lu N, et al. Berberine inhibits proliferation and down-regulates epidermal growth factor receptor through activation of Cbl in colon tumor cells. PloS one. 2013;8(2):e56666. doi: 10.1371/journal.pone.0056666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Washington MK, Powell AE, Sullivan R, et al. Pathology of rodent models of intestinal cancer: progress report and recommendations. Gastroenterology. 2013;144(4):705–717. doi: 10.1053/j.gastro.2013.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saud SM, Young MR, Jones-Hall YL, et al. Chemopreventive activity of plant flavonoid isorhamnetin in colorectal cancer is mediated by oncogenic Src and beta-catenin. Cancer research. 2013;73(17):5473–5484. doi: 10.1158/0008-5472.CAN-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawley SA, Fullerton MD, Ross FA, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336(6083):918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature reviews Molecular cell biology. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer science. 2003;94(11):965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young MR, Ileva LV, Bernardo M, et al. Monitoring of tumor promotion and progression in a mouse model of inflammation-induced colon cancer with magnetic resonance colonography. Neoplasia. 2009;11(3):237–246. 231. doi: 10.1593/neo.81326. following 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong T, Yang Z, Lv CF, Zhang Y. Suppressive effect of berberine on experimental dextran sulfate sodium-induced colitis. Immunopharmacology and immunotoxicology. 2012;34(3):391–397. doi: 10.3109/08923973.2011.609887. [DOI] [PubMed] [Google Scholar]
- 24.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18(55):7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 25.Williams C, Shattuck-Brandt RL, DuBois RN. The role of COX-2 in intestinal cancer. Annals of the New York Academy of Sciences. 1999;889:72–83. doi: 10.1111/j.1749-6632.1999.tb08725.x. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka T, Kohno H, Yoshitani S, et al. Ligands for peroxisome proliferator-activated receptors alpha and gamma inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer research. 2001;61(6):2424–2428. [PubMed] [Google Scholar]
- 27.Lee YS, Kim WS, Kim KH, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55(8):2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 28.Hawley SA, Boudeau J, Reid JL, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. Journal of biology. 2003;2(4):28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woods A, Johnstone SR, Dickerson K, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Current biology : CB. 2003;13(22):2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 30.Shaw RJ, Kosmatka M, Bardeesy N, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(10):3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 32.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer research. 2006;66(3):1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishikawa TO, Herschman HR. Tumor formation in a mouse model of colitis-associated colon cancer does not require COX-1 or COX-2 expression. Carcinogenesis. 2010;31(4):729–736. doi: 10.1093/carcin/bgq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoogendijk AJ, Pinhancos SS, van der Poll T, Wieland CW. AMP-activated protein kinase activation by 5-aminoimidazole-4-carbox-amide-1-beta-D-ribofuranoside (AICAR) reduces lipoteichoic acid-induced lung inflammation. The Journal of biological chemistry. 2013;288(10):7047–7052. doi: 10.1074/jbc.M112.413138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baba Y, Nosho K, Shima K, et al. Prognostic significance of AMP-activated protein kinase expression and modifying effect of MAPK3/1 in colorectal cancer. British journal of cancer. 2010;103(7):1025–1033. doi: 10.1038/sj.bjc.6605846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer research. 2007;67(14):6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 37.Kim HJ, Hawke N, Baldwin AS. NF-kappaB and IKK as therapeutic targets in cancer. Cell death and differentiation. 2006;13(5):738–747. doi: 10.1038/sj.cdd.4401877. [DOI] [PubMed] [Google Scholar]
- 38.Mantena SK, Sharma SD, Katiyar SK. Berberine inhibits growth, induces G1 arrest and apoptosis in human epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin cascade, disruption of mitochondrial membrane potential and cleavage of caspase 3 and PARP. Carcinogenesis. 2006;27(10):2018–2027. doi: 10.1093/carcin/bgl043. [DOI] [PubMed] [Google Scholar]
- 39.Goto H, Kariya R, Shimamoto M, et al. Antitumor effect of berberine against primary effusion lymphoma via inhibition of NF-kappaB pathway. Cancer science. 2012;103(4):775–781. doi: 10.1111/j.1349-7006.2012.02212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.James MA, Fu H, Liu Y, Chen DR, You M. Dietary administration of berberine or Phellodendron amurense extract inhibits cell cycle progression and lung tumorigenesis. Molecular carcinogenesis. 2011;50(1):1–7. doi: 10.1002/mc.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kottakis F, Bardeesy N. LKB1-AMPK axis revisited. Cell research. 2012;22(12):1617–1620. doi: 10.1038/cr.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493(7432):346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 43.Carlson CL, Winder WW. Liver AMP-activated protein kinase and acetyl-CoA carboxylase during and after exercise. Journal of applied physiology. 1999;86(2):669–674. doi: 10.1152/jappl.1999.86.2.669. [DOI] [PubMed] [Google Scholar]
- 44.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circulation research. 2007;100(3):328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 45.Hezel AF, Bardeesy N. LKB1; linking cell structure and tumor suppression. Oncogene. 2008;27(55):6908–6919. doi: 10.1038/onc.2008.342. [DOI] [PubMed] [Google Scholar]
- 46.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer cell. 2007;12(1):9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Svensson RU, Shaw RJ. Cancer metabolism: Tumour friend or foe. Nature. 2012;485(7400):590–591. doi: 10.1038/485590a. [DOI] [PubMed] [Google Scholar]
- 48.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 49.Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer cell. 2004;6(1):91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Yan F, Wang L, Shi Y, et al. Berberine promotes recovery of colitis and inhibits inflammatory responses in colonic macrophages and epithelial cells in DSS-treated mice. American journal of physiology Gastrointestinal and liver physiology. 2012;302(5):G504–514. doi: 10.1152/ajpgi.00312.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118(3):285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 52.Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell metabolism. 2009;9(5):407–416. doi: 10.1016/j.cmet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Hawley SA, Ross FA, Chevtzoff C, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell metabolism. 2010;11(6):554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin J, Gao Z, Liu D, Liu Z, Ye J. Berberine improves glucose metabolism through induction of glycolysis. American journal of physiology Endocrinology and metabolism. 2008;294(1):E148–156. doi: 10.1152/ajpendo.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.