Abstract
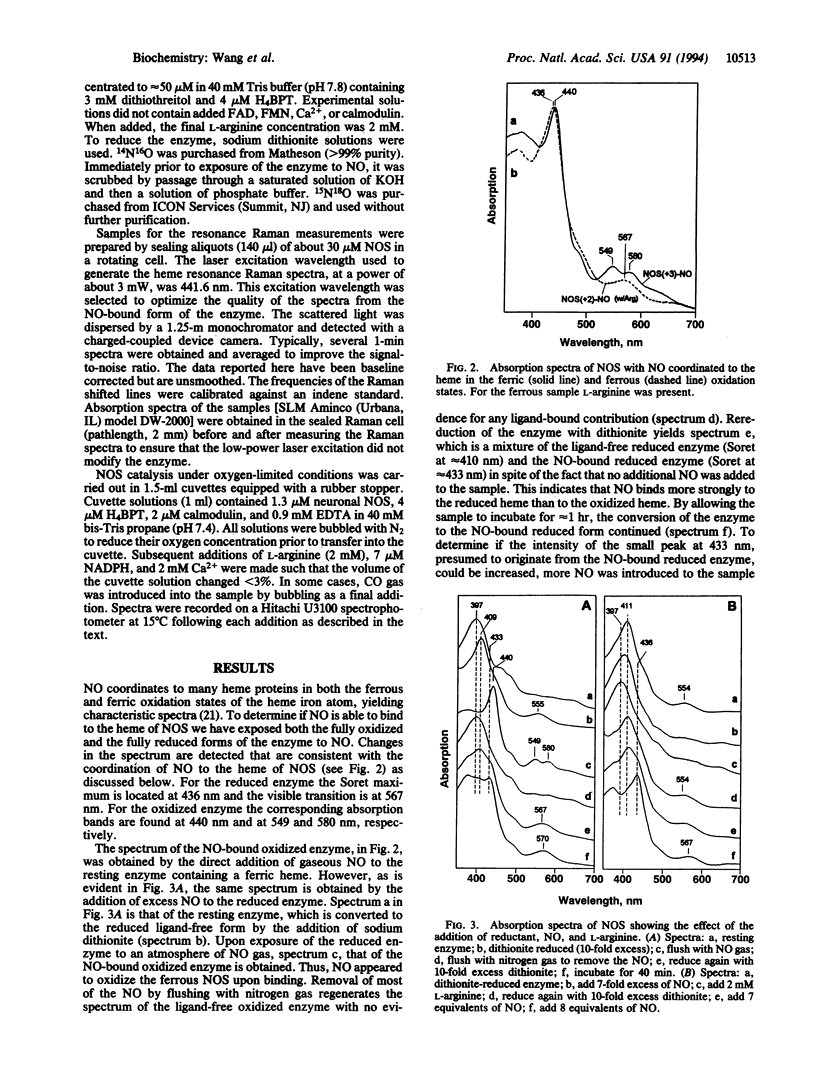
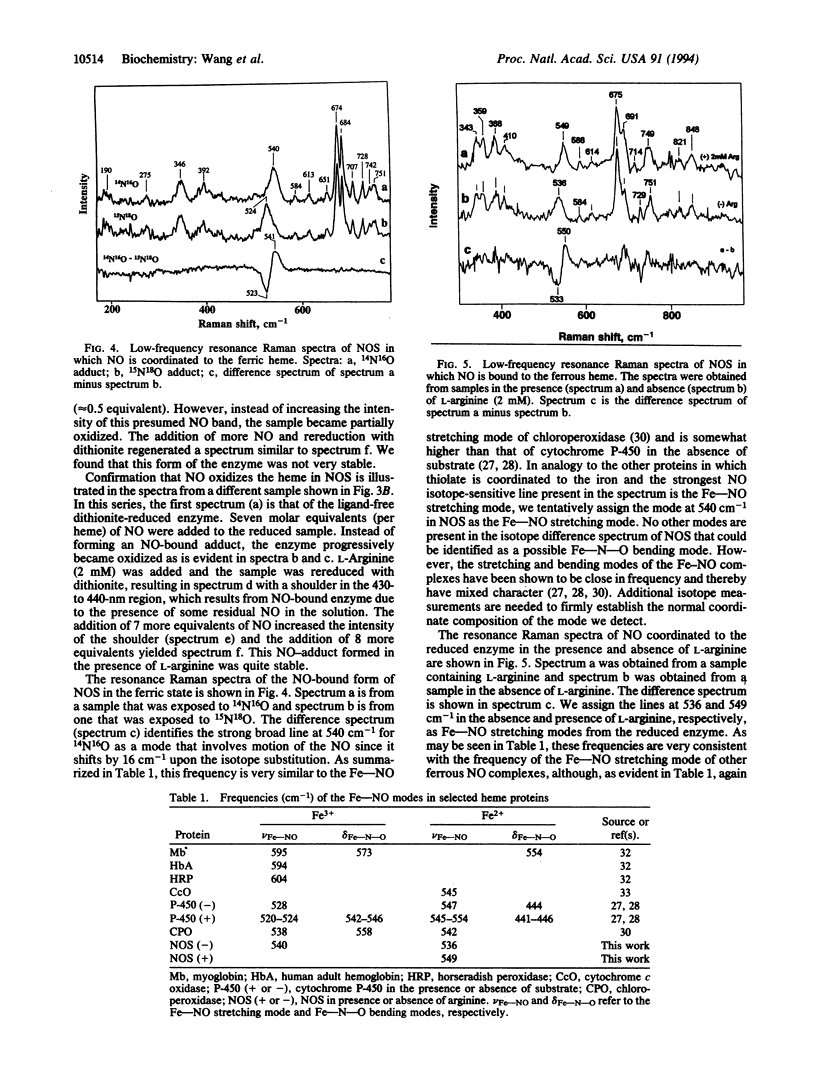
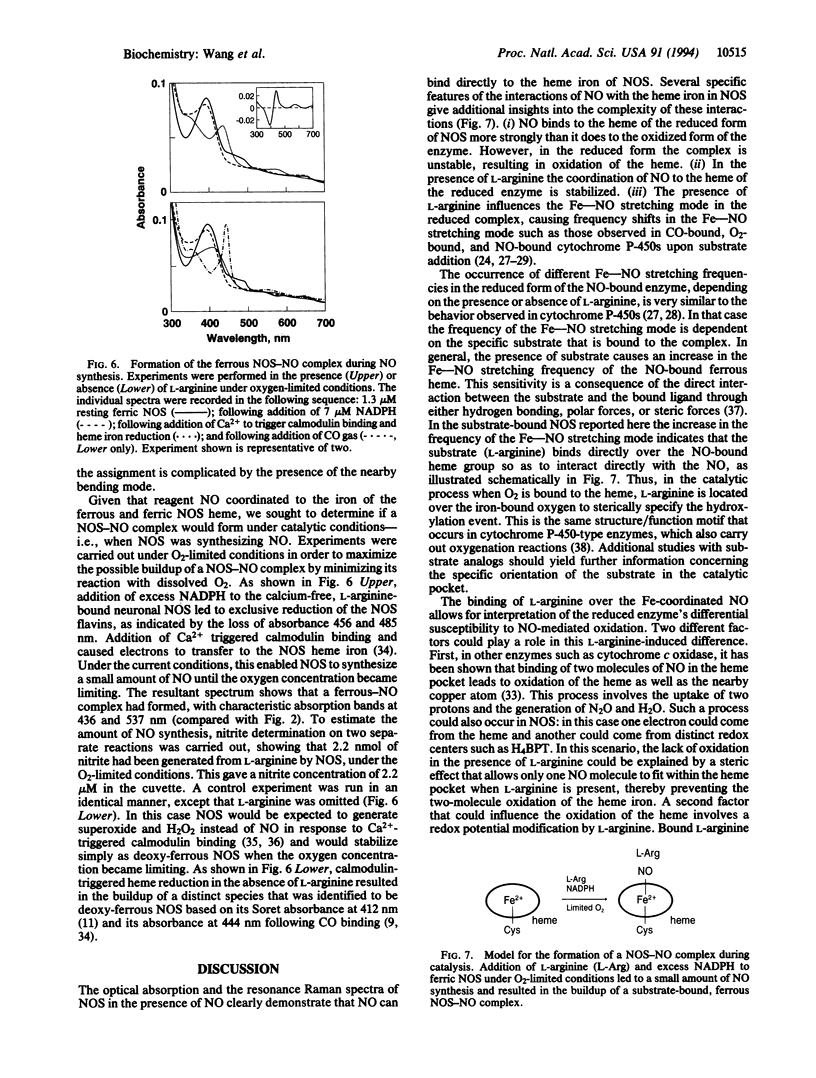
A current question in nitric oxide (NO) biology is whether NO can act as a feedback inhibitor of NO synthase (NOS). We have approached this problem by examining the interaction of NO with neuronal NOS by optical absorption and resonance Raman scattering spectroscopies. Under an inert atmosphere NO coordinated to the heme iron in both the oxidized and reduced forms of NOS. The Soret and visible optical absorption transitions are detected at 436 and at 567 nm, respectively, in the Fe(2+)-NO heme complex and at 440 nm and at 549 and 580 nm, respectively, in the Fe(3+)-NO heme complex. In the resonance Raman spectrum of the ferrous complex the Fe-NO stretching mode is located at 549 cm-1 in the presence of L-arginine and at 536 cm-1 in the absence of L-arginine, whereas in the ferric enzyme the mode is located at 540 cm-1 (in the absence of L-arginine). The interaction between bound L-arginine and the NO indicates that L-arginine binds directly over the heme just as do the substrates in cytochrome P-450s. In the absence of L-arginine, NO readily oxidized the ferrous heme iron. The oxidation was prevented by the presence of bound L-arginine and enabled NOS to form a stable ferrous NO complex. Under oxygen-limited conditions, NO generated by neuronal NOS coordinated to its heme iron and formed a spectrally detectable ferrous-NO complex. Taken together, our results show that NO can bind to both ferric and ferrous NOS and may inhibit NO synthesis through its binding to the heme iron during catalysis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu-Soud H. M., Stuehr D. J. Nitric oxide synthases reveal a role for calmodulin in controlling electron transfer. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10769–10772. doi: 10.1073/pnas.90.22.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benko B., Yu N. T. Resonance Raman studies of nitric oxide binding to ferric and ferrous hemoproteins: detection of Fe(III)--NO stretching, Fe(III)--N--O bending, and Fe(II)--N--O bending vibrations. Proc Natl Acad Sci U S A. 1983 Nov;80(22):7042–7046. doi: 10.1073/pnas.80.22.7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buga G. M., Griscavage J. M., Rogers N. E., Ignarro L. J. Negative feedback regulation of endothelial cell function by nitric oxide. Circ Res. 1993 Nov;73(5):808–812. doi: 10.1161/01.res.73.5.808. [DOI] [PubMed] [Google Scholar]
- Culotta E., Koshland D. E., Jr NO news is good news. Science. 1992 Dec 18;258(5090):1862–1865. doi: 10.1126/science.1361684. [DOI] [PubMed] [Google Scholar]
- Griscavage J. M., Rogers N. E., Sherman M. P., Ignarro L. J. Inducible nitric oxide synthase from a rat alveolar macrophage cell line is inhibited by nitric oxide. J Immunol. 1993 Dec 1;151(11):6329–6337. [PubMed] [Google Scholar]
- Han S., Ching Y. C., Rousseau D. L. Ferryl and hydroxy intermediates in the reaction of oxygen with reduced cytochrome c oxidase. Nature. 1990 Nov 1;348(6296):89–90. doi: 10.1038/348089a0. [DOI] [PubMed] [Google Scholar]
- Heinzel B., John M., Klatt P., Böhme E., Mayer B. Ca2+/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem J. 1992 Feb 1;281(Pt 3):627–630. doi: 10.1042/bj2810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Kincaid J. R. Heme active-site structural characterization of chloroperoxidase by resonance Raman spectroscopy. J Biol Chem. 1993 Mar 25;268(9):6189–6193. [PubMed] [Google Scholar]
- Klatt P., Schmidt K., Mayer B. Brain nitric oxide synthase is a haemoprotein. Biochem J. 1992 Nov 15;288(Pt 1):15–17. doi: 10.1042/bj2880015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt P., Schmidt K., Uray G., Mayer B. Multiple catalytic functions of brain nitric oxide synthase. Biochemical characterization, cofactor-requirement, and the role of N omega-hydroxy-L-arginine as an intermediate. J Biol Chem. 1993 Jul 15;268(20):14781–14787. [PubMed] [Google Scholar]
- Matsuoka A., Stuehr D. J., Olson J. S., Clark P., Ikeda-Saito M. L-arginine and calmodulin regulation of the heme iron reactivity in neuronal nitric oxide synthase. J Biol Chem. 1994 Aug 12;269(32):20335–20339. [PubMed] [Google Scholar]
- McMillan K., Bredt D. S., Hirsch D. J., Snyder S. H., Clark J. E., Masters B. S. Cloned, expressed rat cerebellar nitric oxide synthase contains stoichiometric amounts of heme, which binds carbon monoxide. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11141–11145. doi: 10.1073/pnas.89.23.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keeffe D. H., Ebel R. E., Peterson J. A. Studies of the oxygen binding site of cytochrome P-450. Nitric oxide as a spin-label probe. J Biol Chem. 1978 May 25;253(10):3509–3516. [PubMed] [Google Scholar]
- Porter T. D., Coon M. J. Cytochrome P-450. Multiplicity of isoforms, substrates, and catalytic and regulatory mechanisms. J Biol Chem. 1991 Jul 25;266(21):13469–13472. [PubMed] [Google Scholar]
- Pou S., Pou W. S., Bredt D. S., Snyder S. H., Rosen G. M. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992 Dec 5;267(34):24173–24176. [PubMed] [Google Scholar]
- Pufahl R. A., Marletta M. A. Oxidation of NG-hydroxy-L-arginine by nitric oxide synthase: evidence for the involvement of the heme in catalysis. Biochem Biophys Res Commun. 1993 Jun 30;193(3):963–970. doi: 10.1006/bbrc.1993.1719. [DOI] [PubMed] [Google Scholar]
- Rogers N. E., Ignarro L. J. Constitutive nitric oxide synthase from cerebellum is reversibly inhibited by nitric oxide formed from L-arginine. Biochem Biophys Res Commun. 1992 Nov 30;189(1):242–249. doi: 10.1016/0006-291x(92)91550-a. [DOI] [PubMed] [Google Scholar]
- Rousseau D. L., Singh S., Ching Y. C., Sassaroli M. Nitrosyl cytochrome c oxidase. Formation and properties of mixed valence enzyme. J Biol Chem. 1988 Apr 25;263(12):5681–5685. [PubMed] [Google Scholar]
- Sharma V. S., Traylor T. G., Gardiner R., Mizukami H. Reaction of nitric oxide with heme proteins and model compounds of hemoglobin. Biochemistry. 1987 Jun 30;26(13):3837–3843. doi: 10.1021/bi00387a015. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Bredt D. S. Biological roles of nitric oxide. Sci Am. 1992 May;266(5):68-71, 74-7. doi: 10.1038/scientificamerican0592-68. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Griffith O. W. Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Ikeda-Saito M. Spectral characterization of brain and macrophage nitric oxide synthases. Cytochrome P-450-like hemeproteins that contain a flavin semiquinone radical. J Biol Chem. 1992 Oct 15;267(29):20547–20550. [PubMed] [Google Scholar]
- Stuehr D. J., Kwon N. S., Nathan C. F., Griffith O. W., Feldman P. L., Wiseman J. N omega-hydroxy-L-arginine is an intermediate in the biosynthesis of nitric oxide from L-arginine. J Biol Chem. 1991 Apr 5;266(10):6259–6263. [PubMed] [Google Scholar]
- Tsubaki M., Hiwatashi A., Ichikawa Y. Effects of cholesterol and adrenodoxin binding on the heme moiety of cytochrome P-450scc: a resonance Raman study. Biochemistry. 1986 Jun 17;25(12):3563–3569. doi: 10.1021/bi00360a014. [DOI] [PubMed] [Google Scholar]
- Wang J., Stuehr D. J., Ikeda-Saito M., Rousseau D. L. Heme coordination and structure of the catalytic site in nitric oxide synthase. J Biol Chem. 1993 Oct 25;268(30):22255–22258. [PubMed] [Google Scholar]
- Wells A. V., Li P., Champion P. M., Martinis S. A., Sligar S. G. Resonance Raman investigations of Escherichia coli-expressed Pseudomonas putida cytochrome P450 and P420. Biochemistry. 1992 May 12;31(18):4384–4393. doi: 10.1021/bi00133a002. [DOI] [PubMed] [Google Scholar]
- White K. A., Marletta M. A. Nitric oxide synthase is a cytochrome P-450 type hemoprotein. Biochemistry. 1992 Jul 28;31(29):6627–6631. doi: 10.1021/bi00144a001. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Yamamoto H., Erman J. E., Leigh J. S., Jr, Reed G. H. Electromagnetic properties of hemoproteins. V. Optical and electron paramagnetic resonance characteristics of nitric oxide derivatives of metalloporphyrin-apohemoprotein complexes. J Biol Chem. 1972 Apr 25;247(8):2447–2455. [PubMed] [Google Scholar]


