Abstract
The number of patients infected with H7N9 influenza virus has been increasing since 2013. We examined the efficacy of neuraminidase (NA) inhibitors and the efficacy of a vaccine against an H7N9 influenza virus, A/Anhui/1/2013 (H7N9), isolated from a patient in a cynomolgus macaque model. NA inhibitors (oseltamivir and peramivir) barely reduced the total virus amount because of the emergence of resistant variants with R289K or I219T in NA [residues 289 and 219 in N9 of A/Anhui/1/2013 (H7N9) correspond to 292 and 222 in N2, respectively] in three of the six treated macaques, whereas subcutaneous immunization of an inactivated vaccine derived from A/duck/Mongolia/119/2008 (H7N9) prevented propagation of A/Anhui/1/2013 (H7N9) in all vaccinated macaques. The percentage of macaques in which variant H7N9 viruses with low sensitivity to the NA inhibitors were detected was much higher than that of macaques in which variant H5N1 highly pathogenic influenza virus was detected after treatment with one of the NA inhibitors in our previous study. The virus with R289K in NA was reported in samples from human patients, whereas that with I219T in NA was identified for the first time in this study using macaques, though no variant H7N9 virus was reported in previous studies using mice. Therefore, the macaque model enables prediction of the frequency of emerging H7N9 virus resistant to NA inhibitors in vivo. Since H7N9 strains resistant to NA inhibitors might easily emerge compared to other influenza viruses, monitoring of the emergence of variants is required during treatment of H7N9 influenza virus infection with NA inhibitors.
INTRODUCTION
Emerging H7N9 avian influenza A virus infection in humans has been reported since 2013 (657 cases having been reported to WHO as of 1 May 2015) (1, 2). Some patients infected with the H7N9 virus showed severe morbidity, with pneumonia and acute respiratory distress syndrome (3), indicating significant pathogenicity in humans. Since H7 hemagglutinin (HA) of the virus has affinity to the sialic acid-galactose combination with an α2–6 linkage expressed on the surface of human upper respiratory epithelial cells (4–7) and since serological studies showed that no human had a neutralizing antibody response to the H7N9 virus before the outbreak of H7N9 virus infection (4, 8), development of antiviral agents and vaccines against H7N9 virus is an urgent issue.
First, we examined the efficacy of neuraminidase (NA) inhibitors against H7N9 virus isolated from a human in a macaque model. Several NA inhibitors are currently available for treatment of seasonal influenza virus infection. The effects of NA inhibitors against H7N9 virus have been examined in mice, but effects of NA inhibitors in mice infected with H7N9 virus were controversial in previous studies (4, 9–11). Furthermore, many patients infected with H7N9 virus have been treated with NA inhibitors, but clinical outcomes after treatment were not always satisfactory, probably due to late initiation of antiviral therapy, coexisting medical complications, and emergence of a virus that is resistant to antiviral drugs (3, 10, 12–14). However, the frequency of emergence of a resistant virus has not been determined in human patients. Therefore, assessment of the efficacy of NA inhibitors and of an emerging virus resistant to NA inhibitors in macaques is required under controlled conditions with administration after regulated time points and without coexisting complications since cynomolgus macaques showed symptoms similar to those seen in humans infected with influenza virus (15–18).
Next, we examined the efficacy of a vaccine against H7N9 influenza virus using macaques. We previously established an influenza A virus library that contains virus strains with 144 combinations of 16 HAs and 9 NAs to provide vaccine strains for pandemic preparedness (19). H5N1, H7N7, and H1N1 vaccines selected from the library, especially a formulation of whole particles of each virus inactivated by formalin, conferred protective immunity against H5N1 and H7N7 highly pathogenic avian and H1N1 pandemic influenza viruses in cynomolgus macaques (15–17, 20, 21). This suggests that vaccines in the virus library are useful for production of vaccines against emerging influenza virus infection in humans. Therefore, we selected an H7N9 vaccine strain from the library to assess the efficacy of a whole-particle test vaccine against H7N9 virus isolated from humans by the use of mice (22) and macaques.
Although no escape mutant was detected in vaccinated macaques, we found variant H7N9 viruses that had low sensitivity to NA inhibitors in three of the six macaques treated with NA inhibitors. Since no resistant H7N9 virus was found in previous studies using mice (4, 9), the results of our macaque study suggest the contribution of not only viral factors but also host factors to the emergence of resistant variants and the importance of nonhuman primate models for evaluation of antiviral drugs.
MATERIALS AND METHODS
Ethics statement.
This study was carried out in strict accordance with the Guidelines for the Husbandry and Management of Laboratory Animals of the Research Center for Animal Life Science at Shiga University of Medical Science and Standards Relating to the Care and Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology, Japan. The protocols were approved by the Shiga University of Medical Science Animal Experiment Committee (permit numbers 2013-4-11 and 2013-7-9). The Research Center for Animal Life Science at Shiga University of Medical Science has a permit for importation of cynomolgus macaques. Regular veterinary care and monitoring, balanced nutrition, and environmental enrichment were provided by the Research Center for Animal Life Science at Shiga University of Medical Science. The macaques were euthanized at the endpoint of 7 days after virus inoculation using ketamine followed by intravenous injection of pentobarbital (200 mg/kg of body weight). Animals were monitored every day during the study to be clinically scored as described in Table S3 in the supplemental material (23) and to undergo veterinary examinations to help alleviate suffering. Animals would be scheduled to be euthanized if their clinical scores reached 15 (a humane endpoint).
Animals.
Female cynomolgus macaques (6 to 8 years of age) from Vietnam were used. The cynomolgus macaques used in the present study were healthy adults. Sample collection and virus inoculation were performed under conditions of ketamine (5 mg/kg) and xylazine (1 mg/kg) anesthesia, and all efforts were made to minimize suffering. Food pellets of CMK-2 (CLEA Japan, Inc., Tokyo, Japan) were given once a day after recovery from anesthesia, and drinking water was available ad libitum. The animals were singly housed under conditions of controlled humidity (47% to 54%), temperature (23 to 24°C), and light (12-h light/12-h dark cycle; lights on at 8:00 a.m.). In the text and figures, individual macaques are distinguished by the following identification numbers: S1, S2, and S3, macaques left unvaccinated or untreated with NA inhibitors but injected with saline solution; O1, O2, and O3, macaques treated with oseltamivir phosphate; P1, P2, and P3, macaques treated with peramivir; and V1, V2, and V3, vaccinated macaques. Two weeks before virus inoculation, a telemetry probe (TA10CTA-D70; Data Sciences International, St. Paul, MN) was implanted in the peritoneal cavity of each macaque under conditions of ketamine/xylazine anesthesia followed by isoflurane inhalation to monitor body temperature. The macaques used in this study were free from herpes B virus, hepatitis E virus, Mycobacterium tuberculosis, Shigella spp., Salmonella spp., and Entamoeba histolytica. Under conditions of ketamine/xylazine anesthesia, two cotton sticks were used to collect fluid samples in nasal cavities, oral cavities, and tracheas every day from day 0 to day 7, and the sticks were subsequently immersed in 1 ml of Eagle's minimal essential medium containing 0.1% bovine serum albumin and antibiotics. A bronchoscope (MEV-2560; Machida Endoscope Co. Ltd., Tokyo, Japan) and cytology brushes (BC-203D-2006; Olympus Co., Tokyo, Japan) were used to obtain bronchial samples (17). Blood samples were collected from vaccinated and control macaques before vaccination and 2, 3, and 4 weeks after the first vaccination under conditions of ketamine/xylazine anesthesia.
Viruses and vaccine.
A vaccine strain, influenza virus A/duck/Mongolia/119/2008 (H7N9) (Mong/119; National Center for Biotechnology Information [NCBI] taxonomy database identifier [ID] 600649), was propagated in allantoic cavities of 10-day-old embryonated hen's eggs at 35°C for 48 h (22). An inactivated whole-particle vaccine was prepared using 0.2% formalin as described previously (17). The whole-particle vaccine (1 mg/dose) was inoculated subcutaneously into macaques using syringes twice with a 3-week interval between injections under conditions of ketamine/xylazine anesthesia.
A challenge strain, A/Anhui/1/2013 (H7N9) (Anhui/1; kindly provided by Eri Nobusawa, Kazuya Nakamura, and Masato Tashiro, National Institute of Infectious Disease [NIID], Japan), was isolated from a human patient (2, 24). Anhui/1 was propagated in chicken embryonated eggs once at 35°C for 48 h at the Shiga University of Medical Science (4, 9). The macaques were challenged with Anhui/1 virus (3 × 106 50% tissue culture infective doses [TCID50] in 7 ml Hanks buffered saline solution medium). The virus was inoculated into nostrils (0.5 ml for each nostril), oral cavity (1 ml), and trachea (5 ml) with pipettes and catheters. Virus titers in samples were determined using Madin-Darby canine kidney (MDCK) cells as described previously (15). Experiments using the challenge virus were performed in the biosafety level 3 facility of the Research Center for Animal Life Science, Shiga University of Medical Science.
Compounds.
Oseltamivir phosphate (Chugai Pharmaceutical Co., Ltd., Tokyo, Japan) and peramivir hydrate (Shionogi & Co., Ltd., Osaka, Japan) were purchased from Alfresa Pharma Corporation, Otsu, Japan. Oseltamivir phosphate was dissolved in 0.5% methylcellulose solution at 10 mg/ml and administered into the stomach with catheters (30 mg/kg) once a day for 5 days. With this protocol, the maximum concentration of drug in plasma (Cmax) and the area under the concentration-time curve (AUC) for oseltamivir carboxylate in macaques were larger than those in adult humans administered oseltamivir phosphate twice a day (75 mg × 2) (25, 26). Peramivir hydrate (10 mg/ml, 30 mg/kg) was intravenously injected into macaques once a day for 5 days (27). Oseltamivir carboxylate (ChemScene, LLC, Monmouth Junction, NJ) was purchased from Funakoshi Co., Ltd., Tokyo, Japan.
Sequence analysis of NA genes by the Sanger method.
Viral RNA was extracted from suspensions of swab samples using a Qiagen viral RNA minikit (Qiagen, Hilden, Germany) and reverse transcribed with Uni12 primers (28) and Moloney murine leukemia virus (MMLV) reverse transcriptase (Invitrogen Corporation, Carlsbad, CA). A partial NA region that included nucleotides corresponding to amino acid position 289 (Anhui/1 N9 numbering; 292 by N2 numbering) was amplified using two primers, forward primer 5′-GTGGAATGCATTGGGTGGTC-3′ and reverse primer 5′-ATATCGTCTCGTATTAGTAGAAACAAGGGTCTT-3′, and Ex Taq DNA polymerase (TaKaRa Bio Inc., Otsu, Japan). After denaturation at 94°C for 2 min, the reaction was performed with 35 cycles of denaturation at 94°C for 20 s, annealing at 55°C for 30 s, and extension at 72°C for 90 s, followed by extension at 72°C for 4 min. The sequencing reaction consisted of 25 cycles of denaturation at 96°C for 10 s, annealing at 50°C for 5 s, and extension at 60°C for 90 s. Purified PCR products were sequenced using a BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA). Sequences of DNA templates were determined using a 3500 genetic analyzer (Applied Biosystems). Sequencing data were analyzed using GENETYX version 10 (Genetyx Corporation, Tokyo, Japan).
NA gene allele frequency analysis by deep sequencing.
Viral RNA and cDNA were prepared as described above. The NA region of influenza virus was amplified using two primers, forward primer 5′-TGCACTTCAGCCACTGCTAT-3′ and reverse primer 5′-ATATCGTCTCGTATTAGTAGAAACAAGGGTCTT-3′, and KOD plus-neo DNA polymerase (Toyobo Co. Ltd., Osaka, Japan) in 35 cycles of denaturation at 98°C for 10 s, annealing at 60°C for 30 s, and extension at 68°C for 60 s, followed by final extension at 68°C for 10 min. For bead-bound cDNA prepared as described above from swab samples, emulsion PCR was performed using an Ion Personal Genome Machine (PGM) Template OT2 400 kit (Thermo Fisher Scientific Inc., Waltham, MA) according to the manufacturer's instructions. After bead recovery and enrichment, beads were sequenced using an Ion PGM Sequencing 400 kit and an Ion PGM system (Thermo Fisher Scientific Inc.) according to the appropriate instrument run protocol. The resulting reads were sorted and assembled using CLC Genomics Workbench software, version 7.5 (CLC bio, Aarhus, Denmark).
Neuraminidase inhibition assay.
Each plaque-purified variant that had an amino acid substitution of T at 219 or K at 289 in NA of Anhui/1 was propagated in MDCK cells for one passage. The NA activity of the cloned viruses was determined with an EnzyChrom neuraminidase assay kit (BioAssay Systems, Hayward, CA) according to the manufacturer's instructions. After the NA activity was adjusted at 0.2 to 2.5 U/liter, the NA activity of viruses was determined in the presence of NA inhibitors (0.01 to 100,000 nM). After curves showing the relationship between concentrations of NA inhibitors and percentages of colorimetric inhibition were drawn, 50% inhibitory concentrations (IC50s) were calculated.
Molecular dynamics simulations.
The initial coordinates of wild-type Anhui N9 with oseltamivir were taken from the cocrystal structure (Protein Data Bank [PDB] code 4MWQ) (29). The structures of NA-I219T and NA-R289K with oseltamivir were generated in silico by replacing I at position 219 and R at 289 in the wild-type complex with T and K, respectively, using the LEaP module in the AMBER 14 software suite (Conflex USA, San Diego, CA) (30, 31). Protonation states of the ionizable residues were assigned at pH 6.5 using the PDB2PQR web server (32). The geometry and electrostatic potential of oseltamivir were calculated at the HF/6-31G (d) level with Gaussian 09 (revision A.1.; Gaussian, Inc., Wallingford, CT) (33). Binding free energies were calculated using the script of the molecular mechanics/generalized Born surface area (MM/GBSA) method in AMBER 14 (MMPBSA.py). Detailed procedures are described in Text S1 in the supplemental material.
Detection of antibody specific for virus antigens by ELISA and virus neutralization assay.
The antibody titers of plasma and swab samples against Mong/119 antigens were determined using an enzyme-linked immunosorbent assay (ELISA). Results were calculated after subtraction of the optical density (OD) at 620 nm from the OD at 450 nm. Neutralization titers were determined using the Anhui/1 or Mong/119 virus as described previously (17).
Histopathological examination and detection of viral antigen by immunohistochemical staining.
Lungs obtained at autopsy were immersed in 10% neutral buffered formalin, embedded in paraffin, and cut into 3-μm-thick sections. The sections were stained with hematoxylin and eosin (H&E). For immunohistochemical staining, lung tissues were cut into 4-μm-thick sections. After treatment with trypsin for 15 min at 37°C and blocking with normal goat serum, the sections were incubated with anti-influenza A virus nucleoprotein antibody (AAH5; AbD Serotec, Kidlington, United Kingdom) (1:500 dilution) for 1 h at 37°C, followed by incubation with alkaline phosphatase-conjugated anti-mouse Ig and 5-bromo-4-chloro-3-indoxyl phosphate and nitro blue tetrazolium chloride substrate (Nichirei Bioscience Inc., Tokyo, Japan). After washing with H2O and treatment in boiled water for 15 min, anti-calprotectin antibody (MAC387) (1:200 dilution) reacting with macrophages was added, followed by the addition of alkaline phosphatase-conjugated anti-mouse Ig and new fuchsin (Nichirei Bioscience Inc., Tokyo, Japan). After treatment in boiled water for 15 min, anti-cytokeratin antibody (AE1/AE3) (1:50 dilution) was added to stain epithelial cells, followed by the addition of horseradish peroxidase-conjugated anti-mouse Ig and 3,3′-diaminobenzidine (Nichirei Biosciences Inc.).
RESULTS
Efficacy of NA inhibitors against H7N9 influenza virus in cynomolgus macaques.
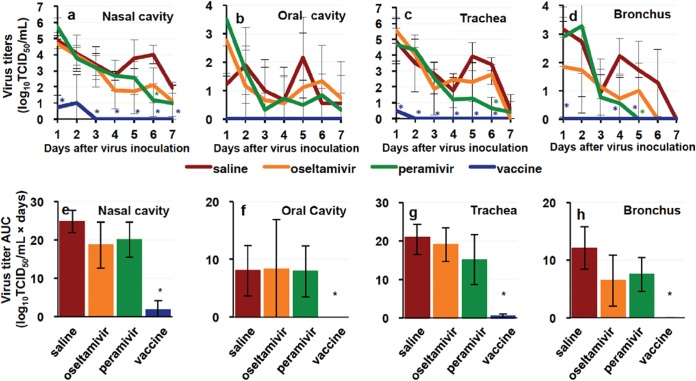
We examined the efficacy of the NA inhibitors, namely, oseltamivir phosphate and peramivir hydrate, that were initially recommended by the WHO for use against H7N9 influenza virus infection (34), since patients with respiratory symptoms might have difficulty in the use of other NA inhibitors for inhalation. All macaques were infected with A/Anhui/1/2013 (H7N9) (Anhui/1) isolated from a patient in China. Average body temperatures of subjects in three unvaccinated groups from 6 p.m. on day 0 to 10 a.m. on day 1 after infection were 1.38 to 2.14°C higher than those before infection (Fig. 1a; see also Fig. S1 in the supplemental material), indicating successful virus inoculation. Body temperatures of the untreated macaques administered saline solution instead of NA inhibitors did not return to a basal level during the study. As shown in Table S3, clinical scores of the untreated macaques increased after virus inoculation due to loss of appetite and temperature changes (Fig. 1b). (All of the macaques survived until day 7 after infection, an endpoint of this study.) After the onset of high body temperature, the NA inhibitors were administered to macaques once a day from day 1 to day 5. The average body temperature change in macaques treated with peramivir on day 4 was significantly lower than that in the untreated macaques (Fig. 1a), but the clinical scores, including loss of appetite, were not significantly ameliorated by treatment with the NA inhibitors (Fig. 1b). The virus was detected in nasal, oral, tracheal, and bronchial samples from the untreated macaques until day 5 to day 7 (Fig. 2; see also Table S1). Significant reductions of virus titers in nasal and tracheal samples on day 6 and in bronchial samples on day 5 were seen in the macaques treated with peramivir compared with the virus titers in control macaques, but no significant difference of virus titers in respiratory samples from the macaques treated with oseltamivir or peramivir was observed on the other days. Summations of virus titers (virus titer areas under the concentration-time curves [AUCs]) in samples from the treated macaques were not significantly reduced by the presence of the NA inhibitors (Fig. 2e to h).
FIG 1.
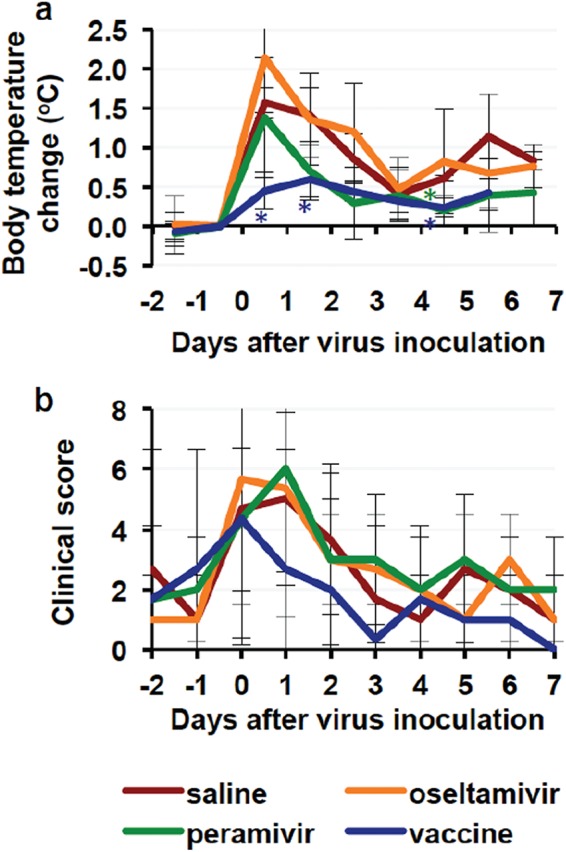
Body temperature changes and clinical scores of macaques infected with Anhui/1. Cynomolgus macaques (n = 3 in each group) were inoculated with influenza virus A/Anhui/1/2013 (H7N9) isolated from a patient in China on day 0. (a) Average temperatures from 6 p.m. to 10 a.m. the next day were calculated on the basis of data for individual macaques (see Fig. S1 in the supplemental material). Average temperatures on each day were compared with those from 6 p.m. on the day before virus inoculation (day −1) to 10 a.m. on the day of virus inoculation (day 0). Averages and standard deviations of data from three macaques are shown in the graph except for temperatures of the saline solution and vaccine groups on day 7 due to failure of a recording computer. Asterisks indicate significant differences from the saline solution group data in the group treated with peramivir on day 4 and the vaccinated group on days 0, 1, and 4 (P < 0.05, Student's t test). (b) Clinical scores were determined by daily observation and body temperature (see Table S3). Averages and standard deviations of the data are shown. There was no statistically significant difference between the saline solution group and the other groups. Red, macaques injected with saline solution; orange, macaques treated with oseltamivir; green, macaques treated with peramivir; blue, macaques with vaccination.
FIG 2.
Virus titers in swab samples from macaques infected with Anhui/1. Cynomolgus macaques (n = 3 in each group) were inoculated with Anhui/1 on day 0. Nasal, oral, tracheal, and bronchial samples were collected on the indicated days. Virus titers in the samples were determined as shown in Table S1 in the supplemental material. Averages of virus titer values (a to d) and averages of virus titer AUC values (e to h) from nasal (a and e), oral (b and f), tracheal (c and g), and bronchial (d and h) samples were calculated on the basis of the titers listed in Table S1. Virus titers under the detection limit were calculated as 0. Averages and standard deviations of the data from three macaques are shown. Red, macaques injected with saline solution; orange, macaques administered oseltamivir; green, macaques administered peramivir; blue, macaques with vaccination. Asterisks indicate significant differences between the saline solution group and the vaccinated group and between the saline solution group and the group treated with peramivir (P < 0.05, Student's t test).
NA gene mutations in virus from macaques treated with NA inhibitors.
Since treatment with the NA inhibitors did not result in a significant reduction of virus titer AUCs in macaques and since the virus was detected in some samples from four of the six macaques on day 7 even after treatment with the NA inhibitors (see Table S1 in the supplemental material), we used Sanger nucleotide sequencing to determine nucleotide sequences of NA of the virus recovered on day 7 after infection. First, we focused on nucleotide sequences corresponding to amino acid position 289 (Anhui N9 numbering; 292 in N2 numbering and 294 in consensus N9 numbering) (24), a position at which variants have been reported for viruses resistant to oseltamivir (35, 36). Viruses in nasal samples from all three untreated macaques on day 7 showed a nucleotide sequence coding for arginine at position 289 (289R) of NA as seen in the virus inoculated into macaques on day 0 (Table 1). On the other hand, a virus with lysine at position 289 (289K; a nucleotide change from AGG to AAG), which was reported to be a virus less sensitive than 289R virus to NA inhibitors (4, 35, 36), was dominant in nasal and oral samples from one of the macaques treated with oseltamivir (O3) and in nasal and tracheal samples from one of the macaques treated with peramivir (P1). The virus in the other samples from treated macaques on day 7 had R at position 289 in NA.
TABLE 1.
Amino acids at position 289 of NA of virus recovered after treatment with NA inhibitorsa
| Inoculum or sample | Treatment | Monkey | Amino acid at NA289 |
|---|---|---|---|
| Inoculumb | R | ||
| Nasal swab | Saline solution | S1 | R |
| Saline solution | S2 | R | |
| Saline solution | S3 | R | |
| Oseltamivir | O2 | R | |
| Oseltamivir | O3 | K | |
| Peramivir | P1 | K | |
| Peramivir | P3 | R | |
| Oral swab | Saline solution | S1 | R |
| Oseltamivir | O3 | K | |
| Peramivir | P1 | R | |
| Tracheal swab | Saline solution | S3 | R |
| Peramivir | P1 | K |
Nucleotide sequences determined by Sanger sequencing were translated to amino acids. Amino acid position 289 of NA was determined on the basis of an N9 neuraminidase of Anhui/1. R, arginine; K, lysine. All samples indicated in the table were collected 7 days after virus inoculation. Viral RNAs were extracted from swab samples in which virus was detected on day 7 as shown in Table S1 in the supplemental material. Samples without detection of virus on day 7 as shown in Table S1 were not examined.
Viral genes were purified from the virus in the same stock lot as that used in inoculation of macaques on day 0.
Next, we examined the frequency of the nucleotide sequence corresponding to K at 289 in NA of the virus in nasal samples from O3 and P1 macaques and nucleotides in the other part of NA using deep sequencing. In the virus gene used in inoculation into macaques, the proportion of the NA gene with A at 866 coding for K at 289 was 0.06% (see Text S1 and Table S2 in the supplemental material). We detected 4 nonsynonymous and 5 synonymous mutations in the NA gene of virus isolated from 7 nasal samples on day 7 after virus infection (Table 2). The proportions of NA genes with a G-to-A mutation at position 866 in nasal samples from O3 and P1 (AGG to AAG, R to K at amino acid position 289) were 85% and 91%, respectively. Nucleotide changes with A to T at 814 (isoleucine [I] to phenylalanine [F] at amino acid position 272) and A to G at 1379 (K to R at amino acid position 460) in nasal samples from untreated macaques S2 and S3 and with T to C at 656 (ATA to ACA and I to threonine [T] at amino acid position 219) in the nasal sample from O2 were detected by deep sequencing. Thus, virus with changes in amino acids of NA compared to the inoculum virus was propagated in three of the six macaques treated with NA inhibitors 7 days after virus infection.
TABLE 2.
Amino acid changes in NA of virus isolated from nasal samples after treatment with NA inhibitors
| Treatment | Monkey | Nucleotide positiona | Inoculum nucleotideb | Sample allele nucleotidec | No. of readsd | Total no. of readse | % allelef | Amino acid positiong | AA in inoculumh | AA in samples |
|---|---|---|---|---|---|---|---|---|---|---|
| Saline solution | S1 | 96 | C | T | 19,642 | 49,403 | 39.76 | 32 | N | Synonymousi |
| Saline solution | S2 | 814 | A | T | 32,571 | 84,385 | 38.60 | 272 | I | F |
| Saline solution | S2 | 825 | C | T | 51,298 | 85,879 | 59.73 | 275 | C | Synonymous |
| Saline solution | S2 | 1379 | A | G | 57,056 | 103,490 | 55.13 | 460 | K | R |
| Saline solution | S3 | 510 | G | A | 28,761 | 127,797 | 22.51 | 170 | V | Synonymous |
| Saline solution | S3 | 825 | C | T | 10,690 | 68,710 | 15.56 | 275 | C | Synonymous |
| Saline solution | S3 | 1242 | C | T | 5,821 | 31,400 | 18.54 | 414 | C | Synonymous |
| Saline solution | S3 | 1379 | A | G | 3,666 | 31,033 | 11.81 | 460 | K | R |
| Oseltamivir | O2 | 656 | T | C | 102,728 | 103,880 | 98.89 | 219 | I | T |
| Oseltamivir | O3 | 866 | G | A | 85,405 | 100,094 | 85.32 | 289 | R | K |
| Peramivir | P1 | 866 | G | A | 79,827 | 88,187 | 90.52 | 289 | R | K |
| Peramivir | P3 | 858 | C | T | 39,120 | 39,491 | 99.06 | 286 | C | Synonymous |
Nucleotide position 1 is the first nucleotide A of the ATG translation start point for methionine.
Viral genes were purified from the virus in the same stock lot as that used in inoculation of macaques on day 0.
Nucleotides different from those in the inoculum virus. All nasal swab samples indicated in the table were collected 7 days after virus inoculation.
Number of sequences with indicated nucleotide changes at each nucleotide position.
Number of sequences analyzed at each nucleotide position.
Percentages of alleles different from the inoculum were calculated as percent allele = 100 × (number of alleles/total read number). Allele frequencies that were higher than 10% are shown in the table.
Amino acid (AA) positions were determined on the basis of an N9 neuraminidase sequence of Anhui/1.
C, cysteine; I, isoleucine; F, phenylalanine; K, lysine; N, asparagine; R, arginine; T, threonine; V, valine.
Synonymous, amino acids were not changed by nucleotide changes.
To examine whether the changes in amino acids in NA affected enzyme activity in the virus isolated after treatment, the 50% inhibitory concentration (IC50) of oseltamivir carboxylate and that of peramivir hydrate against NA of cloned viruses were determined in vitro. The IC50 of oseltamivir against NA of the inoculum virus was 1.87 nM, whereas those of oseltamivir against NA with T at 219 and NA with K at 289 were 38.0 nM and 23,196.5 nM, respectively (Table 3). IC50s of peramivir against NA with T at 219 and K at 289 were 24.5 nM and 8,980.5 nM, which were approximately 30 times and 10,000 times higher than that of the inoculum virus, respectively. Thus, treatment with the NA inhibitors allowed propagation of viruses less sensitive to NA inhibitors in the treated macaques.
TABLE 3.
IC50s of oseltamivir carboxylate and peramivir hydrate against virus isolated from macaques after treatment
| Sample or inoculuma | Amino acids at NA219 and NA289b | IC50 (nM)c |
|
|---|---|---|---|
| Oseltamivir | Peramivir | ||
| O2 | 219T/289R | 38.0 | 24.5 |
| O3 | 219I/289K | 23,196.5 | 8,980.5 |
| Inoculum | 219I/289R | 1.87 | 0.83 |
Virus was isolated from nasal samples and purified with plaque cloning.
Amino acid positions were determined on the basis of an N9 neuraminidase sequence of Anhui/1.
Averages of IC50s against indicated virus were calculated using the results of two or three experiments.
Molecular dynamics simulations of the structures of N9 neuraminidase with amino acid changes and oseltamivir.
To determine whether changes of amino acids in the N9 NA affect drug sensitivity on a molecular basis, we made in silico molecular dynamics simulation models of NA with T at 219 and NA with K at 289 based on the crystal structure of wild-type NA of Anhui/1 reported previously (29). First, to estimate the binding affinity of NA inhibitors to NA with T at 219 and NA with K at 289 (37), the binding free energies (ΔGbinding, calc) of oseltamivir carboxylate in wild-type NA, NA with T at 219, and NA with K at 289 were calculated to be −34.87, −32.36, and −27.05 kcal/mol, respectively (Table 4; see also Text S1 in the supplemental material). The differences in the calculated binding free energy between the wild type and each variant (ΔΔGbinding, calc) were qualitatively in good agreement with ΔΔGbinding, exp, which was estimated from experimental values of IC50 shown in Table 3. Thus, the energy analysis indicates that the substitutions of T for I at position 219 and of K for R at position 289 decrease the binding affinity of oseltamivir carboxylate to NA.
TABLE 4.
Binding free energies and relative binding free energies for the wild-type and variant NAs complexed with oseltamivir
| Contribution | Energy (kcal/mol) |
||
|---|---|---|---|
| Wild-type NA (219I/289R) | NA-219T | NA-289K | |
| ΔEeleca | −163.14 | −145.20 | −143.17 |
| ΔEvdWb | −24.38 | −25.49 | −21.07 |
| ΔGsolv, polarc | 157.55 | 142.98 | 141.27 |
| ΔGsolv, nonpolard | −4.90 | −4.65 | −4.08 |
| ΔGbinding, calce | −34.87 | −32.36 | −27.05 |
| ΔGbinding, expf | −11.98 | −10.19 | −6.36 |
| ΔΔGbinding, calcg | 2.51 | 7.82 | |
| ΔΔGbinding, exph | 1.79 | 5.62 | |
ΔEelec, electrostatic interaction energy.
ΔEvdW, van der Waals interaction energy.
ΔGsolv, polar, polar component of solvation free energy.
ΔGsolv, nonpolar, nonpolar component of solvation free energy.
ΔGbinding, calc, binding free energies = ΔEelec + ΔEvdW + ΔGsolv, polar + ΔGsolv, nonpolar − TΔS (total). − TΔS (total) (conformational entropy) was not considered in the present study due to the high computational cost and low prediction accuracy.
ΔGbinding, exp, binding free energies were calculated from the half-maximal inhibitory concentration (IC50) data in Table 3 using the following formula: ΔGbinding,exp = RT ln (Kdissociation) = RT ln(IC50 + 0.5 Cenzyme) = ∼RT ln IC50. R, ideal gas constant; T, temperature in K; Cenzyme, concentration of the enzyme, which is very small after equilibration and can be omitted in most cases.
ΔΔGbinding, calc, relative binding free energies = ΔGbinding, calc of NA-219T/NA-289K − ΔGbinding, calc of wild-type NA.
ΔΔGbinding, exp, relative binding free energies = ΔGbinding, exp of NA-219T/NA-289K − ΔGbinding, exp of wild-type NA.
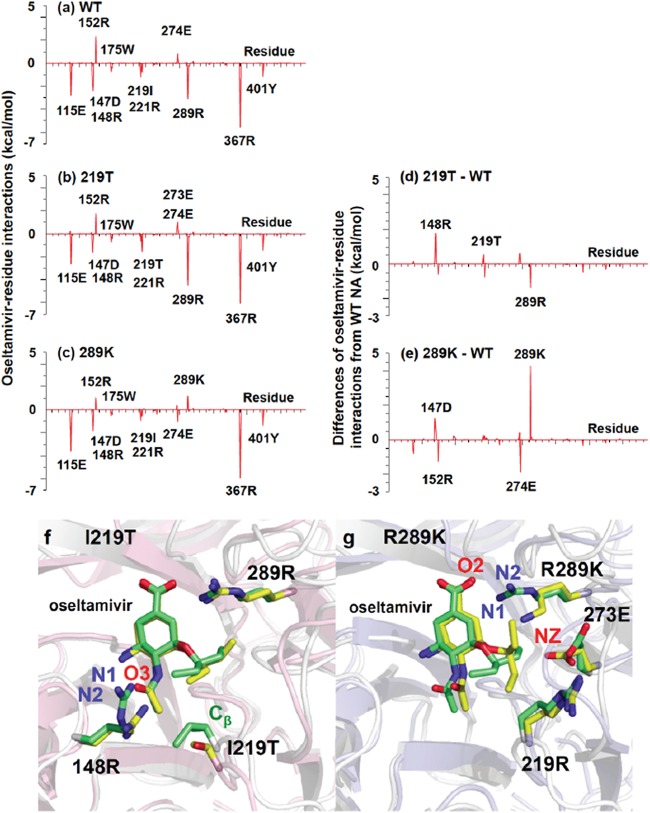
We determined the contribution of individual residues in NA to the interaction with oseltamivir. The total binding free energies were decomposed on each residue using the MM/GBSA free energy decomposition procedure (Fig. 3) (38). Both I at position 219 and R at 289 of the wild-type NA as well as E at 115, D at 147, R at 148, R at 221, R at 367, and Y at 401 of the wild-type NA, NA with T at 219, and NA with K at 289 were responsible for the interaction with oseltamivir (Fig. 3a to c). Furthermore, to clarify the effects of substitutions of T at 219 and K at 289, differences in the binding free energy at each residue between the wild-type NA and NA with T at 219 and between the wild-type NA and NA with K at 289 were calculated (Fig. 3d and e). In the simulation of NA with T at 219, there was no significant change in the binding free energy contribution from the substituted residue 219, whereas R at 148 caused an increase in the binding free energy due to the substitution of T at 219 (Fig. 3d). The substitution of K at 289 caused an increase in the binding free energy at 289, giving rise to a repulsive interaction with oseltamivir (Fig. 3e).
FIG 3.
Binding free energies between oseltamivir and N9 neuraminidase with substitutions at individual residues and simulated molecular structures of NA with T at 219 or K at 289 and oseltamivir. (a to c) Binding free energies between oseltamivir and individual amino acid residues (vertical axes) were calculated using the MM/GBSA free energy decomposition procedure. Residue numbers of amino acids in horizontal axes are indicated as residues in Anhui/1 N9 numbering. (a) Wild-type N9. (b) N9 with 219T. (c) N9 with 289K. (d and e) Changes of binding free energies resulting from amino acid substitution (vertical axes) were calculated by subtraction of the value determined for wild-type N9 from those determined for N9 with mutations. (d) N9 with 219T − wild-type N9 (= b − a). (e) N9 with 289K − wild-type N9 (= c − a). (f and g) Snapshots of simulated structures of the binding pockets in NA and oseltamivir were taken at 50 ns. Oseltamivir is shown in the middle of the figures. (f) Overlay of wild-type N9 (green) and N9 with 219T (Anhui N9 numbering) (yellow). (g) Overlay of wild-type NA (green) and N9 with 289K (yellow).
Structure models of NA with T at 219 and NA with K at 289 bound to oseltamivir were simulated to visualize the interaction between variant NA and oseltamivir (Fig. 3f and g). In the simulation models, residue 219 had no direct contact with oseltamivir in either wild-type N9 or N9 with T at 219 (Fig. 3f). However, since the side chain of T is smaller than that of I, the substitution of T for I at position 219 caused distortion of the conformation, with an increase in the size of the oseltamivir-binding pocket near residue 219 (yellow in Fig. 3f). This distortion resulted in a change in the relative orientation of the side chain of R at 148 to oseltamivir and loss of the hydrogen bonds between R at 148 and oseltamivir. The hypothesis of a loss of the interaction of R at 148 with oseltamivir was also supported by the simulations, i.e., the mean distances between oxygen in the acetamide group of oseltamivir and one of the terminal guanidine nitrogen atoms of R at 148 [O3(otv)-NH1(148R)] and between the identical oxygen of oseltamivir and another terminal nitrogen atom of R at 148 [O3(otv)-NH2(148R)] in the wild-type N9 were 2.97 and 5.09 Å, respectively (see Fig. S2a in the supplemental material), whereas those of O3(otv)-NH1(148R) and O3(otv)-NH2(148R) in the 219T model were 5.94 and 6.48 Å, respectively (see Fig. S2b). In addition, the average distance between the carbon atom (Cβ) of I at 219 and one of the terminal guanidine nitrogen atoms of R at 148 [Cβ(219I)-NH1(148R)] and between Cβ(219I) and another terminal nitrogen atom of R at 148 [Cβ(219I)-NH2(148R)] in the wild-type N9 (5.59 Å) (see Fig. S2c) was almost equal to that of Cβ(219T)-NH1(148R) and Cβ(219T)-NH2(148R) in N9 with T at 219 (5.67 Å) (see Fig. S2d), indicating that substitution of T at 219 does not significantly affect the distance between T at 219 and R at 148 but affects the oscillation among oseltamivir, R at 148, and T at 219. As a result, loss of the hydrogen bonds between residue 148 and oseltamivir would lead to reduction of susceptibility to oseltamivir.
Regarding the substitution at position 289, the average distance between an oxygen atom in the carboxylate group of oseltamivir [O2(otv)] and a terminal nitrogen atom of K at 289 [NZ(289K)] in the NA with K at 289 (5.28 Å) (see Fig. S2f) was much greater than that between O2(otv) and terminal guanidine nitrogen atoms of R at 289 [NH1/2(289R)] in the wild type (3.05 Å) (see Fig. S2e). On the other hand, the average distance between oxygen atoms in the carbonyl group of E at 273 [OE1/2(273E)] and NZ(289K) in the NA with K at 289 (4.45 Å) (see Fig. S2h) was shorter than those between OE1/2(273E) and NH1/2(289R) in the wild type (6.28 Å) (see Fig. S2g). This means that the substitution of K at 289 resulted in the formation of a hydrogen bond between K at 289 and E at 273 instead of loss of the hydrogen bond between K at 289 and oseltamivir (Fig. 3g). The distortion of the oseltamivir-binding site due to the substitution of K at 289 would cause significantly reduced susceptibility to oseltamivir. This molecular mechanism was in excellent agreement with that from the crystal structure (29).
Immunogenicity and protective effects of a vaccine selected from a virus library against H7N9 influenza virus isolated from a human patient.
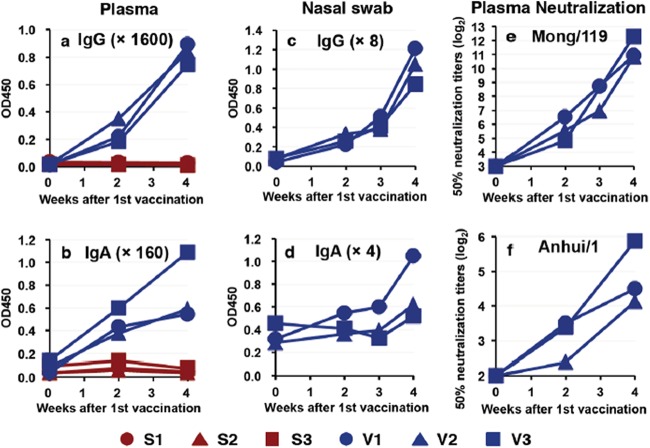
Next, we prepared another countermeasure against H7N9 influenza virus. We selected A/duck/Mongolia/119/2008 (H7N9) (Mong/119) as a vaccine candidate from the virus library (19, 22). Subcutaneous injection of whole virus particles inactivated by formalin without an adjuvant induced IgG and IgA antibodies specific for vaccine antigens in blood and nasal swab samples (Fig. 4a to d). Plasma samples from vaccinated macaques showed neutralizing activity against Mong/119 and Anhui/1 (Fig. 4e and f). Thus, the whole-particle vaccine of H7N9 was immunogenic to induce a neutralizing antibody response in macaques.
FIG 4.
Antibody responses specific for H7N9 virus after vaccination. Cynomolgus macaques were subcutaneously immunized twice (in weeks 0 and 3) with an inactivated whole-particle vaccine of Mong/119. Plasma samples (a, b, e, and f) and nasal swab samples (c and d) were collected during the indicated weeks after the first vaccination. Lines indicate results for individual animals. (a to d) IgG (a and c) and IgA (b and d) antibodies specific for the vaccine antigen at the indicated dilution were analyzed using ELISA. Red, macaques injected with saline solution; blue, macaques injected with the vaccine. (e and f) Neutralization activities against the vaccine strain, Mong/119 (e), and challenge strain, Anhui/1 (f), in plasma are shown as titers that reduced virus plaque levels 50% compared with control culture. At week 0, 50% neutralization titers in all samples were under the detection limit.
The vaccinated macaques and control macaques injected with saline solution were inoculated with Anhui/1 3 weeks after the second vaccination. The average body temperatures of the vaccinated macaques on days 0, 1, and 4 were significantly lower than those of control macaques (Fig. 1a; see also Fig. S1 in the supplemental material). The average of clinical scores in vaccinated macaques after virus infection except on day 4 was lower than that in unvaccinated macaques, although the differences were not statistically significant (Fig. 1b). The virus was detected in nasal and tracheal swab samples from the vaccinated macaques until day 2 and day 1 after infection, respectively, but was not detected in oral and bronchial samples (see Table S1). Virus titer AUCs in the vaccinated macaques, as well as average virus titers on each day, were significantly lower than the virus titer AUCs in the unvaccinated macaques (P < 0.05 by Student's t test) (Fig. 2). Therefore, the whole-particle vaccine derived from the virus library was effective for prevention of virus propagation in macaques infected with Anhui/1.
Viral pneumonia caused by H7N9 virus infection in macaques.
Inflammatory responses in lungs of the treated or vaccinated macaques were compared with those in the untreated and unvaccinated macaques. In lungs of the unvaccinated and untreated macaques, bronchopneumonia accompanying reduction of air contents and thickening of interstitial septa was observed (Fig. 5a). Lymphocytes infiltrated the edematous alveolar space and septa. Influenza A virus NP antigen was partly detected in clusters of both flat and cuboidal epithelial cells (type I and type II alveolar epithelial cells, respectively) but not in macrophages in the lungs of unvaccinated and untreated macaques (Fig. 5b). Severe pneumonia with lymphoid and neutrophilic infiltration was seen in the lungs of macaques treated with oseltamivir or peramivir such as was seen in macaques without treatment (Fig. 5c, e, g, and i). Alveolar epithelial cells positive for the virus antigen showed a tendency to be distributed solitarily in the lungs of macaques treated with NA inhibitors, O1 and P2 macaques, in which no live virus was detected in swab samples on day 7 (Fig. 5d and h), whereas alveolar epithelial cells positive for the virus antigen had accumulated focally in the lungs of macaques treated with NA inhibitors, O3 and P1 macaques, in which virus with K at 289 in NA was detected in swab samples on day 7 (Fig. 5f and j). Therefore, the reduction in clusters of cells infected with the virus indicates improvement with respect to histological pneumonia mediated by NA inhibitors in some of the macaques.
FIG 5.
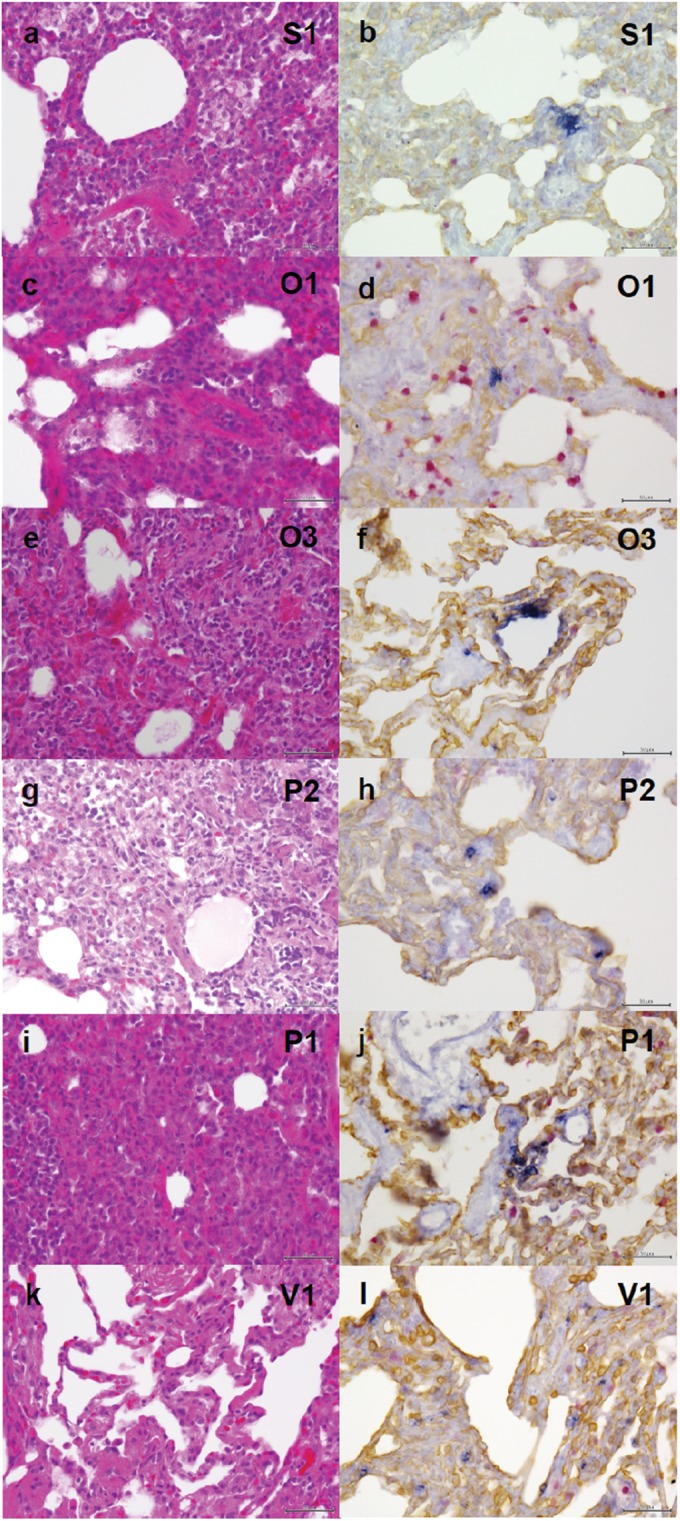
Viral pneumonia in macaques infected with Anhui/1. Unvaccinated and untreated macaques, macaques treated with NA inhibitors, and vaccinated macaques were autopsied 7 days after virus infection. Representative photos from each group are shown. (a and b) Tissue from an unvaccinated and untreated macaque injected with saline solution (S1). (c to f) Tissue from two macaques treated with oseltamivir. (c and d) Tissue from macaque O1, in which no virus was detected on day 7. (e and f) Tissue from macaque O3, in which virus with K at 289 in NA was detected in nasal and oral samples. (g to j) Tissue from two macaques treated with peramivir. (g and h) Tissue from macaque P2, in which no virus was detected on day 7. (i and j) Tissue from macaque P1, in which virus with K at 289 in NA was detected in nasal, oral, and tracheal samples. (k and l) Tissue from a vaccinated macaque (V1). Lung tissues were subjected to H&E staining (a, c, e, g, i, and k) or immunohistochemical staining for influenza virus NP (blue), epithelial cells (brown), and macrophages (red) (b, d, f, h, j, and l). Bars, 50 μm.
In the vaccinated macaques, lymphoid infiltration was observed, but air space was preserved with thin septa, unlike the results seen in the unvaccinated macaques (Fig. 5k versus a). Cells positive for the virus antigen were solitarily detected in the collapsed alveoli of vaccinated macaques (Fig. 5l), although live virus was not detected in swab samples and lung tissues (data not shown), suggesting that the spread of virus from infected cells to other cells was prevented in the vaccinated macaques.
DISCUSSION
H7N9 subtype influenza A virus isolated from a human in 2013 caused viral pneumonia in cynomolgus macaques in the present study that was comparable to pneumonia reported previously in humans and other mammals (3, 4, 39). These results indicate that the H7N9 virus propagates sufficiently to induce respiratory symptoms in nonhuman primates and humans, although the virus has not obtained characteristics of sustained transmission among humans. Before the virus gains gene mutations for transmission among humans, development of antiviral agents and vaccines is required. To extrapolate the efficacy of these measures against viruses, especially when the number of patients infected with an emerging virus is limited, animal models that reflect patients' symptoms and antiviral immune responses are required. However, in mouse models of H7N9 virus infection, since reduction of virus titers by NA inhibitors varied in previous studies, i.e., since the inhibitors were insignificantly effective, effective in the late phase of infection, or effective against only a small amount of virus inoculum (4, 9, 10), use of an alternative model may be encouraged. Thus, the cynomolgus macaque model of influenza virus infection is suitable since macaques develop symptoms similar to those seen in humans and their inflammatory responses are detectable with reagents reactive to human molecules (15, 18, 40).
In the present study, efficacy of NA inhibitors oseltamivir and peramivir in reduction of H7N9 influenza virus titers was observed in some of the macaques. However, there was no statistically significant reduction of virus titers during treatment from day 2 to day 5 or of average virus titer AUCs in the macaques, due to propagation of the virus with known and novel amino acid substitutions in NA—R289K and I219T, respectively. Virus with 289K in NA was found in patients treated with oseltamivir (12), which showed a high IC50 (35, 36) as shown in the present study. On the other hand, virus with 219T in NA was reported for the first time here, though substitutions at position 219, I219R and I219K (I222R and I222K in N2 numbering), were found in a patient infected with H7N9 virus and treated with oseltamivir (10). The IC50s of NA inhibitors against virus with 289K in NA and virus with 219T in NA were up to 12,000 times and 30 times higher than those of NA inhibitors against the inoculum virus, respectively. Structural simulations and calculation of binding free energies predicted that substitution of K at functional residue 289 caused significantly weaker binding to NA inhibitors due to a concomitant conformational change at position 273 in NA (29, 41–43) and that substitution of T at the framework residue 219 made the distance between residue 148 of NA and oseltamivir greater than that between residue 148 of NA with I at 219 and oseltamivir. Thus, the molecular dynamics approach confirmed that amino acid substitutions of T at 219 and K at 289 in NA of viruses isolated from the treated macaques were responsible for the changes in sensitivity to NA inhibitors.
Macaques infected with H5N1 highly pathogenic avian influenza virus were treated with peramivir in a previous study using the same protocol as that in the present study (27). In that H5N1 study, no propagation of a virus that was less sensitive to peramivir corresponding to N9-289K and N9-219T viruses was detected in the macaques after treatment. This might have been due to the absence of or a very low frequency of virus with NA inhibitor resistance-conferring mutations in the inoculum virus that we used. Actually, no mutant virus was detected in a related virus isolated from an identical patient (31). On the other hand, the frequency of the pandemic (H1N1) 2009 virus with substitution of Y for H at 274 in NA, which allowed low sensitivity to NA inhibitors, was only 1% to 2% in Japan, where NA inhibitors are used most frequently in the world (44), and H3N2 seasonal influenza virus resistant to oseltamivir was isolated predominantly from children and immunocompromised patients (45–47). Therefore, an H7N9 virus less sensitive to NA inhibitors might propagate easily in primates compared with other influenza A viruses (12). This speculation is supported by the results of a study revealing that virus with 289K in NA (292 in N2 numbering) showed virulence in mice and guinea pigs similar to that of virus with 289R (36). Therefore, viral propagation in vivo, “replicative fitness,” might not be affected by substitutions of K at 289 and T at 219 in N9 compared to amino acid substitutions in N1 and N2 (11, 48). However, controversial studies indicate low replicative fitness of virus with 289K, replication of which was dominated by that of virus with 289R in ferrets infected with a mixed population of virus with 289R and virus with 289K, and virus with 289K caused less weight loss in mice than did virus with 289R (10, 49). This was due to low NA activity of NA with K at 289, thus being less effective with respect to digestion of sialic acids and release of the virus from infected cells, compared to the activity of NA with R at 289. In the present study, since the virus with 289K in NA was detected after termination of treatment with NA inhibitors, the replicative fitness of the virus with 289K in the macaques might not have been impaired.
On the other hand, the substitution of T at 219 might have a minor effect on NA activity compared with the effect of R-to-K substitution at 289 as seen in H1N1 virus with a D-to-N substitution at 344, which was less sensitive to NA inhibitors and had NA activity similar to that of wild-type seasonal H1N1 virus from 2007 to 2008 (50). Therefore, the virus with 219T was expected to propagate as efficiently as the wild-type virus. No H7N9 strain resistant to oseltamivir with a substitution at 219 was found after treatment of infected mice or ferrets in previous studies, although H2N2 viruses resistant to oseltamivir and H7N9 virus with 289K were reported after treatment in mice and ferrets, respectively (4, 9, 51, 52). Therefore, host factors as well as viral factors might make some contribution to selection and propagation of viruses resistant to NA inhibitors. Thus, to predict the emergence of a virus less sensitive to antiviral drugs and the replicative fitness of mutant viruses, a nonhuman primate model is preferable to mouse and ferret models.
We have used macaques as well as mice to reveal that inactivated whole-particle vaccines were more effective against H5N1 highly pathogenic avian influenza virus and pandemic (H1N1) 2009 virus than were ether-split vaccines (16, 53). Therefore, we prepared the H7N9 whole-particle vaccine selected from our virus library (19), in which vaccine strains were efficiently propagated in embryonated eggs without adaptation to minimize the time required for vaccine production. Although low immunogenicity of H7 virus was a concern for vaccination in mammals (54, 55), we demonstrated that the H7N9 whole-particle vaccine was sufficiently immunogenic in macaques to induce a neutralizing antibody response to both vaccine and challenge strains, resulting in inhibition of virus propagation in the vaccinated macaques. The immunogenicity of Mong/119 might be due to the presence of epitopes for helper T cells that promote antibody responses of B cells (56). Thus, the vaccine prepared from the virus library is expected to be effective against the H7N9 virus emerging in humans (57–59).
In the present study, no macaque infected with H7N9 influenza virus died of infection without vaccination despite showing significant symptoms accompanying viral pneumonia caused by virus inoculation through multiple routes. In addition, we used the same inoculation routes and virus dose of Anhui/1 as those used with other virus strains in previous studies to compare the pathogenicities of the viruses and the efficacies of vaccines and treatments (17, 23, 27). As a result, the pathogenicity of H7N9 influenza virus isolated in humans seems to be lower than that of H5N1 highly pathogenic avian influenza virus in primates and humans under an immunocompetent condition (4, 17, 39, 60). Although the H7N9 vaccine in the present study conferred sufficient protective immunity to prevent severe viral pneumonia with virus inoculation through the trachea, we need to pay attention to the emergence and containment of resistant viruses during treatment with NA inhibitors even in immunocompetent patients, as confirmed in the present study using healthy macaques. Furthermore, development of antiviral drugs with an alternative mode of action and combinational therapy using those drugs might be required for treatment of H7N9 virus infection (11, 61) in addition to research of host factors that allow the emergence of resistant viruses in primates.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Regional Research and Development Resources Utilization Program, Japan Science and Technology Agency, and by a grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan, for a Joint Research Program of the Research Center for Zoonosis Control, Hokkaido University, and the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID).
We thank Eri Nobusawa, Kazuya Nakamura, and Masato Tashiro for providing the virus, Aiko Ohnuma for analyzing deep sequence results, Mayumi Sasada and Mayumi Endo for technical assistance, Kazuo Teramoto for helping with surgery, and Takahiro Nakagawa and Ikuo Kawamoto for animal care.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00793-15.
REFERENCES
- 1.WHO. 2015. Monthly risk assessment summary. Influenza at the human-animal interface. 1 May 2015. http://www.who.int/influenza/human_animal_interface/HAI_Risk_Assessment/en/ Accessed 22 May 2015.
- 2.Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y. 2013. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med 368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 3.Gao HN, Lu HZ, Cao B, Du B, Shang H, Gan JH, Lu SH, Yang YD, Fang Q, Shen YZ, Xi XM, Gu Q, Zhou XM, Qu HP, Yan Z, Li FM, Zhao W, Gao ZC, Wang GF, Ruan LX, Wang WH, Ye J, Cao HF, Li XW, Zhang WH, Fang XC, He J, Liang WF, Xie J, Zeng M, Wu XZ, Li J, Xia Q, Jin ZC, Chen Q, Tang C, Zhang ZY, Hou BM, Feng ZX, Sheng JF, Zhong NS, Li LJ. 2013. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med 368:2277–2285. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe T, Kiso M, Fukuyama S, Nakajima N, Imai M, Yamada S, Murakami S, Yamayoshi S, Iwatsuki-Horimoto K, Sakoda Y, Takashita E, McBride R, Noda T, Hatta M, Imai H, Zhao D, Kishida N, Shirakura M, de Vries RP, Shichinohe S, Okamatsu M, Tamura T, Tomita Y, Fujimoto N, Goto K, Katsura H, Kawakami E, Ishikawa I, Watanabe S, Ito M, Sakai-Tagawa Y, Sugita Y, Uraki R, Yamaji R, Eisfeld AJ, Zhong G, Fan S, Ping J, Maher EA, Hanson A, Uchida Y, Saito T, Ozawa M, Neumann G, Kida H, Odagiri T, Paulson JC, Hasegawa H, Tashiro M, Kawaoka Y. 2013. Characterization of H7N9 influenza A viruses isolated from humans. Nature 501:551–555. doi: 10.1038/nature12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiong X, Martin SR, Haire LF, Wharton SA, Daniels RS, Bennett MS, McCauley JW, Collins PJ, Walker PA, Skehel JJ, Gamblin SJ. 2013. Receptor binding by an H7N9 influenza virus from humans. Nature 499:496–499. doi: 10.1038/nature12372. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, Wang D, Gao R, Zhao B, Song J, Qi X, Zhang Y, Shi Y, Yang L, Zhu W, Bai T, Qin K, Lan Y, Zou S, Guo J, Dong J, Dong L, Wei H, Li X, Lu J, Liu L, Zhao X, Huang W, Wen L, Bo H, Xin L, Chen Y, Xu C, Pei Y, Yang Y, Zhang X, Wang S, Feng Z, Han J, Yang W, Gao GF, Wu G, Li D, Wang Y, Shu Y. 2013. Biological features of novel avian influenza A (H7N9) virus. Nature 499:500–503. doi: 10.1038/nature12379. [DOI] [PubMed] [Google Scholar]
- 7.Tharakaraman K, Jayaraman A, Raman R, Viswanathan K, Stebbins NW, Johnson D, Shriver Z, Sasisekharan V, Sasisekharan R. 2013. Glycan receptor binding of the influenza A virus H7N9 hemagglutinin. Cell 153:1486–1493. doi: 10.1016/j.cell.2013.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai T, Zhou J, Shu Y. 2013. Serologic study for influenza A (H7N9) among high-risk groups in China. N Engl J Med 368:2339–2340. doi: 10.1056/NEJMc1305865. [DOI] [PubMed] [Google Scholar]
- 9.Baranovich T, Burnham AJ, Marathe BM, Armstrong J, Guan Y, Shu Y, Peiris JM, Webby RJ, Webster RG, Govorkova EA. 2014. The neuraminidase inhibitor oseltamivir is effective against A/Anhui/1/2013 (H7N9) influenza virus in a mouse model of acute respiratory distress syndrome. J Infect Dis 209:1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marjuki H, Mishin VP, Chesnokov AP, Jones J, De La Cruz JA, Sleeman K, Tamura D, Nguyen HT, Wu HS, Chang FY, Liu MT, Fry AM, Cox NJ, Villanueva JM, Davis CT, Gubareva LV. 2015. Characterization of drug-resistant influenza A(H7N9) variants isolated from an oseltamivir-treated patient in Taiwan. J Infect Dis 211:249–257. doi: 10.1093/infdis/jiu447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marjuki H, Mishin VP, Chesnokov AP, De La Cruz JA, Fry AM, Villanueva J, Gubareva LV. 2014. An investigational antiviral drug, DAS181, effectively inhibits replication of zoonotic influenza A virus subtype H7N9 and protects mice from lethality. J Infect Dis 210:435–440. doi: 10.1093/infdis/jiu105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Y, Lu S, Song Z, Wang W, Hao P, Li J, Zhang X, Yen HL, Shi B, Li T, Guan W, Xu L, Liu Y, Wang S, Tian D, Zhu Z, He J, Huang K, Chen H, Zheng L, Li X, Ping J, Kang B, Xi X, Zha L, Li Y, Zhang Z, Peiris M, Yuan Z. 2013. Association between adverse clinical outcome in human disease caused by novel influenza A H7N9 virus and sustained viral shedding and emergence of antiviral resistance. Lancet 381:2273–2279. doi: 10.1016/S0140-6736(13)61125-3. [DOI] [PubMed] [Google Scholar]
- 13.Sleeman K, Guo Z, Barnes J, Shaw M, Stevens J, Gubareva LV. 2013. R292K substitution and drug susceptibility of influenza A(H7N9) viruses. Emerg Infect Dis 19:1521–1524. doi: 10.3201/eid1909.130724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin PH, Chao TL, Kuo SW, Wang JT, Hung CC, Lin HC, Yang ZY, Ho SY, Chang CK, Huang MS, Chen HH, Chen YC, Lai HS, Chang SY, Chang SC, Yang PC. 2014. Virological, serological, and antiviral studies in an imported human case of avian influenza A(H7N9) virus in Taiwan. Clin Infect Dis 58:242–246. doi: 10.1093/cid/cit638. [DOI] [PubMed] [Google Scholar]
- 15.Itoh Y, Ozaki H, Tsuchiya H, Okamoto K, Torii R, Sakoda Y, Kawaoka Y, Ogasawara K, Kida H. 2008. A vaccine prepared from a non-pathogenic H5N1 avian influenza virus strain confers protective immunity against highly pathogenic avian influenza virus infection in cynomolgus macaques. Vaccine 26:562–572. doi: 10.1016/j.vaccine.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 16.Arikata M, Itoh Y, Okamatsu M, Maeda T, Shiina T, Tanaka K, Suzuki S, Nakayama M, Sakoda Y, Ishigaki H, Takada A, Ishida H, Soda K, Pham VL, Tsuchiya H, Nakamura S, Torii R, Shimizu T, Inoko H, Ohkubo I, Kida H, Ogasawara K. 2012. Memory immune responses against pandemic (H1N1) 2009 influenza virus induced by a whole particle vaccine in cynomolgus monkeys carrying Mafa-A1*052:02. PLoS One 7:e37220. doi: 10.1371/journal.pone.0037220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakayama M, Shichinohe S, Itoh Y, Ishigaki H, Kitano M, Arikata M, Pham VL, Ishida H, Kitagawa N, Okamatsu M, Sakoda Y, Ichikawa T, Tsuchiya H, Nakamura S, Le QM, Ito M, Kawaoka Y, Kida H, Ogasawara K. 2013. Protection against H5N1 highly pathogenic avian and pandemic (H1N1) 2009 influenza virus infection in cynomolgus monkeys by an inactivated H5N1 whole particle vaccine. PLoS One 8:e82740. doi: 10.1371/journal.pone.0082740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, Muramoto Y, Tamura D, Sakai-Tagawa Y, Noda T, Sakabe S, Imai M, Hatta Y, Watanabe S, Li C, Yamada S, Fujii K, Murakami S, Imai H, Kakugawa S, Ito M, Takano R, Iwatsuki-Horimoto K, Shimojima M, Horimoto T, Goto H, Takahashi K, Makino A, Ishigaki H, Nakayama M, Okamatsu M, Warshauer D, Shult PA, Saito R, Suzuki H, Furuta Y, Yamashita M, Mitamura K, Nakano K, Nakamura M, Brockman-Schneider R, Mitamura H, Yamazaki M, Sugaya N, Suresh M, Ozawa M, Neumann G, Gern J, Kida H, Ogasawara K, Kawaoka Y. 2009. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460:1021–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kida H, Sakoda Y. 2006. Library of influenza virus strains for vaccine and diagnostic use against highly pathogenic avian influenza and human pandemics. Dev Biol (Basel) 124:69–72. [PubMed] [Google Scholar]
- 20.Itoh Y, Ozaki H, Ishigaki H, Sakoda Y, Nagata T, Soda K, Isoda N, Miyake T, Ishida H, Okamoto K, Nakayama M, Tsuchiya H, Torii R, Kida H, Ogasawara K. 2010. Subcutaneous inoculation of a whole virus particle vaccine prepared from a non-pathogenic virus library induces protective immunity against H7N7 highly pathogenic avian influenza virus in cynomolgus macaques. Vaccine 28:780–789. doi: 10.1016/j.vaccine.2009.10.067. [DOI] [PubMed] [Google Scholar]
- 21.Miyake T, Soda K, Itoh Y, Sakoda Y, Ishigaki H, Nagata T, Ishida H, Nakayama M, Ozaki H, Tsuchiya H, Torii R, Kida H, Ogasawara K. 2010. Amelioration of pneumonia with Streptococcus pneumoniae infection by inoculation with a vaccine against highly pathogenic avian influenza virus in a non-human primate mixed infection model. J Med Primatol 39:58–70. doi: 10.1111/j.1600-0684.2009.00395.x. [DOI] [PubMed] [Google Scholar]
- 22.Chu DH, Sakoda Y, Nishi T, Hiono T, Shichinohe S, Okamatsu M, Kida H. 2014. Potency of an inactivated influenza vaccine prepared from A/duck/Mongolia/119/2008 (H7N9) against the challenge with A/Anhui/1/2013 (H7N9). Vaccine 32:3473–3479. doi: 10.1016/j.vaccine.2014.04.060. [DOI] [PubMed] [Google Scholar]
- 23.Pham VL, Nakayama M, Itoh Y, Ishigaki H, Kitano M, Arikata M, Ishida H, Kitagawa N, Shichinohe S, Okamatsu M, Sakoda Y, Tsuchiya H, Nakamura S, Kida H, Ogasawara K. 2013. Pathogenicity of pandemic H1N1 influenza A virus in immunocompromised cynomolgus macaques. PLoS One 8:e75910. doi: 10.1371/journal.pone.0075910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kageyama T, Fujisaki S, Takashita E, Xu H, Yamada S, Uchida Y, Neumann G, Saito T, Kawaoka Y, Tashiro M. 2013. Genetic analysis of novel avian A(H7N9) influenza viruses isolated from patients in China, February to April 2013. Euro Surveill 18:20453 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20453. [PMC free article] [PubMed] [Google Scholar]
- 25.Kitano M, Itoh Y, Kodama M, Ishigaki H, Nakayama M, Ishida H, Baba K, Noda T, Sato K, Nihashi Y, Kanazu T, Yoshida R, Torii R, Sato A, Ogasawara K. 2011. Efficacy of single intravenous injection of peramivir against influenza B virus infection in ferrets and cynomolgus macaques. Antimicrob Agents Chemother 55:4961–4970. doi: 10.1128/AAC.00412-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wattanagoon Y, Stepniewska K, Lindegardh N, Pukrittayakamee S, Silachamroon U, Piyaphanee W, Singtoroj T, Hanpithakpong W, Davies G, Tarning J, Pongtavornpinyo W, Fukuda C, Singhasivanon P, Day NP, White NJ. 2009. Pharmacokinetics of high-dose oseltamivir in healthy volunteers. Antimicrob Agents Chemother 53:945–952. doi: 10.1128/AAC.00588-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitano M, Itoh Y, Ishigaki H, Nakayama M, Ishida H, Pham VL, Arikata M, Shichinohe S, Tsuchiya H, Kitagawa N, Kobayashi M, Yoshida R, Sato A, Le QM, Kawaoka Y, Ogasawara K. 2014. Efficacy of repeated intravenous administration of peramivir against highly pathogenic avian influenza A (H5N1) virus in cynomolgus macaques. Antimicrob Agents Chemother 58:4795–4803. doi: 10.1128/AAC.02817-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desselberger U, Racaniello VR, Zazra JJ, Palese P. 1980. The 3′ and 5′-terminal sequences of influenza A, B and C virus RNA segments are highly conserved and show partial inverted complementarity. Gene 8:315–328. doi: 10.1016/0378-1119(80)90007-4. [DOI] [PubMed] [Google Scholar]
- 29.Wu Y, Bi Y, Vavricka CJ, Sun X, Zhang Y, Gao F, Zhao M, Xiao H, Qin C, He J, Liu W, Yan J, Qi J, Gao GF. 2013. Characterization of two distinct neuraminidases from avian-origin human-infecting H7N9 influenza viruses. Cell Res 23:1347–1355. doi: 10.1038/cr.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Case DA, Babin V, Berryman JT, Betz RM, Cai Q, Cerutti DS, Cheatham TE III, Darden TA, Duke RE, Gohlke H, Goetz AW, Gusarov S, Homeyer N, Janowski P, Kaus J, Kolossváry I, Kovalenko A, Lee TS, LeGrand S, Luchko T, Luo R, Madej B, Merz KM, Paesani F, Roe DR, Roitberg A, Sagui C, Salomon-Ferrer R, Seabra G, Simmerling CL, Smith W, Swails J, Walker RC, Wang J, Wolf RM, Wu X, Kollman PA. 2014. AMBER 14. University of California, San Francisco, San Francisco, California, USA. [Google Scholar]
- 31.Takano R, Kiso M, Igarashi M, Le QM, Sekijima M, Ito K, Takada A, Kawaoka Y. 2013. Molecular mechanisms underlying oseltamivir resistance mediated by an I117V substitution in the neuraminidase of subtype H5N1 avian influenza A viruses. J Infect Dis 207:89–98. doi: 10.1093/infdis/jis633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolinsky TJ, Czodrowski P, Li H, Nielsen JE, Jensen JH, Klebe G, Baker NA. 2007. PDB2PQR: expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res 35:W522–W525. doi: 10.1093/nar/gkm276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, et al. . 2004. Gaussian 03, revision C.02. Gaussian, Inc., Wallingford, CT, USA. [Google Scholar]
- 34.WHO. 18 to 24 April 2013. China—WHO joint mission on human infection with avian influenza A(H7N9) virus. Mission report. http://www.who.int/influenza/human_animal_interface/influenza_h7n9/ChinaH7N9JointMissionReport2013u.pdf?ua=1 Accessed 20 May 2014.
- 35.Yen HL, McKimm-Breschkin JL, Choy KT, Wong DD, Cheung PP, Zhou J, Ng IH, Zhu H, Webby RJ, Guan Y, Webster RG, Peiris JS. 2013. Resistance to neuraminidase inhibitors conferred by an R292K mutation in a human influenza virus H7N9 isolate can be masked by a mixed R/K viral population. mBio 4:e00396-13. doi: 10.1128/mBio.00396-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hai R, Schmolke M, Leyva-Grado VH, Thangavel RR, Margine I, Jaffe EL, Krammer F, Solorzano A, Garcia-Sastre A, Palese P, Bouvier NM. 2013. Influenza A(H7N9) virus gains neuraminidase inhibitor resistance without loss of in vivo virulence or transmissibility. Nat Commun 4:2854. doi: 10.1038/ncomms3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Z, Yang G, Zhou L. 2013. Mutation effects of neuraminidases and their docking with ligands: a molecular dynamics and free energy calculation study. J Comput Aided Mol Des 27:935–950. doi: 10.1007/s10822-013-9691-1. [DOI] [PubMed] [Google Scholar]
- 38.Gohlke H, Kiel C, Case DA. 2003. Insights into protein-protein binding by binding free energy calculation and free energy decomposition for the Ras-Raf and Ras-RalGDS complexes. J Mol Biol 330:891–913. doi: 10.1016/S0022-2836(03)00610-7. [DOI] [PubMed] [Google Scholar]
- 39.de Wit E, Rasmussen AL, Feldmann F, Bushmaker T, Martellaro C, Haddock E, Okumura A, Proll SC, Chang J, Gardner D, Katze MG, Munster VJ, Feldmann H. 2014. Influenza virus A/Anhui/1/2013 (H7N9) replicates efficiently in the upper and lower respiratory tracts of cynomolgus macaques. mBio 5:e01331-14. doi: 10.1128/mBio.01331-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baskin C. 2013. The role and contributions of systems biology to the non-human primate model of influenza pathogenesis and vaccinology. Curr Top Microbiol Immunol 363:69–85. [DOI] [PubMed] [Google Scholar]
- 41.Huang L, Cao Y, Zhou J, Qin K, Zhu W, Zhu Y, Yang L, Wang D, Wei H, Shu Y. 2014. A conformational restriction in the influenza A virus neuraminidase binding site by R152 results in a combinational effect of I222T and H274Y on oseltamivir resistance. Antimicrob Agents Chemother 58:1639–1645. doi: 10.1128/AAC.01848-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woods CJ, Malaisree M, Long B, McIntosh-Smith S, Mulholland AJ. 2013. Computational assay of H7N9 influenza neuraminidase reveals R292K mutation. Sci Rep 3:3561. doi: 10.1038/srep03561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran-To Su C, Ouyang X, Zheng J, Kwoh CK. 2013. Structural analysis of the novel influenza A (H7N9) viral neuraminidase interactions with current approved neuraminidase inhibitors oseltamivir, zanamivir, and peramivir in the presence of mutation R289K. BMC Bioinformatics 14:S7. doi: 10.1186/1471-2105-14-S16-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takashita E, Fujisaki S, Kishida N, Xu H, Imai M, Tashiro M, Odagiri T. 2013. Characterization of neuraminidase inhibitor-resistant influenza A(H1N1)pdm09 viruses isolated in four seasons during pandemic and post-pandemic periods in Japan. Influenza Other Respir Viruses 7:1390–1399. doi: 10.1111/irv.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiso M, Mitamura K, Sakai-Tagawa Y, Shiraishi K, Kawakami C, Kimura K, Hayden FG, Sugaya N, Kawaoka Y. 2004. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet 364:759–765. doi: 10.1016/S0140-6736(04)16934-1. [DOI] [PubMed] [Google Scholar]
- 46.Okomo-Adhiambo M, Demmler-Harrison GJ, Deyde VM, Sheu TG, Xu X, Klimov AI, Gubareva LV. 2010. Detection of E119V and E119I mutations in influenza A (H3N2) viruses isolated from an immunocompromised patient: challenges in diagnosis of oseltamivir resistance. Antimicrob Agents Chemother 54:1834–1841. doi: 10.1128/AAC.01608-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamura D, Sugaya N, Ozawa M, Takano R, Ichikawa M, Yamazaki M, Kawakami C, Shimizu H, Uehara R, Kiso M, Kawakami E, Mitamura K, Kawaoka Y. 2011. Frequency of drug-resistant viruses and virus shedding in pediatric influenza patients treated with neuraminidase inhibitors. Clin Infect Dis 52:432–437. doi: 10.1093/cid/ciq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Butler J, Hooper KA, Petrie S, Lee R, Maurer-Stroh S, Reh L, Guarnaccia T, Baas C, Xue L, Vitesnik S, Leang SK, McVernon J, Kelso A, Barr IG, McCaw JM, Bloom JD, Hurt AC. 2014. Estimating the fitness advantage conferred by permissive neuraminidase mutations in recent oseltamivir-resistant A(H1N1)pdm09 influenza viruses. PLoS Pathog 10:e1004065. doi: 10.1371/journal.ppat.1004065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yen HL, Zhou J, Choy KT, Sia SF, Teng O, Ng IH, Fang VJ, Hu Y, Wang W, Cowling BJ, Nicholls JM, Guan Y, Peiris JS. 2014. The R292K mutation that confers resistance to neuraminidase inhibitors leads to competitive fitness loss of A/Shanghai/1/2013 (H7N9) influenza virus in ferrets. J Infect Dis 210:1900–1908. doi: 10.1093/infdis/jiu353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collins PJ, Haire LF, Lin YP, Liu J, Russell RJ, Walker PA, Martin SR, Daniels RS, Gregory V, Skehel JJ, Gamblin SJ, Hay AJ. 2009. Structural basis for oseltamivir resistance of influenza viruses. Vaccine 27:6317–6323. doi: 10.1016/j.vaccine.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 51.Ison MG, Mishin VP, Braciale TJ, Hayden FG, Gubareva LV. 2006. Comparative activities of oseltamivir and A-322278 in immunocompetent and immunocompromised murine models of influenza virus infection. J Infect Dis 193:765–772. doi: 10.1086/500464. [DOI] [PubMed] [Google Scholar]
- 52.Marjuki H, Mishin VP, Chesnokov AP, De La Cruz JA, Davis CT, Villanueva JM, Fry AM, Gubareva LV. 2015. Neuraminidase mutations conferring resistance to oseltamivir in influenza A(H7N9) viruses. J Virol 89:5419–5426. doi: 10.1128/JVI.03513-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sawai T, Itoh Y, Ozaki H, Isoda N, Okamoto K, Kashima Y, Kawaoka Y, Takeuchi Y, Kida H, Ogasawara K. 2008. Induction of cytotoxic T-lymphocyte and antibody responses against highly pathogenic avian influenza virus infection in mice by inoculation of apathogenic H5N1 influenza virus particles inactivated with formalin. Immunology 124:155–165. doi: 10.1111/j.1365-2567.2007.02745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Couch RB, Decker WK, Utama B, Atmar RL, Niño D, Feng JQ, Halpert MM, Air GM. 2012. Evaluations for in vitro correlates of immunogenicity of inactivated influenza A H5, H7 and H9 vaccines in humans. PLoS One 7:e50830. doi: 10.1371/journal.pone.0050830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Groot AS, Ardito M, Terry F, Levitz L, Ross TM, Moise L, Martin W. 2013. Low immunogenicity predicted for emerging avian-origin H7N9: implication for influenza vaccine design. Hum Vaccin Immunother 9:950–956. doi: 10.4161/hv.24939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Groot AS, Moise L, Liu R, Gutierrez AH, Terry F, Koita OA, Ross TM, Martin W. 2014. Cross-conservation of T-cell epitopes: now even more relevant to (H7N9) influenza. Hum Vaccin Immunother 10:256–262. doi: 10.4161/hv.28135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith GE, Flyer DC, Raghunandan R, Liu Y, Wei Z, Wu Y, Kpamegan E, Courbron D, Fries LF III, Glenn GM. 2013. Development of influenza H7N9 virus like particle (VLP) vaccine: homologous A/Anhui/1/2013 (H7N9) protection and heterologous A/chicken/Jalisco/CPA1/2012 (H7N3) cross-protection in vaccinated mice challenged with H7N9 virus. Vaccine 31:4305–4313. doi: 10.1016/j.vaccine.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 58.Fries LF, Smith GE, Glenn GM. 2013. A recombinant viruslike particle influenza A (H7N9) vaccine. N Engl J Med 369:2564–2566. doi: 10.1056/NEJMc1313186. [DOI] [PubMed] [Google Scholar]
- 59.Klausberger M, Wilde M, Palmberger D, Hai R, Albrecht RA, Margine I, Hirsh A, Garcia-Sastre A, Grabherr R, Krammer F. 2014. One-shot vaccination with an insect cell-derived low-dose influenza A H7 virus-like particle preparation protects mice against H7N9 challenge. Vaccine 32:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muramoto Y, Shoemaker JE, Mai LT, Itoh Y, Tamura D, Sakai-Tagawa Y, Imai H, Uraki R, Takano R, Kawakami E, Ito M, Okamoto K, Ishigaki H, Mimuro H, Sasakawa C, Matsuoka Y, Noda T, Fukuyama S, Ogasawara K, Kitano H, Kawaoka Y. 2014. Disease severity is associated with differential gene expression at the early and late phases of infection in non-human primates infected with different H5N1 highly pathogenic avian influenza viruses. J Virol 88:8981–8997. doi: 10.1128/JVI.00907-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Itoh Y, Yoshida R, Shichinohe S, Higuchi M, Ishigaki H, Nakayama M, Pham VL, Ishida H, Kitano M, Arikata M, Kitagawa N, Mitsuishi Y, Ogasawara K, Tsuchiya H, Hiono T, Okamatsu M, Sakoda Y, Kida H, Ito M, Le QM, Kawaoka Y, Miyamoto H, Ishijima M, Igarashi M, Suzuki Y, Takada A. 2014. Protective efficacy of passive immunization with monoclonal antibodies in animal models of H5N1 highly pathogenic avian influenza virus infection. PLoS Pathog 10:e1004192. doi: 10.1371/journal.ppat.1004192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.