Abstract
Orthopedic foreign body-associated infections are often treated with rifampin-based combination antimicrobial therapy. We previously observed that rifampin-resistant and methicillin-resistant Staphylococcus aureus (MRSA) isolates were present 2 days after cessation of rifampin therapy in experimental foreign body osteomyelitis. Unexpectedly, only rifampin-susceptible isolates were detected 14 days after the completion of treatment. We studied two rifampin-resistant isolates recovered 2 days after treatment and one rifampin-susceptible isolate recovered 14 days after treatment. Growing these isolates alone in vitro or in vivo demonstrated no fitness defects; however, in mixed culture, rifampin-susceptible bacteria outcompeted rifampin-resistant bacteria. In vivo, two courses of rifampin treatment (25 mg/kg of body weight every 12 h for 21 days) yielded a greater decrease in bacterial quantity in the bones of treated animals 14 days following treatment than that in animals receiving a single course of treatment (P = 0.0398). In infections established with equal numbers of rifampin-resistant and rifampin-susceptible bacteria, one course of rifampin treatment did not affect bacterial quantities. Rifampin-resistant and rifampin-susceptible isolates were recovered both 2 days and 14 days following treatment completion; however, the proportion of animals with rifampin-resistant isolates was lower at 14 days than that at 2 days following treatment completion (P = 0.024). In untreated animals infected with equal numbers of rifampin-resistant and rifampin-susceptible bacteria for 4 weeks, rifampin-susceptible isolates were exclusively recovered, indicating the outcompetition of rifampin-resistant by rifampin-susceptible isolates. The data presented imply that although there is no apparent fitness defect in rifampin-resistant bacteria when grown alone, they are outcompeted by rifampin-susceptible bacteria when the two are present together. The findings also suggest that selected rifampin resistance may not persist in initially rifampin-susceptible infections following the discontinuation of rifampin.
INTRODUCTION
As a result of the aging population and increased life expectancy, knee, hip, shoulder, and ankle arthroplasties are some of the most common surgical procedures performed (1). Prosthetic joint infection (PJI) is a devastating complication of arthroplasty, occurring in 1 to 2% of prosthetic joints. The annual monetary burden of hip- and knee-related PJIs increased from $320 million in 2001 to $566 million in 2009, and it is expected to surpass $1.62 billion by 2020 (2). Staphylococcus aureus, along with coagulase-negative staphylococci, causes more than half of PJIs (3), with other causative organisms including streptococci (10%), enterococci (3 to 7%), Gram-negative bacilli (10 to 17%), anaerobes (2 to 4%), and fungi (1 to 3%) (4). The foreign material used in prosthetics is conducive to the formation of microbial biofilms. Microbial biofilms associated with PJIs provide an environment in which bacteria are severalfold more resistant to antimicrobial treatment compared to their planktonic counterparts (5). Bacterial growth in biofilms is slow, rendering growth-dependent antimicrobials ineffective (5). Additionally, bacteria in biofilms are surrounded by a polymeric matrix consisting of nucleic acids, polysaccharides, and/or proteins (6), which mechanically contribute to resistance to some antimicrobial agents. Furthermore, the decreased microcirculation surrounding foreign bodies as a result of altered anatomy leads to decreased effectiveness of host defenses and antimicrobial delivery (6).
Treatment of PJIs can be time-consuming and expensive. PJI management increasingly involves debridement, antibiotics, and implant retention (DAIR) (5). The appropriate duration of antimicrobial therapy has not been comparatively studied and is therefore varied (7). Treatment of PJI, particularly staphylococcal PJI, is notoriously challenging, and recurrence is common. It is imperative that all microorganisms in the joint space be eradicated. Antimicrobial therapy of staphylococcal PJI managed with a DAIR strategy almost always involves rifampin (7), due to its bactericidal activity against slow-growing staphylococci (6, 8). Unfortunately, resistance to rifampin, caused by one of several base pair mutations in the RNA polymerase β-subunit gene, rpoB, is easily selected (9). S. aureus carrying rpoB mutations may have decreased susceptibility to other antimicrobial agents, such as vancomycin (10, 11), further complicating treatment. As a result of the ease with which rifampin resistance is selected, rifampin is never administered alone.
We recently reported the emergence and subsequent “disappearance” of rifampin resistance in a rat model of foreign body osteomyelitis treated with rifampin (12). Briefly, animals were infected with methicillin-resistant S. aureus (MRSA), and infection was established over 4 weeks, followed by 21 days of rifampin treatment (25 mg/kg of body weight every 12 h). Animals were sacrificed 2 days and 14 days after treatment was complete. Isolates recovered from animals at 2 days following treatment were rifampin resistant, whereas those recovered 14 days following treatment were rifampin susceptible. These results provided the groundwork for the data presented in this paper. Specifically, we sought to determine why rifampin-resistant MRSA disappeared after the completion of treatment.
MATERIALS AND METHODS
Microorganisms.
The parental MRSA isolate (IDRL-6169) was recovered from a patient with a prosthetic hip infection and is part of the clinical isolate stock in the Infectious Diseases Research Laboratory at the Mayo Clinic, Rochester, MN. MRSA isolates 4B (rifampin resistant), 4Bw (rifampin resistant), and 7B (rifampin susceptible) were recovered from bone (4B and 7B) or a foreign body (4Bw) of animals identically infected with IDRL-6169 and treated with rifampin monotherapy (12). 4B and 4Bw were recovered from the same animal 2 days following treatment completion, and 7B was recovered from an animal 14 days following treatment completion (12). The rifampin MIC of IDRL-6169 and 7B was <0.25 μg/ml and that of 4B and 4Bw was 32 μg/ml.
Antimicrobial agent.
Lyophilized rifampin for intravenous administration (Rifadin; Sanofi-Aventis, Bridgewater, NJ) was obtained from the Mayo Clinic Pharmacy and resuspended in 10 ml of sterile water, according to the manufacturer's instructions, to make a stock concentration of 60 mg/ml.
In vitro fitness.
Growth of the parental isolate (IDRL-6169), rifampin-resistant isolates 4B and 4Bw, and rifampin-susceptible isolate 7B in Trypticase soy broth (TSB) at 37°C was monitored in triplicate at A600 over 12 h to determine growth rates. Growth rates were calculated using the following formula: [log(X5) − log(X0)]/0.301t, where X5 is the optical density at 600 nm (OD600) at 5 h, X0 is the initial OD600 (t = 0 h), and t = 5.
In vitro competitive growth.
Equal amounts of overnight cultures of rifampin-resistant (4B or 4Bw) and -susceptible (7B) isolates (1 μl of each) in TSB were combined (4B:7B and 4Bw:7B) in 10 ml of TSB and grown for ∼8 h at 37°C. After ∼8 h, 1 μl of the combined culture was added to 10 ml of fresh TSB and incubated overnight at 37°C. This dilution was repeated every morning and evening, followed by quantitative culture and serial dilutions. Serial dilutions were cultured on Mueller-Hinton agar plates with and without rifampin (4 μg/ml) to monitor the disappearance of rifampin-resistant bacteria. Testing was performed for 14 days.
DNA sequencing.
Genomic DNA was isolated from IDRL-6169, 4B, 4Bw, and 7B using the QIAamp DNA minikit (Qiagen, Valencia, CA). The primers 5′-AGTCTATCACACCTCAACAA-3′ and 5′-TAATAGCCGCACCAGAATCA-3′ targeting S. aureus rpoB were obtained from previous work by Aubry-Damon et al. (9). PCR was performed using the LightCycler FastStart DNA master SYBR green I kit (Roche, Indianapolis, IN). Amplification was conducted at 95°C for 10 min, followed by 40 cycles of 95°C for 10 s, 50°C for 5 s, and 72°C for 30 s, and then at 40°C for 30 s. Sequencing was performed at the Medical Genome Facility at the Mayo Clinic. Purified PCR amplicons were bidirectionally sequenced by Sanger sequencing using BigDye Terminator version 1.1 cycle sequencing chemistry (Applied Biosystems, Carlsbad, CA) on a 3130xl genetic analyzer (Applied Biosystems) equipped with a 50-cm by 96-capillary array and POP7 polymer (Applied Biosystems). Sequences were analyzed using Sequencher (Gene Codes, Ann Arbor, MI).
Experimental rat model.
Chronic foreign body osteomyelitis was established in male Wistar rats (Charles River Laboratories, Wilmington, MA), weighing 250 to 300 g, as previously described (13). Briefly, general anesthesia was induced by intramuscular administration of ketamine (60 mg/kg) and xylazine (6 mg/kg). The left leg of each animal was shaved and prepared with Hibiclens (4% chlorhexidine gluconate) (Mölnlycke Health Care, Norcross, GA). Under sterile conditions, the proximal third of the left tibia was surgically exposed and a 1.5-mm hole drilled into the medullary cavity. Fifty microliters of a 106 CFU suspension of MRSA was injected into the bone. Subsequently, a 5-mm by 1-mm stainless steel wire (Zimmer, Warsaw, IN) was implanted into the bone. The hole was covered with dental gypsum and the muscle closed with 3-0 vicryl (Ethicon, Inc., Somerville, NJ). The skin was closed with Tissuemend II (VPL, Phoenix, AZ) and surgical clips. The wound was sprayed with AluSpray (Neogen Corporation, Lansing, MI) and Chew-Guard (Summit Hill Laboratories, Tinton Falls, NJ). Buprenorphine (slow release at 60 mg/kg) was administered subcutaneously for analgesia. The study was approved by the Mayo Clinic Institutional Animal Care and Use Committee.
In vivo fitness.
To assess in vivo fitness differences, foreign body osteomyelitis was established with 4B, 4Bw, or 7B alone for 4 weeks, after which animals were sacrificed, and the involved tibia was extracted under sterile conditions.
In vivo competitive growth.
To assess competition between rifampin-resistant and -susceptible MRSA, foreign body osteomyelitis was established with a 1:1 ratio of rifampin-resistant and rifampin-susceptible MRSA (4B:7B or 4Bw:7B). Four weeks later, the animals were sacrificed, and the involved tibias were extracted under sterile conditions.
Studies using two courses of rifampin treatment.
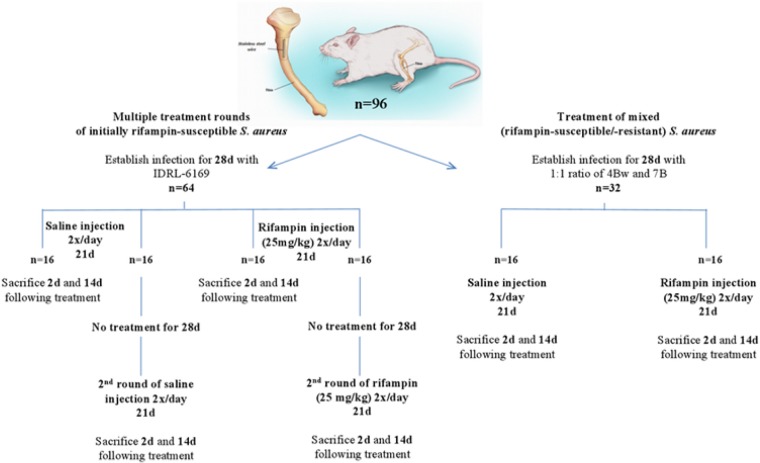
To determine the effect of two courses of rifampin treatment on the emergence or remittance of rifampin resistance, animals were infected with MRSA IDRL-6169 (Fig. 1). Following a 4-week infection, animals were separated into two groups of 32 animals each: rifampin (25 mg/kg intraperitoneally every 12 h for 21 days) monotherapy and control (equivalent volume of sterile saline intraperitoneally every 12 h for 21 days). Eight animals each in the control and treatment groups were sacrificed 2 days after treatment completion, and eight animals each in the control and treatment groups were sacrificed 14 days after the completion of treatment. The remaining 16 animals in each group were untreated for 4 weeks, after which they received a second course of either saline or rifampin monotherapy, delivered as in the first course, for 21 days. Eight animals each from the control and treatment groups were sacrificed 2 days after the completion of therapy, and the final eight animals each from the control and treatment groups were sacrificed 14 days following treatment completion.
FIG 1.
Schematic of in vivo experiments involving treatment with rifampin (illustration reproduced from reference 13).
Competition studies with rifampin monotherapy.
To determine the effect of rifampin monotherapy on infection established with a mixture of rifampin-resistant and -susceptible MRSA, a 1:1 mixture of 4Bw and 7B was used as the inoculum in the foreign body osteomyelitis model. Infection was established for 4 weeks; thereafter, animals were separated into two groups, control (saline injections) and rifampin monotherapy, administered as described above for 21 days. Following treatment completion, half of the animals in each group were sacrificed 2 days after treatment completion, with the remaining animals sacrificed 14 days after the completion of treatment.
For all animal experiments, the animals were sacrificed using CO2, and the left tibias were aseptically removed and frozen at −70°C. Bone was cut within 5 mm of the implanted stainless steel wire, weighed, and pulverized to separate the bone and wire. Pulverized bone and wire were separately suspended in 2 and 1 ml, respectively, of TSB, vortexed for 30 s, sonicated at 40 kHz for 5 min, vortexed for 30 s, serially diluted, and plated onto both Trypticase soy agar plates containing 5% sheep blood (TSA II) (Becton Dickinson, Franklin Lakes, NJ) and Mueller-Hinton agar plates containing 4 μg/ml rifampin. The quantitative culture results of bone and wire on blood agar plates were recorded after incubation for 2 days at 37°C and expressed as log10 CFU per gram of bone or log10 CFU/centimeter squared of wire surface. Qualitative cultures were performed by incubating TSB containing pulverized bone or wire for 24 h, followed by subculture to assess for the presence or absence of MRSA.
Statistical methods.
For statistical purposes, we considered absence of any growth to be 0.1 log10 CFU/g of bone or cm2 of wire. We considered growth in qualitative broth culture (but not in quantitative cultures) to be 0.5 log10 CFU/g of bone or cm2 of wire. Comparisons of the control versus treatment for various durations for bone and wire were performed using the Wilcoxon rank sum test. Proportions of animals with isolates that were resistant to rifampin at 14 days compared with 2 days posttreatment were compared using Fisher's exact test. Analysis was performed using SAS version 9.3 (SAS, Inc., Cary, NC). All tests were 2-sided; P values of <0.05 were considered significant.
RESULTS
In vitro fitness.
There were no growth defects in either of the rifampin-resistant isolates (4B or 4Bw) compared with the rifampin-susceptible (7B) or the parental (IDRL-6169) isolate (Fig. 2). The growth rates (A600) were measured to be 0.89 (4B), 0.87 (4Bw), and 0.89 (7B), suggesting that resistance to rifampin in these isolates does not result in a fitness defect when the bacteria are grown alone in vitro.
FIG 2.
Fitness of rifampin-resistant (4B and 4Bw) MRSA compared with rifampin-susceptible (7B) and parental rifampin-susceptible (IDRL-6169) MRSA (shown as 6169). Error bars indicate standard deviations. P > 0.05 for all time points.
In vitro competitive growth.
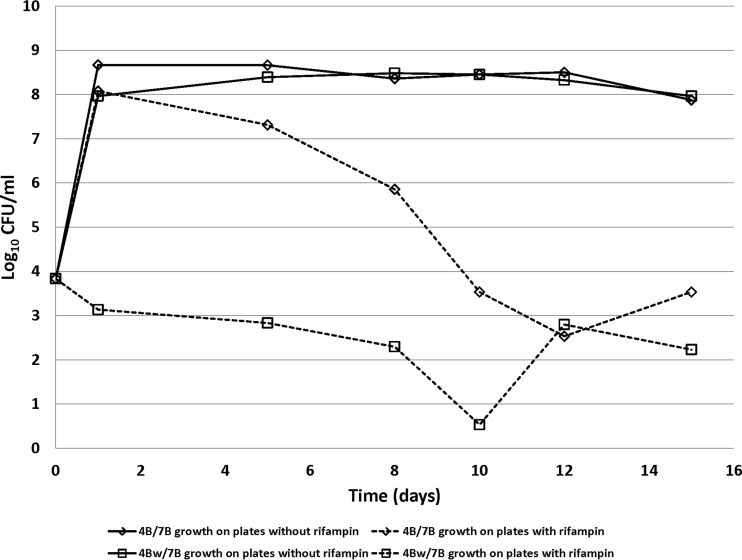
The competition studies revealed that over time, the numbers of rifampin-resistant isolates decrease, while the numbers of rifampin-susceptible isolates remain steady. This indicates that rifampin-susceptible isolates outcompete rifampin-resistant isolates in vitro (Fig. 3).
FIG 3.
In vitro growth of rifampin-resistant and -susceptible isolates combined in equal ratios. The solid lines represent quantities of both resistant and susceptible bacteria, while the dotted lines represent quantities of rifampin-resistant bacteria only. The experiments were repeated twice, with similar findings; the data shown are from one experiment.
DNA sequencing.
Sequencing results indicated that both 4B and 4Bw have a mutation in cluster I of rpoB that is commonly associated with rifampin resistance (9). Strain 4B has an A477D mutation in rpoB, while 4Bw has an H481Y mutation in rpoB. Neither the rifampin-susceptible isolate (7B) nor the parental strain (IDRL-6169) has a mutation in rpoB.
In vivo fitness.
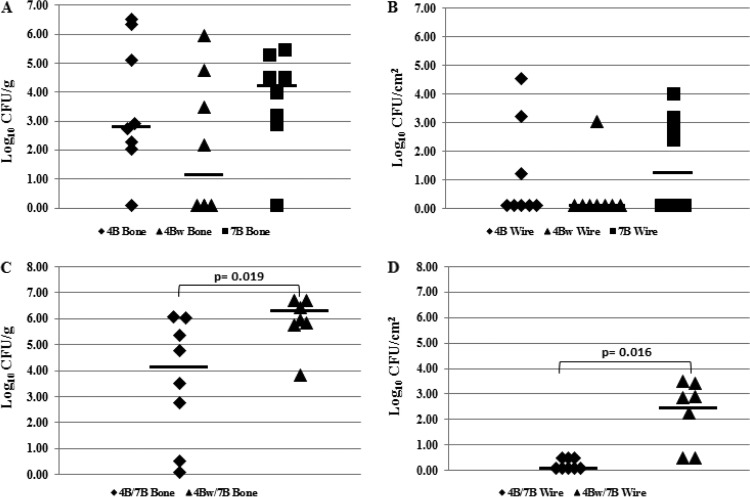
Osteomyelitis established with either 4B, 4Bw, or 7B alone did not result in any growth defects between the rifampin-resistant and rifampin-susceptible MRSA. Bone cultures showed a median value of 2.82 log10 CFU/g of bone for 4B, 1.14 log10 CFU/g of bone for 4Bw, and 4.23 log10 CFU/g of bone for 7B (Fig. 4A). There was no difference in the numbers of organisms recovered from bones between rifampin-resistant and rifampin-susceptible MRSA (4B versus 7B, P = 0.636; 4Bw versus 7B, P = 0.201). The median values for wire cultures were 0.10 log10 CFU/cm2 of wire for 4B, 0.10 log10 CFU/cm2 of wire for 4Bw, and 1.25 log10 CFU/cm2 of wire for 7B (Fig. 4B). There was no difference in the bacterial quantities recovered from wires between rifampin-resistant and rifampin-susceptible MRSA (4B versus 7B, P = 0.729; 4Bw versus 7B, P = 0.123).
FIG 4.
In vivo fitness results. Bacterial quantities in animals infected with either a single MRSA isolate (A and B) (n = 8) or a 1:1 ratio of rifampin-resistant and -susceptible MRSA isolates (C and D) (n = 8 for 4B/7B, n = 7 for 4Bw/7B) after 4 weeks. (A and C) Results of quantitative cultures of bones. (B and D) Results of quantitative cultures of wires. Log10 CFU/g and CFU/cm2 values reported are from growth on blood agar plates without rifampin. The horizontal bars indicate the median values.
In vivo competitive growth.
Competition studies, in which osteomyelitis was established with a 1:1 ratio of rifampin-susceptible to rifampin-resistant MRSA, yielded exclusively rifampin-susceptible isolates from all experiments, suggesting that as in the in vitro studies, rifampin-susceptible isolates outcompete rifampin-resistant isolates in vivo. Quantitative cultures of the bone revealed median values of 4.14 log10 CFU/g and 6.32 log10 CFU/g for the 4B-7B and 4Bw-7B combinations, respectively (Fig. 4C). Quantitative cultures of the wire revealed median values of 0.10 log10 CFU/cm2 and 2.45 log10 CFU/cm2 for the 4B/7B and 4Bw/7B combinations, respectively (Fig. 4D). The competitive mixture of 4B and 7B yielded lower quantities of bacteria than the mixture of 4Bw and 7B (P = 0.0109 for bone and P = 0.0016 for wire), even though the same rifampin-susceptible isolate (7B) was presumably recovered alone at the end of both experiments.
Studies with two courses of rifampin treatment.
The weight of each animal was monitored over the two courses of rifampin treatment, and there was no difference between animals treated with saline and those treated with rifampin (data not shown). After the first course of treatment, wires were missing from three control animals sacrificed at 2 days, one treated animal sacrificed at 2 days, one control animal sacrificed at 14 days, and one treated animal sacrificed at 14 days. Following the second course of treatment, wires were missing from one treated animal sacrificed at 2 days and one control animal sacrificed at 14 days. Wires were presumably lost into the surrounding musculature of the left tibia, since it was noted that in a few animals, the wire had been pushed out of the bone (most likely from new bone formation/remodeling) and was in the muscle fascia.
First course of rifampin treatment.
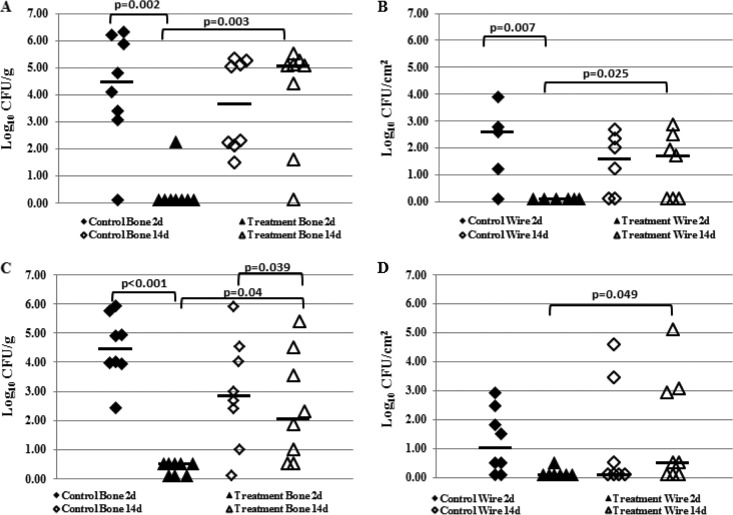
Animals that received saline injections and were sacrificed 2 days after the first course of treatment completion had a median bacterial load of 4.47 log10 CFU/g of bone (Fig. 5A) and 2.59 log10 CFU/cm2 of wire (Fig. 5B). Animals treated with rifampin and sacrificed 2 days after treatment completion had a median bacterial load of 0.10 log10 CFU/g of bone (Fig. 5A) and 0.10 log10 CFU/cm2 of wire (Fig. 5B). All bacteria recovered from rifampin-treated animals were resistant to rifampin at this time point. Among animals sacrificed 14 days after the first course of treatment, saline-injected animals had a median bacterial load of 3.60 log10 CFU/g of bone (Fig. 5A) and 1.40 log10 CFU/cm2 of wire (Fig. 5B). Animals treated with rifampin and sacrificed at 14 days had a median bacterial load of 5.08 log10 CFU/g of bone (Fig. 5A) and 1.69 log10 CFU/cm2 of wire (Fig. 5B). All bacteria recovered from rifampin-treated animals at 14 days were susceptible to rifampin.
FIG 5.
In vivo results following one (A and B) and two (C and D) courses (21 days) of treatment with 25 mg/kg rifampin twice daily. The parental strain IDRL-6169 was used to establish infections. Shown are the bacterial loads recovered from control (saline) animals (⬥) and treated (rifampin) animals (▲). (A and C) Results of quantitative cultures of bone. (B and D) Results of quantitative cultures of wire. Log10 CFU/g and CFU/cm2 values reported are from growth on blood agar plates without rifampin. The horizontal bars indicate the median values.
Bacterial loads in control versus treated animals 2 days after treatment completion showed a difference in quantities for both bone and wire (P = 0.002 and 0.007, respectively). The bacterial quantities in control versus treated bone and wire 14 days after treatment completion were similar (P = 0.713 and 0.883 for bone and wire, respectively). Bacterial quantities in both bone and wire in rifampin-treated animals were lower at 2 days versus 14 days after the completion of treatment (P = 0.003 and 0.025 for bone and wire, respectively).
Second course of rifampin treatment.
Two days following the second course of treatment, the control animals had a median bacterial load of 4.46 log10 CFU/g of bone (Fig. 5C) and 1.01 log10 CFU/cm2 of wire (Fig. 5D), and the rifampin-treated animals had a median bacterial load of 0.50 log10 CFU/g of bone (Fig. 5C) and 0.10 log10 CFU/cm2 of wire (Fig. 5D). Bacteria were recovered from the bones of five and the wire of one rifampin-treated animal. Surprisingly, rifampin-resistant bacteria were recovered from the bones of only two animals; bacteria recovered from the remaining three bones and one wire were susceptible to rifampin. Fourteen days after the completion of treatment, the control animals had a median bacterial load of 2.84 log10 CFU/g of bone (Fig. 5C) and 0.10 log10 CFU/cm2 of wire (Fig. 5D), whereas animals receiving a second course of rifampin treatment had a median bacterial load of 2.07 log10 CFU/g of bone (Fig. 5C) and 0.50 log10 CFU/cm2 of wire (Fig. 5D). All bacteria recovered 14 days after the completion of treatment were susceptible to rifampin.
Following the second course of rifampin treatment, fewer bacteria were recovered from the bones and wires of treated animals than from control animals 2 days after the completion of treatment (P < 0.001 and 0.017 for bone and wire, respectively), whereas after 14 days, the amounts of bacteria on the wires were similar (P = 0.807), although there were fewer bacteria in the treated than in the control bones (P = 0.04). Fewer bacteria were recovered in treated animals at 2 days versus 14 days after treatment completion (P = 0.023 and 0.049 for bone and wire, respectively).
Comparing bacterial quantities following one and two courses of rifampin only showed a difference in the amount of bacteria recovered from bone 14 days after treatment (P = 0.0398). Bacterial quantities recovered from bone at 2 days following treatment completion were similar (P = 0.097), as were bacterial quantities recovered from wires 2 days and 14 days following one or two courses of treatment (P = 0.317 and 0.675, respectively).
Competition studies with rifampin monotherapy.
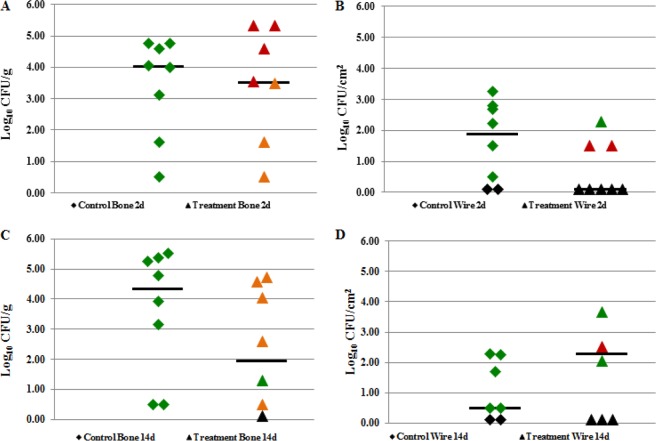
The weight of each animal was monitored over the course of treatment; there was no difference in weight between animals treated with saline and those treated with rifampin (data not shown). One wire was irretrievable in a control animal sacrificed 14 days after the completion of treatment. Animals that received saline injections and were sacrificed 2 days after treatment completion had a median bacterial load of 4.01 log10 CFU/g of bone and 1.87 log10 CFU/cm2 of wire (Fig. 6A and B). Animals treated with rifampin and sacrificed 2 days after treatment completion had a median bacterial load of 3.51 log10 CFU/g of bone and 0.10 log10 CFU/cm2 of wire (Fig. 6A and B). At this time point, bacteria were recovered from seven animals treated with rifampin, of which three had bacteria recovered from both bones and wires, and four had bacteria recovered from bones only. Among these treated animals, bacteria recovered from 4/7 bones and 2/3 wires were uniformly resistant to rifampin. Partial resistance (∼50% of bacteria recovered being resistant and 50% being susceptible to rifampin) was present in 3/7 bones; uniform rifampin susceptibility was present in 1/3 wires of the treated animals. All bacteria recovered from animals receiving saline injections were susceptible to rifampin, indicating that the originally present rifampin-resistant isolates were outcompeted by rifampin-susceptible isolates.
FIG 6.
In vivo results following one course (21 days) of rifampin treatment (25 mg/kg, twice daily) on an infection established with a 1:1 ratio of rifampin-resistant (4Bw) and rifampin-susceptible (7B) MRSA. Shown are the bacterial loads recovered from control (saline) animals (◆) and treated (rifampin) animals (▲). Rifampin resistance is color coded (green, susceptible; orange, partially resistant; red, resistant; black, no growth in culture). (A) Results of quantitative cultures of bone 48 h after treatment completion. (B) Results of quantitative cultures of wire 48 h after treatment completion. (C) Results of quantitative cultures of bone 14 days after treatment completion. (D) Results of quantitative cultures of wire 14 days after treatment completion. Log10 CFU/g and CFU/cm2 values reported are from growth on blood agar plates without rifampin. The horizontal bars indicate the median values.
Animals treated with saline and sacrificed 14 days after the completion of treatment had a median bacterial load of 4.35 log10 CFU/g of bone and 0.50 log10 CFU/cm2 of wire (Fig. 6C and D). Animals treated with rifampin and sacrificed 14 days after treatment completion had a median bacterial load of 1.95 log10 CFU/g of bone and 1.84 log10 CFU/cm2 of wire (Fig. 6C and D). At this time point, bacteria were recovered from six animals treated with rifampin. Of the six, five had bacteria recovered from both bones and wires, and one had bacteria recovered from bone only. From these treated animals, bacteria recovered from 0/6 bones and 1/5 wires were uniformly resistant to rifampin. Partial resistance was present in bacteria recovered from 5/6 bones and 2/5 wires, and exclusively rifampin-susceptible bacteria were recovered from 1/6 bones and 2/5 wires. All bacteria recovered from animals receiving saline injections were susceptible to rifampin, again indicating outcompetition by rifampin-susceptible isolates.
There were no differences in the bacterial quantities recovered from bones between control and treated animals at 2 days or 14 days following treatment (P = 0.752 and 0.139, respectively). Bacterial quantities from control and treated wires were similar at 2 days and 14 days following the completion of treatment (P = 0.099 and 0.345, respectively). A comparison of the bacterial quantities from treated bone at 48 h and 14 days following treatment completion revealed no differences (P = 0.460), nor did a comparison of the bacterial quantities recovered from wires at 2 days and 14 days following treatment completion (P = 0.104). There was a decrease in the proportion of treated animals with rifampin-resistant bacteria recovered at 14 days posttreatment compared with that recovered at 2 days posttreatment (P = 0.024). There was no difference in the proportion of animals with partially resistant bacteria (P = 0.198) and animals with exclusively susceptible bacteria (P = 0.587) in a comparison of the two time points.
DISCUSSION
From our initial in vitro and in vivo experiments, it appeared that rifampin-resistant MRSA isolates, resulting from the selected mutations detailed here, did not have a fitness or growth disadvantage compared to their susceptible counterparts when grown alone. However, when grown with their rifampin-susceptible clones, they were outcompeted by those clones. Interestingly, the in vivo competitive mixture of 4B and 7B yielded lower quantities of bacteria than did the mixture of 4Bw and 7B, despite their both having only rifampin-susceptible S. aureus detectable at the end of the experiments. This raises the possibility that 4Bw enhances early colonization of the foreign body prior to being outcompeted by rifampin-susceptible S. aureus. Further experiments investigating the role of rifampin-resistant isolates in establishing early-stage infections are needed to address this hypothesis. Fitness disadvantages in bacteria with the same rpoB mutations as our isolates have been reported (9, 10); our findings regarding the fitness of rifampin-resistant and rifampin-susceptible isolates when grown alone in vitro and in vivo contradict the published data.
The observation that rifampin-susceptible bacteria outcompete rifampin-resistant bacteria following rifampin treatment raises the question as to whether or not this could be exploited to expand treatment options using rifampin. To this end, we examined the implications of two courses of rifampin treatment. Following one course of treatment with rifampin, bacterial quantities were decreased in bones and on wires of treated animals 2 days after treatment completion compared to those in the controls, an observation that was not unexpected. Also, as expected, all bacteria recovered from treated animals were resistant to rifampin. Fourteen days after the completion of treatment, bacterial quantities from bones and wires of treated animals were similar to those of the controls. Bacteria recovered from treated animals at this time point were susceptible to rifampin, confirming what was previously observed (12); we hypothesize that this occurs due to outcompetition of rifampin-resistant bacteria by rifampin-susceptible bacteria. After the second course of rifampin treatment, bacterial quantities on bones and wires of the treated animals were decreased 2 days after treatment completion compared with those of the controls. However, rifampin-resistant bacteria were recovered from only 2/6 treated animals. Bacterial quantities were decreased in the bones of treated animals 14 days after completion compared to those of the controls. All bacteria recovered from treated animals at this time point were susceptible to rifampin. Bacterial quantities were also lower in bones and on wires in a comparison of treated animals at 2 days and 14 days after the completion of treatment.
Regarding the lower proportion of detectable rifampin-resistant bacteria 2 days posttreatment after the second compared to the first course of rifampin treatment, we hypothesize that the first course of rifampin selected for rifampin-resistant bacteria, with additional mutations compromising their fitness, rendering them less fit to survive. These bacteria may have been present, but undetectable, 14 days after the first course of rifampin treatment. During the second course, they may have reemerged but were quickly outcompeted by susceptible bacteria following treatment completion, resulting in a lower proportion of rifampin-resistant bacteria detected at 2 days following the second course of treatment. It may be that bacterial populations previously resistant to rifampin have a survival disadvantage while undergoing treatment with rifampin a second time.
Additionally, the effectiveness of rifampin throughout the bacterial biofilm in a foreign body infection can be called into question. Previous studies have shown that rifampin is fully able to penetrate Staphylococcus epidermidis biofilms (14, 15). Despite this, the authors of those studies reported bactericidal effects only at the surface of the biofilm. Previous studies have also compared the rifampin susceptibilities of planktonic versus biofilm-associated bacteria and found a decrease in susceptibility in biofilm-associated bacteria. This decreased susceptibility is not a result of rpoB mutation-associated rifampin-resistant bacteria in the biofilm (15). Taking this information into consideration, it is possible that the effectiveness of rifampin is not uniform throughout the biofilm, leaving behind bacteria that would be rifampin susceptible in the planktonic state. The susceptible bacteria are then able to quickly outcompete resistant bacteria once rifampin is removed, even more so following the second course of treatment.
Second, we wanted to determine the effects of rifampin treatment on foreign body osteomyelitis established with a combination of rifampin-resistant and rifampin-susceptible MRSA. No differences were observed among treated and untreated animals at 2 days and 14 days in both bone and wire. These results were not unexpected, due to the initial presence of rifampin-resistant bacteria. Notably, rifampin resistance may have been in the process of disappearing at 14 days posttreatment, as rifampin resistance was present in a smaller proportion of rats than that at 2 days posttreatment. We hypothesize that the decrease in resistant bacteria is due to competition by rifampin-susceptible organisms. Further studies that examine additional time points following treatment completion will better inform this process.
One interesting observation was the presence of mixed populations of rifampin-resistant and -susceptible isolates following rifampin treatment when infections were established with a 1:1 ratio of resistant and susceptible organisms. At first glance, this was not surprising, since the initial infection was a mixed population. However, in the absence of rifampin treatment, resistance was not detectable 4 weeks after establishing the infection with a 1:1 ratio of resistant and susceptible organisms. Based on earlier experiments, we had hypothesized that prior to the start of rifampin treatment, all bacteria would be rifampin susceptible, with the resistant organisms being outcompeted over the 4-week period of infection. From there, the trend would continue in a similar way to infections established with rifampin-susceptible organisms and treated with rifampin, with resistant organisms detectable at 2 days posttreatment and susceptible organisms detectable 14 days posttreatment. The presence of mixed populations at both time points following rifampin treatment indicated that something different occurred. Establishing infection with a mixed resistant/susceptible population compared to a purely susceptible population with subsequent selection of resistance resulted in different outcomes. Further studies are needed to better understand the mechanisms underlying this difference.
Clinically, if rifampin resistance is observed following treatment with rifampin, treatment utilizing a non-rifamycin-based antimicrobial regimen would likely be pursued. Taking into consideration the activity of rifampin against biofilms and the limited availability of alternative antimicrobials, the observations here detailing the weak durability of rifampin resistance in initially rifampin-susceptible infections may enable the use of rifampin despite observed emergence of resistance. Further studies are needed before this strategy can be adopted in clinical practice. Future studies are planned involving whole-genome sequencing to determine what, if any, other changes occur in rifampin-resistant populations that might allow them to be outcompeted by susceptible isolates. We hypothesize that there may be compensatory mutations that allow for a high level of fitness when grown alone in vitro or in vivo. There may be other mutations in virulence factors or gene regulators that contribute to the disappearance of rifampin-resistant bacteria when grown with rifampin-susceptible bacteria. Additionally, we plan to catalog mutations, including those responsible for rifampin resistance, which appear in vivo over time, along with the associated rifampin MIC.
The contrived scenario of rifampin monotherapy is a limitation of this study. Rifampin monotherapy is intentionally avoided in the treatment of osteomyelitis in the clinical setting because of the well-known phenomenon of rapidly emerging rifampin resistance. Our experiments were specifically designed to select for rifampin resistance to examine the consequences of doing so and are not intended to suggest that rifampin monotherapy be used clinically. Experiments examining the appearance of rifampin resistance in the setting of combination therapy are planned in future studies comparing the outcomes of animals previously treated with rifampin followed by a rifampin-containing combination therapy regimen versus animals treated with combination therapy only. This will determine if treatment with a rifampin-containing regimen might be feasible, even if rifampin resistance was previously selected. The animal model used in these experiments is another limitation because of the use of a high inoculum to establish infection and spontaneous healing in some animals. However, this seems reasonable for a study focusing on the kinetics of the emergence of rifampin resistance. A larger animal model may also be beneficial in order to sample bacterial populations from various areas of an infection (i.e., bone distant from the site of infection and bone adjacent to the site of infection and wire). Another limitation is the limited time points at which animals were sacrificed and rifampin resistance was assessed, especially following the second course of rifampin treatment. At this time point, we observed decreased numbers of rifampin-resistant bacteria, which is different from our observations after the first course of treatment. Additional work is needed to fully understand the kinetics of rifampin resistance during and after treatment.
The data reported here suggest that rifampin resistance may not endure following rifampin monotherapy in infections established with rifampin-susceptible S. aureus, because rifampin-resistant isolates may be outcompeted by rifampin-susceptible isolates. Following a second course of rifampin treatment, we observed a decrease in rifampin resistance, supporting the possibility that repeated courses of rifampin treatment for foreign body osteomyelitis may be associated with decreased numbers of rifampin-resistant organisms, even in the setting of prior treatment-associated emergence of rifampin resistance.
ACKNOWLEDGMENT
We received funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases for the Musculoskeletal Research Training Program (T32AR56950).
REFERENCES
- 1.Shuman EK, Urquhart A, Malani PN. 2012. Management and prevention of prosthetic joint infection. Infect Dis Clin North Am 26:29–39. doi: 10.1016/j.idc.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. 2012. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 27:61–65.e1. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Leone S, Borre S, Monforte A, Mordente G, Petrosillo N, Signore A, Venditti M, Viale P, Nicastri E, Lauria FN, Carosi G, Moroni M, Ippolito G, GISIG (Gruppo Italiano di Studio sulle Infezioni Gravi) Working Group on Prosthetic Joint Infections. 2010. Consensus document on controversial issues in the diagnosis and treatment of prosthetic joint infections. Int J Infect Dis 14(Suppl 4):S67–S77. doi: 10.1016/j.ijid.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Corvec S, Portillo ME, Pasticci BM, Borens O, Trampuz A. 2012. Epidemiology and new developments in the diagnosis of prosthetic joint infection. Int J Artif Organs 35:923–934. doi: 10.5301/ijao.5000168. [DOI] [PubMed] [Google Scholar]
- 5.Esposito S, Leone S. 2008. Prosthetic joint infections: microbiology, diagnosis, management and prevention. Int J Antimicrob Agents 32:287–293. doi: 10.1016/j.ijantimicag.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N Engl J Med 351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerli W. 2006. Infection and musculoskeletal conditions: prosthetic-joint-associated infections. Best Pract Res Clin Rheumatol 20:1045–1063. doi: 10.1016/j.berh.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. 1998. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA 279:1537–1541. [DOI] [PubMed] [Google Scholar]
- 9.Aubry-Damon H, Soussy CJ, Courvalin P. 1998. Characterization of mutations in the rpoB gene that confer rifampin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 42:2590–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuo M, Hishinuma T, Katayama Y, Cui L, Kapi M, Hiramatsu K. 2011. Mutation of RNA polymerase beta subunit (rpoB) promotes hVISA-to-VISA phenotypic conversion of strain Mu3. Antimicrob Agents Chemother 55:4188–4195. doi: 10.1128/AAC.00398-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe Y, Cui L, Katayama Y, Kozue K, Hiramatsu K. 2011. Impact of rpoB mutations on reduced vancomycin susceptibility in Staphylococcus aureus. J Clin Microbiol 49:2680–2684. doi: 10.1128/JCM.02144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vergidis P, Schmidt-Malan SM, Mandrekar JN, Steckelberg JM, Patel R. 2015. Comparative activities of vancomycin, tigecycline and rifampin in a rat model of methicillin-resistant Staphylococcus aureus osteomyelitis. J Infect 70:609–615. doi: 10.1016/j.jinf.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Vergidis P, Rouse MS, Euba G, Karau MJ, Schmidt SM, Mandrekar JN, Steckelberg JM, Patel R. 2011. Treatment with linezolid or vancomycin in combination with rifampin is effective in an animal model of methicillin-resistant Staphylococcus aureus foreign body osteomyelitis. Antimicrob Agents Chemother 55:1182–1186. doi: 10.1128/AAC.00740-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunne WM Jr, Mason EO Jr, Kaplan SL. 1993. Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm. Antimicrob Agents Chemother 37:2522–2526. doi: 10.1128/AAC.37.12.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Z, Stewart PS. 2002. Penetration of rifampin through Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother 46:900–903. doi: 10.1128/AAC.46.3.900-903.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]