Abstract
We report here a dehydropeptidase-deficient murine model of tuberculosis (TB) infection that is able to partially uncover the efficacy of marketed broad-spectrum β-lactam antibiotics alone and in combination. Reductions of up to 2 log CFU in the lungs of TB-infected mice after 8 days of treatment compared to untreated controls were obtained at blood drug concentrations and time above the MIC (T>MIC) below clinically achievable levels in humans. These findings provide evidence supporting the potential of β-lactams as safe and mycobactericidal components of new combination regimens against TB with or without resistance to currently used drugs.
TEXT
The widespread assumption that β-lactams, one of the safest types of antibiotics, are not adequate drugs for the treatment of tuberculosis (TB) infection is grounded on (i) the constitutive production of BlaC, a broad-spectrum class A β-lactamase that protects Mycobacterium tuberculosis from β-lactam action (1), (ii) the impermeable nature of the mycobacterial cell wall, rendering most β-lactams weakly efficacious in vitro (2), (iii) their potentially limited penetration into macrophages, (iv) their failure to show significant levels of efficacy in commonly employed TB animal models of infection (3, 4), and (v) the unconvincing clinical proof of efficacy reported to date (5, 6). These arguments, together with further limitations associated with the lack of active and orally bioavailable β-lactams with long half-lives have profoundly affected the state of opinions in the TB research and development (R&D) community to the point where research in this area has virtually stalled for the best part of the last 3 decades.
β-Lactams have a long-standing record of clinical safety and a low potential for interaction with other antitubercular and antiretroviral compounds. As a new class of antitubercular drugs, β-lactams would be the ideal complement to other novel agents to form a completely new antitubercular treatment regimen for treating multidrug-resistant (MDR) or extensively drug-resistant (XDR) TB. In summary, recently published data have produced the following findings. First, they have shown that the combination of some β-lactam carbapenems with clavulanate is efficacious (7) against M. tuberculosis in vitro against both drug-sensitive and MDR-TB strains. Activity was also shown against hypoxic M. tuberculosis cultures. Second, orally bioavailable BlaC-resistant faropenem matches the combination meropenem-clavulanate in terms of potency and bactericidal behavior in vitro against M. tuberculosis (8). Third, a synthetic lethality screen has shown how mycobacteria can become hypersusceptible to β-lactam action through a number of resistance mechanisms commonly induced by current antitubercular therapy (e.g., cell wall biosynthesis and efflux systems) (9). Last, MDR-TB strains are hypersensitive to the meropenem-clavulanate combination in vitro (10). Additionally, to date, it has not been possible to isolate spontaneous mutants resistant to the combination of meropenem and clavulanate or faropenem under laboratory conditions, hinting at a low propensity for the development of resistance. Finally, an increasing number of clinical reports have shown that the combination of meropenem and clavulanate contributes to sputum conversion and secession of symptoms in MDR/XDR therapeutically destitute TB patients (11–13).
Only recently, a European Development Clinical Trials Partnership (EDCTP)-funded early bactericidal activity (EBA) study on the use of different β-lactam combinations in drug-sensitive TB patients in South Africa was initiated (https://clinicaltrials.gov/ct2/show/NCT02349841) (14). In order to be able to support the previously mentioned meropenem- or faropenem-amoxicillin-clavulanate clinical study (14), we chose to focus our efforts on the development of a mouse model of TB infection that could allow a closer reproduction of the human blood pharmacokinetic profiles of β-lactams in mice. The model should be able to address the ubiquitous and high-level expression of dehydropeptidase-I (DHP-I) (15), one of the main enzymes responsible for the cleavage of the lactam ring and hence the extremely quick clearance of β-lactam antibiotics in rodent tissues (including lungs) in comparison to that in humans (16).
The lack of significant efficacy encountered in different animal models of TB infection (4, 5, 17) has been a great source of frustration among TB researchers and is one of the reasons accounting for the very limited number of translational studies published to date in support of an eventual clinical evaluation. Such a model would need to address one of the main limitations associated with the use of rodent species for TB efficacy studies, i.e., the high activity and high level of expression of dehydropeptidase-I, an enzyme that acts as a major contributor to lactam ring cleavage in most tissue types of mice, rats, guinea pigs, and rabbits (16). The model should also be able to sustain both acute and chronic infections and generate significant and robust β-lactam efficacy responses to allow eventual drug-to-drug comparisons. While never specifically used for the purpose of antibacterial drug discovery, such a model has been reported to be deficient in membrane-bound dipeptidase (MBD) (EC 3.4.13.19, also known as DHP-I), an enzyme hypothetically responsible for the conversion of leukotriene D4 (LTD4) to leukotriene E4 (LTE4) (18). This same enzyme was also shown to be responsible for the cleavage of cystinyl-bis-glycine (cys-bis-gly), and its null mutation led to the absence of β-lactamase activity in the lungs, kidneys, small intestines, and hearts of mice.
A preliminary evaluation of the model in terms of its capacity to sustain a viable M. tuberculosis H37Rv infection and improve the whole-blood exposure levels of meropenem showed how the progression of disease in terms of lung CFU counts was identical to that in the reference mouse strain C57BL/6 (see Fig. S1 in the supplemental material). Additionally, significant increases of meropenem both in terms of the maximum concentration of drug (Cmax) and area under the concentration-time curve (AUC) (Table 1) were measured. Most importantly, based on single-dose pharmacokinetic (PK) parameters, the time above the MIC (T>MIC) simulated in the DHP-I knockout (KO) mice increased significantly for the three considered meropenem MIC scenarios (0.23, 0.62, and 1.25 μg/ml) (Fig. 1). Once the principles of reduced β-lactam degradation and viability of the infection in vivo had been validated, a quick and preliminary evaluation of the effectiveness of β-lactam combination treatment to control TB infection in DHP-I-deficient mice was performed in a survival efficacy study (see Fig. S2 in the supplemental material). These combinations were selected while keeping in mind the clinical trial design that was implemented in the ongoing EDCTP-sponsored β-lactam phase IIa study (14) and previous knowledge regarding the in vitro antitubercular activities of their main components (MICs, 0.3, 1.25, 2.5, and 1.25 μ/ml for meropenem-clavulanate, faropenem, cefdinir, and amoxicillin-clavulanate, respectively) (9). The experiment resulted in an increased mean survival time (>39 versus 17.3 days) for β-lactam-treated groups of mice up to the time of final sacrifice (see Fig. S3 in the supplemental material). These experiments, while providing only qualitative evidence of the potential efficacy of β-lactams in a DHP-I-deficient murine model, were used as a basis for further evaluation of the model for the quantitative generation of efficacy signals. This second goal was addressed through the establishment of a new experimental design (see Fig. S4 in the supplemental material). This model showed how different β-lactam combination regimens gave raise to robust and significant CFU reductions in the lungs of infected DHP-I-deficient mice, with faropenem medoxomil showing the most significant reductions (−2 log CFU), followed by meropenem (−1.7 log CFU) and cefdinir (−1.3 log CFU), in comparison to the untreated control (Fig. 2). These results are in contrast to previously reported data for the meropenem-clavulanate combination (4, 5), albeit under different administration schedules. Most importantly, once-a-day treatment with the faropenem medoxomil-containing regimen was also able to produce a 1.3-log CFU reduction, further highlighting the benefits associated with DHP-I deficiency in terms of decreased pharmacokinetic clearance (Table 1). This observation also addresses one of the shortcomings thought to limit the potential of β-lactams for the treatment of human TB, i.e., the likely requirement for multiple daily administrations covering extensive periods of time (T>MIC) to drive efficacy. The benefits of the DHP-I-deficient model were further confirmed by the lack of observable β-lactam antitubercular effect in the reference C57BL/6 strain under the same experimental design (see Fig. S5 in the supplemental material).
TABLE 1.
Whole-blood pharmacokinetic AUC and Cmax parameters of meropenem after subcutaneous administration of 300 mg/kg of body weight in combination with amoxicillin-clavulanate (s.c. at 200/50 mg/kg) in three different murine genetic backgroundsa
| Mouse genetic background | Cmax (μg/ml) | AUC (μg · h/ml) | T>MIC (h)b |
|---|---|---|---|
| TF3157 | 1.1 | 1.7 | 73, 36, 0 |
| C57BL/6 | 0.03c | 0.05c | 0, 0, 0 |
| TF 3157 DHP-I KO | 6.6 | 8.8 | 66, 49, 39 |
All groups were pretreated with probenecid (orally [p.o.] at 200 mg/kg). s.c., subcutaneous.
Results shown are for MICs 1 to 3, which are 230 ng/mol, 625 ng/mol, and 1,250 ng/mol, respectively. The time over MIC (T>MIC) was calculated taking as a reference the simulated profile of the compound after 3-times-a-day administration for 8 days, and simulation was extended to 200 h.
The concentration measured was close to the threshold of detection. The wild-type and DHP-I KO mouse strains corresponded to a TF3157 genetic background.
FIG 1.
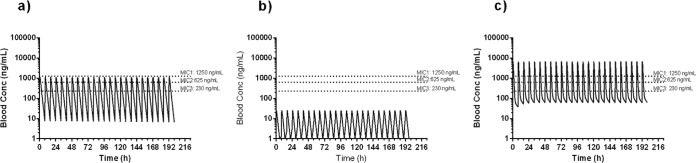
Whole-blood pharmacokinetic profiles simulated from a monocompartmental model after fitting of single-dose profiles of meropenem in TF3157 (a) and C57BL/6 (b). (c) A bicompartmental model was used for TF 3157 DHP-I KO mice. conc, concentration.
FIG 2.
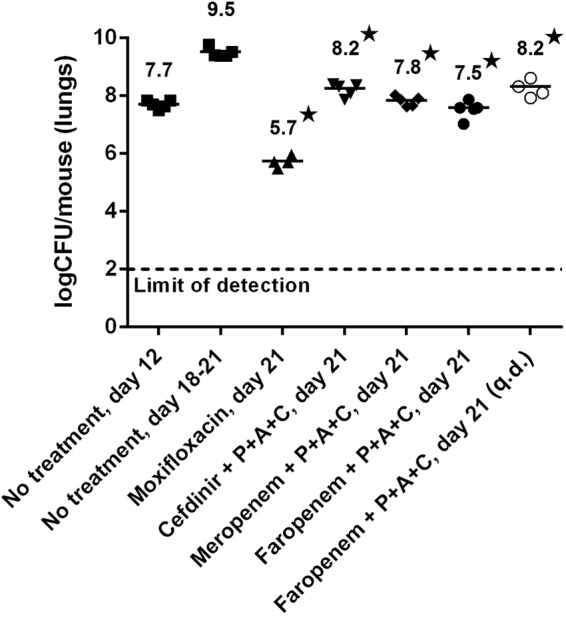
The efficacy response was quantified by measuring the bacterial load in the lungs of infected mice for different combinations of β-lactams in a DHP-I KO acute TB model. Drugs administered subcutaneously were prepared in phosphate-buffered saline (PBS) buffer (300 mg/kg of body weight meropenem three times a day [t.i.d.]. Drugs administered orally were prepared in 1% methylcellulose (500 mg/kg of faropenem medoxomil t.i.d., 200 mg/kg of amoxicillin t.i.d., 50 mg/kg of clavulanate t.i.d., and moxifloxacin q.d.). All administrations (except for moxifloxacin group) were preceded by an oral dose of probenecid (P) (200 mg/kg, 1% methylcellulose). Only the moxifloxacin and the faropenem medoxomil–amoxicillin-clavulanate q.d. mouse groups were administered once-daily regimens and were composed of 4 mice (5 mice for the other groups). Treatments were administered from days 12 to 20 after infection. *, P < 0.05, analysis of variance (ANOVA) using Dunnett's posttest compared to untreated mice (day 21).
In summary, based on the preliminary evidence presented here, we have been able to show how a DHP-I-deficient TF3157 murine model capable of sustaining significant β-lactam compound blood concentrations can be applicable to β-lactam drug discovery against TB. This model gives robust and significant efficacy signals measured as CFU reductions after treatment and can be used to discern between different β-lactam drugs. We believe that these data can modestly contribute to countering some of the past objections to the antitubercular potential of β-lactams. These results pave the way for the systematic in vivo characterization of both old and novel β-lactams as antitubercular leads and for the selection and eventual inclusion of safe and efficacious β-lactam drug components in clinical trials as potential combination partners in novel TB regimens.
All animal studies were reviewed and carried out in accordance with European Directive 2010/63/EU and the GSK Policy on the Care, Welfare, and Treatment of Animals.
Supplementary Material
ACKNOWLEDGMENT
This research was funded in part by a grant from the European Commission Seventh Framework Programme ORCHID project no. 261378.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01063-15.
For this virtual institution, see http://projectorchid.org.
REFERENCES
- 1.Wang F, Cassidy C, Sacchettini JC. 2006. Crystal structure and activity studies of the Mycobacterium tuberculosis β-lactamase reveal its critical role in resistance to β-lactam antibiotics. Antimicrob Agents Chemother 50:2762–2771. doi: 10.1128/AAC.00320-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers HF, Moreau D, Yajko D, Miick C, Wagner C, Hackbarth C, Kocagöz S, Rosenberg E, Hadley WK, Nikaido H. 1995. Can penicillins and other beta-lactam antibiotics be used to treat tuberculosis? Antimicrob Agents Chemother 39:20–24. doi: 10.1128/AAC.39.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.England K, Boshoff HIM, Arora K, Weiner D, Dayao E, Schimel D, Via LE, Barry CE III. 2012. Meropenem-clavulanic acid shows activity against Mycobacterium tuberculosis in vivo. Antimicrob Agents Chemother 56:3384–3387. doi: 10.1128/AAC.05690-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veziris N, Truffot C, Mainardi J-L, Jarlier V. 2011. Activity of carbapenems combined with clavulanate against murine tuberculosis. Antimicrob Agents Chemother 55:2597–2500. doi: 10.1128/AAC.01824-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donald PR, Sirgel FA, Venter A, Parkin DP, Van de Wal BW, Barendse A, Smit E, Carman D, Talent J, Maritz J. 2001. Early bactericidal activity of amoxicillin in combination with clavulanic acid in patients with sputum smear-positive pulmonary tuberculosis. Scand J Infect Dis 33:466–469. doi: 10.1080/00365540152029954. [DOI] [PubMed] [Google Scholar]
- 6.Chambers HF, Kocagöz T, Sipit T, Turner J, Hopewell PC. 1998. Activity of amoxicillin/clavulanate in patients with tuberculosis. Clin Infect Dis 26:874–877. doi: 10.1086/513945. [DOI] [PubMed] [Google Scholar]
- 7.Hugonnet J-E, Tremblay LW, Boshoff HI, Barry CE III, Blanchard JS. 2009. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 323:1215–1218. doi: 10.1126/science.1167498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhar N, Dubée V, Ballell L, Cuinet J, Hugonnet J-E, Signorino-Gelo F, Barros D, Arthur M, McKinney JD. 2015. Rapid cytolysis of Mycobacterium tuberculosis by faropenem, an orally bioavailable β-lactam antibiotic. Antimicrob Agents Chemother 59:1308–1319. doi: 10.1128/AAC.03461-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lun S, Miranda D, Kubler A, Guo H, Maiga MC, Winglee K, Pelly S, Bishai WR. 2014. Synthetic lethality reveals mechanisms of Mycobacterium tuberculosis resistance to β-lactams. mBio 5(5):e01767-14. doi: 10.1128/mBio.01767-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horita Y, Maeda S, Kazumi Y, Doi N. 2014. In vitro susceptibility of Mycobacterium tuberculosis isolates to an oral carbapenem alone or in combination with β-lactamase inhibitors. Antimicrob Agents Chemother 58:7010–7014. doi: 10.1128/AAC.03539-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dauby N, Muylle I, Mouchet F, Sergysels R, Payen MC. 2011. Meropenem/clavulanate and linezolid treatment for extensively drug-resistant tuberculosis. Pediatr Infect Dis J 30:812–813. doi: 10.1097/INF.0b013e3182154b05. [DOI] [PubMed] [Google Scholar]
- 12.De Lorenzo S, Alffenaar JW, Sotgiu G, Centis R, D'Ambrosio L, Tiberi S, Bolhuis MS, van Altena R, Viggiani P, Piana A, Spanevello A, Migliori GB. 2013. Efficacy and safety of meropenem–clavulanate added to linezolid-containing regimens in the treatment of MDR-/XDR-TB. Eur Respir J 41:1386–1392. doi: 10.1183/09031936.00124312. [DOI] [PubMed] [Google Scholar]
- 13.Payen MC, De Wit S, Martin C, Sergysels R, Muylle I, Van Laethem Y, Clumeck N. 2012. Clinical use of the meropenem-clavulanate combination for extensively drug-resistant tuberculosis. Int J Tuberc Lung Dis 16:558–560. doi: 10.5588/ijtld.11.0414. [DOI] [PubMed] [Google Scholar]
- 14.Keener AB. 2014. Oldie but goodie: repurposing penicillin for tuberculosis. Nat Med 20:976–978. doi: 10.1038/nm0914-976. [DOI] [PubMed] [Google Scholar]
- 15.Knopp H, Sundelof JG, Haydu R, Kahan FM. 1982. Metabolism of thienamycin and related carbapenem antibiotics by the renal dipeptidase, dehydropeptidase I. Antimicrob Agents Chemother 22:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukasawa M, Sumita Y, Harabe ET, Tanio T, Nouda H, Kohzuki T, Okuda T, Matsumura H, Sunagawa M. 1992. Stability of meropenem and effect of 1,-methyl substitution on its stability in the presence of renal dehydropeptidase I. Antimicrob Agents Chemother 36:1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chambers HF, Turner J, Schecter GF, Kawamura M, Hopewell PC. 2005. Imipenem for treatment of tuberculosis in mice and humans. Antimicrob Agents Chemother 49:2816–2821. doi: 10.1128/AAC.49.7.2816-2821.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habib GM, Shi ZZ, Cuevas AA, Guo Q, Matzuk MM, Lieberman MW. 1998. Leukotriene D4 and cystinyl-bis-glycine metabolism in membrane-bound dipeptidase-deficient mice. Proc Natl Acad Sci U S A 95:4859–4863. doi: 10.1073/pnas.95.9.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


