Background: Germ cells are critical for any species that multiplies through sexual reproduction.
Results: We found 173 candidate key genes and 18 key signaling pathways that are differentially activated.
Conclusion: Our results showed the crucial genes and pathways involved in the regulation of chicken male germ cell differentiation.
Significance: This study narrows the range of functional genes and pathways during ESC differentiation.
Keywords: bioinformatics, cell differentiation, gene expression, microarray, stem cells
Abstract
Male germ cell differentiation is a subtle and complex regulatory process. Currently, its regulatory mechanism is still not fully understood. In our experiment, we performed the first comprehensive genome and transcriptome-wide analyses of the crucial genes and signaling pathways in three kinds of crucial cells (embryonic stem cells, primordial germ cell, and spermatogonial stem cells) that are associated with the male germ cell differentiation. We identified thousands of differentially expressed genes in this process, and from these we chose 173 candidate genes, of which 98 genes were involved in cell differentiation, 19 were involved in the metabolic process, and 56 were involved in the differentiation and metabolic processes, like GAL9, AMH, PLK1, and PSMD7 and so on. In addition, we found that 18 key signaling pathways were involved mainly in cell proliferation, differentiation, and signal transduction processes like TGF-β, Notch, and Jak-STAT. Further exploration found that the candidate gene expression patterns were the same between in vitro induction experiments and transcriptome results. Our results yield clues to the mechanistic basis of male germ cell differentiation and provide an important reference for further studies.
Introduction
The germ cell holds a singular fascination for cellular, reproductive, and developmental biologists because it is the only cell type that can penetrate from one generation to the next generation. Germ cells, without a doubt, are critical for any species that reproduce through sexual reproduction. It is important for fundamental research to understand the details of development and growth of the underlying germ line cells. The germ cell is an important cell type in which either gene expression and/or suppression were regulated temporally and spatially during embryonic development, according to gene expression switching triggered by interaction with the environment.
However, there were few reports about a transcriptome study of the germ stem cell in the chicken, especially in the early embryonic developmental stages because of technical difficulties for collecting early embryonic germ cells. Several previous studies have been reported finding some regulators (genes and/or pathways) that control the process of germ stem cell specification and differentiation. Saitou et al. (1) found that Ifitm3, Nanos2, Stella, Dppa4, Dnmt3l, and Piwil2 were involved in the early differentiation of germ cells. Blimp1/Prdm1 played an important role in the early stages of embryonic PGCs specialization. Genetic lineage tracing confirmed that almost all Blimp1 positive cells in early embryonic developmental stages would be eventually developed into Stella positive PGCs. BMP signal from the embryonic ectoderm can induce the two key regulatory genes (Blimp1 and Prdm14) that are responsible for the PGC specialization (2). Dazl (deleted in azoospermia) is a major controlling gene of mouse germ cell differentiation, and its expression promotes ESC differentiation to gametes in vitro (3). Dann et al. (4) used shRNA to inhibit Pou5f1, which resulted in the cloning reduction of the recipient mice SSCs after transplantation.
Ewen and Koopman (5) reported that Kit/KitL, FGFs pathways, and LIF cytokine factor have a positive regulatory role in proliferation and survival of PGCs, but TGFβ-activin/nodal signal has an inhibitory effect on PGCs proliferation. Saitou et al. (6) found that Wnt3a can affect BMP signaling pathways, also the ERK, MAPK, PI3K/AKT, Smad, and hedgehog signaling pathways were involved in the process of germ cell development. Rao (7) reported that basic FGF with tyrosine kinase receptor can activate multiple intracellular signaling pathways such as Ras/raf/mek, p38/MAPK, PKC, and PI3K pathways that are required for mammalian SSCs self-renewal and development. These findings suggested that there are some genes and pathways that may be responsible for investigation of germ cell development and differentiation, but its regulatory mechanism was not fully understood until now.
Here, we analyzed all the gene expression patterns of the three kinds of chicken stem cells throughout the whole genome. We identified thousands of differentially expressed genes (DEGs)4 in this process, and from these we chose 173 candidate genes, including 98 genes involved in cell differentiation, 19 involved in the metabolic process, 56 genes involved in the differentiation and metabolic processes, like GAL9, AMH, PLK1, PSMD7, and so on. In addition, we found that there were 18 key signaling pathways mainly involved in cell proliferation, differentiation, and signal transduction process like TGF-β, Notch, and Jak-STAT. Further exploration found that the candidate gene expression patterns were the same between in vitro induction experiments and transcriptome results. Our results yield clues to the mechanistic basis of male germ cell differentiation and provide an important reference for further studies.
Experimental Procedures
Samples
Procedures involving animals and their care were confirmed according to the U.S. National Institute of Health guidelines (publication no. 85-23, revised 1996) and approved by the laboratory animal management and experimental animal ethics committee of Yangzhou University.
This experiment was done using 18,340 freshly fertilized eggs of Suqin yellow chicken (Gallus gallus domesticus) that were obtained from Poultry Institute, Chinese Academy of Agricultural Sciences (Yangzhou, China). There were 10,540 (4,845 male and 4,854 female; lost 841) eggs at stage X used for the isolation of ESCs. PGCs were isolated from gonads of 3,400 eggs (1,594 male and 1,556 female; lost 250) that incubated for 72 h (stage 27) at 37 °C with 60% relative humidity, while 4,400 eggs were incubated for 18 days to isolate SSCs from the testis. The sex of the cells was determined using PCR, and then the cells with the same sex in each stage were collected for further experiments. Each experiment was repeated three times.
FACS Sorting of ESCs, PGCs, and SSCs and RNA Extraction
Different cell surface markers were used to isolate different types of cells by the FACS. SSEA-1 and SOX2 were used for ESCs, SSEA-1 and c-KIT were used for PGCs, and Integrinα6 and Integrinβ1 were used for SSCs isolation. Total RNA was extracted by TRIzol (Invitrogen), and its quality was evaluated with Nanodrop 2000 before the microarray and Illumina RNA sequencing assays.
Microarray and RNA-seq Assays
RNA Libraries pools of the three kinds of cells were established following the protocols of the Agilent microarray and Illumina mRNA-seq with 50 ng of RNA, and the experiments were performed in the Oebiotech Company.
Data Analysis
Filtering and quality control checks of the raw reads from RNA-seq had been done by FastQC. The clean reads were mapped to reference sequences using SOAP2 aligner. The gene expression levels were calculated using RPKM method (reads per kb transcriptome per million reads). GO and pathway analyses of DEGs based on DAVID, FunNet, and WEGO databases were performed to analyze the regulating network of the candidate key genes.
Quantitative Real Time PCR (qRT-PCR)
Microarray and RNA-seq results were validated by quantitative real time PCR. One microgram of RNA was reverse transcribed to cDNA using the Takara reverse transcriptase Moloney murine leukemia virus (RNase H−) (Takara, Dalian, China). Quantitative real time PCR was performed on ABI PRISM 7500 HT sequence detection system (Applied Biosystems, Carlsbad, CA). Cycle number values were normalized against two housekeeping genes, β-Actin and GAPDH.
In Vitro Induced Differentiation
For further confirmation of the chosen key genes involved in the regulation of the male germ cell differentiation, several molecules including retinoic acid (RA), BMP4 (bone morphogenic protein 4), testosterone, and follicle-stimulating hormone were used to induce the differentiation of chicken ESCs toward male germ cells in vitro. The third generation of ESCs were seeded into the 24-well plate with supporting feeder cells with the density of 105 cells/well. RA was added to the medium at a final concentration of 10−5 mol/liter, BMP4 with a final concentration of 40 ng/ml, testosterone with a final concentration of 15 ng/ml, and follicle-stimulating hormone with a final concentration of 25 ng/mg. The cells were collected every 2 days after incubation, and qRT-PCR was used to identify ESCs, PGCs, and SSCs by detecting the specific candidate gene markers and also to detect the key genes expression levels.
Data Access
Sequencing reads are available in the NCBI Sequence GEO accession numbers under accession number GSE57213.
Results
Cell Sorting and Culture
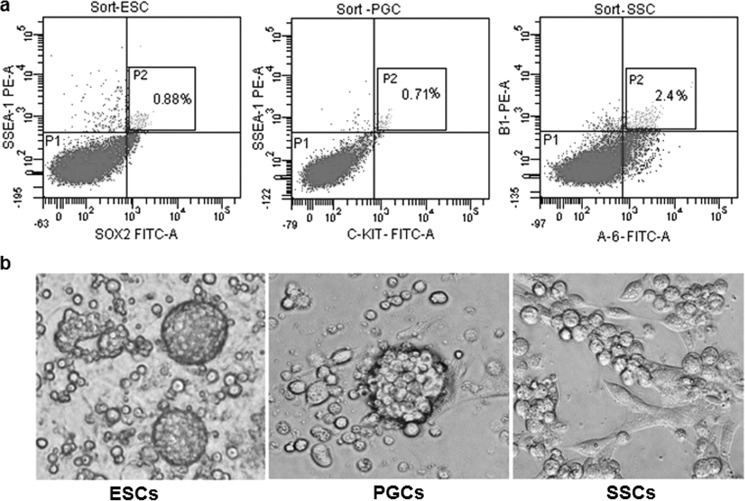
The purity of FAC-sorted ESCs, PGCs, and SSCs was demonstrated as shown in Fig. 1a as according to the results, 0.88% ESCs were SSEA1 and SOX2 positive, 0.71% PGCs were SSEA1- and C-kit-positive, 2.43% SSCs were integrin α6- and integrin β1-positive, respectively. After FACS enrichment, the morphology of the three types of cells were shown in Fig. 1b. The ESCs were small and became bird nest-like clones after culture by 5–7 days, the PGCs were bigger and became mulberry-like clones after culture for 2–4 days, and the SSCs also were bigger than ESCs and became grape-like clones after culture for 5–6 days.
FIGURE 1.
Cell sorting and culture. a, the purity of FAC-sorted ESCs, PGCs, and SSCs. b, the morphological characters of the three types of cells.
Analysis of Differentially Expressed Genes in Microarray Assay
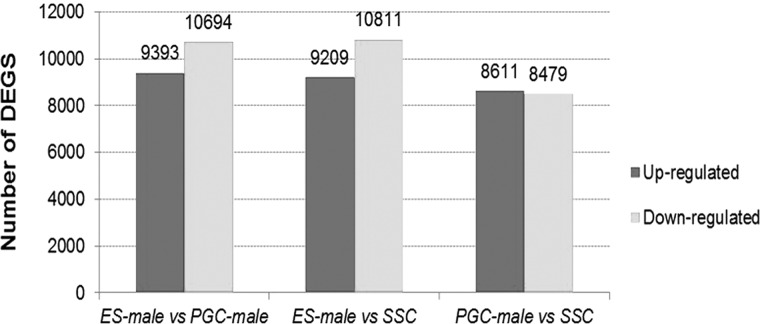
Through microarray data difference analysis using |logFC| ≥ 1 as a standard for differential gene screening, there were 20,087 DEGs in the ESC versus the PGC group: 20,020 DEGs and 17,090 DEGs in the ESC versus the SSC group and PGC versus the SSC groups, respectively (Fig. 2). Concerning the up-regulated DEGs, there were 17 genes with more than 10-fold expression change in the ESC versus the PGC group, 33 genes in the ESC versus the SSC group, and 4 genes in the PGC versus the SSC group. In the down-regulated DEGs, there were 3, 13, and 11 genes detected in ESCs versus PGCs, ESCs versus SSCs, and PGCs versus SSCs, respectively. Most of DEGs belonged to the 2 < |logFC| ≤ 4 and logFC ≤ 2 groups, and only a few DEGs were related to the |logFC| > 10 group, including AMH, HOXD8, GAL10, GAL9, and so on (Tables 1–3).
FIGURE 2.
DEGs in microarry.
TABLE 1.
DEGs related to |logFC| > 10 in ESCs versus PGCs of microarray
LHRH, luteinizing hormone-releasing hormone; PRL, prolactin.
| Probe name | Log FC (ESCs vs. PGCs) | Regulation | Gene symbol | Function | References |
|---|---|---|---|---|---|
| A_87_P054786 | 16.293575 | Up | GAL10 | A hypothalamic-hypophysiotropic hormone and a neuromodulator of LHRH secretion; action galanin is unlikely to influence PRL secretion via inhibition of dopaminergic tone | Refs. 21 and 22 |
| A_87_P078616 | 15.038605 | Up | NPHS2 | Essential for the integrity of the glomerular filter | Ref. 23 |
| A_87_P054986 | 13.116768 | Up | HBZ | Activates erythroid-specific, globin gene expression | Ref. 24 |
| A_87_P274543 | 12.136964 | Up | HBG2 | Gamma chains make up the fetal hemoglobin F, in combination with alpha chains | Ref. 25 |
| A_87_P009555 | 12.067309 | Up | HBAA | ||
| A_87_P009368 | 11.491803 | Up | LECT2 | A liver-specific protein that is thought to be linked to hepatocyte growth | Ref. 26 |
| A_87_P035104 | 11.244352 | Up | GAL9 | A hypothalamic-hypophysiotropic hormone and is a neuromodulator of LHRH secretion and action | Refs. 21 and 22 |
| A_87_P057441 | 10.826411 | Up | HOXD8 | Controls lateral line cell migration | Ref. 27 |
| A_87_P152088 | 10.653027 | Up | TSPAN8 | Encodes a cell surface glycoprotein defined by the monoclonal antibody CO-029, a 27–34-kDa membrane protein expressed in gastric, colon, rectal, and pancreatic carcinomas but not in most normal tissues | Ref. 28 |
| A_87_P108903 | 10.637115 | Up | COL8A1 | A major component of the hexagonal lattice in the Descemet membrane | Ref. 29 |
| A_87_P146353 | 10.3792095 | Up | LUM | Interacts with collagen and limits growth of fibrils in diameter | Ref. 30 |
| A_87_P014692 | 10.170226 | Up | ACTA2 | Important regulators of smooth muscle cell differentiation | Ref. 31 |
| A_87_P132478 | −10.0271225 | Down | OTX2 | A key regulatory gene in photoreceptor cell development | Ref. 32 |
| A_87_P037684 | −10.859593 | Down | ENS-3 | Nucleic acid binding; RNA-DNA hybrid ribonuclease activity; DNA integration | Ref. 33 |
TABLE 2.
DEGs related to |logFC| > 10 in PGCs versus SSCs of microarray
LHRH, luteinizing hormone-releasing hormone.
| Probe name | Log FC (PGCs vs. SSCs) | Regulation | Gene symbol | Function | References |
|---|---|---|---|---|---|
| A_87_P008801 | 13.369993 | Up | AMH | Prevents the development of the Müllerian ducts into the uterus and other Müllerian structures. regulate production of sex hormones | Refs. 34 and 35 |
| A_87_P260443 | 10.933807 | Up | SLCO1A2 | It mediate transport of estrone-3-sulfate and, more weakly, prostaglandin E2. | Refs. 36 and 37 |
| A_87_P150703 | −10.657406 | Down | ATP5A1W | Cessation of recombination between avian sex chromosomes | Ref. 38 |
| A_87_P078616 | −11.816776 | Down | NPHS2 | The association of podocin with specialized lipid raft microdomains of the plasma membrane was a prerequisite for recruitment of nephrin into rafts | Refs. 39 and 40 |
| A_87_P054986 | −12.876568 | Down | HBZ | The zeta-globin polypeptide is synthesized in the yolk sac of the early embryo, while alpha-globin is produced throughout fetal and adult life | Ref. 41 |
| A_87_P058761 | −14.45761 | Down | HINTW | Is a W-chromosome HINT gene in chick, is expressed ubiquitously and is a robust female cell marker applicable in intraspecific chimera studies | Refs. 42 and 43 |
TABLE 3.
DEGs related to |logFC| > 10 in ESCs versus SSCs of microarray
LHRH, luteinizing hormone-releasing hormone; PRL, prolactin.
| Probe name | Log FC (ESCs vs. SSCs) | Regulation | Gene symbol | Function | References |
|---|---|---|---|---|---|
| A_87_P009368 | 15.350238 | Up | LECT2 | Is a liver-specific protein that is thought to be linked to hepatocyte growth | Ref. 26 |
| A_87_P274543 | 13.708621 | Up | HBG2 | The gamma globin genes (HBG1 and HBG2) are normally expressed in the fetal liver, spleen, and bone marrow | Ref. 41 |
| A_87_P054786 | 13.304759 | Up | GAL10 | A hypothalamic-hypophysiotropic hormone and a neuromodulator of LHRH secretion; action galanin is unlikely to influence PRL secretion via inhibition of dopaminergic tone | Refs. 21 and 22 |
| A_87_P129033 | 12.866734 | Up | GAL6 | Has bactericidal activity | Ref. 44 |
| A_87_P100606 | 12.3010845 | Up | GAL7 | Has bactericidal activity | Ref. 44 |
| A_87_P017854 | 11.8544855 | Up | POSTN | Supports adhesion and migration of epithelial cells | Ref. 45 |
| A_87_P009700 | 11.636215 | Up | CATHL2 | Has hemolytic activity and may play a role in the innate immune response | Ref. 46 |
| A_87_P007819 | 11.55645 | Up | RGS5 | Involved in the regulation of heterotrimeric G proteins by acting as GTPase activators | Ref. 47 |
| A_87_P037929 | 11.287436 | Up | KCNMB1 | Modulates the calcium sensitivity and gating kinetics | |
| A_87_P008896 | 11.176561 | Up | LYG2 | Lysozyme activity | Ref. 48 |
| A_87_P059356 | 11.127887 | Up | GAL2 | Potent antibacterial activity against | Ref. 49 |
| A_87_P146353 | 11.08161 | Up | LUM | Regulate collagen fibril organization and circumferential growth, corneal transparency, and epithelial cell migration and tissue repair | |
| A_87_P191933 | 10.969956 | Up | CCDC80 | Promotes cell adhesion and matrix assembly (by similarity); may play a role in eye formation | Ref. 50 |
| A_87_P014692 | 10.948489 | Up | ACTA2 | Involved in various types of cell motility and ubiquitously expressed in all eukaryotic cells | |
| A_87_P263263 | 10.806091 | Up | RSFR | ||
| A_87_P023107 | 10.69445 | Up | CTSG | May participate in the killing and digestion of engulfed pathogens and in connective tissue remodeling at sites of inflammation | |
| A_87_P008907 | 10.515071 | Up | ALDH1A1 | Involved in the regulation of the metabolic responses | |
| A_87_P282153 | 10.302805 | Up | COL1A2 | Matrix integrity | |
| A_87_P035106 | 10.2099 | Up | GAL4 | ||
| A_87_P214693 | 10.184647 | Up | DCN | Plays a role in matrix assembly; capable of suppressing the growth of various tumor cell lines | |
| A_87_P008852 | −10.54513 | Down | GSC | May play a role in spatial programing within discrete embryonic fields or lineage compartments during organogenesis | |
| A_87_P004087 | −10.636513 | Down | LOC770611 | ||
| A_87_P151998 | −10.693583 | Down | LOC430910 | ||
| A_87_P037684 | −10.859767 | Down | ENS-3 | Pol-like protein ENS-3 | |
| A_87_P102041 | −10.96241 | Down | EOMES | Playing a crucial role during development. Functions in trophoblast differentiation and later in gastrulation, regulating both mesoderm delamination and endoderm specification. Plays a role in brain development being required for the specification and the proliferation of the intermediate progenitor cells | |
| A_87_P009011 | −11.044117 | Down | CDX2 | Important in broad range of functions from early differentiation to maintenance of the intestinal epithelial lining of both the small and large intestine | |
| A_87_P150703 | −11.242624 | Down | ATP5A1W | Cessation of recombination between avian sex chromosomes | Ref. 38 |
| A_87_P053051 | −11.601723 | Down | CNOT2 | Involved in the maintenance of embryonic stem (ES) cell identity | Ref. 51 |
| A_87_P058761 | −14.244124 | Down | HINTW | Strongly expressed in the gonads and other tissues of female chicken embryos |
Gene Ontology (GO) analysis of these DEGs showed that more than 30% of DEGs related to the regulation of transcription according to the biological processes classification and more than 30% of DEGs belonged to cell nucleus in the cellular component classification. The molecular function assessment of these DEGs revealed that more than 40% of DEGs associated with ATP binding, nucleotide binding, and metal ion binding.
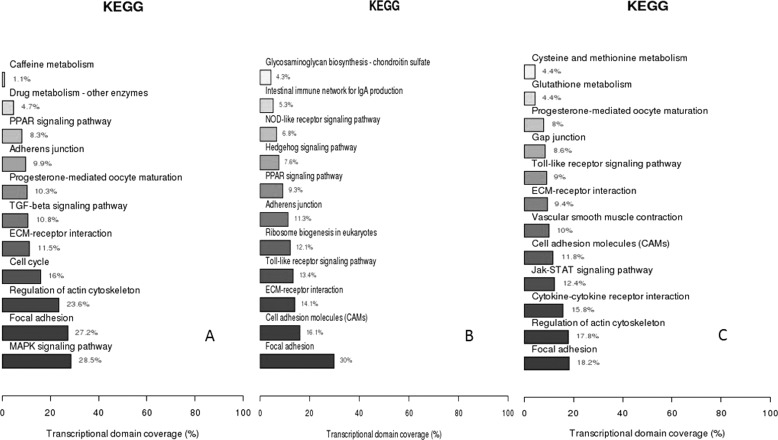
KEGG pathway assay for DEGs indicated that 11 signaling pathways were significantly enriched in the ESC versus the PGC group with enrichment in the MAPK signaling pathway (28.5%) and the focal adhesion pathway (27.2%) (Fig. 3A), although in the ESC versus the SSC group, 11 signaling pathways were significantly enriched with enrichment in the focal adhesion pathway (30%) and cell adhesion attached molecule pathway (16.1%) (Fig. 3B). In PGCs versus SSCs, in the other hand, 12 signaling pathways were enriched, and the most significantly enriched pathways were the focal adhesion (18.2%) and the cytoskeleton regulation (17.8%) (Fig. 3C).
FIGURE 3.
KEGG classification of DEGs in microarray. A, ESCs versus PGCs. B, ESCs versus SSCs. C, PGCs versus SSCs.
Analysis of Differentially Expressed Genes in RNA-seq Assay
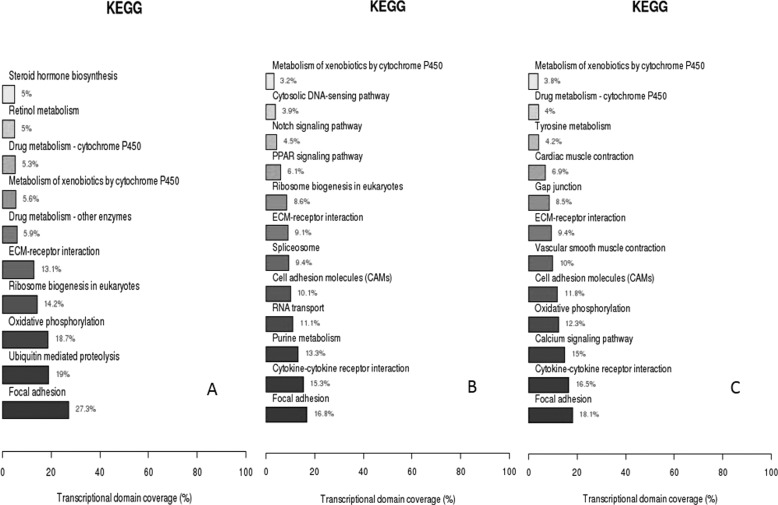
The RNA-seq results indicated that there were 7,697 DEGs in the ESC versus the PGC group, 7,868 DEGs in the ESC versus the SSC group, and 6,226 in the PGC versus the SSC group (Fig. 4). In the up-regulated DEGs, there were six genes with a significant difference more than 10-fold in the ESC versus the PGC set, 13 genes in the ESC versus the SSC pair, and 4 genes in the PGC versus the SSC pair, whereas there were 7 genes down-regulated in ESCs versus PGCs, 4 genes in ESCs versus SSCs, and 2 genes in PGCs versus SSCs. Most of the DEG fold change distributed in 2 < log2 ≤ 4 and log2 ≤ 2, and there were a few DEGs more than 10-fold, including PRDM14, KPNA7, HOXB6, GAL9, and TWIST2, etc. (Tables 4–6). In these DEGs, more than 15% related to the multicellular development within the biological processes classification and more than 30% belonged to cell membrane within the cellular component classification, but the molecular functional assessment of these DEGs revealed that more than 30% associated with ATP binding. KEGG pathway assay indicated that 10 signaling pathways were significantly enriched in ESCs versus PGCs with the most enrichments in the focal adhesion signaling pathway (27.3%) and the ubiquitin-mediated proteolysis (19%) (Fig. 5A), whereas in the ESCs versus SSCs, 12 signaling pathways were significantly enriched in the focal adhesion pathway (16.8%) and cytokine-cytokine receptor interaction pathway (15.3%) (Fig. 5B). On the other hand, there were 12 enriched signaling pathways in the PGCs versus SSCs within the focal adhesion (18.1%) and the cytokine-cytokine receptor interaction pathway (16.5%) (Fig. 5C).
FIGURE 4.
DEGs in RNA-seq.
TABLE 4.
DEGs related to |log2| > 10 in ESCs versus PGCs of RNA-seq
FDR, false discovery rate; HPC, hematopoietic progenitor cell; MSCs, mesenchymal stem cells.
| Gene identification | Description | log2 (ESCs vs. PGCs) | Regulation | p value | FDR | Function | Reference |
|---|---|---|---|---|---|---|---|
| NM_205335.2 | TTR | 11.83347797 | Up | 1.49E-211 | 1.13E-210 | Initiates myoblast differentiation | Ref. 52 |
| NM_206989.1 | UTS2B | 10.86825931 | Up | 5.91E-170 | 3.76E-169 | Induces phenotypic differentiation, migration, and collagen synthesis | Ref. 53 |
| XM_001236989.1 | LOC777548 | 10.76811631 | Up | 5.96E-17 | 1.29E-16 | Restricted to the early phases of HPC differentiation with down-modulation at intermediate/late stages of maturation | |
| XM_426327.2 | ENPEP | 10.7588003 | Up | 0 | 0 | Contribute to the development of renal and hypertensive disorders | |
| NM_001001611.2 | GAL9 | 10.4579734 | Up | 9.20E-85 | 3.75E-84 | Induction of differentiation of MSCs into chondrocytes | Ref. 54 |
| XM_414212.2 | LOC415852 | 10.08034791 | Up | 5.30E-50 | 1.67E-49 | Regulating the responsiveness of cells to adrenal androgens | |
| XM_414795.2 | LOC398026 | −10.0212089 | Down | 0 | 0 | Required for normal fertility and fecundity | Ref. 16 |
| XM_430154.2 | LOC424460 | −10.12099411 | Down | 0 | 0 | ||
| XM_416906.2 | HIST1H2AH | −10.42067022 | Down | 5.27E-77 | 2.03E-76 | Responsible for the nucleosome structure of the chromosomal fiber in eukaryotes | |
| XM_001234742.1 | BPIL2 | −10.52057903 | Down | 0 | 0 | Plays an essential role in host defense | Ref. 55 |
| XM_001231344.1 | LOC768589 | −10.54284209 | Down | 3.90E-115 | 1.91E-114 | Prevents apoptotic cell death | |
| NM_204675.1 | WNT3A | −11.68307778 | Down | 0 | 0 | Facilitates clonal plating of hESCs exhibiting functional hepatic differentiation | Ref. 56 |
| XM_426984.2 | PRDM14 | −12.82024346 | Down | 1.61E-66 | 5.77E-66 | Involved in the maintenance of the self-renewal of human ESCs by suppression of gene expression | Ref. 14 |
TABLE 5.
DEGs related to |log2| > 10 in PGCs versus SSCs of RNA-seq
| Gene identification | Description | log2 (PGCs vs. SSCs) | Regulation | p value | FDR | Function | Reference |
|---|---|---|---|---|---|---|---|
| XM_429858.1 | LOC421502 | 11.21551 | Up | 2.7E-286 | 3.9E-285 | ||
| XM_416467.2 | SMC1B | 10.64341 | Up | 0 | 0 | Required for chromatid cohesion and DNA recombination during meiosis and mitosis | Ref. 57 |
| XM_427005.2 | AAK1 | 10.14017 | Up | 4.61E-20 | 1.14E-19 | Reported as a positive regulator of the Notch pathway | Ref. 58 |
| NM_205030.1 | AMH | 10.04345 | Up | 0 | 0 | Prevents the development of the Müllerian ducts into the uterus and other Müllerian structures; regulates production of sex hormones | Refs. 34 and 35 |
| XM_428866.1 | S-KER | −10.7443 | Down | 4.09E-54 | 1.61E-53 |
TABLE 6.
DEGs related to |log2| > 10 in ESCs versus SSCs of RNA-seq
| Gene identification | Description | log2 (ESCs/SSCs) | Regulation | p value | FDR | Function | References |
|---|---|---|---|---|---|---|---|
| XM_422203.2 | LOC424360 | 11.05914 | Up | 9.10E-245 | 7.40E-244 | ||
| NM_001039453.1 | AQP1 | 11.01037 | Up | 1.20E-141 | 6.30E-141 | ||
| XM_421648.2 | PBIP | 10.53929 | Up | 0 | 0 | Induces endochondral bone formation in adult animals | Ref. 59 |
| NM_213579.1 | GEM | 10.53732 | Up | 0 | 0 | Regulation of Ca2+ channel expression at the cell surface | Ref. 60 |
| XM_419553.2 | LOC421508 | 10.52041 | Up | 0 | 0 | Defective regulation in failing hearts | Ref. 61 |
| XM_001232973.1 | LCAT | 10.51207 | Up | 4.39E-22 | 1.00E-21 | ||
| NM_204897.1 | MENT-1 | 10.43276 | Up | 0 | 0 | Associated with intranuclear foci of condensed chromatin | Ref. 62 |
| NM_204679.1 | CDERMO-1 | 10.21531 | Up | 1.60E-103 | 7.00E-103 | A role during avian skin and feather development | Ref. 63 |
| XM_427005.2 | AAK1 | 10.14017 | Up | 4.94E-19 | 1.08E-18 | Reported as a positive regulator of the Notch pathway | Ref. 58 |
| NM_205259.2 | LOC396194 | 10.11269 | Up | 1.24E-91 | 5.13E-91 | ||
| XM_426613.2 | LOC429057 | 10.1068 | Up | 0 | 0 | Regulates the function of the alternative complement pathway in fluid phase and on cellular surfaces | Ref. 64 |
| NM_001030541.1 | POSTN | 10.09206 | Up | 0 | 0 | Periostin as a mediator of matrix remodeling by cushion mesenchyme towards a mature valve structure | Ref. 65 |
| NM_204311.1 | CDX2 | −12.264 | Down | 0 | 0 | Critical for establishing the trophoectoderm, the precursor of the placenta | Ref. 66 |
| XM_415985.2 | LOC417741 | −10.6646 | Down | 3.10E-160 | 1.80E-159 | Encodes a secreted antagonist of Wnt signaling likely involved in inhibiting Xwnt8 and XmyoD ventrally | Ref. 67 |
| XM_001231344.1 | LOC768589 | −10.5428 | Down | 1.20E-107 | 5.30E-107 | Prevents apoptotic cell death | |
| XM_001234907.1 | LOC771651 | −10.3586 | Down | 4.05E-11 | 7.60E-11 |
FIGURE 5.
KEGG classification of DEGs in RNA-seq. A, ESCs versus PGCs. B, ESCs versus SSCs. C, PGCs versus SSCs.
The Combination Analysis of Microarray and RNA-seq
The combined analysis of both microarray and RNA-seq results of the total DEGs revealed that there were 19 genes with expression differences more than 8-fold in the ESCs versus PGCs including GAL10, HBB, HBZ, and HBA1 genes and GAL9, which showed the highest fold change with successively increased expression in the three kinds of cells. In the ESCs versus SSCs, there were 31 genes with expression changes more than 8-fold including SRY, GAL6, GAL7, and GAL9 genes and the CDX2 that had the highest fold change in the three types of cells. In the PGCs versus SSCs, six genes were found to have more than 8-fold changes in expression, and AMH, HBZ, and HINTW showed highly specific expression in SSCs (Tables 7–9).
TABLE 7.
DEGs related to |log2| > 10 in ESCs versus PGCs of microarray and RNA-seq
LHRH, luteinizing hormone-releasing hormone.
| Gene identification | Description | Function | References |
|---|---|---|---|
| NM_205335.2 | Gallus gallus transthyretin (TTR), mRNA | Transports thyroxine from the bloodstream to the brain | |
| NM_206989.1 | Gallus gallus prepro-urotensin II-related peptide (LOC404534), mRNA | Induces phenotypic differentiation, migration, and collagen synthesis | Ref. 53 |
| XM_001236989.1 | PREDICTED: Gallus gallus hypothetical protein LOC773980 (LOC777548), partial mRNA | Required for the correct speed and extent of migration | Ref. 27 |
| XM_426327.2 | PREDICTED: Gallus gallus similar to aminopeptidase A (LOC428771), mRNA | Probably plays a role in regulating growth and differentiation of early B-lineage cells | |
| NM_001001611.2 | Gallus gallus Gal 9 (GAL9), mRNA | GAL is a hypothalamic-hypophysiotropic hormone and is a neuromodulator of LHRH secretion and action | Ref. 21 and 22 |
| XM_414212.2 | PREDICTED: Gallus gallus hypothetical LOC415852 (LOC415852), mRNA | Regulating the responsiveness of cells to adrenal androgens | |
| XM_426984.2 | PREDICTED: Gallus gallus hypothetical LOC429428 (LOC429428), partial mRNA | Involved in the maintenance of the self-renewal of human ES cells by suppression of gene expression | Ref. 14 |
| NM_204675.1 | Gallus gallus wingless-type MMTV integration site family, member 3A (WNT3A), mRNA | Facilitates clonal plating of hESCs exhibiting functional hepatic differentiation | Ref. 56 |
| XM_001231344.1 | PREDICTED: Gallus gallus hypothetical protein LOC768589 (LOC768589), mRNA | Prevents apoptotic cell death | |
| XM_001234742.1 | PREDICTED: Gallus gallus similar to bactericidal/permeability-increasing protein-like 2 (LOC771461), mRNA | Plays an essential role in host defense | Ref. 55 |
| XM_416906.2 | PREDICTED: Gallus gallus hypothetical LOC418708 (LOC418708), mRNA | Responsible for the nucleosome structure of the chromosomal fiber in eukaryotes | |
| XM_430154.2 | PREDICTED: Gallus gallus hypothetical LOC424460 (LOC424460), mRNA | ||
| XM_414795.2 | PREDICTED: Gallus gallus similar to LOC398026 protein (LOC416488), mRNA | Required for normal fertility and fecundity | Ref. 16 |
TABLE 8.
DEGs related to |log2| > 10 in ESCs versus SSCs of microarray and RNA-seq
| Gene identification | Description | Function | References |
|---|---|---|---|
| XM_422203.2 | PREDICTED: Gallus gallus hypothetical LOC424360 (LOC424360), mRNA | ||
| NM_001039453.1 | Gallus gallus aquaporin 1 (AQP1), mRNA | ||
| XM_421648.2 | PREDICTED: Gallus gallus similar to prepro bone inducing protein (LOC423776), mRNA | Has a key role in physiological and pathological angiogenesis | Ref. 68 |
| NM_213579.1 | Gallus gallus GTP-binding protein overexpressed in skeletal muscle (GEM), mRNA | Participates in receptor-mediated signal transduction at the plasma membrane | Ref. 69 |
| XM_419553.2 | PREDICTED: Gallus gallus similar to cardiac muscle ryanodine receptor (LOC421508), mRNA | ||
| XM_001232973.1 | PREDICTED: Gallus gallus similar to lecithin-cholesterol acyltransferase (LOC769683), mRNA | ||
| NM_204897.1 | Gallus gallus heterochromatin-associated protein MENT (MENT-1), mRNA | Associated with intranuclear foci of condensed chromatin | Ref. 62 |
| NM_204679.1 | Gallus gallus Dermo protein (CDERMO-1), mRNA | A role during avian skin and feather development | Ref. 63 |
| XM_427005.2 | PREDICTED: Gallus gallus similar to AP2-associated protein kinase 1 (adaptor-associated kinase 1) (LOC429449), partial mRNA | Reported as a positive regulator of the Notch pathway | Ref. 58 |
| NM_205259.2 | Gallus gallus leukocyte ribonuclease A-1 (LOC396194), mRNA | ||
| XM_426613.2 | PREDICTED: Gallus gallus similar to complement regulator factor H (LOC429057), mRNA | Regulates the function of the alternative complement pathway in fluid phase and on cellular surfaces | Ref. 64 |
| XM_001235325.1 | PREDICTED: Gallus gallus similar to AP2-associated protein kinase 1 (adaptor-associa1ted kinase 1) (LOC772149), partial mRNA | ||
| NM_001030541.1 | Gallus gallus periostin, osteoblast specific factor (POSTN), mRNA | Mediator of matrix remodeling by cushion mesenchyme towards a mature valve structure | Ref. 65 |
| NM_204311.1 | Gallus gallus caudal type homeobox transcription factor 2 (CDX2), mRNA | Critical for establishing the trophoectoderm, the precursor of the placenta | Ref. 66 |
| XM_415985.2 | PREDICTED: Gallus gallus similar to secreted Xwnt8 inhibitor sizzled (LOC417741), mRNA | Encodes a secreted antagonist of Wnt signaling likely involved in inhibiting Xwnt8 and XmyoD ventrally | Ref. 67 |
| XM_001231344.1 | PREDICTED: Gallus gallus hypothetical protein LOC768589 (LOC768589), mRNA | Prevents apoptotic cell death | |
| XM_001234907.1 | PREDICTED: Gallus gallus hypothetical protein LOC771651 (LOC771651), partial mRNA |
TABLE 9.
DEGs related to |log2| > 10 in PGCs versus SSCs of microarray and RNA-seq
| Gene identification | Description | Function | References |
|---|---|---|---|
| XM_429858.1 | PREDICTED: Gallus gallus hypothetical LOC421502 (LOC421502), mRNA | ||
| XM_416467.2 | PREDICTED: Gallus gallus structural maintenance of chromosomes 1B (SMC1B), mRNA | Required for meiotic chromosome dynamics | Ref. 70 |
| XM_427005.2 | PREDICTED: Gallus gallus similar to AP2-associated protein kinase 1 (adaptor-associated kinase 1) (LOC429449), partial mRNA | Reported as a positive regulator of the Notch pathway | Ref. 58 |
| NM_205030.1 | Gallus gallus anti-Mullerian hormone (AMH), mRNA | Has a critical role in regression of the mullerian duct system during development | Ref. 71 |
| XM_428866.1 | PREDICTED: Gallus gallus similar to Scale keratin (S-KER) (S-ker) (LOC431315), mRNA | ||
| XM_001232144.1 | PREDICTED: Gallus gallus similar to Wpkci (LOC771438), mRNA | Involved in triggering the differentiation of ovary | Ref. 72 |
Screening of Candidate Genes Involved in Male Germ Cell Development
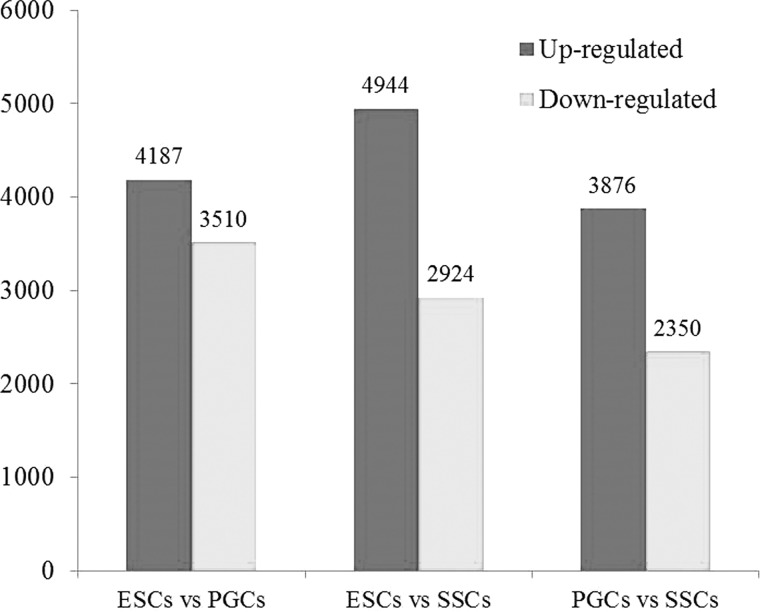
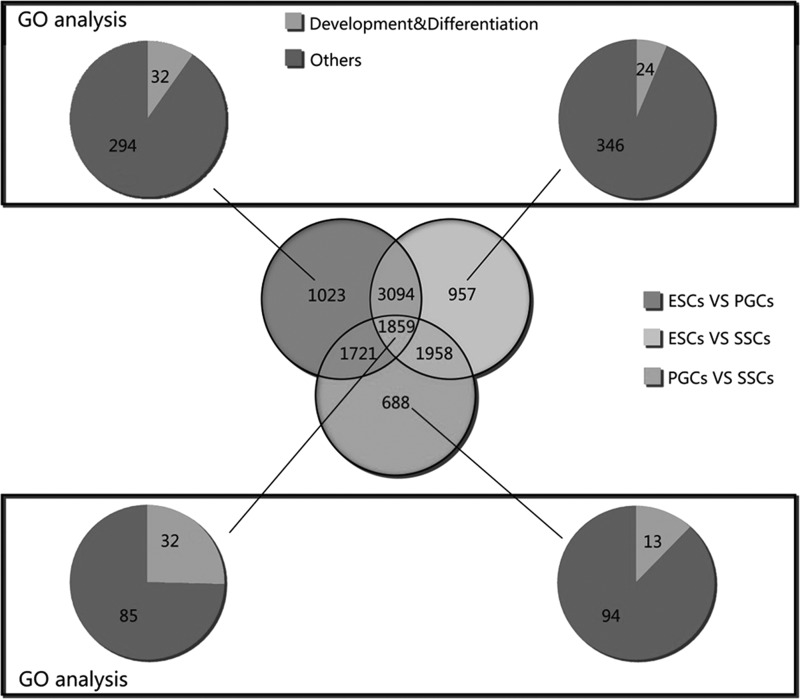
Venny analysis was used to find the specifically expressed genes in these three types of cells. The results showed that there were 1,023 DEGs in ESCs versus PGCs, 957 in ESCs versus SSCs, and 688 in the PGC versus the SSC group. GO analysis found that DEGs in ESCs versus PGCs were mainly enriched in 326 GO terms, of which 32 were associated with development and differentiation. In the ESC versus the SSC group, The DEGs were mainly enriched in 370 GO terms, of which 24 were associated with development and differentiation, whereas in PGCs versus SCCs, DEGs were mainly enriched in 107 GO terms, of which 13 genes were associated with development and differentiation. All DEGs in three groups were mainly enriched in 117 GO terms, of which 32 were associated with development and differentiation (Fig. 6).
FIGURE 6.
Venn diagram comparing DEGs among the three analyses.
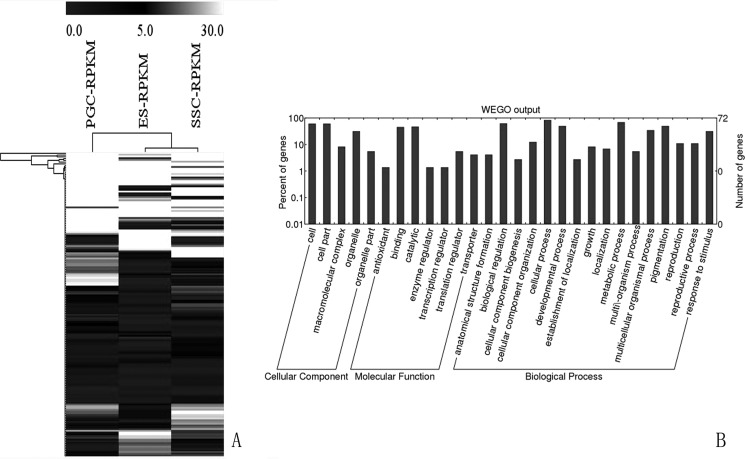
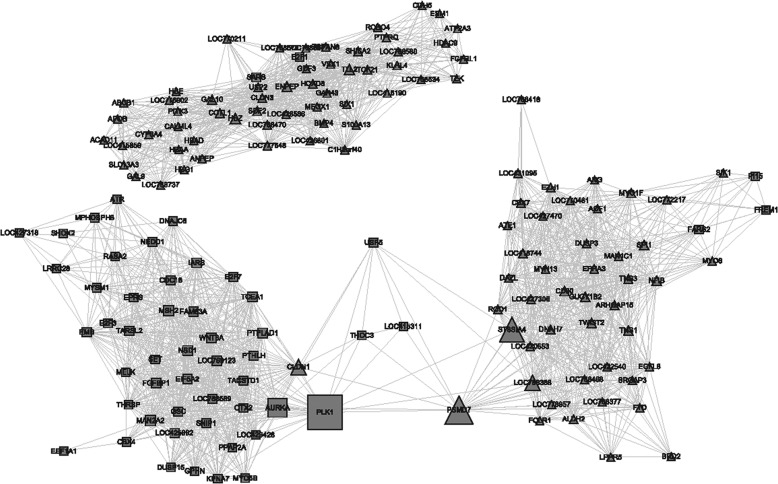
Further analyzes revealed that there are 173 genes are related to development, differentiation, and metabolism, according to their GO classification as shown in the heat map in Fig. 7. Of these genes, 25 were successively up-regulated, and 14 were down-regulated; 18 genes were specifically expressed in ESCs, 58 genes were specifically expressed in PGCs, 16 genes were specifically expressed in SSCs, and 46 genes were specifically expressed in both types of cells. Ontological analysis indicated that 98 DEGs (57%) associated with cell differentiation, 19 genes (11%) were accompanied to metabolic process, and 56 (32%) genes were related to both processes. When we paid attention to the highly expressed 33 genes as candidate players involved in male germ cell development (Table 10), we found that there were 11 DEGs in the three types of cells (9 were up-regulated, and 2 were down-regulated), although 11 genes were specifically expressed only in PGCs, and 2 genes were only specifically expressed in SSCs. We also identified that IARS, TARSL2, EPRS, and THRSP4 genes were specifically expressed in ESCs, but SARS, SLC13A3, TLL2, and SDF2 were specifically expressed in PGCs, whereas other genes were expressed in both two types of cells. Network analysis of 173 candidate genes associated with differentiation and development revealed the regulatory network and the interaction of DEGs. The FunNet analysis found that DEGs were mainly clustered in the three groups (Fig. 8), in which PLK1 and PSMD7 were two key nodes of regulatory networks. The results showed that PLK1 and PSMD7 expressions were decreased from ESCs to SSCs and that GAL9, AMH, PLK1, PSMD7, SDF2, DNAH7, MYH13, PRDM14, KPNA7, HOXB6, TWIST2, SHISA2, SIX1, USP2, MH13, and PR3 might be candidate genes related to chicken male germ cell development, differentiation, and cell metabolism processes.
FIGURE 7.
Heat map representation (left) and GO classification (right) of differentiation-related genes among ESCs, PGCs, and SSCs.
TABLE 10.
Development and differentiation-related genes among three kinds stem cells of chicken
| Gene identification | Gene name | Pattern | Function | References |
|---|---|---|---|---|
| XM_001231469.1 | Hypothetical protein LOC768468 | Up-regulate | ||
| XM_001231338.1 | Similar to pol; hypothetical protein LOC769775 | Up-regulate | ||
| NM_204283.1 | ST8 α-N-acetyl-neuraminide α-2,8-sialyltransferase 4 (ST8SIA4) | Up-regulate | Critical gene for the formation of neural cell adhesion molecule | Ref. 73 |
| NM_205254.1 | Myosin IF (MYO1F) | Up-regulate | Have a role to play in cell motility | Ref. 74 |
| NM_204894.1 | ATP-binding cassette, subfamily B (MDR/TAP), member 1 (ABCB1) | Up-regulate | Involved in the cell type-specific transport or release of estrogen that is essential for avian follicular development | Ref. 75 |
| NM_204735.1 | Myosin VI (MYOVI) | Up-regulate | Plays a role in the maintenance of Golgi morphology and in exocytosis | Ref. 76 |
| NM_001008476.1 | Histone deacetylase 9 (HDAC9) | Up-regulate | ||
| XM_001231534.1 | CD80 | Up-regulate | ||
| NM_205430.1 | EPH receptor A3 (EPHA3) | Up-regulate | Play an important role during development and in signal transduction pathways | Ref. 77 |
| XM_418915.2 | E2F transcription factor 3 (E2F3) | Down-regulate | Critical regulators of the genes responsible for cell cycle progression and growth | Ref. 78 |
| XM_427876.2 | Myosin VB (MYOVB) | Down-regulate | ||
| XM_425527.2 | Growth-associated protein 43 (GAP43) | PGCs specific | ||
| XM_001231480.1 | Similar to Wpkci; histidine triad nucleotide binding protein W | PGCs specific | Involved in triggering the differentiation of ovary | Ref. 72 |
| NM_204926.1 | Ubiquitin-specific peptidase 2 (SUP2) | PGCs specific | ||
| NM_204501.1 | Transmembrane protein 46; hypothetical protein LOC771239 | PGCs specific | ||
| NM_204769.1 | Visual system homeobox 1 (VSX1) | PGCs specific | Retinal differentiation | Ref. 79 |
| NM_204115.1 | Rho-related BTB domain containing 2 (RHOBTB2) | PGCs specific | ||
| NM_001044685.1 | SIX homeobox 1 (SIX1) | PGCs specific | Controls craniofacial and brain development | Ref. 80 |
| NM_204202.1 | Claudin 3 (CLDN3) | PGCs specific | Promotes tubule formation and expansion of the ureteric bud epithelium | Ref. 81 |
| XM_421631.2 | Paired-like homeodomain 3 | PGCs specific | Pituitary and lens formation | Ref. 82 |
| XM_001234694.1 | Histone deacetylase 9 | PGCs specific | ||
| XM_001231443.1 | Hypothetical protein LOC768737 | PGCs specific | ||
| XR_027197.1 | Dynein, axonemal, heavy chain 7 (DNAH7) | SSCs specific | ||
| XM_001231455.1 | Myosin, heavy chain 13, skeletal muscle (MYH13) | SSCs specific | Early specialization of the superfast myosin | Ref. 83 |
| XM_416513.2 | Ubiquitin-specific peptidase 5 (isopeptidase T) | cESCs and PGCs | ||
| NM_204559.1 | msh homeobox 2 (MSX2) | cESCs and PGCs | Crucial role in directing the growth and patterning of limb mesoderm | Ref. 84 |
| XM_001231369.1 | Leucine-rich repeat containing 28 | cESCs and SSCs | ||
| XM_001231533.1 | Hypothetical protein LOC768631 | cESCs and SSCs | ||
| NM_001012929.1 | RAS p21 protein activator 2 | cESCs and SSCs | ||
| NM_001012927.1 | myb-like, SWIRM and MPN domains 1 | cESCs and SSCs | Essential gene for the growth and the differentiation for various types of cells | Ref. 85 |
| XM_422634.2 | Nicotinamide nucleotide adenylyltransferase 3 | cESCs and SSCs |
FIGURE 8.
Gene regulatory network analysis.
Screening of the Candidate Pathways Involved in Male Germ Cell Development
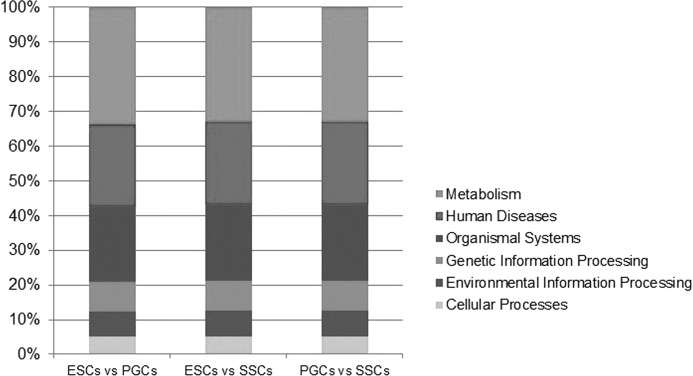
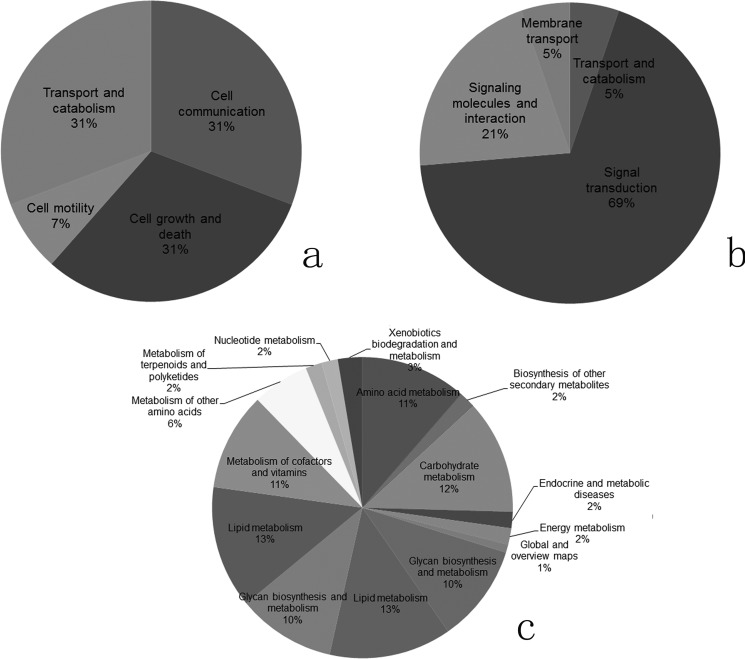
Based on the above KEGG pathway enrichment analysis of the DEGs, further functional classification detected that most of the enriched pathways were related to the metabolism regulation processes (32.94–33.72%), diseases (21.71–21.96%), regulation of genetic information process (8.53–8.63%), environmental information regulatory process (7.36–7.45%), and cellular process regulation (5.04–5.10%) (Fig. 9). Among 13 pathways regulating the cellular processes, there were four pathways involved in cell communication, four pathways in cell growth and apoptosis, one pathway involved in cell motility, and four pathways in the transport and catabolism (Fig. 10a). The participating 19 pathways in the environmental information regulatory process were classified as follows: 1 pathway related to membrane transport, 13 involved in signal transduction, 4 related to signaling molecules interaction, and 1 pathway involved in the transport and catabolism (Fig. 10b). In these pathways, the DEGs were significantly expressed in TGF-β signaling pathway, Notch signaling pathway, Jak-STAT signaling pathway, ErbB signaling pathway, ABC transporter, extracellular matrix receptor interaction, cytokines, and their receptor interaction and cell adhesion molecule pathways. Among the 76 pathways related to the regulation of metabolism, 14 pathways regulate the carbohydrate metabolism, 2 pathways control the energy metabolism, 15 pathways are for lipid metabolism, 2 pathways are for nucleotide metabolism, 20 pathways are for amino acid metabolism, 6 pathways are for polysaccharides biosynthesis and metabolism, 12 pathways are for co-enzyme factor and vitamin metabolism, 2 pathways are for terpenoids and polyketide metabolism, 2 pathways are for biosynthesis of other secondary metabolites, and 3 pathways are related to xenobiotics metabolism and biodegradation (Fig. 10c). Most of the DEGs were significantly expressed in the following 17 metabolic pathways: arginine and proline metabolism, steroid biosynthesis, glutamic acid, serine, threonine metabolism, alanine, aspartic acid, glutamate metabolism, primary bile acid production, purine metabolism, sphingolipid metabolism, mucopolysaccharide metabolism, chondroitin sulfate, tyrosine metabolism, carbohydrate digestion and absorption, retinol metabolism, steroid hormone production, and oxidative phosphorylation pathways.
FIGURE 9.
KEGG pathway enrichment analysis of the DEGs.
FIGURE 10.
Screening of the candidate pathways involved in male germ cell development. a, classification of the 13 enriched pathways related to cellular processes regulation. b, distribution of the participating 19 pathways in environmental information regulatory process. c, percentages of the related pathways to the regulation of metabolism.
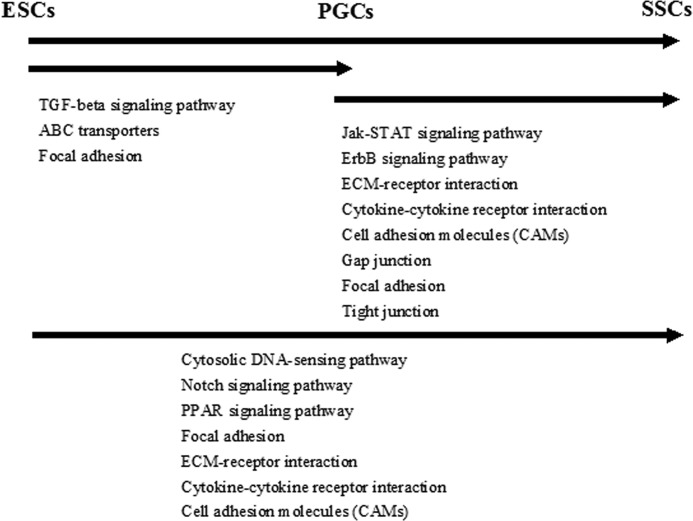
Screening and filtration of the closely related pathways to cell proliferation, differentiation, and signal transduction revealed that TGF-β, focal adhesion signal pathways, and ABC transporter were the most enriched in the ESC versus the PGC group, playing an important role during differentiation of ESCs to PGCs, whereas the most enriched pathways that regulate PGCs to SSC differentiation were Jak-STAT signaling pathway, ErbB signaling pathway, cell adhesion molecules, cytokine receptors and their interactions, extracellular matrix receptor interaction, focal adhesion, tight junctions and gap junctions pathways. In a ESC versus SSC comparison, there were seven significantly enriched pathways that were suspected to be responsible for in vivo differentiation of ESCs to SSCs cells; they are cytoplasmic DNA sensing, Notch signaling, PPAR signaling, the Focal adhesion, extracellular matrix receptor interaction, cytokines and interaction with their receptors, and cell adhesion molecule pathways (Fig. 11).
FIGURE 11.
Signaling regulate different stages of male germ cell.
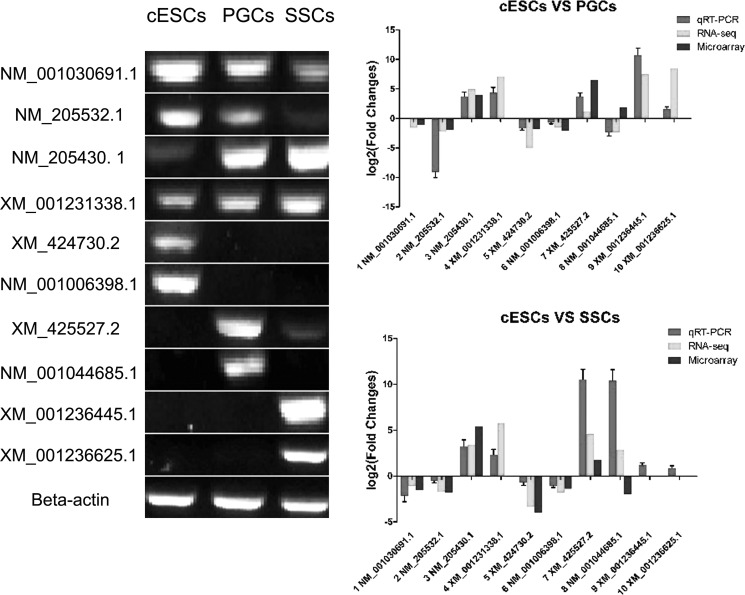
Validation of Microarray and RNA-seq Results by qRT-PCR
qRT-PCR was used to validate gene expression levels by detecting 10 randomly selected DEGs. As shown in Fig. 12, most of the qRT-PCR results were significantly correlated with the microarray and RNA-seq results, indicating the reliability and accuracy of microarray and RNA-seq expression analysis.
FIGURE 12.
Validation of RNA-seq results by quantitative PCR.
Verification of the Microarray and RNA-seq Results by in Vitro Induction Experiments
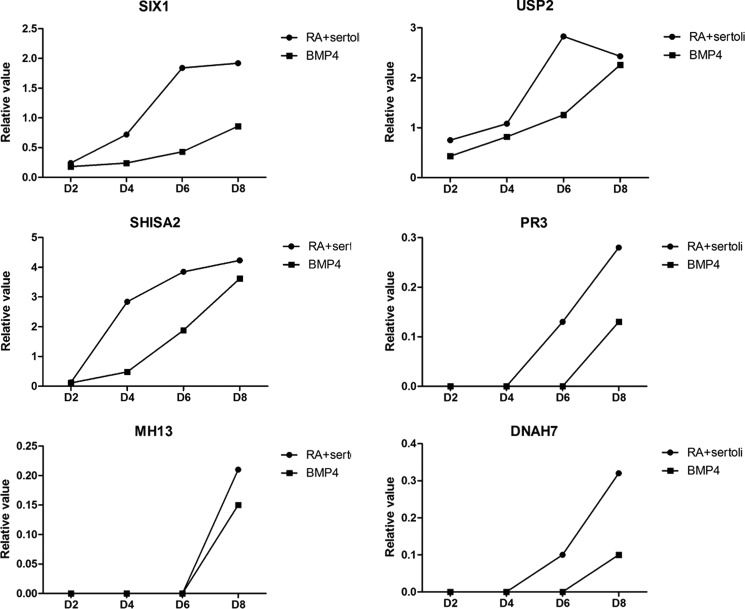
The RA and supporting Sertoli cell induction group showed the embryoid body formation, and PGCs cells appeared after 2 days of induction, accompanying the beginning of SHISA2 gene expression, which was significantly up-regulated from 2–4 days and then increased at a slower rate after 6 days. The BMP4 induction group showed the embryoid body formation and PGCs cell appearance and the SHISA2 gene activation at the sixth day of induction. SIX1 (SIX homeobox 1) gene expression was increased in a linear growth trend until 4–6 days followed by a declined expression at the eighth day of stimulation. BMP4 expression was gradually increased at 2–8 days postinduction, whereas USP2 (ubiquitin specific peptidase 2) expression was gradually increased at 2–4 days and then linearly up-regulated at 4–6 days but began to decline at the day eight after induction. When treated with BMP4, USP2 expression was gradually increased during 2–6 days with a linear upward trend at 6–8 days after induction. The specifically expressed genes in SSCs (MH13, PR3, and DNAH7) were expressed at 6–8 days postinduction, whereas it began to express at day 8 in the BMP4 induction group, consistent with the beginning time of SSCs cell formation. When the induction occurred using the sex hormones in combination with supporting cells and RA, it was observed that EPHA3 expression was continuously increased and reached its highest value at the time of SSC formation, and GAP43 expression was up-regulated during PGC formation, reaching its maximum level of expression at the eighth day postinduction. Then its expression level was declined with the disintegration of embryoid bodies. On the other hand, both LOC773389 and LOC773586 expression showed a continued up-regulation and reached the highest value at SSC formation (Fig. 13).
FIGURE 13.
Express trend of mark genes during differentiation.
Discussion
We have examined, for the first time, the entire gene expression pattern in three kinds of stem cells in chicken through a systematic whole genome and whole transcriptome approaches. Through comparison of the results, we identified that the male germ cell differentiation is a complex regulatory process associated with a lot of genes and signaling pathways. Until now, however, the molecular mechanisms during this process were not fully announced; only some genes and pathways had been confirmed. As an attempt at a complete understanding, two high throughput methods (microarray and RNA-seq) were used to detect the gene expression pattern during male germ cell specification and differentiation.
Light had been shed on some crucial genes that were predicted to be the controller of this process. Our work confirmed the roles of some previously mentioned genes in the differentiation process as SHISA2, AMH, SOX9, ALDH1A1, and others. Boudreau and Jones (8) reported that SHISA2 gene blocks the expression of Wnt signaling pathway. It is necessary to ensure the normal development of mouse PGCs as it was observed by Miles (9). The same findings were obtained in our experiment because the SHISA2 gene was detected in a high expression level in PGCs cells during either the normal in vivo male germ cell differentiation or during its in vitro induction. Also, our results revealed that AMH and SOX9 genes were specifically expressed in SSCs with more than 10-fold difference of the expression changes. These results are consistent with other studies (10) showing that the anti-Mullerian hormone (AMH) was responsible for early male development in vertebrate as AMH, which is a major downstream of SOX9, which boosts the AMH expression through binding to its promoter, leading to stimulation of male germ cell differentiation.
Both ALDH1A1 and CYP26b1 are suspected to be key candidate genes because they are involved in the synthesis, degradation, and maintaining the in vivo homeostasis of RA, which plays an important role in mammalian spermatogenesis process through controlling the activation of the meiotic-related genes, such as STRA8 (stimulated by retinoic acid gene 8) (11–13). At the same time, we observed a continuous up-regulation of ALDH1A1 and CYP26b1 that explain the nonconstant level of RA during the differentiation process caused by the effect of these genes on the RA equilibrium. The maximum levels of ALDH1A1 and CYP26b1 expression were detected in SSCs, indicating its stimulatory role in the meiosis process. A recent study identified the PRDM14 expression by microarray assay, in particular, undifferentiated human ESCs (14), and Chia et al. (15) described it as an important transcription factor in human pluripotency maintenance. HU stated that PLK1, a polo-like kinase family member, was involved in cell mitosis and also expressed in a high significant manner in many of human malignancies so it is considered a carcinogenic gene (16).
Our study observed high expression levels of PRDM14 and PLK1 especially in chicken ESCs in contrast to its decreased levels in the other two types of cells (PGCs and SSCs). As a further confirmation of those previous observations, it also speculated that PRDM14 and PLK1 may play an important role in pluripotency maintenance and their down-regulation and so may be related to the ESC differentiation process. Analysis of candidate genes regulatory network found that PLK1 is located at a key node of the entire regulatory network, as a bridge connecting the entire cell differentiation process.
Studies have shown that ECM (extracellular matrix protein) can provide support for the cell adhesion and also help through the integrin receptor to deliver the extracellular signals that regulate stem cell proliferation, migration, and differentiation. ECM could activate the expression of Integrinα6 and Integrinβ1 that led to cell morphology and the function change (17). In our study, Integrinα6 and Integrinβ1 were expressed in a continuous upward manner in the three types of cells, indicating that the ECM signals may promote the expression of SSCs marker genes and induce the formation of the male germ cells. Cell adhesion molecules could activate FAK signal leading to the reorganization of the actin cytoskeleton and subsequently cause cell differentiation (18). TGF-β signaling could regulate testis formation and male germ cell development (9). Notch signaling was reported antagonistically to regulate germ line stem cell niche formation in Drosophila male embryonic gonads (19) and deletion of Jagged1, a Notch ligand, could lead to the formation of multicystic follicles in the mouse ovary (20). These reports provide support that our selected candidate key signaling pathways play an important role in the differentiation of the male germ cells. p53 signaling pathway and MAPK signaling pathway have been reported to be involved in the cell differentiation.
Some genes were not involved in any signal pathway. GO assay results suggested that these genes have a role in cell differentiation regulation because they showed significant differences among the three types of cells. Therefore the regulatory mechanism and function of these genes and its position in the signal pathways require further researches. The genes that we identified in this study will provide important candidates, which are potential markers identifying ESCs, PGCs, SSCs, and the potential regulators controlling germ cell differentiation.
In this study, we successfully induced differentiation of ESCs into the male germ cells in vitro using an induction system containing RA, supporting cells, BMP4, testosterone, and follicle-stimulating hormone. During these induction procedures, the expression level of the selected crucial genes was detected using quantitative real time PCR. The consistency of microarray and RNA-seq results with our confirmatory experimental observations using this established model indicated its successful ability for induction of in vitro differentiation of ESCs toward male germ cells, and so the candidate crucial genes can be validated based on this system. It was concluded that these candidate crucial genes have the ability of specific expression during male germ cell development either in the normal in vivo differentiation process or in vitro induction system. However, its specific functions remain under further investigation.
Differentiation of male germ cells is an intricate regulatory process that involved a large number of genes and signaling pathways with a lot of unclear mechanisms. Currently, there is not any system or complete comprehensive research report that reveals the variations of these genes, so it cannot be an accurate search for the key regulatory genes or signaling pathways responsible for this process. This study revealed the whole gene expression changes in the entire process of germ cell differentiation to explore the molecular mechanism of its variation and to obtain the candidate crucial genes and signaling pathways, and it was also observed that some of these genes and signaling pathways were first reported in poultry. This research will provide a more reliable reference for the researchers to study the mechanism of germ cell differentiation and narrow the selection of genes or signal pathways. Moreover, it will suggest more precise ideas to be focused on in the future, especially for the in vitro spermatogenesis process because it will support the exploration of promoting induction methods. Currently, we have obtained a partial screening of the critical candidate genes and signaling pathways to start further functional validation experiments for in-depth study and analysis. Using poultry as a research model to study the regulatory mechanism of ESC differentiation toward the male germ cells will contribute a better understanding of cell biology and developmental biology and will be useful for human health-applicable studies.
Our results showed the crucial genes and pathways involved in the regulation of chicken male germ cell differentiation. These results will be helpful for researchers to narrow the range of functional genes and pathways during the ESC differentiation to male germ cells providing an important reference for future research.
Acknowledgments
We thank the researchers who contributed to this work but were not included in the author list.
Footnotes
- DEG
- differentially expressed gene
- ESC
- embryonic stem cell
- PGC
- primordial germ cell
- SSC
- spermatogonial stem cell
- RNA-seq
- RNA sequencing
- qRT-PCR
- quantitative real time PCR
- RA
- retinoic acid
- GO
- Gene Ontology
- KEGG
- Kyoto Encyclopedia of Genes and Genomes.
References
- 1. Saitou M., Barton S. C., Surani M. A. (2002) A molecular programme for the specification of germ cell fate in mice. Nature 418, 293–300 [DOI] [PubMed] [Google Scholar]
- 2. Ohinata Y., Payer B., O'Carroll D., Ancelin K., Ono Y., Sano M., Barton S. C., Obukhanych T., Nussenzweig M., Tarakhovsky A., Saitou M., Surani M. A. (2005) Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 436, 207–213 [DOI] [PubMed] [Google Scholar]
- 3. Yu Z., Ji P., Cao J., Zhu S., Li Y., Zheng L., Chen X., Feng L. (2009) Dazl promotes germ cell differentiation from embryonic stem cells. J. Mol. Cell Biol. 1, 93–103 [DOI] [PubMed] [Google Scholar]
- 4. Dann C. T., Alvarado A. L., Molyneux L. A., Denard B. S., Garbers D. L., Porteus M. H. (2008) Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells 26, 2928–2937 [DOI] [PubMed] [Google Scholar]
- 5. Ewen K. A., Koopman P. (2010) Mouse germ cell development: from specification to sex determination. Mol. Cell. Endocrinol. 323, 76–93 [DOI] [PubMed] [Google Scholar]
- 6. Saitou M., Payer B., O'Carroll D., Ohinata Y., Surani M. A. (2005) Blimp1 and the emergence of the germ line during development in the mouse. Cell Cycle 4, 1736–1740 [DOI] [PubMed] [Google Scholar]
- 7. Rao M. (2004) Conserved and divergent paths that regulate self-renewal in mouse and human embryonic stem cells. Dev. Biol. 275, 269–286 [DOI] [PubMed] [Google Scholar]
- 8. Boudreau N. J., Jones P. L. (1999) Extracellular matrix and integrin signalling: the shape of things to come. Biochem. J. 339, 481–488 [PMC free article] [PubMed] [Google Scholar]
- 9. Miles D. C., Wakeling S. I., Stringer J. M., van den Bergen J. A., Wilhelm D., Sinclair A. H., Western P. S. (2013) Signaling through the TGFβ-activin receptors ALK4/5/7 regulates testis formation and male germ cell development. PLoS One 8, e54606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Western P. S., Harry J. L., Graves J. A., Sinclair A. H. (1999) Temperature-dependent sex determination in the American alligator: AMH precedes SOX9 expression. Dev. Dyn. 216, 411–419 [DOI] [PubMed] [Google Scholar]
- 11. Fan X., Molotkov A., Manabe S., Donmoyer C. M., Deltour L., Foglio M. H., Cuenca A. E., Blaner W. S., Lipton S. A., Duester G. (2003) Targeted disruption of Aldh1a1 (Raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Mol. Cell. Biol. 23, 4637–4648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou Q., Li Y., Nie R., Friel P., Mitchell D., Evanoff R. M., Pouchnik D., Banasik B., McCarrey J. R., Small C., Griswold M. D. (2008) Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol. Reprod. 78, 537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. MacLean G., Abu-Abed S., Dollé P., Tahayato A., Chambon P., Petkovich M. (2001) Cloning of a novel retinoic-acid metabolizing cytochrome P450, Cyp26B1, and comparative expression analysis with Cyp26A1 during early murine development. Mech. Dev. 107, 195–201 [DOI] [PubMed] [Google Scholar]
- 14. Tsuneyoshi N., Sumi T., Onda H., Nojima H., Nakatsuji N., Suemori H. (2008) PRDM14 suppresses expression of differentiation marker genes in human embryonic stem cells. Biochem. Biophys. Res. Commun. 367, 899–905 [DOI] [PubMed] [Google Scholar]
- 15. Chia N. Y., Chan Y. S., Feng B., Lu X., Orlov Y. L., Moreau D., Kumar P., Yang L., Jiang J., Lau M. S., Huss M., Soh B. S., Kraus P., Li P., Lufkin T., Lim B., Clarke N. D., Bard F., Ng H. H. (2010) A genome-wide RNAi screen reveals determinants of human embryonic stem cell identity. Nature 468, 316–320 [DOI] [PubMed] [Google Scholar]
- 16. Hu J., Wang F., Yuan Y., Zhu X., Wang Y., Zhang Y., Kou Z., Wang S., Gao S. (2010) Novel importin-α family member Kpna7 is required for normal fertility and fecundity in the mouse. J. Biol. Chem. 285, 33113–33122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rastegar T., Minaee M. B., Habibi Roudkenar M., Raghardi Kashani I., Amidi F., Abolhasani F., Barbarestani M. (2013) Improvement of expression of α6 and β1 integrins by the co-culture of adult mouse spermatogonial stem cells with SIM mouse embryonic fibroblast cells (STO) and growth factors. Iran. J. basic Med. Sci. 16, 134–139 [PMC free article] [PubMed] [Google Scholar]
- 18. Aplin A. E., Howe A. K., Juliano R. L. (1999) Cell adhesion molecules, signal transduction and cell growth. Curr. Opin. Cell Biol. 11, 737–744 [DOI] [PubMed] [Google Scholar]
- 19. Kitadate Y., Kobayashi S. (2010) Notch and Egfr signaling act antagonistically to regulate germ-line stem cell niche formation in Drosophila male embryonic gonads. Proc. Natl. Acad. Sci. U.S.A. 107, 14241–14246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vanorny D. A., Prasasya R. D., Chalpe A. J., Kilen S. M., Mayo K. E. (2014) Notch signaling regulates ovarian follicle formation and coordinates follicular growth. Mol. Endocrinol. 28, 499–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. López F. J., Merchenthaler I., Ching M., Wisniewski M. G., Negro-Vilar A. (1991) Galanin: a hypothalamic-hypophysiotropic hormone modulating reproductive functions. Proc. Natl. Acad. Sci. U.S.A. 88, 4508–4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grottoli S., Arvat E., Gianotti L., Ramunni J., Di Vito L., Maccagno B., Ciccarelli E., Camanni F., Ghigo E. (1996) Galanin positively modulates prolactin secretion in normal women. J. Endocrinol. Invest. 19, 739–744 [DOI] [PubMed] [Google Scholar]
- 23. Huber T. B., Simons M., Hartleben B., Sernetz L., Schmidts M., Gundlach E., Saleem M. A., Walz G., Benzing T. (2003) Molecular basis of the functional podocin-nephrin complex: mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft microdomains. Hum. Mol. Genet. 12, 3397–3405 [DOI] [PubMed] [Google Scholar]
- 24. Wahlberg K., Jiang J., Rooks H., Jawaid K., Matsuda F., Yamaguchi M., Lathrop M., Thein S. L., Best S. (2009) The HBS1L-MYB intergenic interval associated with elevated HbF levels shows characteristics of a distal regulatory region in erythroid cells. Blood 114, 1254–1262 [DOI] [PubMed] [Google Scholar]
- 25. Foley H. A., Ofori-Acquah S. F., Yoshimura A., Critz S., Baliga B. S., Pace B. S. (2002) Stat3β inhibits gamma-globin gene expression in erythroid cells. J. Biol. Chem. 277, 16211–16219 [DOI] [PubMed] [Google Scholar]
- 26. Marchler-Bauer A., Zheng C., Chitsaz F., Derbyshire M. K., Geer L. Y., Geer R. C., Gonzales N. R., Gwadz M., Hurwitz D. I., Lanczycki C. J., Lu F., Lu S., Marchler G. H., Song J. S., Thanki N., Yamashita R. A., Zhang D., Bryant S. H. (2013) CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Res. 41, D348–D352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Breau M. A., Wilkinson D. G., Xu Q. (2013) A Hox gene controls lateral line cell migration by regulating chemokine receptor expression downstream of Wnt signaling. Proc. Natl. Acad. Sci. U.S.A. 110, 16892–16897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Szala S., Kasai Y., Steplewski Z., Rodeck U., Koprowski H., Linnenbach A. J. (1990) Molecular cloning of cDNA for the human tumor-associated antigen CO-029 and identification of related transmembrane antigens. Proc. Natl. Acad. Sci. U.S.A. 87, 6833–6837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Muragaki Y., Mattei M. G., Yamaguchi N., Olsen B. R., Ninomiya Y. (1991) The complete primary structure of the human α1 (VIII) chain and assignment of its gene (COL8A1) to chromosome 3. Eur. J. Biochem. 197, 615–622 [DOI] [PubMed] [Google Scholar]
- 30. Chakravarti S., Magnuson T. (1995) Localization of mouse lumican (keratan sulfate proteoglycan) to distal chromosome 10. Mamm. Genome 6, 367–368 [DOI] [PubMed] [Google Scholar]
- 31. Kumar M. S., Hendrix J. A., Johnson A. D., Owens G. K. (2003) Smooth muscle α-actin gene requires two E-boxes for proper expression in vivo and is a target of class I basic helix-loop-helix proteins. Circ. Res. 92, 840–847 [DOI] [PubMed] [Google Scholar]
- 32. Nishida A., Furukawa A., Koike C., Tano Y., Aizawa S., Matsuo I., Furukawa T. (2003) Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat. Neurosci. 6, 1255–1263 [DOI] [PubMed] [Google Scholar]
- 33. Frank I., Friedman H. M. (1989) A novel function of the herpes simplex virus type 1 Fc receptor: participation in bipolar bridging of antiviral immunoglobulin G. J. Virol. 63, 4479–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Behringer R. R. (1994) The in vivo roles of mullerian-inhibiting substance. Curr. Top. Dev. Biol. 29, 171–187 [DOI] [PubMed] [Google Scholar]
- 35. Trbovich A. M., Martinelle N., O'Neill F. H., Pearson E. J., Donahoe P. K., Sluss P. M., Teixeira J. (2004) Steroidogenic activities in MA-10 Leydig cells are differentially altered by cAMP and Mullerian inhibiting substance. J. Steroid Biochem. Mol. Biol. 92, 199–208 [DOI] [PubMed] [Google Scholar]
- 36. Kullak-Ublick G. A., Beuers U., Meier P. J., Domdey H., Paumgartner G. (1996) Assignment of the human organic anion transporting polypeptide (OATP) gene to chromosome 12p12 by fluorescence in situ hybridization. J. Hepatol. 25, 985–987 [DOI] [PubMed] [Google Scholar]
- 37. Kullak-Ublick G. A., Hagenbuch B., Stieger B., Schteingart C. D., Hofmann A. F., Wolkoff A. W., Meier P. J. (1995) Molecular and functional characterization of an organic anion transporting polypeptide cloned from human liver. Gastroenterology 109, 1274–1282 [DOI] [PubMed] [Google Scholar]
- 38. Ellegren H., Carmichael A. (2001) Multiple and independent cessation of recombination between avian sex chromosomes. Genetics 158, 325–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boute N., Gribouval O., Roselli S., Benessy F., Lee H., Fuchshuber A., Dahan K., Gubler M. C., Niaudet P., Antignac C. (2000) NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 24, 349–354 [DOI] [PubMed] [Google Scholar]
- 40. Schwarz K., Simons M., Reiser J., Saleem M. A., Faul C., Kriz W., Shaw A. S., Holzman L. B., Mundel P. (2001) Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J. Clin. Invest. 108, 1621–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Higgs D. R., Vickers M. A., Wilkie A. O., Pretorius I. M., Jarman A. P., Weatherall D. J. (1989) A review of the molecular genetics of the human α-globin gene cluster. Blood 73, 1081–1104 [PubMed] [Google Scholar]
- 42. Backström N., Ceplitis H., Berlin S., Ellegren H. (2005) Gene conversion drives the evolution of HINTW, an ampliconic gene on the female-specific avian W chromosome. Mol. Biol. Evol. 22, 1992–1999 [DOI] [PubMed] [Google Scholar]
- 43. Ceplitis H., Ellegren H. (2004) Adaptive molecular evolution of HINTW, a female-specific gene in birds. Mol. Biol. Evol. 21, 249–254 [DOI] [PubMed] [Google Scholar]
- 44. Milona P., Townes C. L., Bevan R. M., Hall J. (2007) The chicken host peptides, gallinacins 4, 7, and 9 have antimicrobial activity against Salmonella serovars. Biochem. Biophys. Res. Commun. 356, 169–174 [DOI] [PubMed] [Google Scholar]
- 45. Takeshita S., Kikuno R., Tezuka K., Amann E. (1993) Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem. J. 294, 271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xiao Y., Cai Y., Bommineni Y. R., Fernando S. C., Prakash O., Gilliland S. E., Zhang G. (2006) Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity. J. Biol. Chem. 281, 2858–2867 [DOI] [PubMed] [Google Scholar]
- 47. Chen C., Zheng B., Han J., Lin S. C. (1997) Characterization of a novel mammalian RGS protein that binds to Gα proteins and inhibits pheromone signaling in yeast. J. Biol. Chem. 272, 8679–8685 [DOI] [PubMed] [Google Scholar]
- 48. Irwin D. M., Gong Z. (2003) Molecular evolution of vertebrate goose-type lysozyme genes. J. Mol. Evol. 56, 234–242 [DOI] [PubMed] [Google Scholar]
- 49. Harwig S. S., Swiderek K. M., Kokryakov V. N., Tan L., Lee T. D., Panyutich E. A., Aleshina G. M., Shamova O. V., Lehrer R. I. (1994) Gallinacins: cysteine-rich antimicrobial peptides of chicken leukocytes. FEBS Lett. 342, 281–285 [DOI] [PubMed] [Google Scholar]
- 50. Mu H., Ohta K., Kuriyama S., Shimada N., Tanihara H., Yasuda K., Tanaka H. (2003) Equarin, a novel soluble molecule expressed with polarity at chick embryonic lens equator, is involved in eye formation. Mech. Dev. 120, 143–155 [DOI] [PubMed] [Google Scholar]
- 51. Smith C. A., Roeszler K. N., Sinclair A. H. (2009) Genetic evidence against a role for W-linked histidine triad nucleotide binding protein (HINTW) in avian sex determination. Int. J. Dev. Biol. 53, 59–67 [DOI] [PubMed] [Google Scholar]
- 52. Lee E. J., Bhat A. R., Kamli M. R., Pokharel S., Chun T., Lee Y. H., Nahm S. S., Nam J. H., Hong S. K., Yang B., Chung K. Y., Kim S. H., Choi I. (2013) Transthyretin is a key regulator of myoblast differentiation. PLoS One 8, e63627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang Y. G., Li J., Li Y. G., Wei R. H. (2008) Urotensin II induces phenotypic differentiation, migration, and collagen synthesis of adventitial fibroblasts from rat aorta. J. Hypertens. 26, 1119–1126 [DOI] [PubMed] [Google Scholar]
- 54. Arikawa T., Matsukawa A., Watanabe K., Sakata K. M., Seki M., Nagayama M., Takeshita K., Ito K., Niki T., Oomizu S., Shinonaga R., Saita N., Hirashima M. (2009) Galectin-9 accelerates transforming growth factor β3-induced differentiation of human mesenchymal stem cells to chondrocytes. Bone 44, 849–857 [DOI] [PubMed] [Google Scholar]
- 55. Weiss J., Olsson I. (1987) Cellular and subcellular localization of the bactericidal/permeability-increasing protein of neutrophils. Blood 69, 652–659 [PubMed] [Google Scholar]
- 56. Si-Tayeb K., Noto F. K., Nagaoka M., Li J., Battle M. A., Duris C., North P. E., Dalton S., Duncan S. A. (2010) Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 51, 297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Handel M. A., Lessard C., Reinholdt L., Schimenti J., Eppig J. J. (2006) Mutagenesis as an unbiased approach to identify novel contraceptive targets. Mol. Cell Endocrinol. 250, 201–205 [DOI] [PubMed] [Google Scholar]
- 58. Gupta-Rossi N., Ortica S., Meas-Yedid V., Heuss S., Moretti J., Olivo-Marin J. C., Israël A. (2011) The adaptor-associated kinase 1, AAK1, is a positive regulator of the Notch pathway. J. Biol. Chem. 286, 18720–18730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. (1988) Novel regulators of bone formation: molecular clones and activities. Science 242, 1528–1534 [DOI] [PubMed] [Google Scholar]
- 60. Béguin P., Nagashima K., Gonoi T., Shibasaki T., Takahashi K., Kashima Y., Ozaki N., Geering K., Iwanaga T., Seino S. (2001) Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature 411, 701–706 [DOI] [PubMed] [Google Scholar]
- 61. Marx S. O., Reiken S., Hisamatsu Y., Jayaraman T., Burkhoff D., Rosemblit N., Marks A. R. (2000) PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101, 365–376 [DOI] [PubMed] [Google Scholar]
- 62. Grigoryev S. A., Bednar J., Woodcock C. L. (1999) MENT, a heterochromatin protein that mediates higher order chromatin folding, is a new serpin family member. J. Biol. Chem. 274, 5626–5636 [DOI] [PubMed] [Google Scholar]
- 63. Scaal M., Fuchtbauer E. M., Brand-Saberi B. (2001) cDermo-1 expression indicates a role in avian skin development. Anat. Embryol. (Berl.) 203, 1–7 [DOI] [PubMed] [Google Scholar]
- 64. Ault B. H. (2000) Factor H and the pathogenesis of renal diseases. Pediatr. Nephrol. 14, 1045–1053 [DOI] [PubMed] [Google Scholar]
- 65. Butcher J. T., Norris R. A., Hoffman S., Mjaatvedt C. H., Markwald R. R. (2007) Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Dev. Biol. 302, 256–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pernaute B., Cañon S., Crespo M., Fernandez-Tresguerres B., Rayon T., Manzanares M. (2010) Comparison of extraembryonic expression of Eomes and Cdx2 in pregastrulation chick and mouse embryo unveils regulatory changes along evolution. Dev. Dyn. 239, 620–629 [DOI] [PubMed] [Google Scholar]
- 67. Salic A. N., Kroll K. L., Evans L. M., Kirschner M. W. (1997) Sizzled: a secreted Xwnt8 antagonist expressed in the ventral marginal zone of Xenopus embryos. Development 124, 4739–4748 [DOI] [PubMed] [Google Scholar]
- 68. Camerino G. M., Nicchia G. P., Dinardo M. M., Ribatti D., Svelto M., Frigeri A. (2006) In vivo silencing of aquaporin-1 by RNA interference inhibits angiogenesis in the chick embryo chorioallantoic membrane assay. Cell Mol. Biol. (Noisy-le-grand) 52, 51–56 [PubMed] [Google Scholar]
- 69. Maguire J., Santoro T., Jensen P., Siebenlist U., Yewdell J., Kelly K. (1994) Gem: an induced, immediate early protein belonging to the Ras family. Science 265, 241–244 [DOI] [PubMed] [Google Scholar]
- 70. Revenkova E., Eijpe M., Heyting C., Hodges C. A., Hunt P. A., Liebe B., Scherthan H., Jessberger R. (2004) Cohesin SMC1β is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat. Cell Biol. 6, 555–562 [DOI] [PubMed] [Google Scholar]
- 71. Johnson P. A., Kent T. R., Urick M. E., Giles J. R. (2008) Expression and regulation of anti-mullerian hormone in an oviparous species, the hen. Biol. Reprod. 78, 13–19 [DOI] [PubMed] [Google Scholar]
- 72. Hori T., Asakawa S., Itoh Y., Shimizu N., Mizuno S. (2000) Wpkci, encoding an altered form of PKCI, is conserved widely on the avian W chromosome and expressed in early female embryos: implication of its role in female sex determination. Mol. Biol. Cell 11, 3645–3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang B., Hu H., Yu B. (2006) Molecular characterization of pig ST8Sia IV: a critical gene for the formation of neural cell adhesion molecule and its response to sialic acid supplement in piglets. Nutr. Neurosci. 9, 147–154 [DOI] [PubMed] [Google Scholar]
- 74. Edgar A. J., Knight A. E., Bennett J. P. (1996) Chicken myosin IB mRNA is highly expressed in lymphoid tissues. J. Anat. 189, 451–456 [PMC free article] [PubMed] [Google Scholar]
- 75. Edelmann H. M., Duchek P., Rosenthal F. E., Föger N., Glackin C., Kane S. E., Kuchler K. (1999) Cmdr1, a chicken P-glycoprotein, confers multidrug resistance and interacts with estradiol. Biol. Chem. 380, 231–241 [DOI] [PubMed] [Google Scholar]
- 76. Sahlender D. A., Roberts R. C., Arden S. D., Spudich G., Taylor M. J., Luzio J. P., Kendrick-Jones J., Buss F. (2005) Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. J. Cell Biol. 169, 285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sajjadi F. G., Pasquale E. B., Subramani S. (1991) Identification of a new eph-related receptor tyrosine kinase gene from mouse and chicken that is developmentally regulated and encodes at least two forms of the receptor. New Biol. 3, 769–778 [PubMed] [Google Scholar]
- 78. Zhou J., Wu M., Xu S., Cheng M., Ding C., Liu Y., Yan H., Biyashev D., Kishore R., Qin G. (2013) Contrasting roles of E2F2 and E2F3 in cardiac neovascularization. PLoS One 8, e65755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen C. M., Cepko C. L. (2000) Expression of Chx10 and Chx10–1 in the developing chicken retina. Mech. Dev. 90, 293–297 [DOI] [PubMed] [Google Scholar]
- 80. Garcez R. C., Le Douarin N. M., Creuzet S. E. (2014) Combinatorial activity of Six1–2-4 genes in cephalic neural crest cells controls craniofacial and brain development. Cell Mol. Life Sci. 71, 2149–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Haddad N., El Andalousi J., Khairallah H., Yu M., Ryan A. K., Gupta I. R. (2011) The tight junction protein claudin-3 shows conserved expression in the nephric duct and ureteric bud and promotes tubulogenesis in vitro. Am. J. Physiol. Renal Physiol. 301, F1057–F1065 [DOI] [PubMed] [Google Scholar]
- 82. Pommereit D., Pieler T., Hollemann T. (2001) Xpitx3: a member of the Rieg/Pitx gene family expressed during pituitary and lens formation in Xenopus laevis. Mech. Dev. 102, 255–257 [DOI] [PubMed] [Google Scholar]
- 83. Briggs M. M., Schachat F. (2000) Early specialization of the superfast myosin in extraocular and laryngeal muscles. J. Exp. Biol. 203, 2485–2494 [DOI] [PubMed] [Google Scholar]
- 84. Sumoy L., Wang C. K., Lichtler A. C., Pierro L. J., Kosher R. A., Upholt W. B. (1995) Identification of a spatially specific enhancer element in the chicken Msx-2 gene that regulates its expression in the apical ectodermal ridge of the developing limb buds of transgenic mice. Dev. Biol. 170, 230–242 [DOI] [PubMed] [Google Scholar]
- 85. Hattori S., Baba H. (1996) [Heterogeneity of GTPase-activating proteins for Ras in the regulation of Ras signal transduction pathway]. Yakugaku Zasshi 116, 21–38 [DOI] [PubMed] [Google Scholar]