ABSTRACT
To date, most therapeutic and vaccine candidates for human immunodeficiency virus type 1 (HIV-1) are evaluated preclinically for efficacy against cell-free viral challenges. However, cell-associated HIV-1 is suggested to be a major contributor to sexual transmission by mucosal routes. To determine if neutralizing antibodies or inhibitors block cell-free and cell-associated virus transmission of diverse HIV-1 strains with different efficiencies, we tested 12 different antibodies and five inhibitors against four green fluorescent protein (GFP)-labeled HIV-1 envelope (Env) variants from transmitted/founder (T/F) or chronic infection isolates. We evaluated antibody/inhibitor-mediated virus neutralization using either TZM-bl target cells, in which infectivity was determined by virus-driven luciferase expression, or A3R5 lymphoblastoid target cells, in which infectivity was evaluated by GFP expression. In both the TZM-bl and A3R5 assays, cell-free virus or infected CD4+ lymphocytes were used as targets for neutralization. We further hypothesized that the combined use of specific neutralizing antibodies targeting HIV-1 Env would more effectively prevent cell-associated virus transmission than the use of individual antibodies. The tested antibody combinations included two gp120-directed antibodies, VRC01 and PG9, or VRC01 with the gp41-directed antibody 10E8. Our results demonstrated that cell-associated virus was less sensitive to neutralizing antibodies and inhibitors, particularly using the A3R5 neutralization assay, and the potencies of these neutralizing agents differed among Env variants. A combination of different neutralizing antibodies that target specific sites on gp120 led to a significant reduction in cell-associated virus transmission. These assays will help identify ideal combinations of broadly neutralizing antibodies to use for passive preventive antibody administration and further characterize targets for the most effective neutralizing antibodies/inhibitors.
IMPORTANCE Prevention of the transmission of human immunodeficiency virus type 1 (HIV-1) remains a prominent goal of HIV research. The relative contribution of HIV-1 within an infected cell versus cell-free HIV-1 to virus transmission remains debated. It has been suggested that cell-associated virus is more efficient at transmitting HIV-1 and more difficult to neutralize than cell-free virus. Several broadly neutralizing antibodies and retroviral inhibitors are currently being studied as potential therapies against HIV-1 transmission. The present study demonstrates a decrease in neutralizing antibody and inhibitor efficiencies against cell-associated compared to cell-free HIV-1 transmission among different strains of HIV-1. We also observed a significant reduction in virus transmission using a combination of two different neutralizing antibodies that target specific sites on the outermost region of HIV-1, the virus envelope. Therefore, our findings support the use of antibody combinations against both cell-free and cell-associated virus in future candidate therapy regimens.
INTRODUCTION
The ability to block human immunodeficiency virus type 1 (HIV-1) transmission remains an elusive goal of AIDS research. A fundamental question is whether lymphocytes harboring the virus in semen, blood, or breast milk have as prominent a role as cell-free virus in initiating infection at mucosal sites (1, 2). Recent studies suggest that cell-associated virus is important in HIV-1 transmission (3–5). Formation of the virological synapse between infected and uninfected cells in close contact is one major mode of cell-to-cell spread of HIV-1 (6–9). It has been suggested that synaptic transmission of cell-associated virus is more efficient and therapeutic resistant than cell-free virus transmission (3, 10–13). Nonetheless, novel immunotherapy, inhibitor, and vaccine candidates have been evaluated preclinically in rhesus macaques for their efficacies against cell-free simian immunodeficiency virus (SIV) and chimeric simian-human immunodeficiency virus (SHIV) blood and mucosal challenges, without consideration of virus transmission by infected lymphocytes (1, 14, 15).
Evidence demonstrating the efficiency of cell-to-cell HIV-1 transmission and the inability to abolish cell-associated virus (3, 13, 16–18) emphasizes the need to determine which therapeutic or preventive agents neutralize cell-associated in addition to cell-free HIV-1. Viral inhibitors used as microbicides and antiretroviral therapy (ART) drugs have been developed to prevent HIV-1 transmission or to treat individuals infected with HIV-1 (19–21). Successful control of HIV-1 replication has been demonstrated using combinations of ART (22–24); nevertheless, ART has proven thus far incapable of eradicating the virus. Strong antibody responses help control viral replication and are important in reducing HIV-1 spread and infection (25). Licensed vaccines, such as that for hepatitis B (26), elicit a robust neutralizing antibody response; however, achievement of similar responses in HIV-1 vaccine studies has proven unsuccessful due to the genetic diversity and high mutation rate of the virus (27). Moreover, the induction of broadly neutralizing antibodies against conserved regions of the HIV-1 envelope glycoprotein (Env) derives from disfavored B cells (28). The only effective HIV-1 vaccine trial to date, RV144, demonstrated modest efficacy attributed to antibodies that targeted the V1/V2 region of Env (29). Unlike the well-characterized combinatorial use of different retroviral inhibitors, little is known about the effect of different neutralizing antibody combinations on HIV-1 transmission in humans (30–32). To our knowledge, no previous studies have combined different neutralizing antibodies and directly measured their effects on cell-to-cell HIV-1 transmission.
Reliable and validated in vitro assays to measure cell-associated HIV-1 transmission of transmitted/founder (T/F) strains in the presence or absence of different neutralizing antibodies or inhibitors have been few (12). Standardized in vitro neutralization assays have been developed for evaluation of the efficacy of neutralizing antibodies against cell-free HIV, SIV, and SHIV transmission (33–35). In these assays, specific cell lines that express CD4 and CCR5, two cell receptors required for HIV-1 cellular infection, are used to measure HIV-1 infectivity. One such epithelial cell-derived recombinant cell line is the HeLa-derived TZM-bl, which expresses luciferase upon infection and therefore enables the measurement of relative HIV-1 infectivity in these cells by quantifying luminescence units (36–38). This neutralization assay has only recently been adapted by Abela et al. for evaluating neutralization of cell-associated HIV-1 (10). It is important to note, however, that the TZM-bl cell line was engineered to express CD4 and CCR5 at higher than physiological levels (39). Hence, although the TZM-bl assay has been validated and is widely used for assessing neutralizing antibodies against HIV-1, TZM-bl cells are not physiologically representative of CD4+ T lymphocytes in vivo (34, 40). Complementary cell-based neutralization assays must also be developed to evaluate and compare cell-associated HIV-1 infectivity using target cells with CD4 and CCR5 expression comparable to what is observed on CD4+ T lymphocytes. For this purpose, we developed a second cell-based assay using A3R5 lymphoblastoid target cells that naturally express CD4 and CXCR4 and are engineered to express CCR5 at lower levels than TZM-bl cells (35, 41). Together, these neutralization assays will enhance the standardized screening and evaluation of several neutralizing antibodies and inhibitors against different strains of both cell-free and cell-associated virus transmission and infection with two different target cell lines.
Our primary objective was to evaluate and rank the relative efficiencies of multiple broadly neutralizing antibodies and HIV-1 inhibitors against the transmission of cell-free versus cell-associated HIV-1 using the two different cell-based neutralization assays described. We also sought to establish whether the comparison of cell-free to cell-associated neutralizing antibody efficiencies differed between the TZM-bl and A3R5 assays or among chronic and T/F Env variants. We hypothesized that cell-associated HIV-1 would exhibit more resistance to neutralizing antibodies and inhibitors than cell-free HIV-1. We further hypothesized that the combined use of neutralizing antibodies targeting HIV-1 Env would more effectively prevent cell-to-cell virus transmission than when used individually. We showed that cell-associated virus was less sensitive to neutralizing antibodies and inhibitors than cell-free virus, particularly with the A3R5 assay. Interestingly, although the rankings of antibodies by neutralizing efficiency differed among Env variants, the order almost never differed for cell-free versus cell-associated virus. Moreover, we found that certain combinations of gp120-specific antibodies at concentrations that demonstrated only a partial effect when used individually were able to improve inhibition of cell-associated virus transmission.
MATERIALS AND METHODS
HIV-1 Env variants.
Four green fluorescent protein (GFP)-reporter proviral plasmids containing the clade B env sequence variants pNLENGli-BaL.ecto (BAL), pNLENGli-WITO.ecto (WITO), pNLENGli-CH040.ecto (CH040), and pNLENGli-CH077.ecto (CH077) were used (kindly provided by Christina Ochsenbauer and John Kappes, University of Alabama at Birmingham). Heterologous env sequences were inserted as previously described (42, 43). Three of these Env variants, including WITO, CH040, and CH077 were derived from T/F HIV-1 strains (44). The fourth env gene variant, BAL, serves as a R5-tropic reference strain and was derived from BaL virus isolated from human infant lung tissue (45). The replication-competent infectious molecular clones also encode a GFP reporter cassette as previously described (43, 46, 47). Plasmids were transfected into 293T cells (American Type Culture Collection, Manassas, VA) with Fugene (Promega, Fitchburg, WI) and supernatant was collected 48 and 72 h posttransfection. Virus was concentrated and propagated in freshly isolated primary peripheral blood mononuclear cells (PBMCs), collected over a 10-day period, and stored at −80°C (38). Each virus was then titrated in a 96-well plate using four replicates of seven 5-fold serial dilutions. To determine the titer of each Env variant, TZM-bl cells (obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID) were added (104 cells/well) in D10 (Dulbecco's minimal essential medium [DMEM] supplemented with 10% fetal bovine serum [FBS] and 1:1,000 gentamicin) containing 20 U/ml interleukin-2 (IL-2) and 10 μg/ml DEAE-dextran (similar to conditions used during later cocultures). Following 72 h of incubation at 37°C in 5% CO2, cells were fixed and stained for β-galactosidase expression using X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) as a substrate (β-galactosidase reporter gene staining kit; Sigma-Aldrich, St. Louis, MO). A positive well was defined as containing one or more blue cells. The 50% tissue culture infective dose (TCID50) per milliliter was calculated using the Spearman-Karber formula (48). The TCID50 per milliliter for each Env variant were as follows: BAL, 1.48 × 106 ± 3.6 × 105 (mean ± standard error of the mean [SEM]); WITO, 1.58 × 106 ± 6.0 × 105; CH040, 2.38 × 106 ± 1.4 × 106; and CH077, 2.3 × 106 ± 5.2 × 105.
Antibodies and inhibitors.
Table S1 in the supplemental material summarizes the antibodies and inhibitors used in this study as well as their target specificities. Antibodies included the gp120-directed binding antibody A32 (49), the gp120 V2-specific binding antibodies CH58 and CH59 (50) (Duke Human Vaccine Institute), the broadly neutralizing antibodies 4E10 (51) (Polymun Scientific, Austria), 10E8 (52, 53) (NIH AIDS Reagent Program), VRC01 (54) (NIH Vaccine Research Center), CH01 (55) (Duke Human Vaccine Institute), PGT126 (56, 57) (The Scripps Research Institute), and PG9 (58) (NIH Vaccine Research Center), and the CD4-directed antibodies huOKT4A and hu5A8 (59) (NIH Nonhuman Primate Reagent Resource). The Ab82 anti-Flu antibody was used as a negative control (60) (Duke Human Vaccine Institute). In the neutralization assays, each antibody was used at a starting concentration of 20 μg/ml. HIV-1 inhibitors were all obtained from the NIH AIDS Reagent Program and dissolved in dimethyl sulfoxide (DMSO). They included the gp41-directed fusion inhibitor T-20 (61), the C-C chemokine receptor 5 (CCR5)-directed entry inhibitor maraviroc (62), the CCR5 receptor antagonist TAK-779 (63, 64), and the nucleoside- and nonnucleoside reverse transcriptase (RT) inhibitors PMPA {9[R-2-(phosphonylmethoxy)propyl] adenine monohydrate} (65) and nevirapine (66), respectively. The starting concentrations of each inhibitor used in the inhibition assays were based on their previously documented half-maximal inhibitory concentrations (IC50s) (61, 62, 64–66).
CD4+ T cell isolation.
Heparinized whole blood from healthy human donors was purchased from Research Blood Components, LLC (Boston, MA) specifically for the purposes of this study. PBMCs were isolated and collected with Ficoll-Paque Plus (GE Healthcare Life Sciences, Pittsburgh, PA) by centrifugation of the fresh whole blood. PBMCs were stimulated for 48 h in R10 (RPMI 1640 supplemented with 10% FBS and 1:1,000 gentamicin) containing 6.25 μg/ml concanavalin A at 37°C in 5% CO2. CD4+ T cells were isolated from stimulated PBMCs using the human CD4+ T cell isolation magnetically activated cell sorting (MACS) kit (Miltenyi Biotec, CA). CD4+ T cells from six different healthy donors were collected and stored in aliquots of 5 × 106 cells in liquid nitrogen.
TZM-bl assay. (i) Cell-associated virus preparation.
One vial of 5 × 106 primary CD4+ T cells was thawed washed twice with 10 ml of R10, resuspended in 5 ml of R10 with 20 U/ml IL-2, and incubated at 37°C in 5% CO2 for 24 h. In each well of a 96-well plate, 5 × 104 CD4+ T cells were seeded in 100 μl of R10 with IL-2. An additional 100 μl of R10 with IL-2 was added to one-quarter of the wells, which remained uninfected as negative controls. The remaining wells were infected with 100 μl of each of the HIV-1 Env variants at the following concentrations: BAL, 7.4 × 104 ± 1.8 × 104 TCID50/ml; WITO, 7.9 × 104 ± 3.0 × 104 TCID50/ml; CH040, 1.19 × 105 ± 7.0 × 104 TCID50/ml; and CH077, 1.15 × 105 ± 2.6 × 104 TCID50/ml. A titration was performed for each of the Env variants to determine the best concentration for optimal infection of primary CD4+ T cells after culture at 37°C at 5% CO2 for 5 days. Each group of infected and uninfected cells was collected and spun down at 300 × g for 5 min. Cells were washed 3 times with 10 ml of prewarmed R10. These infected donor CD4+ T cells represented cell-associated virus. Titrations with each donor's infected cells were again performed to determine the best concentration for optimal infection of TZM-bl target cells after 96 h with each Env variant.
(ii) Cell-free virus preparation.
To prepare cell-free virus, each of the stock Env variants was resuspended 1:480 in D10 containing IL-2 (final dilution of 1:1,600 in 200 μl/well). A titration with each Env variant was performed to determine the concentration that yielded optimal cell-free infection of target cells after a 96-h culture.
(iii) Antibody/inhibitor addition.
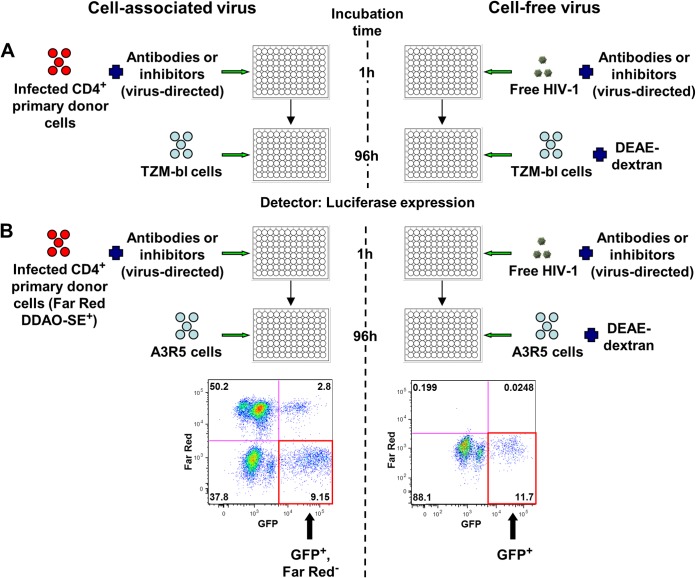
Prior to addition of cell-associated and cell-free virus, antibodies and inhibitors were aliquoted at minimum in triplicate in a 96-well plate in D10 containing IL-2 at the starting concentrations previously mentioned. Most antibodies and inhibitors were serially diluted 10-fold, except maraviroc, which was serially diluted 20-fold. The final volume of antibodies and inhibitors in each well was 90 μl. To each well containing virus-directed antibodies or inhibitors, 60 μl of cell-associated virus or cell-free virus was added, and the mixture was incubated for 1 h at 37°C in 5% CO2. Target cell-directed antibodies (hu5A8 and huOKT4A) and inhibitors (maraviroc and TAK-779) were incubated with TZM-bl target cells prior to virus addition. Positive-control wells contained virus in the absence of antibodies and inhibitors, and negative-control wells contained either uninfected CD4+ T cells (cell-associated virus control) or D10 containing IL-2 (cell-free virus control). Epithelial cell-derived TZM-bl target cells express high levels of CD4 and CCR5 to permit HIV-1 attachment and entry. TZM-bl is a standardized cell line that is routinely used in HIV-1 research for assessment of neutralizing antibody activity (33). Unlike cell-free virus infection of TZM-bl cells, cell-associated virus does not require the polycation DEAE-dextran to infect TZM-bl cells (10). This enables the differentiation of cell-free and cell-associated virus transmission based on the inclusion or omission of this reagent, respectively. A total of 1 × 104 TZM-bl cells were added to bring each well to a final volume of 200 μl. Cell-free or cell-associated virus and target cells in the presence or absence of antibodies and inhibitors were incubated for 96 h at 37°C in 5% CO2. Viral infection of TZM-bl target cells was determined by measuring luminescence with the Steady-Glo luciferase assay system (Promega) (Fig. 1A). The degrees of cell-free and cell-associated virus infection were similar with each Env variant and ranged between 1 × 103 and 1 × 104 relative light units (RLU). The percentage of infectivity with each antibody and inhibitor was calculated by subtracting the average value of the negative-control wells from each sample reading and dividing that result by the average value of the appropriate positive-control wells (cells incubated without antibodies or inhibitors).
FIG 1.
Neutralization assays. (A) Frozen CD4+ cells were thawed, stimulated overnight with IL-2, and infected with one of the four GFP-labeled HIV-1 Env variants to prepare cell-associated virus. Cell-free HIV-1 or HIV-1-infected donor cells were incubated with virus-directed antibodies or inhibitors for 1 h and then added to uninfected TZM-bl target cells. DEAE-dextran was used for cell-free virus infection but not for cell-associated infection. After 96 h, Steady-Glo luciferase expression was used to quantify the amount of infection of TZM-bl cells. (B) Cell-free HIV-1 or HIV-1-infected donor cells stained with Far Red DDAO-SE were incubated with virus-directed antibodies or inhibitors for 1 h and then added to uninfected A3R5 target cells. DEAE-dextran was used for cell-free virus infection only. Flow cytometry was performed to determine the number of newly infected target cells from cell-associated (GFP+ Far Red−) or cell-free (GFP+) virus. FITC, fluorescein isothiocyanate.
A3R5 assay. (i) Cell-associated virus preparation.
Cell-associated virus was prepared and titrated similar to the TZM-bl assay described above. After a 5-day incubation and prior to antibody and inhibitor addition, primary donor cells were divided into two groups: (i) uninfected CD4+ T cells (negative control) and (ii) virus-infected CD4+ T cells. Cells were spun at 300 × g for 5 min. Both groups were stained with 0.3 μM CellTrace Far Red DDAO-SE (Invitrogen Life Technologies) for 15 min in the dark at room temperature and then washed with phosphate-buffered saline (PBS), centrifuged, and resuspended in R10 containing IL-2. Staining of donor CD4+ T cells with Far Red DDAO-SE differentiated donor from target cells. Each donor's stained and infected CD4+ T cells were titrated with A3R5 (A3.01/R5.7 human lymphoblastoid cell line) target cells (kindly provided by Robert McLinden, U.S. Military HIV Research Program) to determine the concentration that yielded optimal infection after a 96-h culture with each Env variant.
(ii) Cell-free virus preparation.
Cell-free virus was prepared by resuspending the CH040, WITO, and CH077 viruses 1:480 in R10 with IL-2 (final dilution of 1:1,600 in a 200-μl well) and the BAL virus to a dilution of 1:30 in 60 μl (final dilution of 1:100 in 200 μl). A titration was performed with each Env variant to determine the concentration that generated optimal infection of A3R5 target cells after a 96-h culture.
(iii) Antibody/inhibitor addition.
Prior to addition of cell-associated or cell-free virus, antibodies and inhibitors were serially diluted in a 96-well plate in R10 containing IL-2 as previously described for the TZM-bl assay. To each well containing virus-directed antibodies or inhibitors, 60 μl of cell-associated virus or cell-free virus was added, and the mixture was incubated for 1 h at 37°C at 5% CO2. The target cell-directed antibody, hu5A8, and inhibitors, maraviroc and TAK-779, were incubated with A3R5 target cells prior to virus addition. Control wells were treated similar to those described for the TZM-bl assay. A3R5 cells are lymphoblastoid cells that express CD4 and have been engineered to express CCR5 at levels observed on primary CD4+ T cells (39, 41, 67). Like the TZM-bl neutralization assay, cell-free versus cell-associated virus transmission was differentiated based on the inclusion or omission, respectively, of DEAE-dextran. A total of 1 × 104 A3R5 cells were added to bring each well to a final volume of 200 μl. Cell-free or cell-associated virus and target cells in the presence or absence of antibodies and inhibitors were incubated for 96 h at 37°C in 5% CO2 and centrifuged for 5 min at 300 × g. All wells except the flow cytometry compensation wells were stained with yellow amine dye (LIVE/DEAD fixable yellow dead cell stain kit; Invitrogen Life Technologies) for 15 min, washed with 2% FBS–PBS, and fixed with 1% formaldehyde. Viral infection of target cells was determined by measuring the amount of single, live GFP+ cells using flow cytometry. With cell-associated virus, newly infected target cells were further differentiated from infected donor cells by their lack of Far Red staining (Far Red− population in Fig. 1B). The percentages of newly cell-free and cell-associated virus-infected cells did not differ by more than 5%. The percentage of infectivity with each antibody and inhibitor was calculated by subtracting the average value of the negative-control wells from each sample reading and dividing that result by the average value of the appropriate positive-control wells (cells incubated without antibodies or inhibitors).
Statistics.
Each experiment was performed used a single Env variant, single antibody or inhibitor, and single assay (TZM-bl or A3R5). The experiments were analyzed by a separate analysis of variance (ANOVA), including the factors (as fixed effects) cell-free or cell-associated virus, donor, and dose. In all experiments, each well containing cell-associated virus was paired with the corresponding well containing cell-free virus for the same dose of reagent. Although only paired observations were used in the ANOVAs, this method of analysis does not maintain pairing. Therefore, a separate stratified (by dose of antibody/inhibitor) exact Wilcoxon signed-rank test, which maintains pairing, was performed on each experiment. As IC50s are often used to compare dose-response curves, relative IC50s were calculated separately for cell-associated and cell-free virus using a nonlinear regression analysis for a four-parameter logistic function in GraphPad Prism, in which two parameters were constrained (i.e., the top and bottom were fixed as the largest and smallest percentages of infectivity observed in that response curve). The relative log IC50s for cell-associated and cell-free virus were compared with F tests in GraphPad Prism; this method also does not maintain pairing. For all three of these methods (ANOVA, stratified Wilcoxon test, and relative IC50), the two-sided significance levels for comparisons between cell-free and cell-associated virus using one of the two assays and one antibody/inhibitor were adjusted for multiple comparisons using Holm's method (68). The qualitative results of the ANOVA and Wilcoxon data sets differed in only 8% of the comparisons, so only Wilcoxon results are noted in the text.
In addition to comparing cell-associated to cell-free inhibition in each of the 100 experiments, we wanted to assess whether all of the experiments combined or all of the experiments within a larger subset of experiments supported the hypothesis that cell-associated virus is more resistant to neutralization than cell-free virus. This was performed by giving each experiment the score of 1 if the hypothesis was true in that experiment and a score of 0 if cell-free virus was more resistant in that experiment. A two-sided exact binomial sign test was used on these scores to assess if the percentage of experiments that demonstrated more resistance of cell-associated virus to neutralization was significantly larger than the null hypothesis of 50% in all 100 experiments and 40 experiments which individually had significant stratified Wilcoxon's tests. There were too few experiments to have adequate sign test power for smaller groups of experiments. The difference between the percentage of all TZM-bl compared to A3R5 experiments in which cell-associated virus was more resistant to neutralization was assessed using a two-sided Fisher's exact test.
The maximum dose of each antibody for each experiment was the same. A stratified (by antibody dose) exact Wilcoxon rank sum test was performed for all possible comparisons of two of the eight antibodies for each combination of cell-free or cell-associated virus, one of the four viruses, and one of the two assays, with P values using the Holm's adjustment for all antibody comparisons from the same experimental conditions. The rank sum test was used instead of the signed-rank test because the antibody experiments were not paired. We did not compare pairs of inhibitors because the doses of each inhibitor differed. Similar exact Wilcoxon rank sum tests (unstratified because only a single dose of each antibody was compared) with Holm's adjustment were performed to compare treatment with a combination of two antibodies to single-antibody treatment. All P values were from two-sided tests. Note that the total number of observations for a single experiment varied from 16 to 187. The number of observations for a single antibody dose was never less than 4, and the median number of observations per dose ranged from 4 to 11 for the A3R5 assays and from 13 to 33 for the TZM-bl assays. Comparisons with more observations are more likely to be significant because the power against a specific alternative increases with sample size. Failure to find a significant difference or failure to reject the null hypothesis of no difference, particularly with the A3R5 assay, does not prove that two groups or dose responses are very similar. For one of the Env variants (WITO), data for each of the eight neutralizing antibodies and five inhibitors were graphed and represented as four-parameter dose-response curves, allowing the visualization of the median values at each dose. In the antibody combination experiments, percentage of infectivity data were represented as box-and-whisker plots, with each box showing the 25th, 50th (median), and 75th percentiles. The whiskers were drawn by the Tukey method in GraphPad Prism.
RESULTS
DEAE-dextran was not required for cell-associated virus infection of TZM-bl or A3R5 cells.
To evaluate the neutralizing activity of several antibodies and inhibitors against both cell-associated and cell-free HIV-1, we used two different cell-based neutralization assays with either TZM-bl or A3R5 target cells (Fig. 1). To determine if cell-associated and/or cell-free chronic BAL and T/F WITO, CH040, and CH077 Env variants required DEAE-dextran for infection in the neutralization assays (36), we compared target cell infectivity with cell-free and cell-associated virus in the presence or absence of DEAE-dextran (Fig. 1; see Fig. S1 in the supplemental material). The concentration of DEAE-dextran was titrated with cell-free virus at 0, 2, 10, and 20 μg/ml to ensure that optimal infection was achieved without dextran-mediated toxicity to the TZM-bl or A3R5 target cells (data not shown). An optimal final DEAE-dextran concentration of 10 μg/ml was determined, consistent with previous reports (10, 36). We demonstrated that DEAE-dextran was necessary for infection with cell-free but not cell-associated virus of both TZM-bl (see Fig. S1A to D) and A3R5 (see Fig. S1E to H) target cells with each Env variant.
A large panel of monoclonal antibodies and inhibitors were evaluated in two functional assays.
HIV-1 infection of both TZM-bl and A3R5 target cells, as measured by luciferase and GFP expression, respectively, was determined in the presence or absence of several different monoclonal antibodies and inhibitors to compare their relative neutralization efficiencies against cell-associated versus cell-free virus transmission. DEAE-dextran was used to distinguish cell-free from cell-associated virus infection (Fig. 1; see Fig. S1 in the supplemental material) (10). Each antibody used had a different target on the virus or target cell, with the exception of two gp41 membrane proximal external region (MPER)-directed antibodies, 4E10 and 10E8, and two Env V2-directed binding antibodies, CH58 and CH59 (see Table S1 in the supplemental material). A maximum antibody concentration of 20 μg/ml was used, and each antibody was diluted a minimum of 3 orders of magnitude until 100% viral infectivity was regained. Four antibodies (anti-Flu, A32, CH58, and CH59) were predicted to be nonneutralizing before any experiments were performed. These four antibodies were the only ones that satisfied the condition that the minimum of the median of percentage of infectivity at the highest dose (20 μg/ml) was 80% or larger. Therefore, the results from these four antibodies were omitted from the analysis as being “nonneutralizing” (Fig. 2; see Fig. S2 in the supplemental material).
FIG 2.
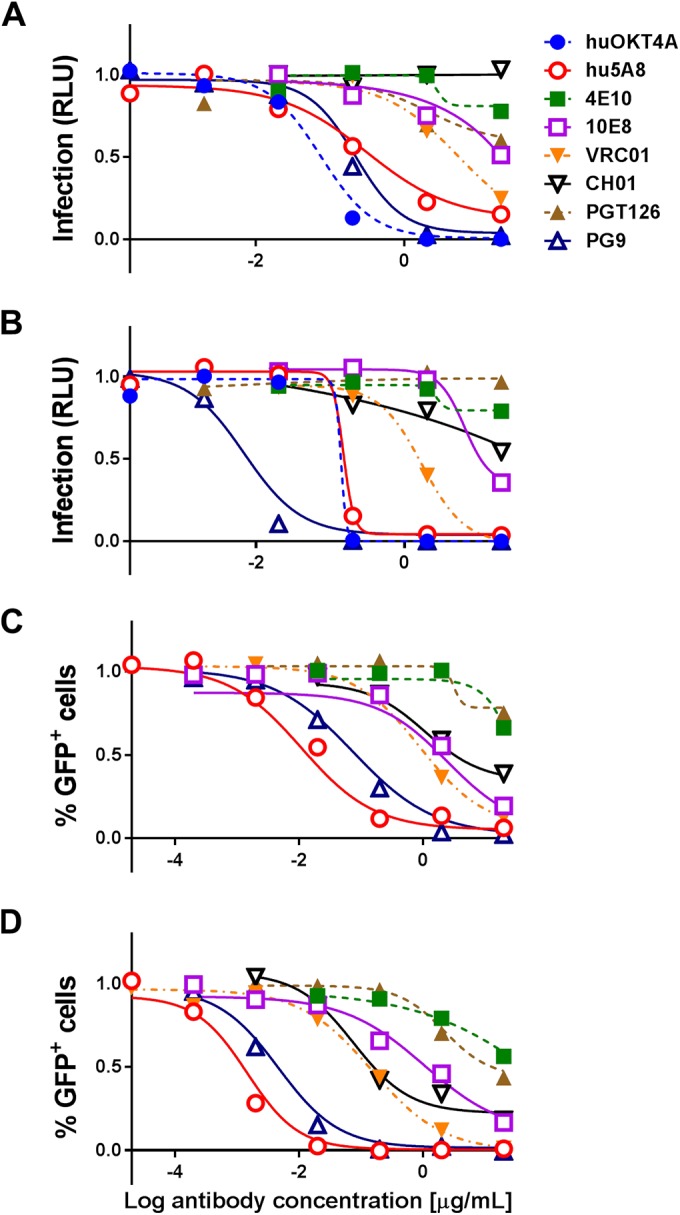
Infection with cell-associated or cell-free WITO ± DEAE-dextran with different antibodies. TZM-bl (A and B) or A3R5 (C and D) target cells were infected with cell-associated (A and C) or cell-free (B and D) WITO virus in the presence of different concentrations (20 to 0.00002 μg/ml) of antibodies, including anti-Flu (Ab82), A32, 4E10, 10E8, VRC01, CH01, PGT126, PG9, CH58, CH59, and the CD4-directed antibodies hu5A8 and huOKT4A. Background (negative control) was subtracted from results, and results were normalized to the no-antibody positive control and summarized as infection based on RLU (A and B) or percentage of GFP+ (Far Red−) cells (C and D), where 1 is equal to 100% infection. Results are expressed as dose-response curves illustrating the median at each log dose.
In addition to neutralizing antibodies, we also evaluated the effects of different retroviral inhibitors on cell-associated compared to cell-free HIV-1 infection. These included the reverse transcriptase (RT) inhibitors PMPA and nevirapine, fusion inhibitor T-20, and CCR5-directed inhibitors TAK-779 and maraviroc (see Table S1 in the supplemental material). Unlike the monoclonal antibodies, the starting concentration of each inhibitor used in the assays was determined individually based on their previously documented IC50s. Due to the potency of the CCR5 inhibitor maraviroc, it was evaluated at 20-fold dilutions compared to the 10-fold dilutions used for each of the other inhibitors. All five of these inhibitors appeared to be effective against the Env variants (Fig. 3; see Fig. S3 in the supplemental material).
FIG 3.
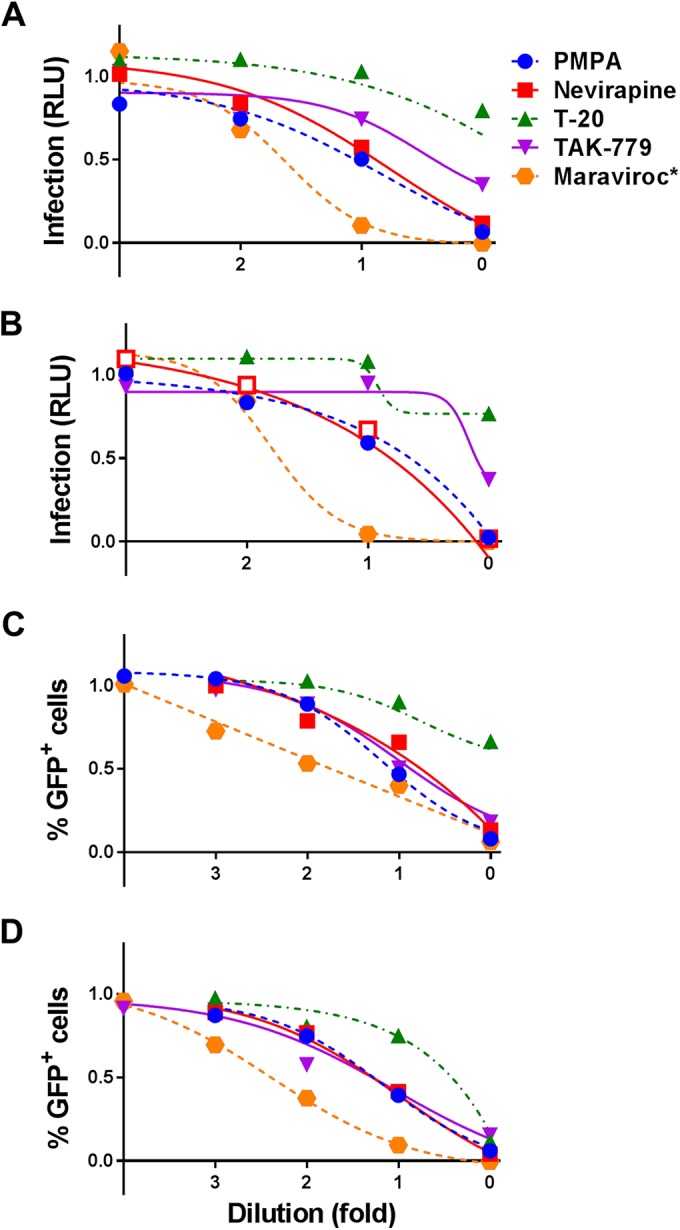
Infection with cell-associated or cell-free WITO ± DEAE-dextran with different inhibitors. TZM-bl (A and B) or A3R5 (C and D) target cells were infected with cell-associated (A and C) or cell-free (B and D) WITO virus in the presence of different concentrations of inhibitors. All inhibitors were serially diluted 10-fold at various ranges of concentration (PMPA, 0.1 to 100 μM; nevirapine, 0.4 to 400 nM; T-20, 0.5 to 500 nM; TAK-779, 1 to 1,000 nM), with the exception of maraviroc, which was serially diluted 20-fold (*, 0.015625 to 2,500 nM). Background was subtracted from results, and results were normalized to the DMSO-positive control and summarized as infection based on RLU (A and B) or percentage of GFP+ (Far Red−) cells (C and D), where 1 is equal to 100% infection. Results are expressed as dose-response curves illustrating the median at each dose dilution.
Differences in neutralization of cell-associated compared to cell-free virus in the TZM-bl and A3R5 assays.
Table 1 shows whether cell-associated or cell-free virus was more resistant to neutralization, based on a nonparametric curve comparison, and if the difference was significant after Holm's adjustment of the stratified exact Wilcoxon signed-rank tests (indicated by bold type) in the 60 antibody comparisons and 40 inhibitor comparisons. When the directions of all experiment comparisons performed were combined, cell-associated virus was more resistant to neutralization than cell-free virus in 65% of the 100 total comparisons (sign test, P = 0.0035) (Table 1; see Table S2 in the supplemental material). The A3R5 assay demonstrated more often (39/48 [81%]; sign test, P = 0.000015) than the TZM-bl assay (26/52 [50%]; sign test, P = 1.00) that cell-associated virus was more resistant to neutralization than cell-free virus. The increased neutralization resistance of cell-associated virus using the A3R5 compared to the TZM-bl assay was not attributable to different treatments or viruses used, nor was it due to the small number of observations per experiment, since the TZM-bl sample size was on average 2.3 times the A3R5 sample size.
TABLE 1.
Comparison of cell-associated and cell-free virus neutralizationa
| Env variant and antibody or inhibitor | TZM-bl assay |
A3R5 assay |
||
|---|---|---|---|---|
| Increased neutralization resistance of virus | P value | Increased neutralization resistance of virus | P value | |
| Antibodies | ||||
| BAL | ||||
| huOKT4A | CF | 6 × 10−2 | ||
| hu5A8 | CA | 4 × 10−2 | CA | 1 × 10−1 |
| 4E10 | CA | 9 × 10−3 | CA | 3 × 10−1 |
| 10E8 | CA | 2 × 10−1 | CA | 7 × 10−2 |
| VRC01 | CA | 2 × 10−7 | CA | 7 × 10−2 |
| CH01 | CF | 3 × 10−1 | CA | 3 × 10−1 |
| PGT126 | CA | 2 × 10−1 | CA | 3 × 10−3 |
| PG9 | CF | 2 × 10−1 | CA | 5 × 10−1 |
| WITO | ||||
| huOKT4A | CA | 1 × 10−4 | ||
| hu5A8 | CA | 2 × 10−3 | CA | 4 × 10−6 |
| 4E10 | CA | 3 × 10−1 | CA | 2 × 10−1 |
| 10E8 | CF | 6 × 10−1 | CA | 8 × 10−1 |
| VRC01 | CA | 5 × 10−7 | CA | 6 × 10−9 |
| CH01 | CA | 2 × 10−6 | CA | 5 × 10−6 |
| PGT126 | CF | 1 × 10−3 | CA | 2 × 10−3 |
| PG9 | CA | 2 × 10−16 | CA | 3 × 10−7 |
| CH040 | ||||
| huOKT4A | CA | 9 × 10−1 | ||
| hu5A8 | CA | 7 × 10−1 | CA | 7 × 10−4 |
| 4E10 | CF | 4 × 10−2 | CF | 7 × 10−1 |
| 10E8 | CF | 7 × 10−1 | CF | 5 × 10−3 |
| VRC01 | CA | 1 × 10−3 | CA | 4 × 10−5 |
| CH01 | CF | 2 × 10−1 | CF | 5 × 10−1 |
| PGT126 | CF | 3 × 10−2 | CA | 9 × 10−4 |
| PG9 | CA | 5 × 10−1 | CF | 9 × 10−1 |
| CH077 | ||||
| huOKT4A | CF | 3 × 10−3 | ||
| hu5A8 | CF | 8 × 10−5 | CA | 9 × 10−6 |
| 4E10 | CF | 1 × 10−2 | CF | 4 × 10−1 |
| 10E8 | CF | 6 × 10−1 | CA | 5 × 10−1 |
| VRC01 | CA | 1 × 10−3 | CA | 3 × 10−4 |
| CH01 | CF | 3 × 10−3 | CA | 3 × 10−1 |
| PGT126 | CF | 4 × 10−2 | CA | 6 × 10−11 |
| PG9 | CA | 2 × 10−6 | CA | 6 × 10−5 |
| Inhibitors | ||||
| BAL | ||||
| PMPA | CF | 7 × 10−2 | CF | 1 |
| Nevirapine | CF | 1 | CA | 8 × 10−1 |
| T-20 | CA | 6 × 10−2 | CF | 8 × 10−1 |
| TAK-779 | CA | 9 × 10−1 | CA | 4 × 10−1 |
| Maraviroc | CA | 1 × 10−1 | CF | 9 × 10−1 |
| WITO | ||||
| PMPA | CA | 3 × 10−1 | CA | 4 × 10−1 |
| Nevirapine | CA | 9 × 10−2 | CA | 9 × 10−4 |
| T-20 | CF | 2 × 10−1 | CA | 7 × 10−3 |
| TAK-779 | CF | 4 × 10−1 | CA | 9 × 10−3 |
| Maraviroc | CA | 5 × 10−1 | CA | 1 × 10−4 |
| CH040 | ||||
| PMPA | CF | 6 × 10−3 | CA | 2 × 10−1 |
| Nevirapine | CF | 1 | CA | 2 × 10−2 |
| T-20 | CA | 1 × 10−4 | CA | 3 × 10−2 |
| TAK-779 | CA | 2 × 10−2 | CA | 2 × 10−3 |
| Maraviroc | CF | 3 × 10−2 | CA | 5 × 10−4 |
| CH077 | ||||
| PMPA | CF | 3 × 10−1 | CF | 5 × 10−1 |
| Nevirapine | CA | 9 × 10−2 | CA | 5 × 10−1 |
| T-20 | CF | 6 × 10−1 | CA | 4 × 10−1 |
| TAK-779 | CF | 4 × 10−3 | CA | 6 × 10−1 |
| Maraviroc | CF | 4 × 10−8 | CA | 5 × 10−1 |
Shown are the P values from the stratified exact Wilcoxon signed-rank test for each combination of 1 of the 13 neutralizing antibodies or inhibitors used against each Env variant in the TZM-bl or A3R5 assay. Boldface entries with P values are significant after Holm's adjustment. Of the 100 comparisons performed, the difference in resistance to neutralization of cell-associated (CA) versus cell-free (CF) virus was significant in 40% of total comparisons. Blank entries represent cases in which an antibody was not used in the A3R5 assay.
The relative IC50s of the neutralizing antibodies are shown in Table 2. The boxes with dashes represent antibodies for which the IC50 could not be calculated with the four-parameter logistic model, which occurred in 22% of the experiments. Most of the significant comparisons of cell-associated to cell-free results in Table 2 were also significant (in the same direction) using the stratified Wilcoxon signed-rank test (Table 1). The three exceptions in which the IC50 for cell-associated virus was significantly different from the IC50 for cell-free virus in Table 2 but not significant in Table 1 were that PGT126 neutralized cell-free greater than cell-associated BAL in the TZM-bl assay, VRC01 neutralized cell-free greater than cell-associated BAL in the A3R5 assay, and PMPA neutralized cell-associated greater than cell-free CH040 in the TZM-bl assay. Wilcoxon results for all three of these comparisons were in the same direction but not significant. In contrast, there were 10 instances in which the Wilcoxon difference was significant and the IC50 difference was not. Among the 22 comparisons in which the IC50 could not be calculated, there were six differences that were significant by the Wilcoxon method. Interestingly, for the stratified Wilcoxon and IC50 analyses, in both the TZM-bl and A3R5 assays for all of the Env variants tested, we found that cell-associated virus demonstrated decreased sensitivity to the gp120-directed antibody VRC01 compared to cell-free virus.
TABLE 2.
Antibody and inhibitor relative IC50s by the TZM-bl and A3R5 assaysa
| Env variant and antibody or inhibitor | TZM-bl assay |
A3R5 assay |
||||||
|---|---|---|---|---|---|---|---|---|
| Increased neutralization resistance of virus | P value | Relative IC50 (antibody, μg/ml; inhibitor, nM) |
Increased neutralization resistance of virus | P value | Relative IC50 (antibody, μg/ml; inhibitor, nM) |
|||
| CA | CF | CA | CF | |||||
| Antibodies | ||||||||
| BAL | ||||||||
| huOKT4A | CF | 0.08 | 0.042 | 0.064 | ||||
| hu5A8 | — | — | — | — | — | — | — | — |
| 4E10 | — | — | — | — | CA | 1 | 3.10 | 3.06 |
| 10E8 | CF | 0.3 | 1.54 | 2.78 | CF | 0.6 | 1.23 | 1.62 |
| VRC01 | CA | 0.0001 | 0.22 | 0.071 | CA | 0.0001 | 0.22 | 0.055 |
| CH01 | — | — | — | — | — | — | — | — |
| PGT126 | CA | 0.0001 | 0.8 | 0.053 | CA | 0.004 | 0.18 | 0.072 |
| PG9 | — | — | — | — | — | — | — | — |
| WITO | ||||||||
| huOKT4A | CF | 0.8 | 0.074 | 0.074 | ||||
| hu5A8 | CA | 0.0008 | 0.29 | 0.080 | CA | 0.0001 | 0.016 | 0.0011 |
| 4E10 | — | — | — | — | CA | 1 | 3.74 | 2.69 |
| 10E8 | CA | 1 | 3.44 | 3.09 | CA | 0.5 | 1.67 | 0.77 |
| VRC01 | CA | 0.0001 | 7.44 | 1.45 | CA | 0.0001 | 1.30 | 0.13 |
| CH01 | — | — | — | — | CA | 0.002 | 1.09 | 0.13 |
| PGT126 | — | — | — | — | CA | 0.3 | 3.16 | 2.03 |
| PG9 | CA | 0.0001 | 0.22 | 0.0070 | CA | 0.0001 | 0.071 | 0.0047 |
| CH040 | ||||||||
| huOKT4A | CF | 0.8 | 0.082 | 0.10 | ||||
| hu5A8 | CF | 1 | 0.21 | 0.21 | CA | 0.0001 | 0.024 | 0.0025 |
| 4E10 | — | — | — | — | — | — | — | — |
| 10E8 | CF | 1 | 2.57 | 3.37 | CF | 0.6 | 0.82 | 1.86 |
| VRC01 | CA | 0.02 | 1.96 | 1.01 | CA | 0.0001 | 3.19 | 0.49 |
| CH01 | — | — | — | — | — | — | — | — |
| PGT126 | — | — | — | — | — | — | — | — |
| PG9 | — | — | — | — | — | — | — | — |
| CH077 | ||||||||
| huOKT4A | CF | 0.8 | 0.031 | 0.036 | ||||
| hu5A8 | CF | 0.5 | 0.078 | 0.13 | CA | 0.009 | 0.012 | 0.0018 |
| 4E10 | — | — | — | — | CF | 0.3 | 0.62 | 3.36 |
| 10E8 | CF | 0.4 | 2.22 | 3.19 | CA | 0.2 | 2.29 | 0.82 |
| VRC01 | CA | 0.0001 | 3.34 | 1.26 | CA | 0.0001 | 2.23 | 0.27 |
| CH01 | — | — | — | — | CA | 0.4 | 1.88 | 0.33 |
| PGT126 | CF | 0.5 | 0.088 | 0.049 | CA | 0.0001 | 1.34 | 0.0049 |
| PG9 | CA | 0.0001 | 0.059 | 0.013 | CA | 0.0001 | 0.35 | 0.0034 |
| Inhibitors | ||||||||
| BAL | ||||||||
| PMPA | CF | 0.002 | 772 | 2,001 | CF | 0.7 | 1,103 | 1,303 |
| Nevirapine | CF | 0.6 | 63.8 | 84.6 | CF | 1 | 53.25 | 54.8 |
| T-20 | — | — | — | — | CA | 1 | 93.0 | 85.4 |
| TAK-779 | CF | 0.7 | 517 | 807 | CA | 0.7 | 89.1 | 60.3 |
| Maraviroc | CA | 0.2 | 15.1 | 6.58 | CF | 0.4 | 1.03 | 4.17 |
| WITO | ||||||||
| PMPA | CF | 0.6 | 1,181 | 2,021 | CF | 0.7 | 436 | 547.5 |
| Nevirapine | CF | 0.7 | 40.3 | 58.4 | CA | 0.4 | 43.5 | 23.5 |
| T-20 | — | — | — | — | CF | 1 | 59.8 | 82.2 |
| TAK-779 | CF | 0.9 | 134 | 194 | CA | 0.2 | 74.4 | 33.2 |
| Maraviroc | CA | 0.3 | 19.4 | 11.4 | CA | 0.0001 | 20.5 | 2.56 |
| CH040 | ||||||||
| PMPA | CF | 0.08 | 505 | 1,288 | CA | 0.6 | 1,327 | 856 |
| Nevirapine | CA | 0.6 | 86.3 | 44.6 | CA | 0.9 | 39.2 | 36.8 |
| T-20 | CA | 0.4 | 92.5 | 52.0 | CA | 1 | 91.4 | 72.8 |
| TAK-779 | CA | 0.05 | 77.9 | 43.3 | CA | 0.04 | 44.8 | 16.6 |
| Maraviroc | CA | 0.009 | 4.44 | 1.12 | CA | 0.0008 | 5.49 | 0.27 |
| CH077 | ||||||||
| PMPA | CF | 0.5 | 1,080 | 1,661 | CF | 0.1 | 284.5 | 716 |
| Nevirapine | CF | 0.7 | 41.6 | 50.9 | CA | 0.9 | 33.6 | 27.1 |
| T-20 | CF | 1 | 58.9 | 68.0 | CF | 0.4 | 12.2 | 38.3 |
| TAK-779 | CF | 0.0005 | 9.77 | 43.1 | CF | 0.6 | 502 | 690 |
| Maraviroc | CF | 0.0001 | 0.088 | 1.60 | CF | 0.5 | 883 | 1,481 |
Antibody and inhibitor relative IC50s against cell-associated (CA) and cell-free (CF) virus are shown for TZM-bl and A3R5 target cells. Values were determined based on the neutralization dose responses from Fig. S2 and S3 in the supplemental material. Significant differences (in bold) between the log IC50s for the cell-associated and cell-free Env variants were assessed with ANOVA following a nonlinear regression analysis with a variable slope equation in GraphPad Prism. Only P values that were significant after Holm's adjustment for multiple comparisons were considered significant. Blank entries represent cases in which an antibody was not used in the A3R5 assay. —, cases in which the IC50 could not be calculated with the four-parameter logistic model.
To further compare the magnitude of difference in neutralization sensitivity between cell-associated and cell-free virus, the ratios of the relative IC50s of each antibody and inhibitor against cell-associated versus cell-free virus were calculated (see Table S3 in the supplemental material). Instances in which cell-associated virus had a smaller relative IC50 and greater sensitivity to neutralization than cell-free virus have ratios below 1; instances in which cell-associated virus had a larger relative IC50 than cell-free virus have ratios above 1. For the TZM-bl assay, the ratios ranged from 0.06 to 32, with a median of 0.8 and quartiles of 0.6 and 2.1. For the A3R5 assay, the ratios ranged from 0.2 to 274, with a median of 1.6 and quartiles of 0.8 and 4.9.
Neutralization efficiencies of all antibodies were ranked for each Env variant tested and assay used.
Table 3 ranks the neutralizing antibodies from most to least potent for each cell-associated or cell-free Env variant tested and assay used. Of the 112 comparisons of antibody pairs used in the TZM-bl assay, 89 (79%) of the cell-associated comparisons and 90 (80%) of the cell-free comparisons were significant after Holm's adjustment. Of the 84 comparisons of antibody pairs used in the A3R5 assay, 58 (69%) of the cell-associated comparisons and 69 (82%) of the cell-free comparisons were significant after Holm's adjustment. Among the four gp120-directed antibodies VRC01, CH01, PGT126, and PG9, the CD4 binding site (CD4bs)-specific antibody VRC01 generally had significantly better neutralizing activity than the other three against BAL and CH040, with the exception that it was not different from PGT126 for cell-associated or cell-free BAL. The anti-V1/V2 antibody PG9 had the best neutralizing activity against WITO and CH077.
TABLE 3.
Comparisons of single-antibody neutralization efficienciesa
| Virus and Env variant | TZM-bl assay |
A3R5 assay |
||
|---|---|---|---|---|
| P value range | Result | P value range | Result | |
| Cell associated | ||||
| BAL | 8 × 10−32 to 1 × 10−5 | huOKT4A > VRC01 > PGT126 > 10E8 > 4E10/PG9/hu5A8/CH01 | 9 × 10−12 to 4 × 10−3 | VRC01/PGT126 > 10E8 > 4E10/PG9/hu5A8/CH01 |
| WITO | 1 × 10−29 to 8 × 10−3 | huOKT4A > hu5A8/PG9 > VRC01 > 10E8/PGT126 > 4E10/CH01 | 2 × 10−15 to 2 × 10−2 | hu5A8 > PG9b > VRC01/10E8/CH01 > 4E10/PGT126 |
| CH040 | 6 × 10−28 to 2 × 10−8 | huOKT4A > hu5A8/VRC01 > 10E8/4E10/PG9/PGT126/CH01 | 2 × 10−13 to 1 × 10−3 | hu5A8/10E8 > VRC01 > 4E10/PG9/PGT126/CH01 |
| CH077 | 3 × 10−35 to 4 × 10−5 | huOKT4A > hu5A8/PG9 > PGT126 > 10E8/VRC01 > 4E10/CH01 | 2 × 10−16 to 4 × 10−3 | hu5A8c > PG9 > PGT126/VRC01 > 4E10/CH01 |
| Cell free | ||||
| BAL | 7 × 10−32 to 5 × 10−3 | VRC01 > huOKT4A > PGT126 > 10E8 > 4E10/hu5A8d > PG9/CH01 | 4 × 10−17 to 9 × 10−3 | PGT126/VRC01 > 10E8 > 4E10/hu5A8e > CH01 > PG9 |
| WITO | 6 × 10−52 to 1 × 10−2 | PG9/huOKT4A > hu5A8 > VRC01 > CH01/10E8f > 4E10 > PGT126 | 3 × 10−23 to 3 × 10−4 | PG9/hu5A8 > VRC01 > CH01/10E8 > 4E10/PGT126 |
| CH040 | 2 × 10−29 to 6 × 10−4 | huOKT4A > hu5A8/VRC01 > PG9/10E8/4E10/CH01 > PGT126g | 8 × 10−17 to 7 × 10−3 | hu5A8 > VRC01 > 10E8 > PGT126/4E10/CH01 > PG9h |
| CH077 | 2 × 10−45 to 3 × 10−3 | huOKT4A > PG9 > hu5A8 > PGT126 > VRC01 > 10E8/4E10/CH01 | 2 × 10−19 to 1 × 10−3 | hu5A8/PG9i > PGT126 > VRC01 > 10E8 > 4E10/CH01 |
A stratified (by antibody dose) exact Wilcoxon rank sum test was done for all possible comparisons of the 8 neutralizing antibodies for each Env variant, assay type, and cell-associated or cell-free virus with P values using the Holm's adjustment for all antibody comparisons from the same experimental conditions. Only two-sided P values that were significant after Holm's adjustment for multiple comparisons were considered significant. The symbol “>” indicates that the antibody to the left has significantly greater neutralization efficiency than the antibody to the right. When an antibody is listed directly after another antibody (e.g., “4E10/PG9”), the antibodies do not differ significantly. The symbol “∼” indicates pairs of antibodies that do not differ significantly.
PG9 ∼ 10E8.
hu5A8 > 10E8 and 10E8 ∼ PG9, PGT126, VRC01, 4E10, and CH01.
hu5A8 ∼ PG9.
hu5A8 ∼ CH01.
10E8 ∼ 4E10.
PGT126 ∼ 10E8, 4E10, and CH01.
PG9 ∼ 4E10 and CH01.
PG9 ∼ PGT126.
When a pair of antibodies differed significantly in neutralization efficiency, the rank order of some of these antibody potencies also differed between assays, cell-associated and cell-free virus, and/or among Env variants (see Table S4 in the supplemental material). Among the 306 total significant differences observed, there were only three instances when cell-associated and cell-free virus both had a significant difference between a particular pair of antibodies, but in the opposite direction. There were also only three instances when the order of a pair of antibodies differed between the A3R5 and TZM-bl assays (see Table S4). However, the rank order in efficiency of two neutralizing antibodies differed between two or more of the Env variants 37 times, which represent 95 pairs of Env variants (see Table S4). Although each neutralizing antibody appeared in at least 1 of these 37 occurrences, PGT126, PG9, VRC01, and hu5A8 were among the most frequently represented (18, 14, 13, and 11 times, respectively). This variability in neutralization efficiency among Env variants is not surprising considering the high variability of antibody binding sites among HIV-1 strains.
To further explore if the neutralization response of the chronic HIV-1 Env variant BAL was different from those of the three T/F Env variants WITO, CH040, and CH077, we examined 32 of the 37 antibody potency rank order differences from Table S4 in the supplemental material that included three or four Env variants, one of which was BAL. Based on scoring of whether the potency rank order of the effective neutralizing antibody for BAL was different from the rank orders of the two or three T/F Env variants, we found that the chronic isolate BAL was more often in the minority than the other three T/F Env variants (P = 0.0089). For comparison, WITO appeared in a similar number of cases in Table S4 and was more often in the majority (P = 0.00019). This suggests that the chronic infection isolate BAL had a significantly different neutralization response than the three T/F Env variants.
Combination of two gp120-directed broadly neutralizing antibodies had an additive inhibitory effect against cell-associated virus in the TZM-bl and A3R5 assays.
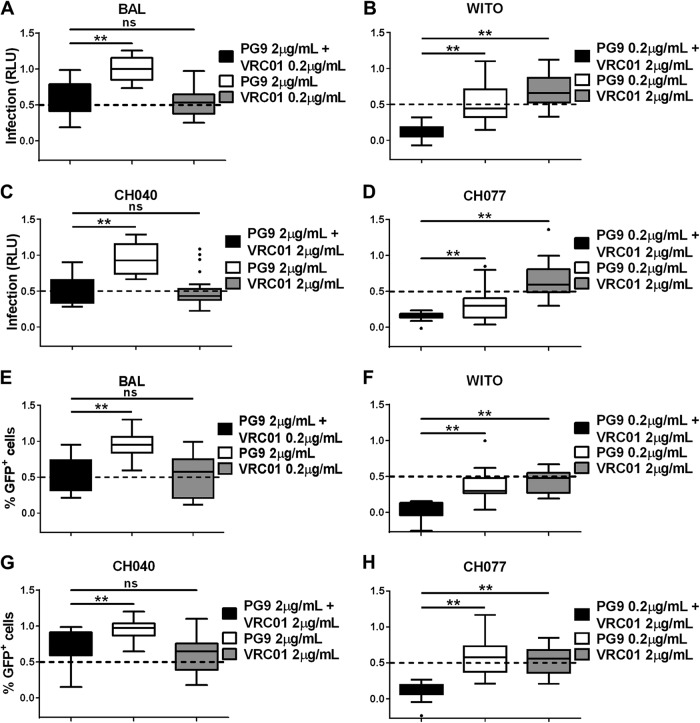
To explore if combinations of different neutralizing antibodies could more potently inhibit cell-associated virus transmission compared to single neutralizing antibodies, we combined two different pairs of antibodies. The first combination we evaluated included two gp120-specific neutralizing antibodies, VRC01 and PG9. VRC01 consistently demonstrated significantly lower neutralization efficiency against cell-associated compared to cell-free virus among all Env variants tested. Differences in cell-associated versus cell-free virus neutralization by PG9 were significant only against the T/F Env variants WITO and CH077 (Table 1; see Table S2 in the supplemental material). When PG9 and VRC01 were combined at concentrations that demonstrated partial inhibition when used individually, cell-associated WITO and CH077 infection of both TZM-bl and A3R5 target cells was significantly reduced (P < 0.01) (Fig. 4). In contrast, the combination of PG9 and VRC01 demonstrated a similar reduction in cell-associated BAL and CH040 infection to VRC01 alone of both TZM-bl and A3R5 target cells. This suggests that improved neutralization of cell-associated virus with a combination of gp120-specific antibodies is dependent on the potency of each antibody used in the combination for a particular virus strain.
FIG 4.
Neutralization of cell-associated virus by combination of two gp120-specific broadly neutralizing antibodies. Two HIV-1-directed antibodies, PG9 and VRC01, were combined at concentrations that have a partial effect to no effect on cell-associated virus infection when used individually. Infection was evaluated in TZM-bl (A to D) or A3R5 (E to H) target cells with cell-associated HIV-1 BAL (A and E), WITO (B and F), CH040 (C and G), and CH077 (D and H). Infection was measured by quantifying the RLU of TZM-bl cells (A to D) or the percentage of GFP+ (Far Red−) A3R5 cells (E to H) for each cell-associated Env variant in the presence of the indicated concentrations of antibodies, used in combination or alone, where 1 is equal to 100% infection. Results were normalized and expressed as box-and-whisker plots illustrating the median, first and third quartiles, and range with outliers (solid circles). Significant differences were assessed with exact Wilcoxon rank sum tests and Holm's adjustment to compare each combination of antibodies to the single-antibody treatment. A significant difference is represented by ** (P < 0.005), and a nonsignificant difference is represented by “ns.”
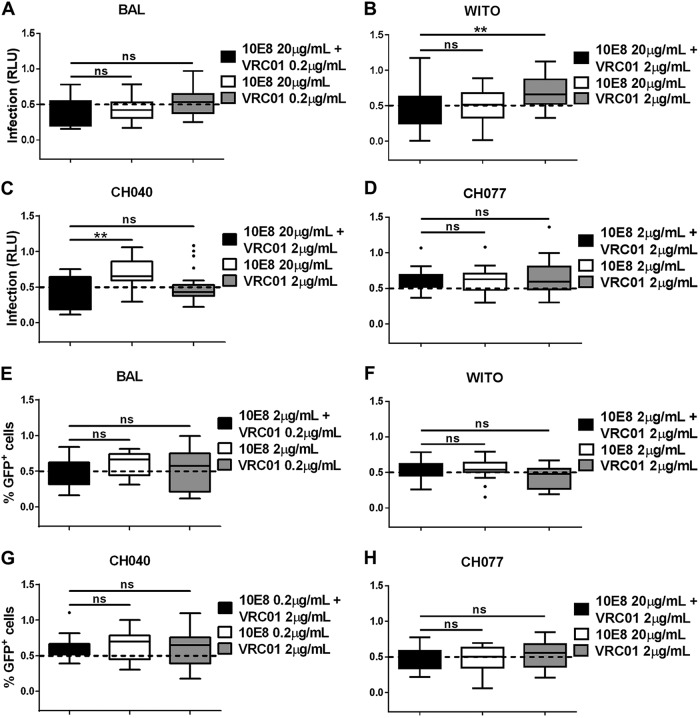
To investigate if this combination effect might be observed with two antibodies that did not target the same Env region, we evaluated the combination of VRC01 and 10E8, the more effective of the two MPER gp41-directed antibodies. Similar to the PG9 and VRC01 combination, 10E8 and VRC01 were combined at concentrations that yielded partial neutralization when used individually against each cell-associated Env variant. Unlike the PG9 and VRC01 combination, however, the 10E8 and VRC01 combination did not elicit any significant additive inhibitory effect against cell-associated virus in either the TZM-bl or A3R5 neutralization assay (Fig. 5).
FIG 5.
Neutralization of cell-associated virus by combination of gp120- and gp41-specific broadly neutralizing antibodies. Two HIV-1-directed antibodies, 10E8 and VRC01, were combined at concentrations that have a partial effect to no effect on cell-associated virus infection when used individually. Infection was evaluated in TZM-bl (A to D) or A3R5 (E to H) target cells with cell-associated HIV-1 BAL (A and E), WITO (B and F), CH040 (C and G), and CH077 (D and H). Infection was measured by quantifying RLU of TZM-bl cells (A to D) or the percentage of GFP+ (Far Red−) A3R5 cells (E to H) for each cell-associated Env variant in the presence of the indicated concentrations of antibodies, used in combination or alone, where 1 is equal to 100% infection. Results were normalized and expressed as in Fig. 4. Significant differences were assessed with exact Wilcoxon rank sum tests and Holm's adjustment to compare each combination of antibodies to the single-antibody treatment. A significant difference is represented by ** (P < 0.0075), and a nonsignificant difference is represented by “ns.”
DISCUSSION
In this study, we demonstrated that cell-associated virus is more resistant to neutralizing antibodies and viral inhibitors than cell-free virus. Overall, the A3R5 assay was more sensitive than the TZM-bl assay at detecting differences in neutralization of cell-free compared to cell-associated virus. Comprehensive statistical analyses, after correction for multiple comparisons, indicated significant differences in potencies of various neutralizing antibodies against cell-associated versus cell-free virus. We also showed extensive and significant variation in neutralizing antibody efficiencies among different chronic and T/F HIV-1 strains, as previously demonstrated (69–71). Moreover, the combination of suboptimal doses of VRC01 and PG9 better neutralized cell-associated Env variants WITO and CH077 than either antibody alone. This improved neutralization of cell-associated virus was not observed when combining suboptimal doses of gp120- and gp41-specific antibodies for any of the four Env variants. These results suggest that further combinations of antiviral antibodies and/or inhibitors need to be evaluated and optimized for immunotherapies and prevention strategies to broadly and more effectively target cell-associated T/F virus strains.
Due to methodological differences among reports studying cell-associated virus infection, it has been difficult to obtain a clear and comprehensive understanding of which epitope-specific broadly neutralizing antibodies are more or less potent against cell-associated and cell-free virus transmission (10, 12, 72, 73). Consequently, we measured antibody and inhibitor efficiencies using two previously validated and standardized neutralization assays with TZM-bl or A3R5 target cells that we adapted for evaluation of cell-associated virus transmission inhibition (33, 35, 41). With each assay, we studied cell-free viruses dependent on the polycation DEAE-dextran. Although the dependence on DEAE-dextran may not apply to all isolated HIV-1 strains, we were able to compare the infectivity of several diverse neutralization-resistant clade B Env variants (40). We demonstrated significant differences in the neutralization profiles of Env glycoproteins expressed in one chronic infection strain compared to three T/F HIV-1 strains. Most studies to date that have investigated cell-associated virus transmission have primarily focused on laboratory-adapted HIV-1 strains with few T/F isolates (10, 12, 74). Due to the recently appreciated importance of T/F viruses in HIV-1 transmission and pathogenesis, more focus has shifted toward studying T/F viral isolates and Env variants (75–77). To our knowledge, this study is one of the first to comprehensively compare the individual potencies of a large panel of broadly neutralizing antibodies and inhibitors, as well as for two pairs in combination, against both cell-associated and cell-free virus transmission of multiple T/F Env variants. Similar to results reported by Sagar et al. examining dendritic cell-to-CD4+ T cell transmission of HIV-1, we also observed differences in neutralizing antibody efficiencies against infected CD4+ T cell-to-CD4+ T cell virus transmission among Env variants (78). These results underscore the importance of evaluating neutralization potencies against several HIV-1 strains, such as dualtropic, macrophage-tropic, and T/F isolates, to gain a deeper understanding of epitope conservation and accessibility among viruses during cell-to-cell spread (12, 77, 79).
Although there is evidence that cell-associated virus plays an important role in HIV-1 transmission (3–5), combinations of broadly neutralizing monoclonal antibodies have not been evaluated in human neutralization assays against cell-associated HIV-1 thus far. Horwitz et al. previously showed a decrease in cell-associated HIV-1 DNA and viral load in the humanized mouse model treated with up to three gp120-specific antibodies. However, cell-to-cell HIV-1 transmission before and after immunotherapy was not measured (30, 31, 80). To determine if a combination of neutralizing antibodies could abolish cell-associated virus transmission as well as cell-free virus transmission, we tested two specific combinations of antibodies using both the TZM-bl and A3R5 neutralization assay. Our results demonstrated that a combination of monoclonal gp120-specific antibodies at submaximal concentrations targeting the CD4bs or V1/V2 Env region neutralized two of four strains of cell-associated virus more efficiently than when used individually. Moreover, the combination of two gp120-specific neutralizing antibodies was more effective against cell-associated virus transmission than a combination of gp41- and gp120-specific antibodies, which had no apparent additive effect. Although these results warrant further in-depth analyses of different antibody combinations against more HIV-1 strains, they do provide a proof of concept that combinations of specific antibodies may better neutralize cell-associated virus, which can be tested effectively in both the TZM-bl and A3R5 cell-based neutralization assays. Our findings may inform the future development of prophylactic vaccines to produce specific broadly neutralizing antibodies capable of neutralizing both cell-free and cell-associated virus.
Previous reports have shown that cell-free transmission and cell-associated virus transmission exhibit different sensitivities to gp120- but not gp41-directed agents (10, 78). It is important to note, however, that these previous findings were primarily based on the IC50 values of neutralizing antibodies and inhibitors. Our additional in-depth statistical analyses comparing antibodies stratified by dose, and not solely by IC50 value, suggest that previously reported differences in neutralizing antibody efficiencies between cell-associated and cell-free virus may be underestimated. Moreover, the MPER-specific antibodies previous reports focused on were 4E10 and 2F5. The non-membrane-binding MPER-specific antibody 10E8 was not tested (10, 12, 78). As this study compares a large, functionally diverse panel of antibodies and inhibitors and their potencies to neutralize multiple cell-associated and cell-free Env variants, the statistical analyses performed had some limitations due to the number of experimental conditions requiring adjustment for multiple comparisons. The sample size of some experiments was not large enough to adequately compare the difference in neutralization efficiencies among different Env variants in various settings. In the future, automation of these functional assays may prove valuable to cost-effectively perform high-throughput screening of therapeutic antibodies and viral inhibitors.
One possible explanation for the differences in neutralization efficiency observed among antibodies may be due to differences in epitope accessibility. Steric hindrance from the coreceptor attachments and compartmental networks that develop during virological synapse formation in cell-to-cell transmission may render the virus less accessible than cell-free virus to certain antibodies or inhibitors (6, 13). In addition, antibody-binding kinetics may contribute to limited epitope accessibility during cell-associated virus transmission. For example, the time that gp120-directed neutralizing antibodies are able to bind cell-associated virus during synaptic formation is limited to the short duration between virus budding and CD4 and CCR5 engagement (12). This is a much shorter epitope exposure period than that of cell-free virus. MPER-specific antibody epitopes are only accessible postattachment upon the fusion-active or fusion-intermediate conformation (53, 81). Finally, differences in antibody or inhibitor binding avidities may also affect their ability to neutralize cell-associated virus efficiently. Malbec et al. recently showed that only a subset of broadly neutralizing antibodies are able to efficiently inhibit transmission of cell-associated HIV-1 by targeting the CD4bs or the V3 loop glycan, albeit less than against cell-free viruses (72). Using immunofluorescence, they demonstrated that these more potent neutralizing antibodies were able to congregate at the virological synapse and impede cell-to-cell transmission (72). Additional studies are necessary to further deduce precisely why some broadly neutralizing antibodies are less efficient against certain cell-associated viruses than others.
This study describes two different neutralization assays that enable the screening of several antibodies and inhibitors against cell-associated and cell-free virus transmission and infection. We not only show that neutralizing antibodies, including VRC01 and PG9, and some inhibitors are less efficient against cell-associated compared to cell-free HIV-1 transmission, but also that neutralization efficiencies widely differ between chronic and T/F HIV-1 strains. Furthermore, combining a particular pair of gp120-specific neutralizing antibodies is more effective at inhibiting cell-associated virus transmission. These findings advocate that cell-associated virus neutralization should be evaluated in addition to cell-free virus to better inform HIV-1 vaccine, immunotherapy, and ART design. Enhanced screening and evaluation of novel, more powerful antibodies and inhibitors and optimal combinations thereof will be useful for prophylactic HIV-1 vaccine and passive immunotherapy development.
Supplementary Material
ACKNOWLEDGMENTS
We thank Christina Ochsenbauer and John C. Kappes (University of Alabama at Birmingham) for the GFP-labeled plasmids expressing each Env variant, Robert McLinden (U.S. Military HIV Research Program) for the A3R5 cells, and Hua-Xin Liao (Duke Human Vaccine Institute), John R. Mascola (NIH Vaccine Research Center), Dennis R. Burton (The Scripps Research Institute), and Keith A. Reimann (Nonhuman Primate Reagent Resource) for supplying human monoclonal antibodies. We acknowledge the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH for 10E8 (catalogue no. 12294) from Jinghe Huang, Leo Laub, and Mark Connors, T-20 (catalogue no. 9409), maraviroc (catalogue no. 11580), TAK-779 (catalogue no. 4983), PMPA (catalogue no. 10199), and nevirapine (catalogue no. 4666). We also thank Evan M. Cale (NIH Vaccine Research Center) for help in manuscript preparation.
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIAID) and by the Duke Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery (CHAVI-ID) grant no. UM1-AI100645. This publication was made possible with help from the Harvard University Center for AIDS Research (CFAR), an NIH funded program (P30-AI060354), which is supported by the following NIH cofunding and participating institutes and centers: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, NIDDK, NIGMS, FIC, and OAR.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We declare no competing interests.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00783-15.
REFERENCES
- 1.Virgin HW, Walker BD. 2010. Immunology and the elusive AIDS vaccine. Nature 464:224–231. doi: 10.1038/nature08898. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DJ, Yunis EJ. 1983. “Trojan Horse” leukocytes in AIDS. N Engl J Med 309:984–985. [PubMed] [Google Scholar]
- 3.Kolodkin-Gal D, Hulot SL, Korioth-Schmitz B, Gombos RB, Zheng Y, Owuor J, Lifton MA, Ayeni C, Najarian RM, Yeh WW, Asmal M, Zamir G, Letvin NL. 2013. Efficiency of cell-free and cell-associated virus in mucosal transmission of human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol 87:13589–13597. doi: 10.1128/JVI.03108-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salle B, Brochard P, Bourry O, Mannioui A, Andrieu T, Prevot S, Dejucq-Rainsford N, Dereuddre-Bosquet N, Le Grand R. 2010. Infection of macaques after vaginal exposure to cell-associated simian immunodeficiency virus. J Infect Dis 202:337–344. doi: 10.1086/653619. [DOI] [PubMed] [Google Scholar]
- 5.Bernard-Stoecklin S, Gommet C, Corneau AB, Guenounou S, Torres C, Dejucq-Rainsford N, Cosma A, Dereuddre-Bosquet N, Le Grand R. 2013. Semen CD4+ T cells and macrophages are productively infected at all stages of SIV infection in macaques. PLoS Pathog 9:e1003810. doi: 10.1371/journal.ppat.1003810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen BK. 2012. T cell virological synapses and HIV-1 pathogenesis. Immunol Res 54:133–139. doi: 10.1007/s12026-012-8320-8. [DOI] [PubMed] [Google Scholar]
- 7.Piguet V, Sattentau Q. 2004. Dangerous liaisons at the virological synapse. J Clin Invest 114:605–610. doi: 10.1172/JCI22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sattentau Q. 2008. Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol 6:815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]
- 9.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, Onfelt B, Sattentau Q, Davis DM. 2008. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol 10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 10.Abela IA, Berlinger L, Schanz M, Reynell L, Gunthard HF, Rusert P, Trkola A. 2012. Cell-cell transmission enables HIV-1 to evade inhibition by potent CD4bs directed antibodies. PLoS Pathog 8:e1002634. doi: 10.1371/journal.ppat.1002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sigal A, Kim JT, Balazs AB, Dekel E, Mayo A, Milo R, Baltimore D. 2011. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature 477:95–98. doi: 10.1038/nature10347. [DOI] [PubMed] [Google Scholar]
- 12.Schiffner T, Sattentau QJ, Duncan CJ. 2013. Cell-to-cell spread of HIV-1 and evasion of neutralizing antibodies. Vaccine 31:5789–5797. doi: 10.1016/j.vaccine.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Chen P, Hubner W, Spinelli MA, Chen BK. 2007. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J Virol 81:12582–12595. doi: 10.1128/JVI.00381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashuba AD, Dyer JR, Kramer LM, Raasch RH, Eron JJ, Cohen MS. 1999. Antiretroviral-drug concentrations in semen: implications for sexual transmission of human immunodeficiency virus type 1. Antimicrob Agents Chemother 43:1817–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein G, Manson K, Tribbick G, Smith R. 2000. Minimization of chronic plasma viremia in rhesus macaques immunized with synthetic HIV-1 Tat peptides and infected with a chimeric simian/human immunodeficiency virus (SHIV33). Vaccine 18:2789–2795. doi: 10.1016/S0264-410X(00)00085-2. [DOI] [PubMed] [Google Scholar]
- 16.Del Portillo A, Tripodi J, Najfeld V, Wodarz D, Levy DN, Chen BK. 2011. Multiploid inheritance of HIV-1 during cell-to-cell infection. J Virol 85:7169–7176. doi: 10.1128/JVI.00231-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaizu M, Weiler AM, Weisgrau KL, Vielhuber KA, May G, Piaskowski SM, Furlott J, Maness NJ, Friedrich TC, Loffredo JT, Usborne A, Rakasz EG. 2006. Repeated intravaginal inoculation with cell-associated simian immunodeficiency virus results in persistent infection of nonhuman primates. J Infect Dis 194:912–916. doi: 10.1086/507308. [DOI] [PubMed] [Google Scholar]
- 18.Mayer KH, Boswell S, Goldstein R, Lo W, Xu C, Tucker L, DePasquale MP, D'Aquila R, Anderson DJ. 1999. Persistence of human immunodeficiency virus in semen after adding indinavir to combination antiretroviral therapy. Clin Infect Dis 28:1252–1259. doi: 10.1086/514775. [DOI] [PubMed] [Google Scholar]
- 19.Vanpouille C, Arakelyan A, Margolis L. 2012. Microbicides: still a long road to success. Trends Microbiol 20:369–375. doi: 10.1016/j.tim.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garg S, Goldman D, Krumme M, Rohan LC, Smoot S, Friend DR. 2010. Advances in development, scale-up and manufacturing of microbicide gels, films, and tablets. Antiviral Res 88(Suppl 1):S19–S29. doi: 10.1016/j.antiviral.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 21.De Clercq E. 2004. Antiviral drugs in current clinical use. J Clin Virol 30:115–133. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Balzarini J, Schols D. 2012. Combination of antiretroviral drugs as microbicides. Curr HIV Res 10:53–60. doi: 10.2174/157016212799304652. [DOI] [PubMed] [Google Scholar]
- 23.Cooper DA, Heera J, Goodrich J, Tawadrous M, Saag M, Dejesus E, Clumeck N, Walmsley S, Ting N, Coakley E, Reeves JD, Reyes-Teran G, Westby M, Van Der Ryst E, Ive P, Mohapi L, Mingrone H, Horban A, Hackman F, Sullivan J, Mayer H. 2010. Maraviroc versus efavirenz, both in combination with zidovudine-lamivudine, for the treatment of antiretroviral-naive subjects with CCR5-tropic HIV-1 infection. J Infect Dis 201:803–813. doi: 10.1086/650697. [DOI] [PubMed] [Google Scholar]
- 24.Montaner JS, Reiss P, Cooper D, Vella S, Harris M, Conway B, Wainberg MA, Smith D, Robinson P, Hall D, Myers M, Lange JM. 1998. A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: the INCAS Trial. Italy, The Netherlands, Canada and Australia Study. JAMA 279:930–937. [DOI] [PubMed] [Google Scholar]
- 25.Friedman J, Alam SM, Shen X, Xia SM, Stewart S, Anasti K, Pollara J, Fouda GG, Yang G, Kelsoe G, Ferrari G, Tomaras GD, Haynes BF, Liao HX, Moody MA, Permar SR. 2012. Isolation of HIV-1-neutralizing mucosal monoclonal antibodies from human colostrum. PLoS One 7:e37648. doi: 10.1371/journal.pone.0037648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gitlin N. 1997. Hepatitis B: diagnosis, prevention, and treatment. Clin Chem 43:1500–1506. [PubMed] [Google Scholar]
- 27.Walker LM, Burton DR. 2010. Rational antibody-based HIV-1 vaccine design: current approaches and future directions. Curr Opin Immunol 22:358–366. doi: 10.1016/j.coi.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haynes BF, Kelsoe G, Harrison SC, Kepler TB. 2012. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat Biotechnol 30:423–433. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, Zang T, Dorner M, Billerbeck E, Labitt RN, Gaebler C, Marcovecchio PM, Incesu RB, Eisenreich TR, Bieniasz PD, Seaman MS, Bjorkman PJ, Ravetch JV, Ploss A, Nussenzweig MC. 2012. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horwitz JA, Halper-Stromberg A, Mouquet H, Gitlin AD, Tretiakova A, Eisenreich TR, Malbec M, Gravemann S, Billerbeck E, Dorner M, Buning H, Schwartz O, Knops E, Kaiser R, Seaman MS, Wilson JM, Rice CM, Ploss A, Bjorkman PJ, Klein F, Nussenzweig MC. 2013. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci U S A 110:16538–16543. doi: 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu P, Williams LD, Shen X, Bonsignori M, Overman RG, Moody MA, Liao HX, Stieh DJ, McCotter KL, French AL, Hope TJ, Shattock R, Haynes BF, Tomaras GD. 2014. Capacity for infectious HIV-1 virion capture differs by envelope antibody specificity. J Virol 88:5165–5170. doi: 10.1128/JVI.03765-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montefiori DC. 2005. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol Chapter 12:Unit 12.11. doi: 10.1002/0471142735.im1211s64. [DOI] [PubMed] [Google Scholar]
- 34.Sarzotti-Kelsoe M, Bailer RT, Turk E, Lin CL, Bilska M, Greene KM, Gao H, Todd CA, Ozaki DA, Seaman MS, Mascola JR, Montefiori DC. 2013. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J Immunol Methods 409:131–146. doi: 10.1016/j.jim.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarzotti-Kelsoe M, Daniell X, Todd CA, Bilska M, Martelli A, LaBranche C, Perez LG, Ochsenbauer C, Kappes JC, Rountree W, Denny TN, Montefiori DC. 2014. Optimization and validation of a neutralizing antibody assay for HIV-1 in A3R5 cells. J Immunol Methods 409:147–160. doi: 10.1016/j.jim.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozaki DA, Gao H, Todd CA, Greene KM, Montefiori DC, Sarzotti-Kelsoe M. 2012. International technology transfer of a GCLP-compliant HIV-1 neutralizing antibody assay for human clinical trials. PLoS One 7:e30963. doi: 10.1371/journal.pone.0030963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polonis VR, Brown BK, Rosa Borges A, Zolla-Pazner S, Dimitrov DS, Zhang MY, Barnett SW, Ruprecht RM, Scarlatti G, Fenyo EM, Montefiori DC, McCutchan FE, Michael NL. 2008. Recent advances in the characterization of HIV-1 neutralization assays for standardized evaluation of the antibody response to infection and vaccination. Virology 375:315–320. doi: 10.1016/j.virol.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother 46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, McLinden R, Tovanabutra S, Laurence-Chenine A, Sanders-Buell E, Moody MA, Bonsignori M, Ochsenbauer C, Kappes J, Tang H, Greene K, Gao H, LaBranche CC, Andrews C, Polonis VR, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Self SG, Berman PW, Francis D, Sinangil F, Lee C, Tartaglia J, Robb ML, Haynes BF, Michael NL, Kim JH. 2012. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J Infect Dis 206:431–441. doi: 10.1093/infdis/jis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown BK, Wieczorek L, Sanders-Buell E, Rosa Borges A, Robb ML, Birx DL, Michael NL, McCutchan FE, Polonis VR. 2008. Cross-clade neutralization patterns among HIV-1 strains from the six major clades of the pandemic evaluated and compared in two different models. Virology 375:529–538. doi: 10.1016/j.virol.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 41.McLinden RJ, Labranche CC, Chenine AL, Polonis VR, Eller MA, Wieczorek L, Ochsenbauer C, Kappes JC, Perfetto S, Montefiori DC, Michael NL, Kim JH. 2013. Detection of HIV-1 neutralizing antibodies in a human CD4+/CXCR4+/CCR5+ T-lymphoblastoid cell assay system. PLoS One 8:e77756. doi: 10.1371/journal.pone.0077756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edmonds TG, Ding H, Yuan X, Wei Q, Smith KS, Conway JA, Wieczorek L, Brown B, Polonis V, West JT, Montefiori DC, Kappes JC, Ochsenbauer C. 2010. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology 408:1–13. doi: 10.1016/j.virol.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ochiel DO, Ochsenbauer C, Kappes JC, Ghosh M, Fahey JV, Wira CR. 2010. Uterine epithelial cell regulation of DC-SIGN expression inhibits transmitted/founder HIV-1 trans infection by immature dendritic cells. PLoS One 5:e14306. doi: 10.1371/journal.pone.0014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ochsenbauer C, Edmonds TG, Ding H, Keele BF, Decker J, Salazar MG, Salazar-Gonzalez JF, Shattock R, Haynes BF, Shaw GM, Hahn BH, Kappes JC. 2012. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. J Virol 86:2715–2728. doi: 10.1128/JVI.06157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pastore C, Ramos A, Mosier DE. 2004. Intrinsic obstacles to human immunodeficiency virus type 1 coreceptor switching. J Virol 78:7565–7574. doi: 10.1128/JVI.78.14.7565-7574.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levy DN, Aldrovandi GM, Kutsch O, Shaw GM. 2004. Dynamics of HIV-1 recombination in its natural target cells. Proc Natl Acad Sci U S A 101:4204–4209. doi: 10.1073/pnas.0306764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gelderblom HC, Vatakis DN, Burke SA, Lawrie SD, Bristol GC, Levy DN. 2008. Viral complementation allows HIV-1 replication without integration. Retrovirology 5:60. doi: 10.1186/1742-4690-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mascola JR. 1999. Neutralization of HIV-1 infection of human peripheral blood mononuclear cells (PBMC): infectivity reduction method. Methods Mol Med 17:317–322. [DOI] [PubMed] [Google Scholar]
- 49.Ferrari G, Pollara J, Kozink D, Harms T, Drinker M, Freel S, Moody MA, Alam SM, Tomaras GD, Ochsenbauer C, Kappes JC, Shaw GM, Hoxie JA, Robinson JE, Haynes BF. 2011. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J Virol 85:7029–7036. doi: 10.1128/JVI.00171-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, Liu P, Lu X, Parks RJ, Montefiori DC, Ferrari G, Pollara J, Rao M, Peachman KK, Santra S, Letvin NL, Karasavvas N, Yang ZY, Dai K, Pancera M, Gorman J, Wiehe K, Nicely NI, Rerks-Ngarm S, Nitayaphan S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Sinangil F, Kim JH, Michael NL, Kepler TB, Kwong PD, Mascola JR, Nabel GJ, Pinter A, Zolla-Pazner S, Haynes BF. 2013. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 38:176–186. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cardoso RM, Zwick MB, Stanfield RL, Kunert R, Binley JM, Katinger H, Burton DR, Wilson IA. 2005. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity 22:163–173. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 52.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J, Frey G, Peng H, Rits-Volloch S, Garrity J, Seaman MS, Chen B. 2014. Mechanism of HIV-1 neutralization by antibodies targeting a membrane-proximal region of gp41. J Virol 88:1249–1258. doi: 10.1128/JVI.02664-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, O'Dell S, Walker LM, Wu X, Guenaga J, Feng Y, Schmidt SD, McKee K, Louder MK, Ledgerwood JE, Graham BS, Haynes BF, Burton DR, Wyatt RT, Mascola JR. 2011. Mechanism of neutralization by the broadly neutralizing HIV-1 monoclonal antibody VRC01. J Virol 85:8954–8967. doi: 10.1128/JVI.00754-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, Marshall DJ, Crump JA, Kapiga SH, Sam NE, Sinangil F, Pancera M, Yongping Y, Zhang B, Zhu J, Kwong PD, O'Dell S, Mascola JR, Wu L, Nabel GJ, Phogat S, Seaman MS, Whitesides JF, Moody MA, Kelsoe G, Yang X, Sodroski J, Shaw GM, Montefiori DC, Kepler TB, Tomaras GD, Alam SM, Liao HX, Haynes BF. 2011. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol 85:9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sadjadpour R, Donau OK, Shingai M, Buckler-White A, Kao S, Strebel K, Nishimura Y, Martin MA. 2013. Emergence of gp120 V3 variants confers neutralization resistance in an R5 simian-human immunodeficiency virus-infected macaque elite neutralizer that targets the N332 glycan of the human immunodeficiency virus type 1 envelope glycoprotein. J Virol 87:8798–8804. doi: 10.1128/JVI.00878-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, Stanfield RL, Julien JP, Ramos A, Crispin M, Depetris R, Katpally U, Marozsan A, Cupo A, Maloveste S, Liu Y, McBride R, Ito Y, Sanders RW, Ogohara C, Paulson JC, Feizi T, Scanlan CN, Wong CH, Moore JP, Olson WC, Ward AB, Poignard P, Schief WR, Burton DR, Wilson IA. 2011. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science 334:1097–1103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O'Dell S, Patel N, Shahzad-ul Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang GY, Diwanji D, Georgiev I, Kwon YD, Lee D, Louder MK, Moquin S, Schmidt SD, Yang ZY, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Burton DR, Koff WC, Walker LM, Phogat S, Wyatt R, Orwenyo J, Wang LX, Arthos J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. 2011. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boon L, Holland B, Gordon W, Liu P, Shiau F, Shanahan W, Reimann KA, Fung M. 2002. Development of anti-CD4 MAb hu5A8 for treatment of HIV-1 infection: preclinical assessment in non-human primates. Toxicology 172:191–203. doi: 10.1016/S0300-483X(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt AG, Xu H, Khan AR, O'Donnell T, Khurana S, King LR, Manischewitz J, Golding H, Suphaphiphat P, Carfi A, Settembre EC, Dormitzer PR, Kepler TB, Zhang R, Moody MA, Haynes BF, Liao HX, Shaw DE, Harrison SC. 2013. Preconfiguration of the antigen-binding site during affinity maturation of a broadly neutralizing influenza virus antibody. Proc Natl Acad Sci U S A 110:264–269. doi: 10.1073/pnas.1218256109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin N, Welsch S, Jolly C, Briggs JA, Vaux D, Sattentau QJ. 2010. Virological synapse-mediated spread of human immunodeficiency virus type 1 between T cells is sensitive to entry inhibition. J Virol 84:3516–3527. doi: 10.1128/JVI.02651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, Mori J, Rickett G, Smith-Burchnell C, Napier C, Webster R, Armour D, Price D, Stammen B, Wood A, Perros M. 2005. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother 49:4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takami S, Minami M, Katayama T, Nagata I, Namura S, Satoh M. 2002. TAK-779, a nonpeptide CC chemokine receptor antagonist, protects the brain against focal cerebral ischemia in mice. J Cereb Blood Flow Metab 22:780–784. doi: 10.1097/00004647-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Baba M, Nishimura O, Kanzaki N, Okamoto M, Sawada H, Iizawa Y, Shiraishi M, Aramaki Y, Okonogi K, Ogawa Y, Meguro K, Fujino M. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci U S A 96:5698–5703. doi: 10.1073/pnas.96.10.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herrera C, Cranage M, McGowan I, Anton P, Shattock RJ. 2009. Reverse transcriptase inhibitors as potential colorectal microbicides. Antimicrob Agents Chemother 53:1797–1807. doi: 10.1128/AAC.01096-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheeseman SH, Hattox SE, McLaughlin MM, Koup RA, Andrews C, Bova CA, Pav JW, Roy T, Sullivan JL, Keirns JJ. 1993. Pharmacokinetics of nevirapine: initial single-rising-dose study in humans. Antimicrob Agents Chemother 37:178–182. doi: 10.1128/AAC.37.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim JH, Pitisuttithum P, Kamboonruang C, Chuenchitra T, Mascola J, Frankel SS, DeSouza MS, Polonis V, McLinden R, Sambor A, Brown AE, Phonrat B, Rungruengthanakit K, Duliege AM, Robb ML, McNeil J, Birx DL. 2003. Specific antibody responses to vaccination with bivalent CM235/SF2 gp120: detection of homologous and heterologous neutralizing antibody to subtype E (CRF01.AE) HIV type 1. AIDS Res Hum Retroviruses 19:807–816. doi: 10.1089/088922203769232601. [DOI] [PubMed] [Google Scholar]
- 68.Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand J Statist 6:65–70. [Google Scholar]
- 69.deCamp A, Hraber P, Bailer RT, Seaman MS, Ochsenbauer C, Kappes J, Gottardo R, Edlefsen P, Self S, Tang H, Greene K, Gao H, Daniell X, Sarzotti-Kelsoe M, Gorny MK, Zolla-Pazner S, LaBranche CC, Mascola JR, Korber BT, Montefiori DC. 2014. Global panel of HIV-1 Env reference strains for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 88:2489–2507. doi: 10.1128/JVI.02853-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hraber P, Korber BT, Lapedes AS, Bailer RT, Seaman MS, Gao H, Greene KM, McCutchan F, Williamson C, Kim JH, Tovanabutra S, Hahn BH, Swanstrom R, Thomson MM, Gao F, Harris L, Giorgi E, Hengartner N, Bhattacharya T, Mascola JR, Montefiori DC. 2014. Impact of clade, geography, and age of the epidemic on HIV-1 neutralization by antibodies. J Virol 88:12623–12643. doi: 10.1128/JVI.01705-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, Bhattacharya T, Lapedes A, Polonis VR, McCutchan FE, Gilbert PB, Self SG, Korber BT, Montefiori DC, Mascola JR. 2010. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol 84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malbec M, Porrot F, Rua R, Horwitz J, Klein F, Halper-Stromberg A, Scheid JF, Eden C, Mouquet H, Nussenzweig MC, Schwartz O. 2013. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. J Exp Med 210:2813–2821. doi: 10.1084/jem.20131244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sourisseau M, Sol-Foulon N, Porrot F, Blanchet F, Schwartz O. 2007. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J Virol 81:1000–1012. doi: 10.1128/JVI.01629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Permanyer M, Ballana E, Ruiz A, Badia R, Riveira-Munoz E, Gonzalo E, Clotet B, Este JA. 2012. Antiretroviral agents effectively block HIV replication after cell-to-cell transfer. J Virol 86:8773–8780. doi: 10.1128/JVI.01044-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salazar-Gonzalez JF, Salazar MG, Keele BF, Learn GH, Giorgi EE, Li H, Decker JM, Wang S, Baalwa J, Kraus MH, Parrish NF, Shaw KS, Guffey MB, Bar KJ, Davis KL, Ochsenbauer-Jambor C, Kappes JC, Saag MS, Cohen MS, Mulenga J, Derdeyn CA, Allen S, Hunter E, Markowitz M, Hraber P, Perelson AS, Bhattacharya T, Haynes BF, Korber BT, Hahn BH, Shaw GM. 2009. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med 206:1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liao HX, Tsao CY, Alam SM, Muldoon M, Vandergrift N, Ma BJ, Lu X, Sutherland LL, Scearce RM, Bowman C, Parks R, Chen H, Blinn JH, Lapedes A, Watson S, Xia SM, Foulger A, Hahn BH, Shaw GM, Swanstrom R, Montefiori DC, Gao F, Haynes BF, Korber B. 2013. Antigenicity and immunogenicity of transmitted/founder, consensus, and chronic envelope glycoproteins of human immunodeficiency virus type 1. J Virol 87:4185–4201. doi: 10.1128/JVI.02297-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sagar M, Akiyama H, Etemad B, Ramirez N, Freitas I, Gummuluru S. 2012. Transmembrane domain membrane proximal external region but not surface unit-directed broadly neutralizing HIV-1 antibodies can restrict dendritic cell-mediated HIV-1 trans-infection. J Infect Dis 205:1248–1257. doi: 10.1093/infdis/jis183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bieniasz PD, Fridell RA, Aramori I, Ferguson SS, Caron MG, Cullen BR. 1997. HIV-1-induced cell fusion is mediated by multiple regions within both the viral envelope and the CCR-5 co-receptor. EMBO J 16:2599–2609. doi: 10.1093/emboj/16.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klein F, Gaebler C, Mouquet H, Sather DN, Lehmann C, Scheid JF, Kraft Z, Liu Y, Pietzsch J, Hurley A, Poignard P, Feizi T, Morris L, Walker BD, Fatkenheuer G, Seaman MS, Stamatatos L, Nussenzweig MC. 2012. Broad neutralization by a combination of antibodies recognizing the CD4 binding site and a new conformational epitope on the HIV-1 envelope protein. J Exp Med 209:1469–1479. doi: 10.1084/jem.20120423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Joyner AS, Willis JR, Crowe JE Jr, Aiken C. 2011. Maturation-induced cloaking of neutralization epitopes on HIV-1 particles. PLoS Pathog 7:e1002234. doi: 10.1371/journal.ppat.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.