Abstract
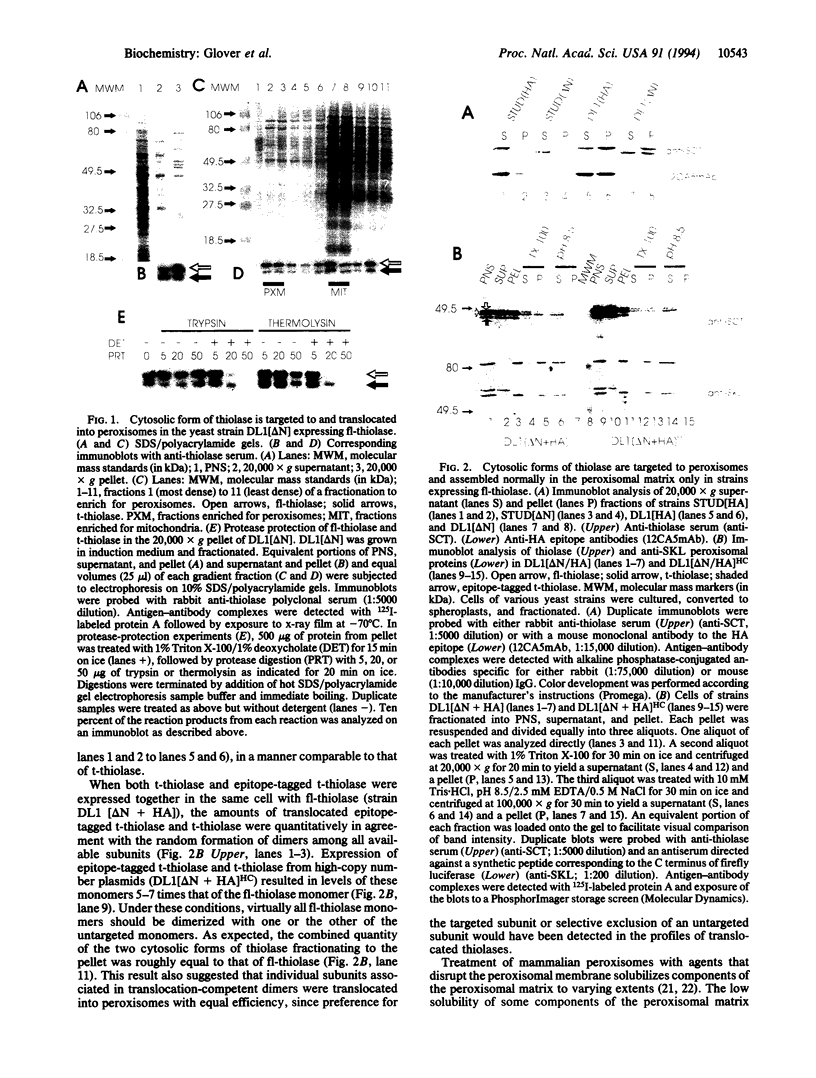
The active conformation of native peroxisomal 3-ketoacyl-CoA thiolases (EC 2.3.1.16) is homodimeric. We have previously shown that a truncated Saccharomyces cerevisiae thiolase lacking its first 16 N-terminal amino acids fails to be translocated into peroxisomes but assembles into an enzymatically active form in the cytoplasm of a strain with a disrupted nuclear thiolase gene. We now report that when truncated thiolase is cosynthesized with full-length thiolase, approximately 50% of truncated thiolase cofractionates with the full-length thiolase to fractions enriched for peroxisomes and is translocated into peroxisomes as shown by its protection from the action of external proteases. We constructed an immunologically distinct cytosolic variant of thiolase by adding an influenza hemagglutinin epitope tag to the N terminus of the truncated thiolase. In a strain simultaneously expressing the full-length, truncated, and epitope-tagged truncated thiolases, we demonstrated that normally untargeted thiolase subunits are efficiently translocated into peroxisomes by dimerization with full-length thiolase subunits. Even though truncated and epitope-tagged truncated thiolase subunits are translocated into peroxisomes in this strain, only the full-length thiolase subunit can be coimmunoprecipitated with the epitope-tagged truncated thiolase subunit from the peroxisomal matrix. This observation suggests that interactions between thiolase subunits are not disrupted during translocation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitchison J. D., Murray W. W., Rachubinski R. A. The carboxyl-terminal tripeptide Ala-Lys-Ile is essential for targeting Candida tropicalis trifunctional enzyme to yeast peroxisomes. J Biol Chem. 1991 Dec 5;266(34):23197–23203. [PubMed] [Google Scholar]
- Aitchison J. D., Nuttley W. M., Szilard R. K., Brade A. M., Glover J. R., Rachubinski R. A. Peroxisome biogenesis in yeast. Mol Microbiol. 1992 Dec;6(23):3455–3460. doi: 10.1111/j.1365-2958.1992.tb01780.x. [DOI] [PubMed] [Google Scholar]
- Alexson S. E., Fujiki Y., Shio H., Lazarow P. B. Partial disassembly of peroxisomes. J Cell Biol. 1985 Jul;101(1):294–304. doi: 10.1083/jcb.101.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brul S., Wiemer E. A., Westerveld A., Strijland A., Wanders R. J., Schram A. W., Heymans H. S., Schutgens R. B., Van den Bosch H., Tager J. M. Kinetics of the assembly of peroxisomes after fusion of complementary cell lines from patients with the cerebro-hepato-renal (Zellweger) syndrome and related disorders. Biochem Biophys Res Commun. 1988 May 16;152(3):1083–1089. doi: 10.1016/s0006-291x(88)80395-4. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chen W. J., Douglas M. G. An F1-ATPase beta-subunit precursor lacking an internal tetramer-forming domain is imported into mitochondria in the absence of ATP. J Biol Chem. 1988 Apr 15;263(11):4997–5000. [PubMed] [Google Scholar]
- Christianson T. W., Sikorski R. S., Dante M., Shero J. H., Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992 Jan 2;110(1):119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Craig E., Kang P. J., Boorstein W. A review of the role of 70 kDa heat shock proteins in protein translocation across membranes. Antonie Van Leeuwenhoek. 1990 Oct;58(3):137–146. doi: 10.1007/BF00548924. [DOI] [PubMed] [Google Scholar]
- Glover J. R., Andrews D. W., Subramani S., Rachubinski R. A. Mutagenesis of the amino targeting signal of Saccharomyces cerevisiae 3-ketoacyl-CoA thiolase reveals conserved amino acids required for import into peroxisomes in vivo. J Biol Chem. 1994 Mar 11;269(10):7558–7563. [PubMed] [Google Scholar]
- Goodman J. M., Scott C. W., Donahue P. N., Atherton J. P. Alcohol oxidase assembles post-translationally into the peroxisome of Candida boidinii. J Biol Chem. 1984 Jul 10;259(13):8485–8493. [PubMed] [Google Scholar]
- Gould S. J., Krisans S., Keller G. A., Subramani S. Antibodies directed against the peroxisomal targeting signal of firefly luciferase recognize multiple mammalian peroxisomal proteins. J Cell Biol. 1990 Jan;110(1):27–34. doi: 10.1083/jcb.110.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F. U., Martin J., Neupert W. Protein folding in the cell: the role of molecular chaperones Hsp70 and Hsp60. Annu Rev Biophys Biomol Struct. 1992;21:293–322. doi: 10.1146/annurev.bb.21.060192.001453. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Hino S., Yamasaki F. Intraparticulate localization of some peroxisomal enzymes related to fatty acid beta-oxidation. Eur J Biochem. 1981 Nov;120(1):47–51. doi: 10.1111/j.1432-1033.1981.tb05668.x. [DOI] [PubMed] [Google Scholar]
- Hiltunen J. K., Wenzel B., Beyer A., Erdmann R., Fosså A., Kunau W. H. Peroxisomal multifunctional beta-oxidation protein of Saccharomyces cerevisiae. Molecular analysis of the fox2 gene and gene product. J Biol Chem. 1992 Apr 5;267(10):6646–6653. [PubMed] [Google Scholar]
- Igual J. C., Matallaná E., Gonzalez-Bosch C., Franco L., Pérez-Ortin J. E. A new glucose-repressible gene identified from the analysis of chromatin structure in deletion mutants of yeast SUC2 locus. Yeast. 1991 May-Jun;7(4):379–389. doi: 10.1002/yea.320070408. [DOI] [PubMed] [Google Scholar]
- Kolodziej P. A., Young R. A. Epitope tagging and protein surveillance. Methods Enzymol. 1991;194:508–519. doi: 10.1016/0076-6879(91)94038-e. [DOI] [PubMed] [Google Scholar]
- Kruse C., Kindl H. Oligomerization of malate synthase during glyoxysome biosynthesis. Arch Biochem Biophys. 1983 Jun;223(2):629–638. doi: 10.1016/0003-9861(83)90627-6. [DOI] [PubMed] [Google Scholar]
- Kurihara T., Ueda M., Tanaka A. Peroxisomal acetoacetyl-CoA thiolase and 3-ketoacyl-CoA thiolase from an n-alkane-utilizing yeast, Candida tropicalis: purification and characterization. J Biochem. 1989 Sep;106(3):474–478. doi: 10.1093/oxfordjournals.jbchem.a122876. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewin A. S., Hines V., Small G. M. Citrate synthase encoded by the CIT2 gene of Saccharomyces cerevisiae is peroxisomal. Mol Cell Biol. 1990 Apr;10(4):1399–1405. doi: 10.1128/mcb.10.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelkoop E., Strijland A., Tager J. M. Does aminotriazole inhibit import of catalase into peroxisomes by retarding unfolding? FEBS Lett. 1991 Feb 11;279(1):79–82. doi: 10.1016/0014-5793(91)80255-2. [DOI] [PubMed] [Google Scholar]
- Miyazawa S., Furuta S., Osumi T., Hashimoto T., Ui N. Properties of peroxisomal 3-ketoacyl-coA thiolase from rat liver. J Biochem. 1981 Aug;90(2):511–519. doi: 10.1093/oxfordjournals.jbchem.a133499. [DOI] [PubMed] [Google Scholar]
- Nye S. H., Scarpulla R. C. In vivo expression and mitochondrial targeting of yeast apoiso-1-cytochrome c fusion proteins. Mol Cell Biol. 1990 Nov;10(11):5753–5762. doi: 10.1128/mcb.10.11.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Neupert W. The mitochondrial protein import apparatus. Annu Rev Biochem. 1990;59:331–353. doi: 10.1146/annurev.bi.59.070190.001555. [DOI] [PubMed] [Google Scholar]
- Rosenkrantz M., Alam T., Kim K. S., Clark B. J., Srere P. A., Guarente L. P. Mitochondrial and nonmitochondrial citrate synthases in Saccharomyces cerevisiae are encoded by distinct homologous genes. Mol Cell Biol. 1986 Dec;6(12):4509–4515. doi: 10.1128/mcb.6.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S. Targeting of proteins into the peroxisomal matrix. J Membr Biol. 1992 Jan;125(2):99–106. doi: 10.1007/BF00233350. [DOI] [PubMed] [Google Scholar]
- Swinkels B. W., Gould S. J., Bodnar A. G., Rachubinski R. A., Subramani S. A novel, cleavable peroxisomal targeting signal at the amino-terminus of the rat 3-ketoacyl-CoA thiolase. EMBO J. 1991 Nov;10(11):3255–3262. doi: 10.1002/j.1460-2075.1991.tb04889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. L., Krisans S. K. Rat liver peroxisomes catalyze the initial step in cholesterol synthesis. The condensation of acetyl-CoA units into acetoacetyl-CoA. J Biol Chem. 1990 Apr 5;265(10):5731–5735. [PubMed] [Google Scholar]
- Van Loon A. P., Van Eijk E., Grivell L. A. Biosynthesis of the ubiquinol-cytochrome c reductase complex in yeast. Discoordinate synthesis of the 11-kd subunit in response to increased gene copy number. EMBO J. 1983;2(10):1765–1770. doi: 10.1002/j.1460-2075.1983.tb01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner K., Schatz G. Protein translocation across membranes. Science. 1988 Sep 9;241(4871):1307–1313. doi: 10.1126/science.2842866. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Schatz G. Mitochondria can import artificial precursor proteins containing a branched polypeptide chain or a carboxy-terminal stilbene disulfonate. J Cell Biol. 1988 Dec;107(6 Pt 1):2045–2049. doi: 10.1083/jcb.107.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton P. A., Gould S. J., Feramisco J. R., Subramani S. Transport of microinjected proteins into peroxisomes of mammalian cells: inability of Zellweger cell lines to import proteins with the SKL tripeptide peroxisomal targeting signal. Mol Cell Biol. 1992 Feb;12(2):531–541. doi: 10.1128/mcb.12.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton P. A., Gould S. J., Rachubinski R. A., Subramani S., Feramisco J. R. Transport of microinjected alcohol oxidase from Pichia pastoris into vesicles in mammalian cells: involvement of the peroxisomal targeting signal. J Cell Biol. 1992 Aug;118(3):499–508. doi: 10.1083/jcb.118.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland M., Subramani S. Cytosol-dependent peroxisomal protein import in a permeabilized cell system. J Cell Biol. 1993 Feb;120(3):675–685. doi: 10.1083/jcb.120.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeelen J. P., Wierenga R. K., Erdmann R., Kunau W. H. Crystallographic studies of 3-ketoacylCoA thiolase from yeast Saccharomyces cerevisiae. J Mol Biol. 1990 Sep 20;215(2):211–213. doi: 10.1016/S0022-2836(05)80338-9. [DOI] [PubMed] [Google Scholar]