Abstract
Niches are local tissue microenvironments that maintain and regulate stem cells. Long-predicted from mammalian studies, these structures have recently been characterized within several invertebrate tissues using methods that reliably identify individual stem cells and their functional requirements. Although, similar single-cell resolution has not usually been achieved in mammalian tissues, principles likely to govern the behavior of niches in diverse organisms are beginning to emerge and considerable progress has been made elucidating the microenvironmental mechanisms that promote stem cell maintenance. These mechanisms may not only guide tissue homeostasis but when altered during adulthood likely contribute to aging and tumorigenesis.
Introduction
Stem cells are emerging as one of the fundamental underpinnings of tissue biology. They allow blood, bone, gametes, epithelia, nervous system, muscle, and myriad other tissues to be replenished by fresh cells throughout life. Additional stem cells lie dormant, but can be activated at particular life cycle stages, or following injury. These potent agents are controlled within restricted tissue microenvironments known as “niches.” Until recently, niches were a theoretical concept strongly supported by the observation that transplanted stem cells survive and grow only in particular tissue locations. The number of such sites could be saturated, after which transferring additional stem cells provided little or no further engraftment. However, in recent years it has become possible to identify stem cells and niches with increasing precision. In this review we summarize progress in delineating stem cells and their niches, as well as in discovering the mechanisms that control stem cell function. Finally, we examine how niches change with age and contribute to cancer and tissue aging.
Identifying stem cells
Accurately identifying stem cells in vivo remains the biggest obstacle to progress in understanding stem cell biology. Normal stem cells and their neighboring cells within tissues can rarely be pinpointed by histological methods. Some properties that have been widely assumed to mark stem cells, such as preferential BrdU label-retention (caused by an expected tendency of stem cells to divide more slowly than many of their progeny) have frequently proven to be unreliable where definitive independent markers are available (Barker et al., 2007; Crittenden et al., 2006; Kiel et al., 2007a; Margolis and Spradling, 1995). Specific stem cell molecular markers have not been found in most tissues. However, within the relatively simple tissues of small invertebrates such as Drosophila, it has been possible to genetically tag individual stem cells and document their ability to self renew for a prolonged period. Seven different types of stem cell have now been identified (Figure 1).
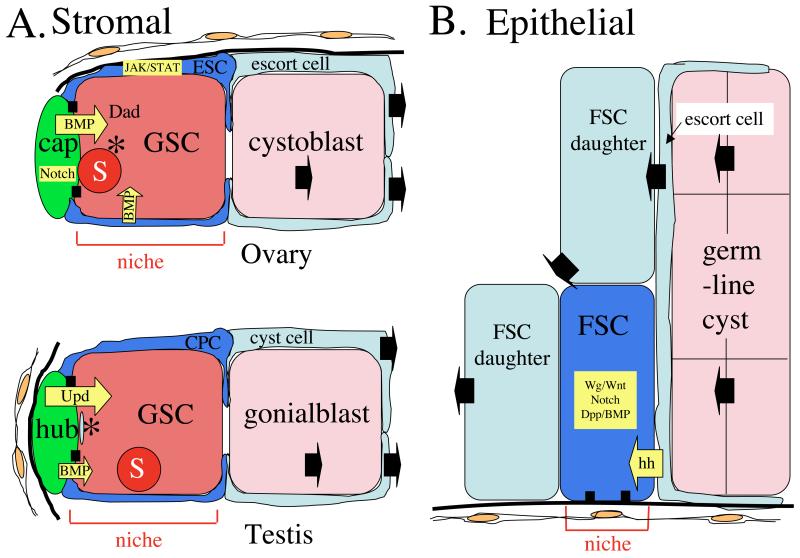
Figure 1. Two general classes of stem cell niche.
A) The Drosophila male and female GSC niches are examples of the stromal niche. Non-dividing stromal cells (green) hold the GSCs (dark pink) in place via adherens junctions (black boxes). GSCs contain a spectrosome (S) and a localized centrosome (*) that in the male is known to be the maternal centrosome. The GSC is surrounded by escort stem cells (ESC) or cyst progenitor stem cells (CPC) whose daughters (light blue) encyst the GSC daughter cell (pink). B) The Drosophila follicle cell stem cell (FSC) is an example an epidermal niche. The FSC is surrounded by FSC daugher cells (light blue), and also contacts the thin escort cells (light blue) that surround developing germline cysts (pink). The FSC does not contact any permanent stromal cells, but remains associated with a region of the basement membrane (thick black line). Intercellular signals are shown in yellow. The movement of cells is indicated by black arrows.
In contrast to the ability to identify invertebrate stem cells and their niches with single-cell resolution, the relative vastness of mammalian tissues and the rarity of stem cells have conspired to make it much more difficult to confidently identify individual stem cells in vivo. Germline stem cells lie within the basal cell layer of the seminiferous tubule (de Rooij, 2001), epithelial stem cells reside within the bulge of hair follicles (Cotsarelis et al., 1990; Taylor et al., 2000; Tumbar et al., 2004), neural stem cells reside within the lateral ventricle subventricular zone of the central nervous system (Doetsch, 2003), muscle stem cells reside among satellite cells under the basal lamina of myofibers (Collins et al., 2005; Kuang et al., 2007), and hematopoietic stem cells (HSCs) reside within the bone marrow, close to endosteum and/or sinusoidal blood vessels (Adams and Scadden, 2006; Kiel et al., 2005). In each case these locations have been described as stem cell niches and the factors that regulate the maintenance of these stem cells are starting to be identified. Yet we have little definitive information about exactly which supporting cells stem cells interact with or which cells produce the key factors that regulate stem cell maintenance. Improvements in imaging technology and more extensive genetic analyses are needed to bring the resolution of invertebrate stem cell studies to mammalian systems.
Stem cell markers
Gene expression markers have long been sought that would distinguish stem cells based on a unique underlying process. Such markers would free researchers from the experimental difficulties of identifying stem cells by lineage and simultaneously provide clues about regulatory mechanisms. Recent studies of invertebrate stem cells generally encourage this view but provide a cautionary perspective. Markers truly specific for one or multiple stem cells, as might be expected if stem cells constitute a distinctive cell “type” sharing stem cell-specific genes, have not been found. At the level of gene expression, stem cells resemble their own daughters and transit cells more than stem cells from a different lineage. However, two types of useful makers have been identified. First, stem cells sometimes contain distinctive structures related to their early state of differentiation, such as the round aggregate of ER-like vesicles (“spectrosome”) in Drosophila germ line stem cells (GSCs). Second, components of the signaling pathways involved in stem cell maintenance and daughter programming, such as Dad (Kai and Spradling, 2003) or Socs36E (Bach et al., 2007) in male and female GSCs, respectively, allow stem cell identification if combined with anatomical information.
Studies of well-characterized stem cells reveal why it is difficult to use markers to initially identify unknown stem cells. Markers of primitive cells are often not fully specific for stem cells. For example, spectrosomes identical to those in GSCs also reside in primordial germ cells, and spectrosome structure does not change fast enough during differentiation to distinguish GSCs from their initial daughters. However, combining spectrosome content and anatomical position together allows GSCs to be accurately identified. Markers reflecting stem cell signaling also must be supplemented with additional information. Daughter cells may briefly retain markers of signal reception such as Dad (Kai and Spradling, 2003). Moreover, stem cells do not simply exhibit constant signaling profiles, but vary depending on the behavior of neighboring cells and their physiological environment. For example, the Notch ligand, Delta, preferentially labels most ISCs compared to surrounding midgut cells (Ohlstein and Spradling, 2007). However, despite the fact that a Delta-mediated signal from the ISC to its daughter programs the daughter to differentiate as an enterocyte, Delta does not reliably mark all ISCs. ISCs are multipotent, and stem cells about to generate enteroendocrine rather than enterocyte daughters, lack cytoplasmic Delta. Consequently, stem cells typically can be recognized using gene markers only after they have been identified by lineage or transplantation, and their behavior under various conditions becomes understood.
Among mammalian tissues, the hematopoietic system is perhaps the most advanced in terms of HSC markers. HSCs are defined based on the ability of single cells to self-renew and give long-term multilineage reconstitution of all major blood cell lineages upon transplantation into irradiated mice. HSCs represent only about 1 out of every 30,000 cells (0.003%) in the bone marrow. Thus, purifying these cells is no mean feat: even combinations of markers that distinguish HSCs from 99.9% of other cells in the bone marrow yield populations that are only 3% pure. Nonetheless, twenty years of work has identified combinations of markers that yield cells by flow-cytometry that are approximately 50% pure for HSCs (Kiel et al., 2005; Matsuzaki et al., 2004; Takano et al., 2004). The problem is that until recently, these combinations of markers were too complex for the identification of HSCs by immunofluorescence in sections from hematopoietic tissues. As a result, the field usually made inferences about the localization of HSCs in vivo using simplified combinations of markers that yielded poor or uncertain stem cell purity. This created uncertainty about the precise location of bona fide HSCs.
Similar issues limit the characterization of stem cell niches in most mammalian tissues. Neural stem cells in the forebrain have been identified based on their ultrastructural characteristics by electron microscopy within the subventricular zone of the lateral ventricle (Doetsch et al., 1999); however, this approach does not allow the purification of live stem cells for transplantation and definitive markers for their purification by flow-cytometry have yet to be identified. Epithelial stem cells within the bulge of the hair follicle have been enriched based on Histone-GFP label retention and CD34 expression (Blanpain et al., 2004), though purity remains uncertain, and these markers do not work as well in certain contexts, such as after stem cell activation (Kobielak et al., 2007). The identification of markers that permit the purification of live stem cells, irrespective of cell cycle status, would make it possible to more fully explore the mechanisms that regulate the function of these cells.
Identification of stem cells through lineage analysis
Recent advances in the application of Cre-recombinase fate mapping in mice have begun to provide insights into the nature of mammalian stem cells. Fate mapping studies of muscle satellite cells (Kuang et al., 2007), spermatogonial stem cells (Nakagawa et al., 2007), epidermal stem cells (Clayton et al., 2007) and intestinal stem cells (Barker et al., 2007) have clarified the relationship between stem cells and their daughters, as well as some of the mechanisms that regulate tissue homeostasis. Hair follicle stem cells have also been fate mapped, demonstrating that cells within the bulge give rise to all of the epithelial cells within the hair follicle (Morris et al., 2004) and can even transiently contribute to wound repair in the epidermis (Ito et al., 2005). Neural stem cells in the forebrain subventricular zone have been fate mapped using a variety of approaches that have demonstrated regional heterogeneity in the embryonic origin, developmental potential, and fate of these cells (Merkle et al., 2007; Young et al., 2007). These studies have provided considerable new insights into the biology of mammalian stem cells, though with certain notable exceptions (Barker et al., 2007; Kuang et al., 2007) these approaches have usually not made it possible to image mammalian stem cells within their niches in a way that clearly distinguishes these cells from their progeny.
The recent identification of intestinal epithelial stem cells by Clevers and colleagues illustrates the power of both single cell resolution and lineage marking for the identification of a mammalian stem cell niche in vivo (Barker et al., 2007). These authors discovered that a Wnt target gene, Lgr5, was restricted in expression within the intestinal epithelium to columnar epithelial cells at the base of the crypts (CBCs). Fate mapping of these cells with a Cre knock-in allele of Lgr5 demonstrated that individual Lgr5-positive CBCs self-renew in vivo as well as giving rise to all intestinal epithelial lineages. This represents a critical advance as studies of intestinal epithelial stem cells have long been hampered by a lack of markers and clear functional assays. Moreover, a large body of older literature had provisionally identified the intestinal epithelial stem cells based on more indirect methods, such as BrdU label-retention, as the ‘+4 cells’ in a different position, just above the columnar cells in the crypts (Potten and Loeffler, 1990). Thus this study clarifies the identity of the stem and implicates a different microenvironment (lower in the crypt) as their niche.
Cell culture assays
The difficulty associated with identifying markers is not the only factor limiting our ability to identify mammalian stem cell niches. In some tissues, the functional definition for what constitutes a stem cell is also uncertain. Central nervous system stem cells have generally been identified based on their ability to self-renew and to form multilineage colonies in culture. However, at least some restricted progenitors in the nervous system can be reprogrammed by relatively short periods of culture to acquire multipotency: some of the cells that undergo multilineage differentiation in culture might not be capable of multilineage differentiation in vivo (Gabay et al., 2003; Kondo and Raff, 2000). Moreover, some neural stem cell populations that have been considered homogeneous based on experiments performed in culture, are quite heterogeneous in terms of fate, and even developmental potential, in vivo (Gabay et al., 2003; Merkle et al., 2007). Culture environments sometimes alter the patterning of cells in ways that modify their fates, and even their developmental potentials (Joseph and Morrison, 2005). Similar concerns apply to other mammalian stem cells that have been identified and studied primarily based upon their behavior in culture, or after expansion in culture.
This problem has been addressed in neural crest stem cells that give rise to the peripheral nervous system by using flow-cytometry to prospectively identify and isolate the neural crest stem cells that are capable of forming multilineage colonies in culture (Bixby et al., 2002; Morrison et al., 1999). Prospective identification means that the uncultured stem cells can be distinguished from other cells based on marker expression, making it possible to study these cells in vitro or in vivo. Prospective identification thus made it possible to inject uncultured rat neural crest stem cells into the neural crest migration pathway of developing chick embryos. The ability of these cells to migrate throughout the chick peripheral nervous system and to give rise to diverse types of rat neurons and glia demonstrated that this broad developmental potential was not acquired in culture (Bixby et al., 2002; Morrison et al., 1999). This work demonstrates that it is possible to prospectively identify and isolate by flow-cytometry highly purified, uncultured stem cells from solid mammalian tissues, making it possible to study the stem cells as they exist in vivo, rather than after they have changed their properties in culture.
Identifying niches
A niche consists of a local tissue microenvironment capable of housing and maintaining one or more stem cells. However, use of the term “niche” continues to vary widely, and in some cases is applied so broadly as to be almost devoid of meaning. Perturbing a precisely identified stem cell or its surroundings allows the existence, size and regulatory properties of a corresponding niche to be revealed. Ideally, a candidate niche should be transiently depleted of its full complement of stem cells and then shown to take up and maintain a newly introduced stem cell. This provides evidence that the niche microenvironment is localized and not a general tissue property. For example, showing that GSCs are maintained at the gonad tips by local signals suggested the existence of a niche (Kiger et al., 2001; Tulina and Matunis, 2001; Xie and Spradling, 1998); but demonstrating that new stem cells can be introduced and maintained there provided the clearest evidence (Brawley and Matunis, 2004; Kai and Spradling, 2004; Xie and Spradling, 2000). While regulation by widely diffusible signals such as insulin are also critically important (Drummond-Barbosa and Spradling, 2001), we suggest that the term “niche” be reserved for the specialized local microenvironments where stem cells reside and that directly promote the maintenance of stem cells. This distinction is highly relevant to mammalian tissues. For example, it remains uncertain whether endosteal cells, such as osteoblasts and osteoclasts, influence HSC numbers in the bone marrow (Calvi et al., 2003; Zhang et al., 2003) by promoting the maintenance of HSCs that reside in direct contact with these cells, or by secreting factors that act at a distance, directly or indirectly regulating HSCs that localize to other nearby microenvironments (Adams and Scadden, 2006; Kiel et al., 2007b).
Two basic types of niche have been recognized. The niches at the tips of Drosophila female and male gonads are examples of “stromal cell” niches (Fig. 1A). These niches develop whether or not stem cells are present, and maintain their morphology after stem cell loss. Distinct “stromal” cell types- cap cells, and hub cells, respectively, initially guide niche morphogenesis and continue to directly contact and signal resident stem cells. In contrast, the stem cells for ovarian follicle cells (FSC) reside in “epithelial” niches devoid of specialized cells (Fig. 1B). Niche-resident FSCs contact only moving, developing cells, including their own progeny (Nystul and Spradling, 2007). Yet, precisely two FSC niches exist within each ovariole at sites that remain constant despite this dynamic environment, possibly due to direct contact between the FSC and a fixed region of basement membrane. Both types of niches depend on cell-cell junctional molecules (Song and Xie, 2002; Song et al., 2002). Epithelial niches may also be limited by the presence of specific molecules within the extracellular matrix or on nearby tissue cells, but this remains to be proven.
Currently well-characterized niches vary in size and complexity (Table 1). The FSC niche contains a single stem cell of a single type (Nystul and Spradling, 2007). In contrast, the niches at the tip of the ovariole and testis are larger and house two types of stem cell. Escort stem cells and cyst progenitor cells are squamous epithelial stem cells that contact the GSCs in the female and male, respectively, and coordinate to produce cysts containing daughters of both stem cell types. The ovarian niche usually contains 2 GSCs and 4-8 escort stem cells (Decotto and Spradling, 2005), while 10-15 GSCs and 20-30 cyst progenitor cells can occupy the testis niche (Gonczy et al., 1997; Wallenfang et al., 2006). The GSC niche at the tip of the C. elegans hermaphrodite gonad appears to be even larger, and may harbor as many as 50 developmentally equivalent GSCs (Crittenden et al., 2006).
Table 1. Characterized invertebrate stem cells and their niches.
| Stem cell | Species | Niche type | Major signal | Additional signals |
RNAi? | Replaced? | Number/nich e |
Targets | Recent Reference |
|---|---|---|---|---|---|---|---|---|---|
| GSC (female) | D. melanogaster | S | JAK- STAT BMP |
Notch | Y | Y | 2 | Bam | (Lopez-Oneiva et al., 2008) |
| GSC (male) | D. melanogaster | S | JAK- STAT BMP |
Y | 7-12 | Bam | (Yamashita et al., 2007) | ||
| Escort stem cell | D. melanogaster | S | JAK STAT |
EGFR | 4-6 | (Gilboa and Lehmann, 2006) | |||
| Cyst progenitor | D. melanogaster | S | JAK- STAT? |
EGFR? | 14- 24 |
(Brawley and Matunis, 2004) | |||
| Follicle stem cell | D. melanogaster | E | Hh | Notch, Dpp, Wg |
Y | Y | 1 | (Nystul and Spradling, 2007) | |
| Intestinal Stem cell |
D. melanogaster | E | Notch | Y? | 1? | (Ohlstein and Spradling, 2007) | |||
| GSC hermaphrodite |
C. elegans | S | Notch | 50? | Gld1 Gld2 Gld3 |
(Kimble and Crittenden, 2007) |
S = stromal niche; E = epithelial niche;
Facultative, distributed niches for mammalian stem cells
The small numbers of stem cells in some invertebrate niches are similar to what has been observed in mammalian skeletal muscle. Muscle satellite cells, which include the stem cells of skeletal muscle, are scattered as individual cells under the basal laminas of different muscle fibers (Collins et al., 2005; Kuang et al., 2007). This suggests that at least some mammalian stem cells can reside as individual cells within niches distributed throughout tissues. In other cases, multiple stem cells are clustered together within relatively small substructures within tissues, like in the bulge of hair follicles (Cotsarelis et al., 1990) or in the forebrain subventricular zone (Doetsch et al., 1999), though the difficulty associated with definitively distinguishing the stem cells from their progeny in these tissues makes it difficult to assess the extent to which stem cells are clustered together as opposed to being interspersed among other cells.
GSCs in Drosophila and C. elegans occupy a single spatially invariant niche throughout adult life. This is also seen in some mammalian tissues, for example in the central nervous system where neural stem cells reside throughout postnatal life in the lateral ventricle subventricular zone (Doetsch, 2003) and dentate gyrus of the hippocampus (Palmer et al., 1997). However, other mammalian tissues are much more dynamic with respect to the locations where stem cells are sustained. A good example is the hematopoietic system. Under steady-state conditions, HSCs reside and undergo hematopoiesis in the bone marrow. SLAM family markers have facilitated the purification of HSCs using simple combinations of markers that can be used to identify HSCs by immunofluorescence in sections (Kiel et al., 2005). Staining of sections through adult hematopoietic tissues using these markers revealed the presence of individual HSCs around bone marrow sinusoids (specialized blood vessels that allow cells to pass in and out of circulation) as well as near the endosteum (the interface of bone and marrow). It remains uncertain whether both locations represent niches, and if so, whether they are spatially distinct niches or whether perivascular cells and endosteal cells collaborate to form a common niche (Figure 2). Histological examination has not yet revealed anatomically specialized regions of sinusoids or endosteum that seem uniquely capable of hosting HSCs. Rather individual HSCs appear to be able to occupy facultative niches scattered over the surface of many sinusoids, and/or near much of the vast endosteal surface of trabecular bone (Arai et al., 2004; Kiel et al., 2005; Nilsson et al., 2001; Sugiyama et al., 2006; Zhang et al., 2003).
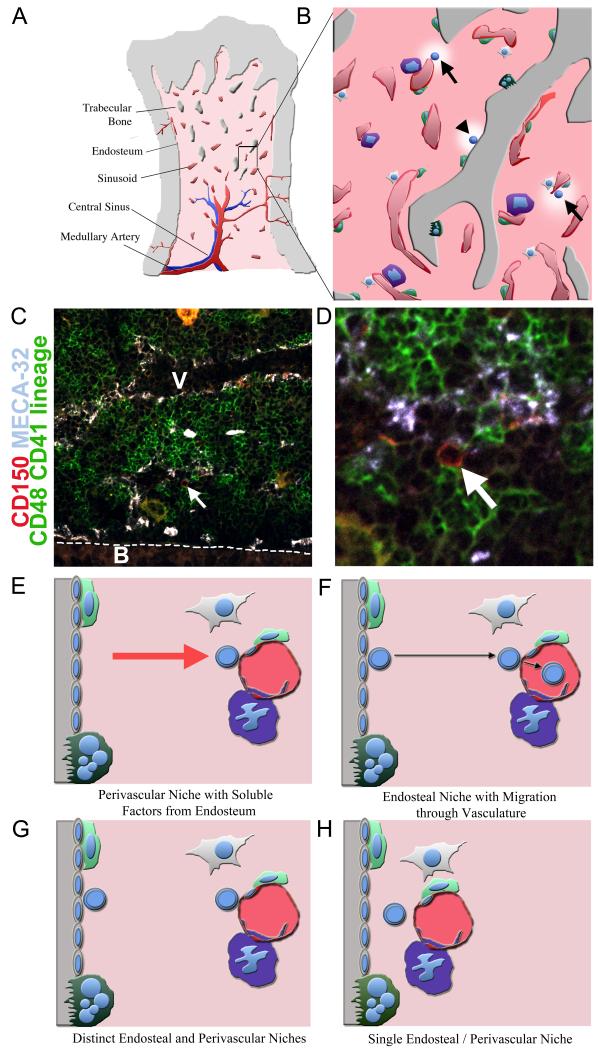
Figure 2. HSC niches.
A) Adult HSCs reside primarily within bone marrow. Bone marrow is a complex organ containing many different hematopoietic and non-hematopoietic cells. Bony trabeculae are found throughout the trabecular zone of bone, such that many cells in this region are close to bone surface. The interface of bone and bone marrow is known as the endosteum. Arteries carry oxygen, nutrients, and hematopoietic growth factors into the bone marrow, before feeding into venous circulation. Sinusoids are specialized venuoles that form a reticular network of fenestrated vessels that allow cells to pass in and out of circulation. B) A close up view of the bone marrow showing sinusoids (red), bone (grey), and hematopoietic areas (light red). Sinusoids are often associated with megakaryocytes (purple), CXCL12-expressing reticular cells (light green), and mesenchymal progenitors (white). The bone surface is covered by bone-resorbing osteoclasts (dark green) as well as bone lining cells that can differentiate into bone-forming osteoblasts. HSCs (highlighted blue cells) are found adjacent to sinusoidal blood vessels (arrows) as well as at or near the endosteum (arrowhead) (Adams and Scadden, 2006; Kiel et al., 2007b; Kiel et al., 2005; Nilsson et al., 2001). Osteoblasts and osteoclasts elaborate factors that regulate HSC maintenance and localization (Adams et al., 2006; Arai et al., 2004; Calvi et al., 2003; Kollet et al., 2006; Zhang et al., 2003). Perivascular reticular cells and mesenchymal progenitors have also been proposed to elaborate factors that regulate HSC maintenance (Sacchetti et al., 2007; Sugiyama et al., 2006). Low (C) and high (D) magnification views of a section through bone marrow showing a CD150+CD48−CD41−Lineage− candidate HSC (approximately 50% of such cells give HSC activity upon transplantation into irradiated mice (Kiel et al., 2005); see arrow; this cell is red but not green) that is adjacent to a sinusoid (outlined by white (C) or light blue (D) Meca-32+ endothelial cells) and that is near but not at the endosteum (highly vascularized and outlined with a dotted line). Bone is marked with a ‘B’ and a large blood vessel lumen is marked with a ‘V’. Most differentiating hematopoietic cells stain green. Large yellow to orange cells are megakaryocytes. Fours possible niche models are consistent with available data. E) HSCs (round blue cells) may reside in perivascular niches in which HSCs adhere to perivascular cells but are influenced by soluble factors released by nearby endosteal cells. F) HSCs may reside in endosteal niches, but frequently migrate through perivascular environments where the cells may be regulated by perivascular cells. G) HSCs may reside in spatially distinct endosteal and perivascular niches that may or may not be functionally equivalent. H) HSCs may reside in a single type of niche that is created by both endosteal and perivascular cells.
The existence of facultative niches in the hematopoietic system may be critical to facilitate the migratory nature of HSCs. HSCs appear to constantly recirculate from one bone marrow compartment to another (i.e. femur to tibia) (Wright et al., 2001). It would appear that these recirculating HSCs move from one facultative niche to another, stochastically selecting among a wide variety of locations that are capable of supporting their maintenance. Of course it remains to be determined whether all of these endosteal and sinusoidal locations actively promote the maintenance of HSCs, or whether HSCs simply pass through some of these locations during their migration.
This ability to activate facultative niches may underlie the remarkable capacity of the hematopoietic system to dramatically expand stem cell numbers and hematopoiesis in response to stress. The spleen and liver contain few stem cells and little hematopoiesis under normal conditions, but stresses that induce increased hematopoiesis can activate high levels of extramedullary hematopoiesis in these organs. For example, hematopoietic malignancies that displace bone marrow hematopoiesis often lead to the relocation of most hematopoiesis to the spleen and liver. When this occurs, greatly expanded numbers of HSCs and other hematopoietic progenitors can be found within these organs. This demonstrates that these organs are able to activate facultative niches that can support the long-term maintenance of HSCs and hematopoiesis. The precise nature of these niches remains largely uncharacterized, but like in bone marrow, HSCs within the hematopoietic spleen are observed primarily around sinusoids (Kiel et al., 2005), raising the possibility of a perivascular niche. The ability to activate facultative niches is not limited to the hematopoietic system as injury of adult skin can lead to the formation of new hair follicles that become colonized by stem cells (Ito et al., 2007). The ability to dynamically redistribute and activate new niches may be an important strategy underlying the regenerative capacity of metazoans.
Niche mechanisms: primary maintenance signals
All three characterized GSC niches maintain resident stem cells in an undifferentiated state using a major short-range intercellular signal, which surprisingly, differs in each case. Local signaling both constrains the total number of stem cells that can be maintained and in the Drosophila GSC niches ensures that one of the two daughters of each stem cell division will lie outside the niche and differentiate (Figure 3). In the testis, JAK/STAT signal reception is restricted primarily by the localized expression of the Unpaired ligand in hub cells (Kiger et al., 2001; Tulina and Matunis, 2001). Similarly, localized Unpaired expression in the terminal filament and cap cells appears to stimulate BMP ligand expression within somatic cells of the ovarian niche, leading to a restricted zone of high BMP signaling capacity (Lopez-Oneiva et al., 2008). C. elegans GSCs require Notch signals from the distal tip cells to be maintained (Kimble and Crittenden, 2007). Since the lag-2 ligand is produced in the distal tip cell, it is available only at the distal end of the gonad, which plausibly accounts for the size of the niche. It is not known if the length of the distal tip cell cytoplasmic processes that extend from the gonad tip are responsible for determining how far the niche extends (reviewed in (Kimble and Crittenden, 2007).
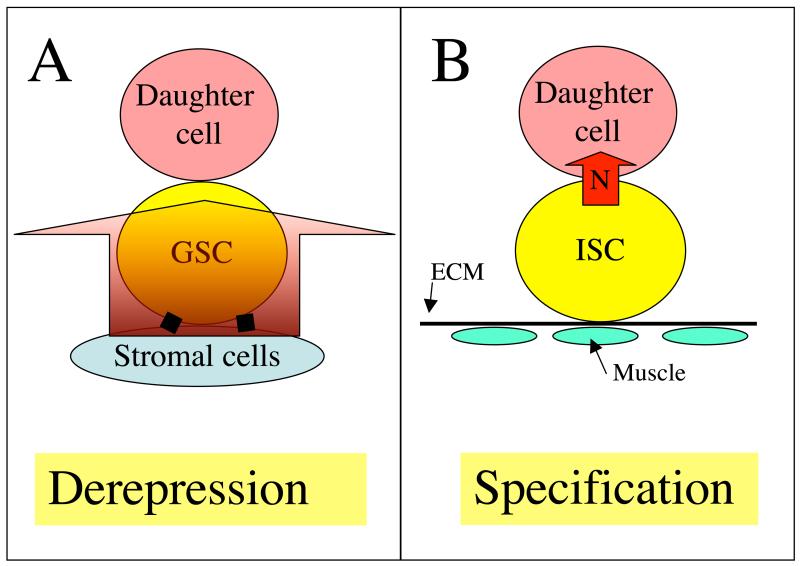
Figure 3. Two general classes of stem cell asymmetry.
A) The preprogrammed differentiation of the first class of stem cell, that includes Drosophila GSCs, is only repressed by a local signal (red arrow) generated by nearby stromal cells. Daughter cells become derepressed by becoming displaced from the repressive signal within the niche and differentiate. Mammalian GSCs may use a related mechanism as they are preferentially found near (but not attached to) hormone-producing interstitial cells (Yoshida et al., 2007a). B) A second class of stem cell requires signals to differentiate, such as the Drosophila intestinal stem cell (ISC) pictured. ISCs divide asymmetrically such that only one daughter receives a (Notch) signal that specifies its fate (Ohlstein and Spradling, 2007). Asymmetric partitioning of Notch activity also maintains Drosophila and mouse neural progenitors (Lee et al., 2006; Petersen et al., 2004).
The identities of the key factors that maintain mammalian stem cells and the cell types that produce them are less well known. For example, hedgehog signaling is required to maintain neural stem cells in the forebrain subventricular zone, but it is unclear exactly what cells are producing hedgehog, whether they are a specialized niche component or whether expression is generalized throughout the subventricular zone (Ahn and Joyner, 2005; Balordi and Fishell, 2007). Complex models are often proposed to describe HSC niches but most elements of these models have not been tested genetically. In contrast, there is strong genetic evidence for the critical roles played by Wnt signaling and BMP signaling in the regulation of epithelial stem cells in the hair follicle, though the sources of Wnts and BMPs remain uncertain (Blanpain and Fuchs, 2006). One limitation is that the lack of single cell resolution in the imaging of most mammalian stem cells in vivo creates uncertainty about the identity of the supporting cells with which stem cells interact. The size and complexity of mammalian tissues also makes it daunting to conditionally delete regulatory genes from each potential supporting cell to directly determine the source of critical signals.
The hematopoietic system poignantly illustrates this challenge as most or all of the factors that regulate HSC maintenance are expressed by multiple cell types in different regions of the bone marrow. CXCL12 is required for the maintenance of bone marrow HSCs and is expressed by both perivascular and endosteal cells (Kollet et al., 2006; Sacchetti et al., 2007; Sugiyama et al., 2006). Are there multiple, redundant sources of CXCL12 in the bone marrow, or is one cell type the major source of CXCL12 for HSC maintenance? Angiopoietin-1, a factor proposed to regulate HSC quiescence (Arai et al., 2004), is another example. Angiopoietin-1 expression has been attributed to both osteoblasts at the endosteum (Arai et al., 2004) and to perivascular mesenchymal progenitors (Sacchetti et al., 2007). The complexity of the bone marrow means that it will ultimately be necessarily to conditionally delete factors from a number of different cell types to determine which cells play major roles in regulating HSC maintenance. So far, none of the factors that are thought to regulate HSC maintenance have been conditionally deleted from particular cell types to determine which cell is the physiologically important source.
Niche mechanisms: additional signals
In addition to a major primary signal that acts directly on stem cells to promote their maintenance, many niches and stem cells have been shown to depend on additional signals whose action is less well understood and which may function indirectly to maintain niche integrity. For example, besides a primary Hh signal (Forbes et al., 1996), the FSC niche requires Wg and BMP signaling (Kirilly et al., 2005; Song and Xie, 2003). The male GSC niche, in addition to JAK/STAT, requires the BMP and Notch pathways (Kawase et al., 2004; Shivdasani and Ingham, 2003; Song et al., 2007; Ward et al., 2006). Detailed studies of the Drosophila GSC niches reveal hierarchies among the multiple signals. JAK/STAT signaling lies at the top, and is used by key niche cells to stimulate other niche cells to signal GSCs via the BMP pathway (Decotto and Spradling, 2005; Lopez-Oneiva et al., 2008). In contrast, Notch-mediated signals are sent from GSCs to niche cells (Song et al., 2007; Ward et al., 2006). Niches inherently involve multiple interacting cell types; there are several conversations going on simultaneously. The male and female GSC niches employ the same two major signaling pathways, but in the male JAK/STAT signaling acts directly on GSCs as well as via other niche cells (López-Onieva et al. 2008). This may represent an example of how niche circuitry has adapted to the different regulatory requirements of male versus female gamete production.
The regulation of secondary niche signals may have implications for the recovery of mammalian tissues from stress. In the hematopoietic system, Notch signaling (Calvi et al., 2003) and Wnt signaling are sufficient to promote adult HSC self-renewal in culture (Reya et al., 2003; Willert et al., 2003). Yet in vivo, conditional deletion of the relevant Notch receptor and ligand (Mancini et al., 2005) or conditional deletion of ß-catenin and γ-catenin (Koch et al., 2007) does not affect adult HSC maintenance. One possibility is that redundant signals promote HSC self-renewal in vivo such that maintenance of the tissue does not depend upon any one signal. Along these lines, Notch and Wnt signaling may be physiologically necessary for recovery from certain stresses but not for adult HSC maintenance under steady state conditions. Distributing responsibility for HSC maintenance across multiple primary and second signals, each of which is more or less important under different conditions, would confer flexibility and robustness.
Niche mechanisms: divisional asymmetry
Niches also share a requirement for a system to ensure that stem cells remain in the niche following stem cell division. In all the characterized invertebrate niches except the C. elegans niche, stem cell divisions are usually asymmetric: one daughter cell remains in the niche and one exits and differentiates. Two basic mechanisms for ensuring asymmetry are known, daughter derepression and daughter induction (Fig. 3). In addition, to maintaining stem cells during division, stem cell niches must prevent external cells from gaining entry and displacing the resident stem cells (Nystul and Spradling, 2007). The molecular basis of this exclusion remains poorly understood, but the need to discharge daughters without admitting competitors may have contributed to the evolution of niche structure as well as self/non-self recognition by the immune system (Laird et al., 2005).
It remains unclear whether mammalian stem cells sustain themselves by undergoing asymmetric divisions that leave one stem cell within the niche while a second daughter exits to differentiate. The divisions of fetal progenitors in the nervous system (Gotz and Huttner, 2005) and skin (Lechler and Fuchs, 2005) have been analyzed, suggesting that these early progenitors undergo symmetric and asymmetric divisions during the course of organogenesis. However, it remains technically difficult to directly image the divisions of rare stem cells within adult niches. For example, in the hematopoietic system, stem cells represent only 0.003% of cells and only 2% of these cells are actively dividing at any one time. Finding these cells is like looking for a needle in a haystack. Nonetheless, a recent fate-mapping study of muscle satellite cells showed that a subset of these cells are Pax7+Myf5− stem cells that undergo asymmetric self-renewing divisions on muscle fibers, giving rise to a basal Pax7+Myf5− stem cell daughter and an apical Pax7+Myf5− satellite cell with a more restricted proliferative potential (Kuang et al., 2007). Overall, the evidence suggests that mammalian stem cells employ both symmetric and asymmetric divisions to regulate their numbers and tissue homeostasis, and that neither mechanism is likely to be constitutively employed as a way of sustaining the niche (Morrison and Kimble, 2006).
Maintaining stem cell vitality: hierarchies, immortal strands and competition
A longstanding goal has been to identify biological processes that promote a stem cell’s ability to self-renew over the long lifespan of metazoans such as humans. DNA damage occurs each time the genome is replicated, and inactivation of DNA repair pathways frequently leads to premature stem cell depletion, resembling premature aging (Ito et al., 2004). Mammalian stem cells have surveillance mechanisms that detect unresolved DNA damage and that eliminate the cells in which this occurs, possibly by activation of p53 or Rb-mediated senescence (Collado et al., 2007). The increased incidence of cancer observed in response to mutations that inactivate these senescence mechanisms suggests that senescence is induced to avoid carcinogenesis.
Organizing cell production into a stem cell hierarchy can greatly decrease the maximum number of cell divisions stem cells must undergo. For example, a set of reserve stem cells might only replicate periodically, while their daughters are used to replenish a set of transit amplifying progenitors that function during the remainder of each cycle. Despite these theoretical advantages, systems that maintain a true hierarchy like that in the hair follicle appear uncommon. The CBCs in the mouse intestine divide at about the same rate as downstream cells and continue throughout life (Barker et al. 2007). Most, if not all, HSCs simultaneously contribute to hematopoiesis (Harrison et al., 1987), though these cells give rise to at least two populations of more rapidly dividing multipotent progenitors with limited self-renewal potential, creating a hierarchy of multipotent progenitors (Morrison et al., 1997). The model invertebrate stem cells studied to date also divide at about the same rate as cells farther down the lineage.
Asymmetrically dividing stem cells have been proposed to slow their accumulation of mutations by selectively segregating newly synthesized DNA strands to differentiating daughter cells, while retaining older DNA strands in stem cell daughters (Cairns, 1975). Experimental evidence has been provided in support of this “immortal strand hypothesis” in a variety of systems (reviewed in (Rando, 2007)), though these have tended to be systems in which it is not yet possible to definitively distinguish stem cells from other types of progenitors. Analyses of highly purified HSCs (Kiel et al., 2007a) and C. elegans germline stem cells (Kimble and Crittenden, 2007) failed to detect any evidence of asymmetric segregation of older and younger DNA strands. These studies indicate that asymmetric strand segregation cannot be a general feature of stem cells, though it may occur in some systems. Critical questions are whether asymmetric strand segregation occurs in stem cells or in other progenitors from these systems and whether this process really serves to slow the accumulation of mutations or whether it exists to serve a very different purpose.
Stem cell replacement has recently been suggested as a mechanism by which damaged or prematurely differentiated stem cells are selectively removed from their niches (Nystul and Spradling, 2007). Some wild type invertebrate stem cell types are regularly displaced (Margolis and Spradling, 1995) and the ability to lineage label mammalian stem cells is revealing what may be similar events (Nakagawa et al., 2007). The stem cell supplying the replacement may lie nearby (Xie and Spradling, 2000; Jin et al. 2008), or a daughter cell may migrate over a distance, suggesting that it can actively home in on an appropriate niche (Nystul and Spradling, 2007). Competence to serve as a replacement stem cell persists in daugher cells for at least three divisions downstream from male and female Drosophila GSCs (Brawley and Matunis, 2004; Kai and Spradling, 2004). However, for stem cell replacement to serve as a damage reduction mechanism it must be shown that wild type daughter cells preferentially replace stem cells with recently acquired deleterious mutations. Differences in adhesivity, and to a lesser extent cellular growth rate, determine the replacement hierarchy of GSCs (Jin et al. 2008). Since adhesivity declines following the onset of differentiation, the system may ensure the replacement of stem cells that begin to prematurely differentiate and slow their division.
Molecular asymmetry during stem cell division
The dramatic divergence in the fates of a stem cell’s two daughters has spurred many searches for molecular asymmetries at mitosis (reviewed in (Morrison and Kimble, 2006). Cells are capable of asymmetrically segregating proteins, RNAs, organelles, DNAs, and damaged molecules. The existence of asymmetric inheritance during stem cell division is not sufficient to demonstrate function, however. The fusome is preferentially retained in the stem cell at division, yet genetic disruption of the fusome does not affect GSC maintenance or division, but does prevent progeny germ cells from differentiating properly. Consequently, the challenge has been to identify asymmetries at stem cell division and show that they are functionally important for the outcome of division. One good example is the asymmetric segregation of atypical protein kinase C among asymmetrically dividing Drosophila neuroblasts: cells that inherit atypical protein kinase C inherit stem cell identity (Lee et al., 2006).
One of the best-established asymmetries of stem cell division with an important functional role is spindle orientation. Regulated spindle orientation during stem cell division ensures that daughters end up in different signaling environments, as in Drosophila GSCs. Programmed changes in divisional orientation figure prominently during neuroblast and SOP development as well (reviewed in (Rogers et al., 1994)). The genetic basis for this orientation has been extensively studied (Siller et al., 2006) and has been shown to play a role in cell fate determination (Egger et al., 2007). In the Drosophila testis the GSC normally divides perpendicular to the hub. This ensures that one cell will remain attached to the hub and will continue as a stem cell, while the other will receive less Upd signal from the hub, and will differentiate as a gonialblast. When the normal GSC spindle orientation is disrupted, daughter cell fates are frequently altered (Yamashita et al., 2003).
Other stem cells regulate spindle orientation using different mechanisms. GSCs and escort stem cells in the Drosophila ovary divide away from the cap cells, either by a specific system of divisional orientation (Deng and Lin, 1997) or by the balance of mechanical and adhesive forces in the vicinity of the cap cells. Such an orientation ensures that the downstream daughter (the prospective cystoblast) receives a lower BMP signal than the cap-cell associated daughter (the prospective GSC) leading to the de-repression of the cystoblast determinant bam. However, the gene products that function in spindle orientation in male GSCs such as APC2 are not observed in female GSCs (reviewed in (Fuller and Spradling, 2007). Drosophila ISCs divide in a programmed direction with respect to the basement membrane (Ohlstein and Spradling, 2007), but orient their spindles about 30±15 degrees from parallel. Neither the genetic basis nor the significance of this directional control is currently known. FSCs also divide away from the basement membrane, and also at an angle less than 90° (Nystul and Spradling, 2007).
The immortal centrosome hypothesis
Spindle orientation in Drosophila depends on epithelial or planar cell polarity, G protein signaling, and the actin cytoskeleton. Specific proteins and RNAs can be segregated cortically to positions where they will be inherited primarily by one daughter or the other. Differences associated with the centrosomes can also be brought into play. In principle, inherent structural differences between the maternal centrosome and daughter centrosomes render every cell division asymmetric. After the contractile ring forms, cell abscission requires that extracellular vesicles generated in just one of the cells fuse to the midbody ring (Gromley et al., 2005). Centriolin anchors complexes required for this vesicle fusion and integrates fusion with abscission. It is easy to see how such differences might be amplified and utilized to differentially program daughter cell fates. For example, the recycling endosome segregates asymmetrically in association with one of the two centrosomes in sensory organ precursors, biasing Notch signal reception (Emery et al., 2005). Such a mechanism might explain the differential stability of Delta in the daughters of ISC division (Ohlstein and Spradling, 2007).
The Drosophila male GSC preferentially inherits and retains the maternal centrosome as long as it remains in the niche, while the daughter centrosome migrates to the opposite pole of the cell during division and it is inherited by the daughter cell fated to differentiate (Yamashita et al., 2007). During this period, the GSC divides perpendicular to the hub, due to the association of the mother centrosome with APC protein located at the GSC-hub cell junction (Yamashita et al., 2003). Maternal centrosomes support a more robust microtubule array that facilitate its association with the hub during interphase, allowing daughter centrosomes to migrate to the opposite side of the cell prior to division. This ensures that one pole of the spindle is adjacent to the hub, and that the spindle orients perpendicular to the niche, such that one daughter cell remains within the niche and the other daughter cell is displaced from the nice (and therefore fated to differentiate). The discovery that male GSCs contain an “immortal” centrosome raises the possibility that preferential centrosome inheritance is a conserved stem cell mechanism. It remains unclear, however, whether other stem cells exhibit this same behavior or whether maternal centrosome retention by GSCs is part of the cause or simply an effect of its spindle orientation mechanism.
Niches in disease: cancer stem cell niches
There is abundant evidence that the normal cells that surround and infiltrate tumors secrete factors that promote the growth and progression of cancer (Tlsty and Coussens, 2006). In this regard, neoplastic cells do not fundamentally differ from normal progenitors in the sense that they remain partially dependent upon environmental factors that are synthesized by other cells. A striking example of this comes from neurofibromatosis in which tumors arise from neurofibromin(Nf1)-deficient Schwann cells in peripheral nerves. Tumorigenesis by Nf1-deficient Schwann cells is greatly facilitated by clonally unrelated Nf1+/− cells in the environment, like fibroblasts and mast cells that are recruited to the tumor (Yang et al., 2006; Yang et al., 2003). Nf1−/− Schwann cells are less tumorigenic in wild-type environments, apparently because wild-type fibroblasts and mast cells are not recruited as efficiently or less able to secrete factors that promote tumor growth (Zhu et al., 2002). Similar phenomena are observed in the hematopoietic system where myeloproliferative disease can arise as a result of mutations that only affect the bone marrow microenvironment (not in the hyperproliferative hematopoietic cells themselves) (Walkley et al., 2007a) or from mutations that are required both in the hyperproliferative hematopoietic cells as well as in non-hematopoietic cells in the environment (Walkley et al., 2007b). Beyond affecting tumor growth, normal cells can also regulate metastasis: mesenchymal cells in or around tumors can increase the metastasis of breast cancer cells (Karnoub et al., 2007). These studies demonstrate profound effects of unrelated cells in the environment on the behavior of cancer cells.
Over the past several years it has become clear that the growth of at least some cancers is driven by cancer stem cells, particularly malignant cancer cells that are more tumorigenic than other cancer cells (Pardal et al., 2003; Reya et al., 2001). This means that many cancers are hierarchically organized, much like normal tissues, with infrequent stem cells at the top of the hierarchy that both self-renew to form more cancer stem cells and undergo epigenetic changes (“differentiate”) to form phenotypically diverse cancer cells with limited proliferative potential. The tumorigenic cancer stem cells often have phenotypic and functional characteristics similar to normal stem cells in the same tissue (Bonnet and Dick, 1997; Lessard and Sauvageau, 2003; Singh et al., 2004). This raises the question of whether cancer stem cells depend upon specialized microenvironments for their maintenance, just like normal stem cells.
For the most part, it has not yet been possible to address this question carefully because we are still trying to identify markers that definitively distinguish cancer stem cells from normal cells and other cancer cells. However, there is some evidence that supports this proposition in the context of brain tumors. Endothelial cells secrete factors that promote the self-renewal of normal neural stem cells in culture (Shen et al., 2004) and dividing neural progenitors sometimes reside close to blood vessels in the brain (Palmer et al., 2000). Recent work on brain tumor stem cells suggests that these cells tend to reside closer to blood vessels than other brain tumor cells and that vascular cells promote the maintenance of brain tumor stem cells in culture and promote tumorigenesis in vivo (Calabrese et al., 2007). These results raise the possibility that anti-cancer therapies might be more effective by targeting the microenvironments in which cancer stem cells reside in addition to the cancer cells themselves. Since cancer cells are characteristically less dependent upon survival factors and less restrained in their expansion than normal stem cells, they are unlikely to obligately depend upon niches. Nonetheless, it is conceivable that supportive niches contribute to therapy resistance by supplying growth factors that enhance the survival of tumorigenic cancer cells during therapy.
Niches in disease: the aging of niches
A fundamental characteristic of aging is the reduced regenerative capacity of aging tissues and this is at least partially attributable to changes in the niche with age. In the Drosophila testis, the number of spermatogonial stem cells, their mitotic activity and the number of progeny they generate all decline significantly with age (Wallenfang et al., 2006). These changes are partially attributable to changes within the niche as hub cells from older males express reduced levels of DE-cadherin and Upd, both of which are necessary for GSC maintenance (Boyle et al., 2007). Forced over-expression of Upd in the hub cells of older males rescues the age-related decline in GSC frequency. In the Drosophila ovary, there is also a decline with age in stem cell frequency and function that is partially attributable to reduced expression of E-cadherin and BMP within the niche, factors required for GSC maintenance (Pan et al., 2007). Additional work will be required to characterize the effect of aging on mammalian stem cell niches.
PERSPECTIVE.
The environmental mechanisms that regulate stem cell function are steadily being elucidated in invertebrate systems where stem cells from a variety of tissues can be imaged and genetically modified. In vertebrate tissues there has also been impressive progress, though technical advances will be required in most vertebrate systems to improve our ability to image stem cells with single cell resolution: that is, with the confidence that individual cells are likely to be bona fide stem cells based on the markers they express rather than simply members of a population that is somewhat enriched for stem cell activity. It will also be important to more systematically test mechanisms that are proposed to regulate stem cell maintenance using genetics: are proposed mechanisms really necessary for stem cell maintenance under physiological conditions in vivo and can niche cells be identified by conditionally deleting potential maintenance factors from specific cell types that reside nearby the stem cells. Until these demanding goals are achieved, models of vertebrate stem cell niches will remain somewhat speculative. By better understanding the physiological mechanisms that regulate stem cell maintenance new strategies can be developed to promote tissue regeneration after injury, to maintain stem cell activity during aging, and to sensitize cancer stem cells to therapy. Clearly, fundamental scientific and medical questions reside within the niche.
Table 2. Some examples of well-characterized mouse stem cell niches.
| Stem cell | Location | Supporting cells | Major signals | Stem cells/niche |
Recent References |
|---|---|---|---|---|---|
| HSCs | Endosteal, perivascular |
Osteoblasts, osteoclasts, mesenchymal progenitors, reticular cells |
CXCL12; SCF; Tpo; Notch; Wnt; SHH; Ang1 |
1 | (Adams and Scadden, 2006) |
| Satellite muscle cell | Under basal lamina on myofiber |
Myofiber? | Wnt; Notch; HGF; CXCL12 |
1 | (Dhawan and Rando, 2005) |
| CNS SVZ stem cell | SVZ | endothelial; ependymal? |
SHH; Notch; Wnt; TGFα; FGF; VEGF; |
many | (Doetsch, 2003) |
| Intestinal epithelium | base of crypt | fibroblasts?, hematopoietic cells? |
Wnt; Notch; BMP | 4-6 | (Barker et al., 2007) |
| Hair follicle bulge | bulge | Vascular? | Wnt; BMP; TGFß | many | (Blanpain and Fuchs, 2006) |
| Matrix stem cells in hair shaft |
basement membrane above dermal papillae |
Dermal papillae | SHH; BMP; Notch; Wnt |
many | (Legue and Nicolas, 2005) |
| Interfollicular epidermis |
basal layer | Dermis | Wnt; Notch | ? | (Clayton et al., 2007) |
| Spermatogonial | basal layer, seminiferous tubules |
Leydig, sertoli, vascular |
BMP4; BMP8b; SCF; FGF; GDNF |
? | (Yoshida et al., 2007a; Yoshida et al., 2007b) |
SVZ = lateral ventricle subventricular zone. Note that the critical signals that maintain mammalian stem cells and the sources of these signals are usually not sufficiently characterized to reliably categorize these niches as stromal or epithelial.
ACKNOWLEDGEMENTS
This work was supported by the Howard Hughes Medical Institute. Thanks to Mark Kiel for assistance with figures. We apologize to authors whose work could not be cited due to space constraints.
REFERENCES
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nature Immunology. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Bach EA, Ekas LA, Ayala-Camargo A, Flaherty MS, Lee H, Perrimon N, Baeg GH. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007;7:323–331. doi: 10.1016/j.modgep.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Balordi F, Fishell G. Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J Neurosci. 2007;27:5936–5947. doi: 10.1523/JNEUROSCI.1040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007 doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 2002;35:643–656. doi: 10.1016/s0896-6273(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Medicine. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Boyle M, Wong C, Rocha M, Jones DL. Decline in self-renewal factors contributes to aging of the stem cell niche. Cell Stem Cell. 2007;1:458–469. doi: 10.1016/j.stem.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, Jones PH. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Crittenden SL, Leonhard KA, Byrd DT, Kimble J. Cellular analyses of the mitotic region in the Caenorhabditis elegans adult germ line. Mol Biol Cell. 2006;17:3051–3061. doi: 10.1091/mbc.E06-03-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction. 2001;121:347–354. doi: 10.1530/rep.0.1210347. [DOI] [PubMed] [Google Scholar]
- Decotto E, Spradling AC. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev Cell. 2005;9:501–510. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Deng W, Lin H. Spectrosomes and fusomes anchor mitotic spindles during asymmetric germ cell divisions and facilitate the formation of a polarized microtubule array for oocyte specification in Drosophila. Dev Biol. 1997;189:79–94. doi: 10.1006/dbio.1997.8669. [DOI] [PubMed] [Google Scholar]
- Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15:666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231:265–278. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- Egger B, Boone JQ, Stevens NR, Brand AH, Doe CQ. Regulation of spindle orientation and neural stem cell fate in the Drosophila optic lobe. Neural Develop. 2007;2:1. doi: 10.1186/1749-8104-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, Gaitan MG, Knoblich JA. Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;122:763–773. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Forbes AJ, Lin H, Ingham PW, Spradling AC. hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development. 1996;122:1125–1135. doi: 10.1242/dev.122.4.1125. [DOI] [PubMed] [Google Scholar]
- Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- Gabay L, Lowell S, Rubin LL, Anderson DJ. Deregulation of dorsoventral patterning by FGF confers trilineage differentiation capacity on CNS stem cells in vitro. Neuron. 2003;40:485–499. doi: 10.1016/s0896-6273(03)00637-8. [DOI] [PubMed] [Google Scholar]
- Gilboa L, Lehmann R. Soma-germline interactions coordinate homeostasis and growth in the Drosophila gonad. Nature. 2006;443:97–100. doi: 10.1038/nature05068. [DOI] [PubMed] [Google Scholar]
- Gonczy P, Matunis E, DiNardo S. bag-of-marbles and benign gonial cell neoplasm act in the germline to restrict proliferation during Drosophila spermatogenesis. Development. 1997;124:4361–4371. doi: 10.1242/dev.124.21.4361. [DOI] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Lerner C, Hoppe PC, Carlson GA, Alling D. Large numbers of primitive stem cells are active simultaneously in aggregated embryo chimeric mice. Blood. 1987;69:773–777. [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Joseph NM, Morrison SJ. Toward and understanding of the physiological function of mammalian stem cells. Developmental Cell. 2005 doi: 10.1016/j.devcel.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kai T, Spradling A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci U S A. 2003;100:4633–4638. doi: 10.1073/pnas.0830856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Kawase E, Wong MD, Ding BC, Xie T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development. 2004;131:1365–1375. doi: 10.1242/dev.01025. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, He S, Ashkenazi R, Gentry SN, Teta M, Kushner JA, Jackson TL, Morrison SJ. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007a;449:238–242. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Radice GL, Morrison SJ. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007b;1:204–217. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM Family Receptors Distinguish Hematopoietic Stem and Progenitor Cells and Reveal Endothelial Niches for Stem Cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- Kimble J, Crittenden SL. Controls of Germline Stem Cells, Entry into Meiosis, and the Sperm/Oocyte Decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol. 2007;23:405–433. doi: 10.1146/annurev.cellbio.23.090506.123326. [DOI] [PubMed] [Google Scholar]
- Kirilly D, Spana EP, Perrimon N, Padgett RW, Xie T. BMP signaling is required for controlling somatic stem cell self-renewal in the Drosophila ovary. Dev Cell. 2005;9:651–662. doi: 10.1016/j.devcel.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci U S A. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F. Simultaneous loss of {beta}- and {gamma}-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2007 doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird DJ, De Tomaso AW, Weissman IL. Stem cells are units of natural selection in a colonial ascidian. Cell. 2005;123:1351–1360. doi: 10.1016/j.cell.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Robinson KJ, Doe CQ. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature. 2006;439:594–598. doi: 10.1038/nature04299. [DOI] [PubMed] [Google Scholar]
- Legue E, Nicolas JF. Hair follicle renewal: organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development. 2005;132:4143–4154. doi: 10.1242/dev.01975. [DOI] [PubMed] [Google Scholar]
- Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- Lopez-Oneiva L, Fernandez-Minan A, Gonzalez-Reyes A. Jak/Stat signalling in niche support cells regulates dpp transcription to contol germline stem cell maintenance in the Drosophila ovary. Development. 2008;135:533–540. doi: 10.1242/dev.016121. [DOI] [PubMed] [Google Scholar]
- Mancini SJ, Mantei N, Dumortier A, Suter U, Macdonald HR, Radtke F. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood. 2005;105:2340–2342. doi: 10.1182/blood-2004-08-3207. [DOI] [PubMed] [Google Scholar]
- Margolis J, Spradling A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development. 1995;121:3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- Matsuzaki Y, Kinjo K, Mulligan RC, Okano H. Unexpectedly efficient homing capacity of purified murine hematopoietic stem cells. Immunity. 2004;20:87–93. doi: 10.1016/s1074-7613(03)00354-6. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997;124:1929–1939. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–749. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293–2299. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- Nystul T, Spradling AC. An epithelial niche in the Drosophila ovary undergoes long range stem cell replacement. Cell Stem Cell. 2007;1:277–285. doi: 10.1016/j.stem.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pan L, Chen S, Weng C, Call G, Zhu D, Tang H, Zhang N, Xie T. Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell. 2007;1:470–478. doi: 10.1016/j.stem.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem cell biology to cancer. Nature Reviews Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- Petersen PH, Zou K, Krauss S, Zhong W. Continuing role for mouse Numb and Numbl in maintaining progenitor cells during cortical neurogenesis. Nat Neurosci. 2004;7:803–811. doi: 10.1038/nn1289. [DOI] [PubMed] [Google Scholar]
- Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Rando TA. The immortal strand hypothesis: segregation and reconstruction. Cell. 2007;129:1239–1243. doi: 10.1016/j.cell.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Rogers MV, Buensuceso C, Montague F, Mahadevan L. Vanadate Stimulates differentiation and neurite outgrowth in rat pheochromocytoma PC12 cells and neurite extension in human neuroblastoma SH-SY5Y cells. NEUROSCIENCE. 1994;60:479–494. doi: 10.1016/0306-4522(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shivdasani AA, Ingham PW. Regulation of stem cell maintenance and transit amplifying cell proliferation by tgf-beta signaling in Drosophila spermatogenesis. Curr Biol. 2003;13:2065–2072. doi: 10.1016/j.cub.2003.10.063. [DOI] [PubMed] [Google Scholar]
- Siller KH, Cabernard C, Doe CQ. The NuMA-related Mud protein binds Pins and regulates spindle orientation in Drosophila neuroblasts. Nat Cell Biol. 2006;8:594–600. doi: 10.1038/ncb1412. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Song X, Call GB, Kirilly D, Xie T. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development. 2007;134:1071–1080. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- Song X, Xie T. DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc Natl Acad Sci U S A. 2002;99:14813–14818. doi: 10.1073/pnas.232389399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Xie T. Wingless signaling regulates the maintenance of ovarian somatic stem cells in Drosophila. Development. 2003;130:3259–3268. doi: 10.1242/dev.00524. [DOI] [PubMed] [Google Scholar]
- Song X, Zhu CH, Doan C, Xie T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296:1855–1857. doi: 10.1126/science.1069871. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Takano H, Ema H, Sudo K, Nakauchi H. Asymmetric division and lineage commitment at the level of hematopoietic stem cells: inference from differentiation in daughter cell and granddaughter cell pairs. J Exp Med. 2004;199:295–302. doi: 10.1084/jem.20030929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annual Review of Pathology-Mechanisms of Disease. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley CR, Olsen GH, Dworkin S, Fabb SA, Swann J, McArthur GA, Westmoreland SV, Chambon P, Scadden DT, Purton LE. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007a;129:1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007b;129:1081–1095. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenfang MR, Nayak R, DiNardo S. Dynamics of the male germline stem cell population during aging of Drosophila melanogaster. Aging Cell. 2006;5:297–304. doi: 10.1111/j.1474-9726.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- Ward EJ, Shcherbata HR, Reynolds SH, Fischer KA, Hatfield SD, Ruohola-Baker H. Stem cells signal to the niche through the Notch pathway in the Drosophila ovary. Curr Biol. 2006;16:2352–2358. doi: 10.1016/j.cub.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315:518–521. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang FC, Chen S, Clegg T, Li X, Morgan T, Estwick SA, Yuan J, Khalaf W, Burgin S, Travers J, et al. Nf1+/− mast cells induce neurofibroma like phenotypes through secreted TGF-beta signaling. Hum Mol Genet. 2006;15:2421–2437. doi: 10.1093/hmg/ddl165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang FC, Ingram DA, Chen S, Hingtgen CM, Ratner N, Monk KR, Clegg T, White H, Mead L, Wenning MJ, et al. Neurofibromin-deficient Schwann cells secrete a potent migratory stimulus for Nf1+/− mast cells. J Clin Invest. 2003;112:1851–1861. doi: 10.1172/JCI19195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Nabeshima YI, Nakagawa T. Stem Cell Heterogeneity: Actual and Potential Stem Cell Compartments in the Mouse Spermatogenesis. Ann N Y Acad Sci. 2007a doi: 10.1196/annals.1411.003. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007b;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 2007;27:8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–922. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]