Abstract
Objectives: To compare the effectiveness of lavender (Lavandula angustifolia) and sleep hygiene versus sleep hygiene alone on sleep quantity and sleep quality and to determine sustained effect at two-week follow-up.
Design: A randomized controlled trial with investigator blinding and steps taken to blind the participants.
Setting: Participants' usual sleep setting.
Subjects: Seventy-nine college students with self-reported sleep issues.
Interventions: The intervention took place over five nights with baseline, postintervention, and two-week follow-up assessments. Both groups practiced good sleep hygiene and wore an inhalation patch on their chest at night. One group wore a patch with 55 μl of lavender essential oil and the other group wore a blank patch.
Outcome measures: Sleep quantity was measured using a Fitbit® tracker and a sleep diary, and sleep quality was measured using the Pittsburgh Sleep Quality Index (PSQI) and the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) sleep disturbance short form.
Results: The lavender and sleep hygiene group demonstrated better sleep quality at postintervention and two-week follow-up (PSQI p=0 .01, <0.001 and PROMIS p=0.04, 0.007, respectively). The sleep-hygiene-only group also demonstrated better sleep quality but to a lesser extent (PSQI p=0.02, 0.06 and PROMIS p=0.03, 0.03, respectively). Additionally, a clinical effect was found for the lavender group at postintervention, along with a significant finding for waking feeling refreshed (p=0.01). Sleep quantity did not differ between groups.
Conclusions: Lavender and sleep hygiene together, and sleep hygiene alone to a lesser degree, improved sleep quality for college students with self-reported sleep issues, with an effect remaining at follow-up.
Introduction
Sleep issues are prevalent in our 24/7, nonstop society, with 25–65% of children, adolescents, and adults having sleep issues, depending on the definition of sleep issues or insomnia.1–3 Difficulty with sleep initiation, sleep maintenance, or daytime sleepiness can affect health, safety, and performance. Health effects associated with sleep problems include decreased well-being, fatigue, anxiety, depression, cardiovascular disease, hypertension, inflammation, obesity, diabetes, and impaired glucose tolerance.4 Costs of sleep issues are in the tens of billions of dollars annually.5–9 Identification of effective self-care sleep interventions is needed and primary care providers can play a key role in recommending self-care sleep interventions as first-line treatments for their patients with sleep issues.
Mild insomnia is frequently self-treated using over-the-counter medications, herbs, or strategies such as sleep hygiene, cognitive behavioral therapy, and sleep restriction therapy that modify its precipitating and contributory factors.10 More severe insomnia is treated with hypnotic drugs that are considered safe for short-term use, but are often prescribed long-term and have many side effects.11–16 Despite the use of a variety of treatments, short-term insomnia frequently becomes chronic insomnia.17 Additionally, many people do not treat their insomnia.1 Both ineffective treatment and lack of treatment contribute to the development of chronic insomnia and the prevalence of sleep issues.
Cost-effective, convenient, accessible, and safe interventions for addressing sleep issues can aid in decreasing the associated wide ranging health effects of lack of sleep. Essential oils with sedative or hypnotic properties are promising as a sleep therapy. Inhaled, their chemical constituents enter the circulatory system through the lungs and the neurochemical pathway via the limbic system.18 A systematic review of the literature on inhaled essential oils and sleep19 and 2 additional studies20,21 identified 17 studies with a wide variety of methodologies. Thirteen of the studies were randomized controlled trials (RCTs).20–32 Lavender essential oil was most frequently studied, with results trending toward a positive effect.20,21,23–26,28–31,33–36 A small to moderate benefit of lavender on sleep was found in a systematic review of the literature specific to lavender and sleep.37
Lavender (Lavandula angustifolia) essential oil was selected for the study intervention based on its documented sedative and hypnotic properties18 and its safety profile.38 College students were selected as the population of the study because early intervention in young adults can offer prevention for chronic insomnia as they become older adults. Sleep hygiene instruction, a cognitive/educational intervention, was included as usual care.
The objective of this study was to investigate the effect of inhaled lavender (L. angustifolia) and sleep hygiene on sleep quality and quantity compared to sleep hygiene alone in college students with self-reported sleep issues and to determine if any effect is sustained at two-week follow-up.
Materials and Methods
Participants and setting
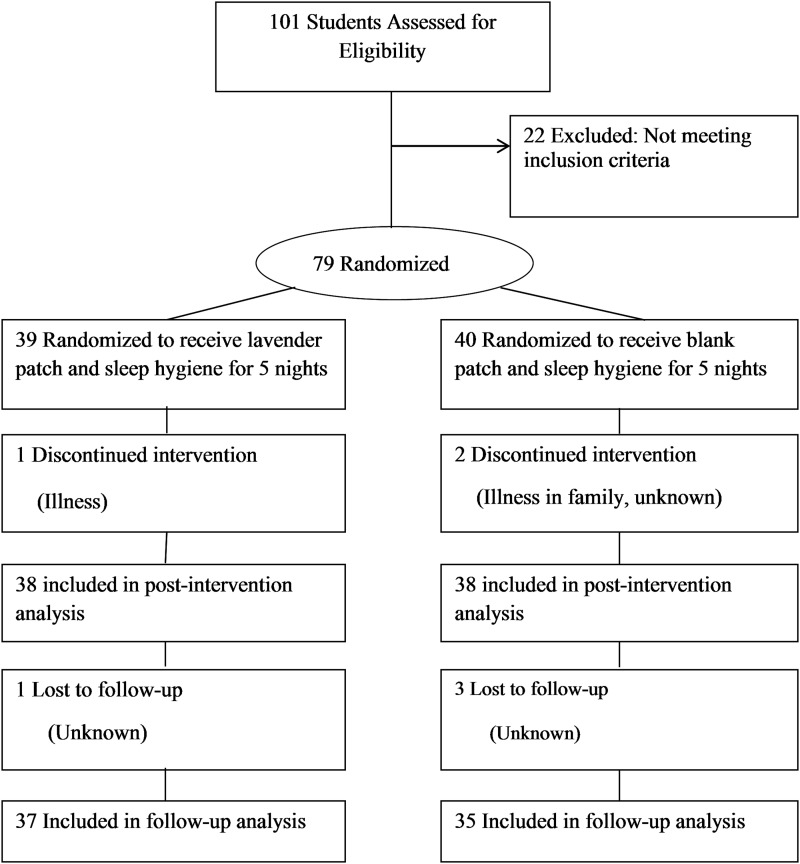
Participants were recruited (fall 2013) using flyers posted around campus and presentations of the study to health advocates in on-campus living facilities. Inclusion criteria were as follows: English-speaking college student, ≥18 years old with self-reported sleep issues (difficulty falling asleep, frequent awakenings during the night, or daytime sleepiness), and able to attend two study visits at the health center. Exclusion criteria were pregnancy, night shift work, or use of prescription sleep medications. Of the 101 individuals who completed the screening for eligibility assessment, 22 individuals were excluded (Fig. 1). The setting was the participants usual sleep setting.
FIG. 1.
Flow diagram of study.
Study design
This was a parallel-group RCT with participant and investigator blinding. Participants were randomized into one of two groups upon enrollment using a 1:1 allocation ratio. Simple randomization was completed by a noninvestigator and envelopes were used to maintain blinding. Group 1 (LSH) was assigned to use lavender patches plus sleep hygiene (n=39) and group 2 (SH) was assigned to use blank patches and sleep hygiene (n=40) for five consecutive nights. Assessments were conducted at baseline, during the intervention, postintervention, and at two-week follow-up. The study was approved by the University of Minnesota human subjects committee.
Outcome measures
Outcome variables measured included both sleep quantity and sleep quality to determine the effect of lavender on each. Participants completed sleep quality surveys (Pittsburgh Sleep Quality Index [PSQI] and the NIH Patient-Reported Outcomes Measurement Information System [PROMIS™] sleep disturbance short form 8b) and a sleep hygiene survey (SHS) at baseline, postintervention, and at two-week follow-up. During the intervention Fitbit® trackers were to be worn at night and online sleep diaries completed in the morning.
Fitbit One™ wireless activity tracking device
This device tracks sleep based on movement. It was shown to have an intradevice reliability of 96.5–99, comparable to both polysomnography and actigraphy. Similar to actigraphy, it was found to misidentify wake as sleep compared to polysomnography.39 Self-report such as a sleep diary has been recommended as a supplement.40
Daily sleep diary
This is a standard means to collect quantitative sleep data and was used to supplement the Fitbit One for sleep quantity information and to provide a daily perspective on sleep quality. The sleep diary for this study was an adaptation of the National Sleep Foundation Sleep Diary. It included questions on sleep quality, sleep disturbances, adverse effects, and adherence to use of the tracker and patch.41 Sleep diaries have been found to be more reliable than actigraphy.42
Pittsburgh Sleep Quality Index
This survey generates seven component scores: sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, sleeping medications use, and daytime functioning. The sum of these components yields a global score of 21, a higher score indicating poorer sleep quality. A PSQI score greater than five distinguishes poor sleepers and a change in three points suggests a clinical effect. This instrument has been found to have a high test–retest reliability and good validity for use with good and poor sleepers.43 It assesses sleep quality and disturbances for up to a one-month period and has been found to have higher test–retest reliability for shorter intervals.44 It is a recommended measure for treatment effectiveness studies for global sleep quality.40
NIH Patient-Reported Outcomes Measurement Information System sleep disturbance short form (SF8b, PROMIS v.1.0 www.nihpromis.org)
This survey served as an additional sleep quality instrument. This short form correlates strongly with the PROMIS sleep disturbance long form. It was developed for samples with or without sleep disorders and measures sleep quality and disturbance over a previous one-week interval. This instrument has been validated for sleep–wake function but not for responsiveness to change. The total possible score is 40, with a higher score indicating more disturbed sleep.45,46
Sleep Hygiene Questionnaire
This survey was administered to assess compliance with the recommended sleep practices.47 Total possible score was 112, with a higher score indicating poorer sleep practices. The instrument was found to have acceptable test–retest reliability, although internal reliability was poor. This may reflect that hygiene practices change over time.48
Materials
Patch
The 3 cm adhesive patch contained a 1 cm disc of absorbent material that contained 55 μl of lavender oil applied consistently by a metered pump for the lavender group and left blank for the placebo group. According to the manufacturer, the patch has a time release function allowing it to last for 6–8 hours. The patch has a skin barrier backing so that essential oils are inhaled but not absorbed standardizing the route of administration (Bioesse Technologies, LLC, Minnetonka, MN).
L. angustifolia essential oil
A gas chromatography/mass spectrometry (GC/MS) chemical analysis for the batch of lavender oil utilized in the study was provided to the PI to ensure treatment integrity. The essential oil used was chemically consistent with the International Organization for Standardization (ISO) for L. angustifolia.49
Procedure
This study was completed in 4 waves of about 20 students each. The principal investigator (PI) conducted both intake and assessments. Screened and consented respondents were scheduled for initial and postintervention sessions. Roommates who were screened and met inclusion/exclusion criteria were scheduled for different waves of the study to maintain participant blinding to the degree possible (n=2). Assessments were administered online via the PROMIS Assessment Center.
Participants were provided with six patches, a Fitbit One tracker, and detailed written and verbal instructions for the patch and the tracker. The participants were instructed to adhere the patch to the mid-upper chest and to place the tracker on their nondominant wrist before going to bed and to remove them in the morning. The patches for the two groups were packaged identically except for group code number. A noninvestigator validated code assignments so the investigator remained blinded to treatment. Participants were informed that the groups differed in the dosage of essential oil. The name of the essential oil and the exact dosages were not provided to the participants. Both groups received sleep hygiene information and were asked to practice the guidelines during the intervention. The sleep hygiene recommendations were (1) maintain a regular sleep schedule, (2) avoid fluid intake before bed and food, caffeine, alcohol, and nicotine late in the day, (3) create a good sleeping environment (e.g., wear ear plugs and a sleep mask and avoid screens and texting), (4) create a relaxing bedtime routine, (5) keep up with school work, and (6) exercise regularly. This list is based on the NIH-recommended list of sleep practices,50 with some modifications for college students.51–53
Participants were instructed to begin the intervention on Sunday evening and end it Friday morning. They received e-mail and text reminders in the evening to use the patch and Fitbit device and in the morning to complete the sleep diary. They were instructed to return the Fitbit device and any remaining patches at their postintervention session. For the follow-up assessment, a link was e-mailed to each participant 14 days after intervention completion with a reminder text message if the surveys were not completed within a day and a half.
Statistical analyses
Sample size determination
Sample size determination was based on analysis of covariance for the main outcome, the PSQI, with a 2-tailed alpha of 0.05 and a power of 80%. A clinically significant effect size was set at three based on results of other studies.43 Sample size was determined to be 25 participants in each group using a standard deviation of 4.57.43
Data analyses
Analysis was performed in SAS version 9.2, SPSS 21, and R version 2.15.1. Linear regression was used when there were single observations on an individual for the specified model and data were approximately normal. Generalized estimating equation (GEE), which accounts for the correlation that occurs on repeated measurements of an individual, was utilized for models with multiple observations over time. The most appropriate covariance structure was selected based on the nature of the correlations between the repeated measurement data and the limitations of the software (SAS allows only independent covariance structure for multinomial outcomes). In summary, appropriate models were chosen based on the distribution of the response variable, whether response variables were repeatedly measured, and the covariance structure between repeated measures.
A series of models were created beginning with full models using all parameters and subsequent models that reduced parameters based on their statistical significance in the full models. Parameters that remained in the reduced models regardless of significance were age, gender, sleep hygiene score, treatment group, patch worn, and time for longitudinal models. Results were considered significant at a 2-sided α=0.05. The NIH Assessment Center provides normalized T-scores for analysis on the PROMIS sleep disturbance questionnaires. Raw scores were used for analysis in this study because the normalized T-scores were calibrated against a sicker population than the general U.S. population, in contrast to the healthy college student sample in this study.54
Missing data were handled appropriately for each statistical method. For summary statistics missing data were ignored, and for data in long format (GEE and regression) estimates were generated for every variable based on the data present. Weighted sums and percentages were used to account for missing data on the daily sleep diary.
Results
The overall sample was two-thirds female and one-third male, the mean age was 21.6, and the majority of participants were white and not Hispanic or Latino (Table 1). The two groups were demographically similar, with race the only factor for which there was a statistically significant difference between groups (p=0.02).
Table 1.
Demographic and Clinical Characteristics of Participants
| Characteristic | Lavender patch+sleep hygiene (LSH group) (n=39) | Blank patch+sleep hygiene (SH group) (n=40) | Total (n=79) | p |
|---|---|---|---|---|
| Age, mean (range), years | 20.9 (18–28) | 22.1 (18–36) | 21.6 (18–36) | 0.09a |
| Gender, n (%) | 9.46b | |||
| Female | 25 (64) | 29 (73) | 54 (69) | |
| Male | 14 (36) | 10 (25) | 24 (30) | |
| NA | 0 (0) | 1 (2) | 1 (1) | |
| Race, n (%) | 0.02c | |||
| White | 24 (62) | 29 (73) | 53 (67) | |
| Black or African American | 0 (0) | 2 (5) | 2 (3) | |
| Asian | 13 (33) | 4 (10) | 17 (22) | |
| American Indian or Alaskan Native | 0 (0) | 1 (2) | 1 (1) | |
| Other | 0 (0) | 1 (2) | 1 (1) | |
| NA | 2 (5) | 3 (8) | 5 (6) | |
| Ethnicity, n (%) | 0.34c | |||
| Not Hispanic or Latino | 36 (92) | 31 (78) | 67 (85) | |
| Hispanic or Latino | 1 (3) | 3 (7) | 4 (5) | |
| NA | 2 (5) | 6 (15) | 8 (10) | |
| Health conditions,dn (%) | 0.18b | |||
| No | 34 (87) | 29 (73) | 63 (80) | |
| Yes | 5 (13) | 11 (27) | 16 (20) |
t-test.
Chi square.
Fisher's exact test.
Health conditions reported: depression (n=4), allergies (n=3), asthma (n=3), anxiety (n=2), ADHD (n=2), overweight (n=1), hypertension (n=1), epilepsy (n=1), cyclothymia (n=1), and vitamin D deficiency (n=1). Some participants reported more than one condition.
Participants reported use of the patch and the Fitbit each morning on the sleep diary. Although subjects reported wearing the Fitbit 92% of the cumulative person-nights (n=365), data were recoverable for only 14% of the person-nights (n=57). Despite assistance from the manufacturer and additional instruction to the participants, technical issues related to the Fitbit device resulted in unacceptable levels of missing data. Participants reported using their patches 93% of the person-nights (n=369). This number was validated by the number of patches returned. Patches were reported to have fallen off during sleep in 37% of the person nights (n=146); however, this was not a significant covariate in any of the models.
Sleep hygiene practice was assessed at baseline, postintervention, and follow-up with the SHS (Table 2); the mean total scores were 42.7 (range 20–76), 23.2 (range 0–54), and 31.5 (range 3–58), respectively, where lower scores indicating better sleep hygiene practices during the intervention than before or after. There were no statistically significant differences between groups for the SHS scores at baseline, postintervention, and follow-up (p=0.64, 0.51, and 0.79, respectively).
Table 2.
SHS Frequencies and Means: Better Sleep Hygiene Scores Posttreatment and No Differences Between Groups
| Group | Assessment | Mean SHS score | SD | Minimum SHS score | Maximum SHS score | N | p-Value between group differences |
|---|---|---|---|---|---|---|---|
| LSH group | Pre | 42.72 | 11.54 | 20 | 76 | 39 | |
| Post | 23.16 | 11.88 | 4 | 45 | 38 | ||
| Follow-up | 31.47 | 11.07 | 7 | 57 | 36 | ||
| SH group | Pre | 41.53 | 10.79 | 22 | 66 | 40 | |
| Post | 21.39 | 11.34 | 0 | 54 | 38 | ||
| Follow-up | 32.23 | 12.53 | 3 | 58 | 35 | ||
| Total | Pre | 42.11 | 11.12 | 20 | 76 | 79 | 0.64 |
| Post | 22.28 | 11.57 | 0 | 54 | 76 | 0.51 | |
| Follow-up | 31.85 | 11.73 | 3 | 58 | 71 | 0.79 |
SHS score range is 0–112, with lower scores indicating better sleep hygiene.
LSH group, lavender plus sleep hygiene group; SH group, sleep-hygiene-only group; SHS, sleep hygiene survey.
Adverse effects
Adverse effects to the intervention were reported on the daily sleep diary. Only four adverse effects of minor skin irritation were reported lasting one night for each report.
Sleep quantity
There were no statistically significant differences in sleep quantity between groups based on the sleep diary (Table 3). For both groups the number of awakenings decreased (p=0.02) and falling asleep easily increased (p=0.001). Little variability was found for sleep efficiency (number of minutes asleep/number of minutes in bed) with a majority of the results falling in the normal range near 100%.
Table 3.
Sleep Quantity Results: Postintervention
| Model | Variable | Estimate | Std. error | p |
|---|---|---|---|---|
| Total time in bed | LSH group | −11.07 | 12.28 | 0.37 |
| SHS score | −0.49 | 0.56 | 0.38 | |
| Time (days) | 3.90 | −2.33 | −0.10 | |
| Age (years) | −3.74 | 1.43 | 0.009 | |
| Gender (female) | 24.08 | 12.46 | 0.05 | |
| Total time asleep | LSH group | −11.02 | 12.19 | 0.37 |
| SHS score | −0.50 | 0.55 | 0.37 | |
| Time | 4.01 | 2.37 | 0.09 | |
| Gender (female) | 23.75 | 12.30 | 0.05 | |
| Age | −3.71 | 1.44 | 0.01 |
| Model | Variable | Relative risk | 95% CI lower/higher | p |
|---|---|---|---|---|
| Times awakened | LSH group | 1.29 | 0.86/1.93 | 0.22 |
| SHS score | 1.00 | 0.99/1.02 | 0.68 | |
| Time | .92 | 0.86/0.99 | 0.02 | |
| Treatment–time interaction | 0.87 | 0.78/0.98 | 0.02 | |
| Age (years) | 0.99 | 0.94/1.04 | 0.73 | |
| Gender (female) | 1.07 | 0.74/1.57 | 0.71 |
| Model | Variable | Odds ratio | 95% CI lower/higher | p |
|---|---|---|---|---|
| Fell asleep easily | LSH group | 1.03 | 0.61/1.73 | 0.92 |
| SHS score | 0.99 | 0.97/1.02 | 0.53 | |
| Time | 1.37 | 1.16/1.62 | 0.001 | |
| Age (years) | 0.99 | 0.94/1.04 | 0.76 | |
| Gender (female) | 0.88 | 0.49/1.59 | 0.67 |
Sleep quality
There was a statistically significant difference between groups for sleep quality, waking feeling refreshed, and daytime fatigue. The LSH group demonstrated improved sleep quality at postintervention compared to the SH group. This effect remained at two-week follow-up. The findings were consistent for both standardized tools, the PSQI (postintervention p=0.01, follow-up p≤0.001) and the PROMIS sleep disturbance tool (postintervention p=0.04, follow-up p=0.007). Those reporting better sleep hygiene practices on the SHS also demonstrated improved sleep quality but to a lesser extent than the LSH group (Table 4). Better sleep hygiene scores were associated with better sleep quality as measured by the PSQI and the PROMIS survey at postintervention (p=0.02 and 0.03, respectively) and by PROMIS at follow-up (p=0.03). The PSQI mean scores did not differ between groups at baseline (p=0.08) and were greater than 5 (LSH=8.2, SH=8.7), indicating poor sleep before the intervention. While both groups had improved sleep quality at postintervention and follow-up, the LSH group at postintervention had a mean PSQI score of 4.9, while the SH group had a mean PSQI score of 6.5. This suggests normal sleep on average at postintervention for the LSH group only. The mean change from baseline to postintervention for the LSH group was 3.2, and so the group receiving lavender demonstrated a clinical effect. No clinical effect was demonstrated for the SH group. The LSH group had less daytime fatigue at postintervention and follow-up (p=0.02 and 0.009, respectively) and was more likely to wake feeling refreshed at postintervention (p=0.01).
Table 4.
Sleep Quality Results: Postintervention and Follow-up
| Model | Variable | Estimate | Std. error | p |
|---|---|---|---|---|
| Post-PSQI global score | LSH group | −1.24 | 0.43 | 0.01 |
| Post-SHS score | 0.05 | 0.02 | 0.02 | |
| Age (years) | 0.02 | 0.05 | 0.77 | |
| Gender (female) | 0.29 | 0.50 | 0.57 | |
| Follow-up PSQI global score | LSH group | −1.65 | 0.49 | <0.001 |
| Follow-up SHS score | 0.03 | 0.02 | 0.06 | |
| Time | 0.002 | 0.03 | 0.94 | |
| Treatment–time interaction | 0.07 | 0.03 | 0.02 | |
| Age (years) | 0.04 | 0.05 | 0.39 | |
| Gender (female) | 0.25 | 0.43 | 0.55 | |
| Post-PROMIS total score | LSH group | −1.78 | 0.83 | 0.04 |
| Post-SHS score | 0.09 | 0.04 | 0.03 | |
| Age (years) | −0.12 | 0.10 | 0.24 | |
| Gender (female) | 0.22 | 0.96 | 0.82 | |
| Follow-up PROMIS total score | LSH group | −1.47 | 0.54 | 0.007 |
| Follow-up SHS score | 0.08 | 0.03 | 0.03 | |
| Time | 0.01 | 0.05 | 0.90 | |
| Age (years) | −0.14 | 0.09 | 0.12 | |
| Gender (female) | 0.66 | 0.69 | 0.34 |
| Model | Variable | Odds ratio | 95% CI lower/higher | p |
|---|---|---|---|---|
| Post-waking refreshed | LSH group | 1.87 | 1.15/3.03 | 0.01 |
| Post-SHS score | 0.99 | 0.97/1.01 | 0.32 | |
| Time | 1.18 | 1.02/1.37 | 0.03 | |
| Age (years) | 1.01 | 0.97/1.05 | 0.56 | |
| Gender (female) | 1.32 | 0.71/2.47 | 0.38 | |
| Post-daytime sleepiness | LSH group | 0.63 | 0.19/2.08 | 0.45 |
| Post-SHS score | 1.07 | 1.01/1.13 | 0.03 | |
| Age (years) | 0.86 | 0.74/0.99 | 0.04 | |
| Gender (female) | 3.22 | 0.76/13.63 | 0.11 | |
| Follow-up daytime sleepiness | LSH group | 0.92 | 0.45/1.89 | 0.83 |
| Follow-up SHS score | 1.03 | 1.00/1.07 | 0.09 | |
| Time | 1.03 | 1.00/1.07 | 0.09 | |
| Age (years) | 0.90 | 0.83/0.98 | 0.01 | |
| Gender (female) | 2.80 | 1.04/7.51 | 0.04 | |
| Post-daytime dysfunction | LSH group | 0.33 | 0.13/0.84 | 0.02 |
| Post-SHS score | 1.01 | 0.96/1.06 | 0.67 | |
| Age (years) | 0.96 | 0.85/1.09 | 0.56 | |
| Gender (female) | 1.56 | 0.52/4.72 | 0.43 | |
| Follow-up daytime dysfunction | LSH group | 0.18 | 0.05/0.66 | 0.009 |
| Follow-up SHS score | 1.03 | 1.00/1.07 | 0.09 | |
| Time | 0.93 | 0.86/1.00 | 0.06 | |
| Treatment–time interaction | 1.10 | 1.01/1.20 | 0.03 | |
| Age (years) | 0.98 | 0.89/1.08 | 0.75 | |
| Gender (female) | 0.84 | 0.38/1.82 | 0.66 |
Discussion
This RCT with participant and investigator blinding examined the effect of inhaled L. angustifolia and sleep hygiene on college students with self-reported sleep issues and found improved sleep quality in the group receiving lavender at postintervention and at two-week follow-up. Better sleep practices were also independently associated with sleep quality but to a lesser degree. The findings were consistent on all assessment tools and with the literature. Due to the variety of methodologies, direct comparison to previous studies cannot be made. Inclusion of follow-up assessment at two weeks added an important new element to the literature. The persistent effect of lavender on sleep quality at two-week follow-up suggests a re-balancing or long-acting effect on the sleep cycle, although the exact mechanism of action is unknown. No difference between groups was found for sleep quantity, although both groups reported falling asleep easily and less awakenings at postintervention. Participants reported high adherence to the study protocol and few side effects. The findings suggest that the use of lavender and sleep hygiene is a safe and effective intervention for sleep in college students with self-reported sleep issues.
The study had several limitations. The impact of the racial difference between groups is unclear. The missing Fitbit data resulted in only one valid measurement of sleep quantity, the sleep diary, confirming the importance of using supporting measures where possible. Newer versions of personnel trackers may be more valid for research. The self-report nature of the data is a limitation; however, the instruments used are well tested in many populations. Although the patches did not uniformly remain adhered to the skin overnight, this did not prevent inhalation, as supported by the results. The specific amount inhaled cannot be guaranteed using these methods, which were chosen to provide a somewhat standardized dose in the home setting without specialized equipment. Blinding to smells is difficult and this may have been a factor. This study took steps to blind participants and the investigator was blinded.
Methodological strengths of this study contribute to the sleep self-care and clinical intervention literature. Product integrity verification of the lavender essential oil through GC/MS analysis validated treatment integrity. If not done, doubt is cast on the results because commercially available essential oils may be adulterated. Very few and minor adverse effects were reported, mainly related to the adhesive on the patches rather than the lavender essential oil, supporting its safety. The use of the online Assessment Center for standard sleep measurement tools and managing study flow likely contributed to the high participant adherence to protocols, and these can be replicated and refined in future studies. The use of the participants' normal sleep setting increased external validity, and a follow-up assessment provided important information on the duration of the effect of the intervention.
Conclusions
This RCT supports the use of lavender and sleep hygiene as safe, accessible, and effective interventions for self-reported sleep issues in college students. Further research to study their effect on other populations and additional studies exploring the duration of intervention effects are needed. Safe, effective, self-care interventions such as lavender (L. angustifolia) and sleep hygiene are viable as a first-line intervention for sleep issues.
Acknowledgments
Financial and material support was provided by funding through the School of Nursing, Wladimir and Paulina Zenkovich Nursing Fellowship, and the Sophia Fund. The Sigma Theta Tau, Zeta Chapter, provided funding for statistical analysis and dissemination. Boynton Health Service, University of Minnesota, provided funding for Fitbit trackers, logistical assistance with recruitment, and space. Wyndmere Naturals, Inc., and Bioesse Technologies, LLC, supplied materials for this study.
Author Disclosure Statement
No competing financial interests exist for any of the authors. None of the sponsors or funders had any involvement in the design or conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the article.
References
- 1.National Sleep Foundation. 2009 Sleep in America Poll. Online document at: www.sleepfoundation.org/sleep-polls-data/sleep-in-america-poll/2009-health-and-safety, accessed October20, 2014
- 2.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; international statistical classification of diseases and related health problems, tenth revision; and research diagnostic Criteria/International classification of sleep disorders, second edition criteria: Results from the America Insomnia Survey. Biol Psychiatry 2011;69:592–600 [DOI] [PubMed] [Google Scholar]
- 3.National Institutes of Health. Sleep disorders research plan. 2011; NIH Publication No. 11–7820.
- 4.Ferrie JE, Kumari M, Salo P, et al. Sleep epidemiology—a rapidly growing field. Int J Epidemiol 2011;40:1431–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahly V, Berglund P, Coulouvrat C, et al. The associations of insomnia with costly workplace accidents and errors: Results from the America Insomnia Survey. Arch Gen Psychiatry 2012;69:1054–1063 [DOI] [PubMed] [Google Scholar]
- 6.Kessler R, Berglund P, Coulouvrat C, et al. Insomnia and the performance of US workers: Results from the America Insomnia Survey. Sleep 2011;34:1161–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarsour K, Kalsekar A, Swindle R, et al. The association between insomnia severity and healthcare and productivity costs in a health plan sample. Sleep 2011;34:443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosekind MR, Gregory KB. Insomnia risks and costs: Health, safety, and quality of life. Am J Manag Care 2010;16:617–626 [PubMed] [Google Scholar]
- 9.Ozminkowski R, Wang S, Walsh J. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep 2007;30:263–273 [DOI] [PubMed] [Google Scholar]
- 10.Leger D, Bayon V. Societal costs of insomnia. Sleep Med Rev 2010;14:379–389 [DOI] [PubMed] [Google Scholar]
- 11.Brandt NJ, Piechocki JM. Treatment of insomnia in older adults: Re-evaluating the benefits and risks of sedative hypnotic agents. J Gerontol Nurs 2013;39:48–54 [DOI] [PubMed] [Google Scholar]
- 12.Ford JA, McCutcheon J. The misuse of Ambien among adolescents: Prevalence and correlates in a national sample. Addict Behav 2012;37:1389–1394 [DOI] [PubMed] [Google Scholar]
- 13.Hair PI, McCormack PL, Curran MP. Eszopiclone: A review of its use in the treatment of insomnia. Drugs 2008;68:1415–1434 [DOI] [PubMed] [Google Scholar]
- 14.Kleykamp BA, Griffiths RR, McCann UD, et al. Acute effects of Zolpidem extended-release on cognitive performance and sleep in healthy males after repeated nightly use. Exp Clin Psychopharmacol 2012;20:28–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swainston Harrison T, Keating GM. Zolpidem: A review of its use in the management of insomnia. CNS Drugs 2005;19:65–89 [DOI] [PubMed] [Google Scholar]
- 16.Zosel A, Osterberg EC, Mycyk MB. Zolpidem misuse with other medications or alcohol frequently results in intensive care unit admission. Am J Ther 2011;18:305–308 [DOI] [PubMed] [Google Scholar]
- 17.Bootzin R, Epstein D. Understanding and treating insomnia. Annu Rev Clin Psychol 2011;7:435–458 [DOI] [PubMed] [Google Scholar]
- 18.Bowles EJ. The Chemistry of Aromatherapeutic Oils. Third edition. Crowns Nest NSW, Australia: Allen & Unwin, 2003 [Google Scholar]
- 19.Lillehei A, Halcon L. A systematic review of the effect of inhaled essential oils on sleep. J Complement Altern Med 2014;20:441–451 [DOI] [PubMed] [Google Scholar]
- 20.Cho M, Min E, Hur M, Lee M. Effects of aromatherapy on the anxiety, vital signs, and sleep quality of percutaneous coronary intervention patients in intensive care units. Evid Based Complement Altern Med 2013;2013:381381–381381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lytle J, Mwatha C, Davis K. Effect of lavender aromatherapy on vital signs and perceived quality of sleep in the intermediate care unit: A pilot study. Am J Crit Care 2014;23:24–29 [DOI] [PubMed] [Google Scholar]
- 22.Goes T, Antunes F, Alves P, Teixeira-Silva F. Effect of sweet orange aroma on experimental anxiety in humans. J Altern Complement Med 2012;18:798–804 [DOI] [PubMed] [Google Scholar]
- 23.Hirokawa K, Nishimoto T, Taniguchi T. Effects of lavender aroma on sleep quality in healthy Japanese students. Percept Mot Skills 2012;114:111–122 [DOI] [PubMed] [Google Scholar]
- 24.Chien L, Cheng S, Liu C. The effect of lavender aromatherapy on autonomic nervous system in midlife women with insomnia. Evid Based Complement Altern Med 2012;2012:740813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arzi A, Sela L, Green A, et al. The influence of odorants on respiratory patterns in sleep. Chem Senses 2010;35:31–40 [DOI] [PubMed] [Google Scholar]
- 26.Moeini M, Khadibi M, Bekhradi R, et al. Effect of aromatherapy on the quality of sleep in ischemic heart disease patients hospitalized in intensive care units of heart hospitals of the Isfahan University of Medical Sciences. Iran J Nurs Midwifery Res 2010;15:234–239 [PMC free article] [PubMed] [Google Scholar]
- 27.Goel N, Lao RP. Sleep changes vary by odor perception in young adults. Biol Psychol 2006;71:341–349 [DOI] [PubMed] [Google Scholar]
- 28.Goel N, Kim H, Lao RP. An olfactory stimulus modifies nighttime sleep in young men and women. Chronobiol Int 2005;22:889–904 [DOI] [PubMed] [Google Scholar]
- 29.Lewith GT, Godfrey AD, Phillip P. A single-blinded, randomized pilot study evaluating the aroma of Lavandula augustifolia as a treatment for mild insomnia. J Altern Complement Med 2005;11:631–637 [DOI] [PubMed] [Google Scholar]
- 30.Raudenbush B, Koon J, Smith J, Zoladz P. Effects of odorant administration on objective and subjective measures of sleep quality, post-sleep mood and alertness, and cognitive performance. North Am J Psychol 2003;5:181–192 [Google Scholar]
- 31.Diego MA, Jones NA, Field T, et al. Aromatherapy positively affects mood, EEG patterns of alertness and math computations. Int J Neurosci 1998;96:217–224 [DOI] [PubMed] [Google Scholar]
- 32.Badia P, Wesensten N, Lammers W, et al. Responsiveness to olfactory stimuli presented in sleep. Physiol Behav 1990;48:87–90 [DOI] [PubMed] [Google Scholar]
- 33.Cannard G. The effect of aromatherapy in promoting relaxation and stress reduction in a general hospital. Complement Ther Nurs Midwifery 1996;2:38–40 [DOI] [PubMed] [Google Scholar]
- 34.Stringer J, Donald G. Aromasticks in cancer care: An innovation not to be sniffed at. Complement Ther Clin Pract 2011;17:116–121 [DOI] [PubMed] [Google Scholar]
- 35.Hudson R. The value of lavender for rest and activity in the elderly patient. Complement Ther Med 1996;4:52–57 [Google Scholar]
- 36.Hardy M, Kirk-Smith MD, Stretch DD. Replacement of drug treatment for insomnia by ambient odour. Lancet 1995;346:701. [DOI] [PubMed] [Google Scholar]
- 37.Fismer K, Pilkington K. Lavender and sleep: A systematic review of the evidence. Eur J Integr Med 2012;4:e436–e447 [Google Scholar]
- 38.Tisserand R, Young R. Essential Oil Safety. Second edition. London: Churchill Livingstone Elsevier, 2014 [Google Scholar]
- 39.Montgomery-Downs HE, Insana SP, Bond JA. Movement toward a novel activity monitoring device. Sleep Breathing 2012;16:913–917 [DOI] [PubMed] [Google Scholar]
- 40.Buysse DJ, Ancolilsrael S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep 2006;29:1155–1173 [DOI] [PubMed] [Google Scholar]
- 41.National Sleep Foundation. Sleep diary. Online document at: www.sleepfoundation.org/tools-for-better-sleep/images/SleepDiaryv6.pdf Updated 2013, accessed October20, 2014
- 42.Levenson J, Troxel W, Begley A, et al. A quantitative approach to distinguishing older adults with insomnia from good sleeper controls. J Clin Sleep Med 2013;9:125–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buysse DJ, Reynolds C, Monk T, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 1988;28:192–213 [DOI] [PubMed] [Google Scholar]
- 44.Backhaus J, Junghanns K, Broocks A, et al. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res 2002;53:737–740 [DOI] [PubMed] [Google Scholar]
- 45.Buysse DJ, Yu L, Moul DE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep 2010;33:781–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS™ sleep disturbance and sleep-related impairment item banks. Behav Sleep Med 2011;10:6–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lack LC. Delayed sleep and sleep loss in university students. J Am Coll Health 1986;35:105–110 [DOI] [PubMed] [Google Scholar]
- 48.Brown FC, Buboltz WC, Jr., Soper B. Development and evaluation of the sleep treatment and education program for students (STEPS). J Am Coll Health 2006;54:231–237 [DOI] [PubMed] [Google Scholar]
- 49.ISO International Standards. Oil of lavender (Lavandula angustifolia mill.).2002;ISO 3515:2002 (E).
- 50.National Heart Lung and Blood Institute. At-A-Glance: Healthy sleep. 2009;09-7426. Online document at: www.nhlbi.nih.gov/files/docs/public/sleep/healthy_sleep_atglance.pdf, accessed October20, 2014
- 51.Taub JM, Tanguay PE, Clarkson D. Effects of daytime naps on performance and mood in a college student population. J Abnorm Psychol 1976;85:210–217 [DOI] [PubMed] [Google Scholar]
- 52.Flausino NH, Da Silva Prado JM, de Queiroz SS, et al. Physical exercise performed before bedtime improves the sleep pattern of healthy young good sleepers. Psychophysiology 2012;49:186–192 [DOI] [PubMed] [Google Scholar]
- 53.Orzech KM, Salafsky DB, Hamilton LA. The state of sleep among college students at a large public university. J Am Coll Health 2011;59:612–619 [DOI] [PubMed] [Google Scholar]
- 54.PROMIS Network. Patient reported outcomes management information systems (PROMIS). Online document at: www.nihpromis.org (updated 2013, accessed October20, 2014