Abstract
Background
Venous thromboembolism (VTE) is an important patient safety issue. We sought to compare the predictive capacity of the 2005 and 2010 Caprini Risk Assessment Models for peri-operative VTE risk.
Methods
We performed a retrospective, observational, cross-over study using an established surgical outcomes database. A total of 3,334 adult plastic surgery patients were identified. Patients were risk-stratified using both the 2005 and 2010 Caprini Risk Assessment Model (RAM). Each patient served as their own control, resulting in precise matching for identified and unidentified confounders. The outcome of interest was 60-day, symptomatic VTE. The predictive capacities of the 2005 and 2010 Caprini risk scores were compared.
Results
Use of the 2010 Caprini RAM resulted in a systematic increase in the aggregate risk score. The median 2010 Caprini score was significantly higher than median 2005 Caprini score (6 vs. 5, P<.001). When compared to the 2010 model, the 2005 Caprini RAM was able to better separate the lowest and highest risk patients from one another. Patients classified as “super-high” risk (Caprini score >8) using the 2005 Caprini RAM were significantly more likely to have a 60-day VTE event when compared to patients classified as “super-high” risk using the 2010 guidelines (5.85% vs. 2.52%, P=.021).
Conclusion
When compared to the 2010 Caprini RAM, the 2005 Caprini RAM provides superior risk stratification. The 2005 Caprini RAM is the more appropriate method to risk-stratify plastic surgery patients for peri-operative VTE risk.
Level of evidence
Risk, II
Keywords: deep venous thrombosis, pulmonary embolus, venous thromboembolism, risk, risk stratification, Caprini, DVT, PE, VTE
INTRODUCTION
Individualized risk stratification for venous thromboembolism (VTE) allows risk to be estimated based on a patient's unique factors. Accurate risk assessment in the peri-operative period helps clinicians to identify both high and low-risk patients. With this information, informed decisions about VTE prophylaxis can be made for an individual patient based on their unique risk profile. Appropriate prophylaxis in high-risk patients is known to prevent VTE, a peri-operative complication that is potentially fatal.
Several individualized risk models have been developed and clinically evaluated 1-4. However, none has been more extensively validated than the individualized risk assessment model (RAM) developed, refined, and popularized by Caprini and colleagues. The Caprini Risk Assessment Model (RAM) is a weighted risk stratification tool initially published in 1991 5. The RAM is described as “weighted” because different risk factors carry different point values. Risk factor weighting is performed because some risk factors (such as personal history of VTE or known thrombophilia) are known to contribute to aggregate risk more than others (such as swollen legs or major surgery within 30 days) 6, 7.
The Caprini RAM has been regularly updated to reflect improved understanding of VTE pathophysiology and risk factors. 8-14. The 2005 version of the Caprini RAM 8 has been validated to predict 30-day or 60-day VTE risk in multiple surgical populations, including plastic and reconstructive surgery patients 6-8. As a result of these validation studies, strata have been identified to report level of risk and response to prophylaxis based on risk level 6, 7, 16, 17.
The most recent version of the Caprini RAM was published in 2010 10. When compared to the 2005 RAM, the 2010 RAM has four distinct, data-driven changes, including addition of new risk factors or re-weighting of old risk factors (Table 1). The changes were based on recently published studies that identified associations between the risk factors in Table 1 and post-operative VTE. Although the associations are real, we do not know if incorporation of these risk factors into a VTE RAM improves risk stratification. Stated another way, we do not know if the 2010 Caprini RAM is a better risk stratification tool than the 2005 Caprini RAM. A “better” risk stratification tool would improve a clinician's ability to separate high from low-risk patients, would provide information on the risk and benefits of treatment based on risk level, and would assist a clinician in making a binary yes/no decision for treatment. We designed this study to evaluate whether the newer 2010 Caprini RAM was a better risk stratification tool than the 2005 Caprini RAM for plastic surgery patients.
Table 1.
Differences between the 2005 and 2010 versions of the Caprini Risk Assessment Models
| 2005 Caprini Model | 2010 Caprini Model | |
|---|---|---|
| Operative time | 0-44 minutes=1 point | 0-59 minutes=1 point |
| ≥45 minutes=2 points | 60-119 minutes=2 points | |
| 120-179 minutes=3 points | ||
| ≥180 minutes=5 points | ||
| Body mass index (kg/m2) | ≥25=1 point | ≥30 & <40=1 point |
| ≥40 & <50=2 points | ||
| ≥50=3 points | ||
| Superficial venous thrombophlebitis (SVT) | Not a risk factor | History of SVT=3 points |
| Cancer | History of cancer=2 points | History of cancer=2 points |
| Current cancer=2 points | Current cancer=3 points | |
As part of the Venous Thromboembolism Prevention Study (VTEPS), we have previously established a multi-center surgery outcomes database of more than 3,300 adult plastic and reconstructive surgery patients. Among the study variables, the VTEPS database contains peri-operative VTE risk factors and 60-day VTE outcomes. We designed a retrospective, observational, cross-over study using the existing VTEPS database. The study's objective was to compare scores derived from the 2005 Caprini RAM to those derived from the 2010 Caprini RAM and examine the two scores’ ability to predict 60-day VTE risk.
METHODS
The VTEPS study was funded by the Plastic Surgery Foundation in 2008. The VTEPS Network consisted of four tertiary care hospitals, including the University of Pittsburgh (Pittsburgh, PA), the University of Texas-Southwestern (Dallas, TX), Regions Hospital (St. Paul, MN), and the University of Michigan (Ann Arbor, MI). The VTEPS study was approved by each site's Institutional Review Board.
All data in the VTEPS database were acquired retrospectively through medical chart review performed by physician-led teams. Inclusion criteria included age ≥18, operation under general anesthesia, and post-operative admission to the hospital for at least one night. All patients were considered to be moderate risk for peri-operative VTE events (Caprini score ≥3 based on the 2005 Caprini RAM) 8. Lower extremity trauma reconstruction patients were excluded from the VTEPS database.
Independent variables
Independent variables included all variables necessary to complete the 2005 Caprini RAM 8. Age, operative time and body mass index were recorded as continuous variables. All other variables were recorded as dichotomous. Additional independent variables (e.g. those not included in the 2005 Caprini score) included the year the procedure was performed, VTEPS site, patient gender, whether multiple operations were performed during the initial hospitalization, surgical procedure type and location, receipt of post-operative enoxaparin per VTEPS protocol, administration of aspirin, and length of hospitalization.
Dependent variables
The outcome variable for this study was symptomatic venous thromboembolism, defined as patients with a newly diagnosed, post-operative, symptomatic DVT or PE within the 60-day VTEPS follow-up period. Screening studies were not performed on asymptomatic patients. All VTE events required confirmation using an objective image method. Appropriate modalities included venous duplex ultrasound, venography, ventilation-perfusion scan, or computed tomography. In addition, DVT or PE detected at autopsy were considered to be post-operative events if they were deemed to be the cause of or a major contributor to the patient's death. Patients whose medical records lacked 60 days of follow-up were excluded.
Comparison of the 2005 and 2010 Caprini risk models
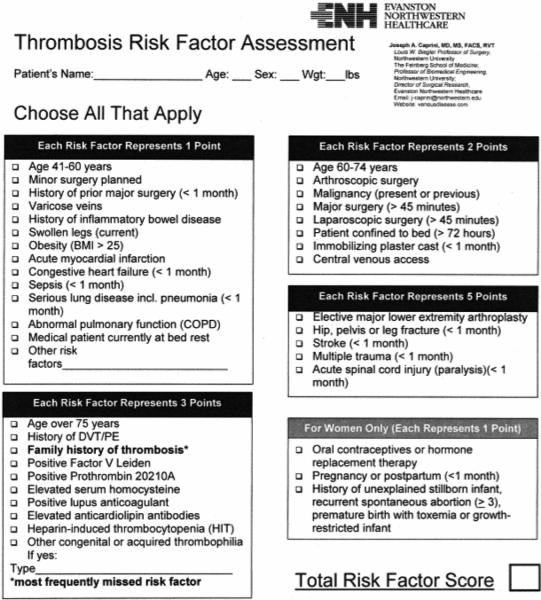
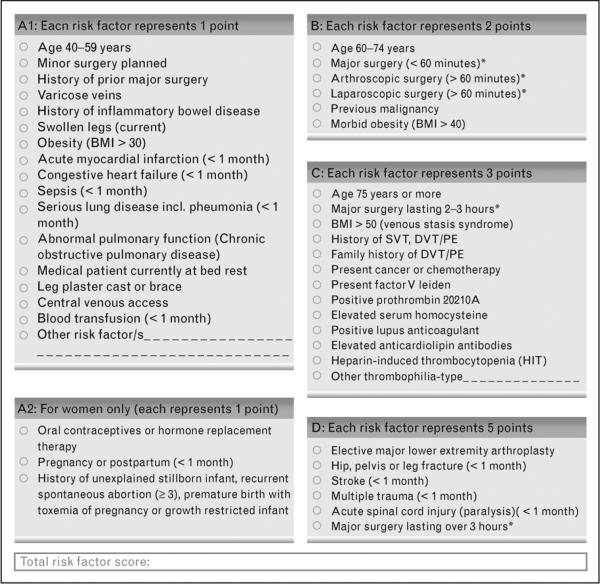
The Caprini RAM is a weighted risk model that produces an aggregate risk score based on the presence or absence of 39 individual risk factors. VTEPS used the 2005 Caprini RAM to risk-stratify patients for peri-operative VTE risk. This model was chosen as it had previously been validated in general surgery patients 6 and was considered the “gold standard” individualized risk assessment tool when VTEPS was designed in 2008. The VTEPS Network published a formal validation of the 2005 Caprini score based on data from 1,126 adult plastic surgery patients who received no chemoprophylaxis 7. The 2005 and 2010 Caprini RAMs are shown in Figures 1 and 2.
Figure 1.
The 2005 Caprini Risk Assessment Model. Adapted from reference 8, with permission.
Figure 2.
The 2010 Caprini Risk Assessment Model. Adapted from reference 10, with permission.
During the VTEPS study, the updated 2010 Caprini RAM was published. Four distinct changes were present in the updated version. When compared to the 2005 model, the 2010 model utilized additional sub-categorizations for the BMI, operative time, and cancer risk factors. Super-morbid obesity and prolonged operative time were more heavily weighted (e.g. received additional points) than in prior versions of the model. A distinction between active cancer and history of cancer was also made, with a corresponding increase in weighting for patients with active cancer. Additionally, personal history of superficial venous thrombophlebitis (SVT) was added as a new, distinct risk factor. New risk factors and weightings are summarized in Table 1. The literature that supports these associations is extensively described in the Discussion section.
Study design and statistical analysis
Statistical analyses were performed using Stata11 (College Station, Texas).
This study was designed as a retrospective, observational, cross-over study. In a cross-over study, the same patient receives a sequence of different exposures or treatments over time. Thus, when data are stratified for analysis by exposure or treatment, the composition of patients in each group is identical. In fact, the only chance for confounding lies in risk factors that may have changed in the interval between exposures or treatment. This source of confounding is eliminated when the exposure or treatment is performed simultaneously. An example of this would be when two sides of the face are treated with different peel solutions during the same operation.
For this study, individual patients were risk-stratified using the 2005 and 2010 Caprini RAM based on data available at the time of operation. Two patient groups were then created. Each group was comprised of the same 3,334 patients but was risk-stratified using a different method. As each patient served as their own control, the two groups were perfectly matched for both identified demographic risk factors (e.g. age, personal history of VTE, known thrombophilia), intra-operative risk factors (e.g. procedure type, procedure duration, use of sequential compression devices), post-operative risk factors or protective measures (e.g. central venous access, development of post-operative sepsis or myocardial infarction, receipt of post-operative enoxaparin) as well as unidentified or unmeasured confounding factors.
VTEPS collected data for all risk factors required to complete the 2005 Caprini RAM (Figure 1). An aggregate 2005 risk score was calculated for each patient. VTEPS data for BMI and operative time were then re-weighted using the 2010 Caprini guidelines and a new 2010 aggregate score was calculated. The VTEPS database did not contain data for active vs. history of cancer or history of superficial venous thrombophlebitis. Thus, the newly calculated score was an approximation, not a true value, for the 2010 Caprini score.
Group differences in 2005 and 2010 Caprini scores were examined using the Wilcoxon Signed Rank test as previous work 6, 7 has shown that the Caprini score is not linearly correlated with risk. For VTE risk analysis, patients were stratified by Caprini score at accepted and published levels (Caprini scores of 1 to 2, 3 to 4, 5 to 6, 7 to 8, and >8) 6, 7, 10. Descriptive statistics that examined VTE incidence by stratified risk score were generated. Differences in observed VTE rate by stratified risk score were examined using Pearson's chi-square test. A P value of less than 0.05 was considered significant.
RESULTS
The VTEPS database contained complete data for 3,334 patients. Prevalence of individual Caprini RAM risk factors is shown in Table 2. Anatomic surgical sites are summarized in Table 3. Among 3,334 patients, 43.7% (n=1,458) received post-operative enoxaparin per the VTEPS protocol and 8.3% (n=275) received intra- or post-operative aspirin.
Table 2.
Demographic data stratified by Caprini score risk factors (N=3,334)
| RISK FACTOR | % OF ALL PATIENTS WITH RISK FACTOR (n) |
|---|---|
| ONE POINT RISK FACTORS | |
| Age 41-59 | 54.2% (1,808) |
| Minor surgery planned | 5.1% (169) |
| Major surgery within 30 days | 13.6% (452) |
| Varicose veins | 1.0% (32) |
| History of IBD | 0.7% (23) |
| Swollen legs (current) | 3.2% (108) |
| Acute myocardial infarction <3 months | 0.2% (5) |
| Congestive heart failure <1 month | 0.7% (24) |
| Sepsis <1 month | 0.5% (15) |
| Serious lung disease (inc. pneumonia) <1 month | 0.5% (15) |
| Chronic obstructive pulmonary disease | 2.2% (72) |
| TWO POINT RISK FACTORS | |
| Age 60-74 years | 16.2% (542) |
| Arthroscopic surgery | 0.2% (5) |
| Malignancy (present or previous) | 37.1% (1,237) |
| Laparoscopic surgery >45 minutes | 0.2% (6) |
| Central venous access | 9.0% (299) |
| THREE POINT RISK FACTORS | |
| Age ≥75 | 4.6% (152) |
| History of DVT/PE | 3.4% (114) |
| Family history of DVT/PE | 1.1% (35) |
| Positive Factor V Leiden | 0.3% (8) |
| Positive Prothrombin 20210A | 0.03% (1) |
| Positive Lupus anticoagulant | 0.1% (3) |
| Heparin induced thrombocytopenia | 0.1% (3) |
| Elevated serum homocysteine | 0 |
| Elevated anticardiolipin antibodies | 0 |
| Other congenital or inherited thrombophilia | 0.2% (7) |
| Polycythemia vera | 0.1% (3) |
| FIVE POINT RISK FACTORS | |
| Elective major lower extremity arthroplasty | 0.5% (18) |
| Hip, pelvis, or leg fracture <1 month | 0.4% (13) |
| Stroke <1 month | 0.03% (1) |
| Multiple trauma <1 month | 2.3% (76) |
| Acute spinal cord injury or paralysis <1 month | 1.0% (3) |
| FEMALE-SPECIFIC RISK FACTOR (N=2,188) | |
| ONE POINT RISK FACTORS | |
| Oral contraceptives | 7.1% (156) |
| Pregnancy or postpartum (<1 month) | 0.2% (4) |
| History of unexplained stillborn infant recurrent spontaneous abortion (≥3), premature birth with toxemia or growth-restricted infant | 0.3% (6) |
Table 3.
VTEPS patients stratified by anatomic surgical site (N=3,334)
| Anatomic site | Percentage of patients (n) |
|---|---|
| Breast | 33.6% (1,120) |
| Chest/back/abdomen | 28.3% (944) |
| Upper extremity | 17.7% (593) |
| Head and neck | 14.8% (494) |
| Lower extremity | 12.6% (421) |
| Genitalia | 1.9% (64) |
| Multiple site | 11.0% (302) |
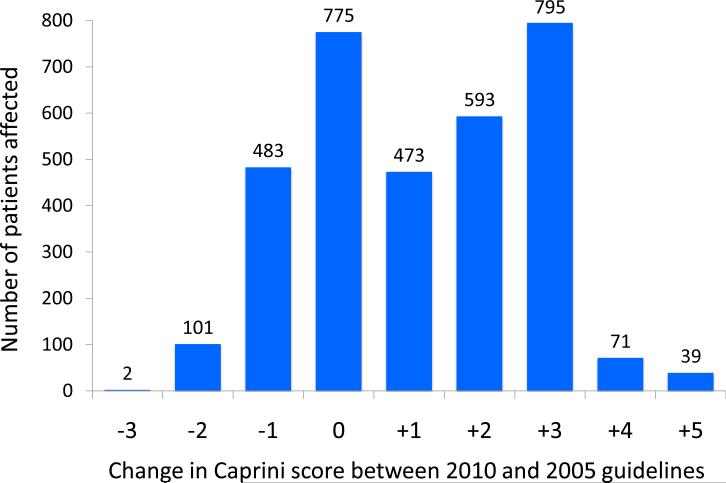
The median 2010 Caprini score was significantly higher than the median 2005 Caprini score (6 vs. 5, P<0.001). When compared to 2005 Caprini scores, 2010 scores were lower in 17.6% of patients (range 1-3 points), unchanged in 23.3% of patients, and increased in 59.2% of patients (range 1-5 points). Among patients with increased scores, 46% were increased by 3 or more points (Figure 3).
Figure 3.
Change in 2010 Caprini score at the individual patient level, as compared to the 2005 Caprini score.
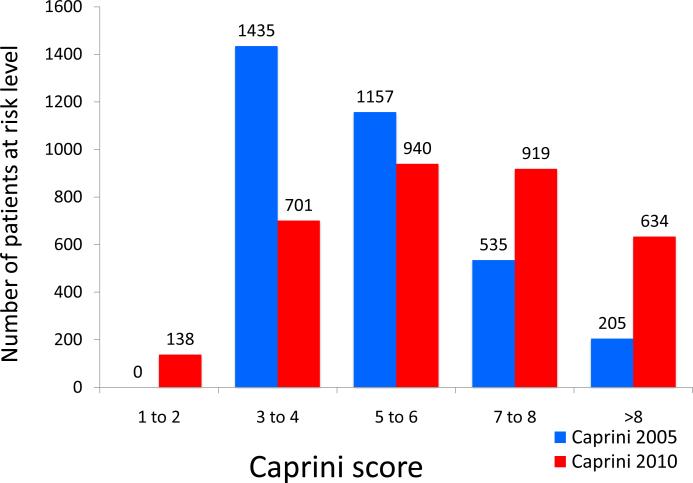
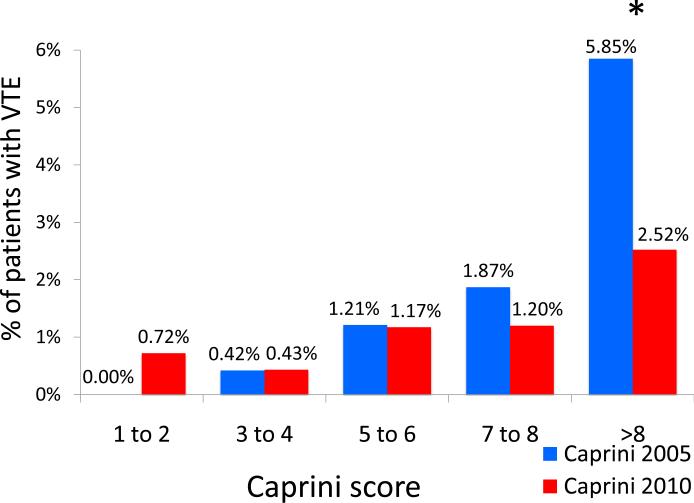
Using the 2005 Caprini scores, the majority of patients were classified as moderate to high risk (Caprini scores of 3-4 or 5-6) with 6% of patients being “super-high” risk (Caprini scores of >8). Among “super-high” risk patients, observed 60-day rate of VTE was 5.85%. Using the 2010 Caprini scores, the number of patients risk-stratified as “super-high” risk increased three-fold to 19% of all patients. Observed VTE rates among this group decreased to 2.52% due to increased number of patients in the denominator without a corresponding increase in number of observed VTE events (Figures 4 and 5). The 2005 Caprini RAM separated high from low risk patients better than the 2010 Caprini RAM.
Figure 4.
Number of patients at each risk level when stratified by the 2005 and 2010 Caprini Risk Assessment Model.
Figure 5.
Observed rate of VTE at each risk level when stratified by the 2005 and 2010 Caprini Risk Assessment Model. * P=.021
Patients classified as “super-high” risk (Caprini score >8) using the 2005 Caprini RAM were significantly more likely to have had a 60-day VTE event when compared to patients classified as “super-high” risk using the 2010 guidelines (5.85% vs. 2.52%, P=.021). There were no significant differences in observed 60-day VTE rate at any other distinct risk level (Table 4).
Table 4.
Comparison of observed VTE rates at stratified risk levels between the 2005 and 2010 Caprini Risk Assessment Models. P values compare observed rates of VTE at individual risk levels using the 2005 and 2010 Caprini RAM.
| 2005 Caprini Model | 2010 Caprini Model | p value | |||
|---|---|---|---|---|---|
| Risk Level | Patients without VTE | Patients with VTE (%) | Patients without VTE | Patients with VTE (%) | |
| Caprini 1-2 | 0 | 0 | 137 | 1 (0.72%) | --- |
| Caprini 3-4 | 1429 | 6 (0.42%) | 698 | 3 (0.43%) | 0.974 |
| Caprini 5-6 | 1143 | 14 (1.21%) | 929 | 11 (1.17%) | 0.933 |
| Caprini 7-8 | 525 | 10 (1.87%) | 908 | 11 (1.20%) | 0.300 |
| Caprini >8 | 193 | 12 (5.85%) | 618 | 16 (2.52%) | 0.021 |
Discussion
Based on available data, the 2005 Caprini RAM is a better predictor of 60-day VTE risk in adult plastic surgery patients. We recommend that the 2005 Caprini RAM be used to risk-stratify adult plastic surgery patients and that the 2005 Caprini score be used to guide decision-making for VTE prophylaxis. Existing incidence and response-to-prophylaxis data derived from the 2005 Caprini RAM cannot necessarily be generalized to scores obtained using the 2010 Caprini RAM. Such generalization will result in inaccurate estimation of VTE risk and will produce results that are in contrast with physician and healthcare provider expectations. This, in turn, will hamper the ability of clinicians to provide data-driven informed consent to their patients regarding peri-operative VTE risk.
These findings should not be taken as a criticism of the 2010 Caprini RAM. Published literature supports that the risk factors newly added or re-weighted in the 2010 Caprini RAM are associated with increased VTE risk. However, this analysis shows that the 2010 RAM does not risk-stratify adult plastic surgery patients as well as the 2005 RAM when risk is categorized by previously published risk cut-points (Caprini scores of 3-4, 5-6, 7-8, and >8). The 2010 Caprini RAM may improve risk stratification if new cut-points are identified and validated. This manuscript serves as a call for formal validation studies of the updated 2010 Caprini RAM. Validation studies will need to identify the clinically relevant breakpoints for the 2010 risk score that will, in turn, improve provider ability to risk-stratify and make prophylaxis decisions for surgical patients.
DATA-DRIVEN CHANGES TO THE 2010 RAM
Body mass index
Morbid obesity is known to alter lower extremity venous hemodynamics through increased intraabdominal pressure with resultant decrease in lower extremity venous outflow. Decreased outflow creates lower extremity venous stasis, which is one of three components of Virchow's Triad. Presence of chronic venous hypertension is believed to damage venous valves with resultant venous reflux and venous stasis ulceration 18-20. Sugerman et al examined VTE risk factors in 1,924 morbidly obese patients who required gastric bypass. Presence of pre-operative venous stasis ulcers (e.g. the venous stasis syndrome) was significantly associated with post-operative fatal PE (4% vs. 0.2%, P<.001) and carried a four-fold increased risk for all PE (4% vs. 0.9%). These data were the impetus for inclusion of BMI >50 as an independent, three point risk factor in the 2010 RAM 18.
Large epidemiologic studies have demonstrated a correlation between increased BMI and increased VTE risk. The Physician's Health Study analyzed 358 VTE events that occurred in 18,662 male physicians over a 20-year period. Their findings indicate that each BMI increase of 1 kg/m2 was associated with a relative risk increase of 1.11 for VTE 21. Tsai et al combined the Atherosclerosis Risk in Communities and Cardiovascular Health Study databases to examine cardiovascular risk factors for VTE. Their analysis showed that, when compared to patients with BMI<25, a stepwise increased hazard for VTE existed in patients with BMI of 25-30, 35-40, and >40 22.
Active vs. history of cancer
Cancer patients are known to have significantly increased risk for VTE 2, 12, 23-26 and regional or distant metastases are known to carry additional risk 23, 25, 27. Chemotherapy is also known to significantly increase VTE 4, 28, 29 but is not a distinct 2010 RAM risk factor. The “active cancer” risk factor may actually serve as a proxy for patient's likelihood to receive chemotherapy. Tamoxifen is commonly provided to patients with active breast cancer and is a recognized risk factor for VTE, though it is not a distinct 2010 RAM risk factor 30.
Analysis of the prospectively collected DVT FREE registry, which includes over 5,000 patients with DVT, shows that 20% of DVT patients had active cancer. Greater than 40% of cancer patients with DVT were receiving chemotherapy at the time of DVT diagnosis 31. When compared to patients with a history of cancer, patients with active cancer are more likely to be hospitalized, be immobile, and/or have surgery, each of which may increase VTE risk.
History of superficial venous thrombosis
The Prospective Observational Superficial Thrombophlebitis (POST) study group examined 844 patients with symptomatic SVT for concurrent DVT. At presentation with SVT, 25% of patients had a concurrent DVT. Among patients without concurrent DVT, 10% developed a thromboembolic complication within the three month follow-up period 32. Heit et al performed a matched case control study of 625 patients with a first lifetime VTE event. Regression analysis identified personal history of SVT as an independent predictor of future DVT or PE 24.
Operative time
Prolonged operative time has many potential confounders as an independent VTE risk factor. For example, patients with malignancy or more advanced malignant disease (both of which are VTE risk factors) might require more extensive operative procedures. One study utilized regression techniques to control for such confounders. Sakon and colleagues 33 conducted a multi-center, prospective epidemiological study of major abdominal surgery patients. Multivariable regression analysis demonstrated that each additional hour of operative time was associated with an odds ratio of 1.32 for VTE. Previous studies in thermal and electrically injured patients have also identified increased number of operations as a VTE risk factor 34, 35.
Limitations
The inherent limitations of the VTEPS database have previously been described 7,16 and are briefly discussed here. Lower extremity trauma reconstruction patients were omitted from the final VTEPS database and thus these results cannot be generalized to this unique, high-risk patient population. No RAM has been developed or validated to predict post-operative VTE risk among pediatric plastic surgery patients, and pediatric patients were excluded from VTEPS. Finally, VTEPS inclusion criteria required that a patient receive general anesthesia and be admitted for at least an overnight stay after surgery. Thus, these findings cannot be generalized to surgery performed under IV sedation or regional block, or surgery performed in the outpatient setting.
The major limitation of this project is that the VTEPS database does not contain data for history vs. active malignancy or for history of superficial venous thrombophlebitis. While these data points would only increase the reported 2010 Caprini score, it is possible that addition of these points would also improve risk discrimination.
Previous work has identified standard risk stratification levels (e.g. Caprini scores of 3-4, 5-6, 7-8, and >8) upon which prophylaxis decisions are based. These levels were based on 2005 Caprini scores from the VTEPS study 7, 16. The focus of this project was to examine whether the same values could be generalized to the Caprini scores from the 2010 guidelines. We have shown that use of the 2010 Caprini model results in a systematic increase in calculated risk score, and thus the model requires formal validation (and likely new risk stratification levels) prior to widespread use in plastic surgery patients. Given that the VTEPS database lacks two key data points necessary to complete the 2010 Caprini RAM, we cannot perform that formal validation using VTEPS data.
Conclusion
The 2005 version of the Caprini RAM is a validated risk prediction tool for 60-day VTE events in adult plastic surgery patients who require post-operative admission. We recommend that the 2005 Caprini RAM be used to risk-stratify plastic surgery patients and guide prophylaxis decisions. The 2010 Caprini RAM requires formal validation with appropriate determination of new risk levels prior to widespread adoption.
Footnotes
Meeting disclosure:
This work has been accepted for presentation at the 2012 American Venous Forum meeting.
FINANCIAL DISCLOSURE AND PRODUCTS PAGE
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript. The Venous Thromboembolsim Prevention Study was funded by the Plastic Surgery Foundation.
Contributor Information
Christopher J. Pannucci, Section of Plastic Surgery, University of Michigan, Ann Arbor, Michigan.
Ruth J. Barta, Department of Plastic and Hand Surgery, Regions Hospital, St. Paul, Minnesota.
Pamela R. Portschy, Department of Plastic and Hand Surgery, Regions Hospital, St. Paul, Minnesota.
George Dreszer, Department of Plastic and Hand Surgery, Regions Hospital, St. Paul, Minnesota.
Ronald E. Hoxworth, Department of Plastic Surgery, University of Texas-Southwestern, Dallas, Texas.
Loree K. Kalliainen, Department of Plastic and Hand Surgery, Regions Hospital, St. Paul, Minnesota.
Edwin G. Wilkins, Section of Plastic Surgery, University of Michigan, Ann Arbor, Michigan.
REFERENCES
- 1.Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352:969–977. doi: 10.1056/NEJMoa041533. [DOI] [PubMed] [Google Scholar]
- 2.Pannucci CJ, Shanks A, Moote MJ, Bahl V, Cederna PS, Naughton NN, Wakefield TW, Henke PK, Campbell DA, Kheterpal S. Identifying patients at high risk for venous thromboembolism requiring treatment after outpatient surgery. Ann Surgery. doi: 10.1097/SLA.0b013e3182519ccf. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yale SH, Medlin SC, Liang H, Peters T, Glurich I, Mazza JJ. Risk assessment model for venothromboembolism in post-hospitalized patients. Int Angiol. 2005;24:250–254. [PubMed] [Google Scholar]
- 4.Rogers SO, Jr, Kilaru RK, Hosokawa P, Henderson WG, Zinner MJ, Khuri SF. Multivariable predictors of postoperative venous thromboembolic events after general and vascular surgery: Results from the patient safety in surgery study. J Am Coll Surg. 2007;204:1211–1221. doi: 10.1016/j.jamcollsurg.2007.02.072. [DOI] [PubMed] [Google Scholar]
- 5.Arcelus JI, Candocia S, Traverso CI, Fabrega F, Caprini JA, Hasty JH. Venous thromboembolism prophylaxis and risk assessment in medical patients. Semin Thromb Hemost. 1991;17(Suppl 3):313–318. [PubMed] [Google Scholar]
- 6.Bahl V, Hu HM, Henke PK, Wakefield TW, Campbell DA, Jr, Caprini JA. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2009 doi: 10.1097/SLA.0b013e3181b7fca6. [DOI] [PubMed] [Google Scholar]
- 7.Pannucci CJ, Bailey SH, Dreszer G, et al. Validation of the Caprini risk assessment model in plastic and reconstructive surgery patients. J Am Coll Surg. 2011;212:105–112. doi: 10.1016/j.jamcollsurg.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70–78. doi: 10.1016/j.disamonth.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9:253–262. doi: 10.1023/a:1018770712660. [DOI] [PubMed] [Google Scholar]
- 10.Caprini JA. Risk assessment as a guide to thrombosis prophylaxis. Curr Opin Pulm Med. 2010;16:448–452. doi: 10.1097/MCP.0b013e32833c3d3e. [DOI] [PubMed] [Google Scholar]
- 11.Zakai NA, Wright J, Cushman M. Risk factors for venous thrombosis in medical inpatients: Validation of a thrombosis risk score. J Thromb Haemost. 2004;2:2156–2161. doi: 10.1111/j.1538-7836.2004.00991.x. [DOI] [PubMed] [Google Scholar]
- 12.Bergqvist D. Risk of venous thromboembolism in patients undergoing cancer surgery and options for thromboprophylaxis. J Surg Oncol. 2007;95:167–174. doi: 10.1002/jso.20625. [DOI] [PubMed] [Google Scholar]
- 13.Deheinzelin D, Braga AL, Martins LC, et al. Incorrect use of thromboprophylaxis for venous thromboembolism in medical and surgical patients: Results of a multicentric, observational and cross-sectional study in Brazil. J Thromb Haemost. 2006;4:1266–1270. doi: 10.1111/j.1538-7836.2006.01981.x. [DOI] [PubMed] [Google Scholar]
- 14.Davison SP, Venturi ML, Attinger CE, Baker SB, Spear SL. Prevention of venous thromboembolism in the plastic surgery patient. Plast Reconstr Surg. 2004;114:43E–51E. doi: 10.1097/01.prs.0000131276.48992.ee. [DOI] [PubMed] [Google Scholar]
- 15.Shuman AG, Hu HM, Pannucci CJ, Jackson CR, Bradford CR, Bahl V. Stratifying the risk of venous thromboembolism in otolaryngology. Otolaryngol Head Neck Surg. doi: 10.1177/0194599811434383. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pannucci CJ, Dreszer G, Wachtman CF, Bailey SH, Portschy PR, Hamill JB, Hume KM, Hoxworth RE, Rubin JP, Kalliainen LK, Pusic AL, Wilkins EG. Post-operative enoxaparin prevents symptomatic venous thromboembolism in high-risk plastic surgery patients. Plast Reconst Surg. 2011;128:1093–1103. doi: 10.1097/PRS.0b013e31822b6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199:S3–10. doi: 10.1016/j.amjsurg.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Sugerman HJ, Sugerman EL, Wolfe L, Kellum JM, Jr, Schweitzer MA, DeMaria EJ. Risks and benefits of gastric bypass in morbidly obese patients with severe venous stasis disease. Ann Surg. 2001;234:41–46. doi: 10.1097/00000658-200107000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willenberg T, Schumacher A, Amann-Vesti B, et al. Impact of obesity on venous hemodynamics of the lower limbs. J Vasc Surg. 2010;52:664–668. doi: 10.1016/j.jvs.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 20.Arfvidsson B, Eklof B, Balfour J. Iliofemoral venous pressure correlates with intraabdominal pressure in morbidly obese patients. Vasc Endovascular Surg. 2005;39:505–509. doi: 10.1177/153857440503900607. [DOI] [PubMed] [Google Scholar]
- 21.Glynn RJ, Rosner B. Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol. 2005;162:975–982. doi: 10.1093/aje/kwi309. [DOI] [PubMed] [Google Scholar]
- 22.Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: The longitudinal investigation of thromboembolism etiology. Arch Intern Med. 2002;162:1182–1189. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- 23.Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: The @RISTOS project. Ann Surg. 2006;243:89–95. doi: 10.1097/01.sla.0000193959.44677.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch Intern Med. 2000;160:809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 25.Blom JW, Doggen CJM, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations and the risk of venous thrombosis. JAMA. 2005;293:715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 26.Osborne NH, Wakefield TW, Henke PK. Venous thromboembolism in cancer patients undergoing major surgery. Ann Surg Oncol. 2008;15:3567–3578. doi: 10.1245/s10434-008-0151-4. [DOI] [PubMed] [Google Scholar]
- 27.Chew HK, Wun T, Harvey DJ, Zhou H, White RH. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol. 2007;25:70–76. doi: 10.1200/JCO.2006.07.4393. [DOI] [PubMed] [Google Scholar]
- 28.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd. Predictors of survival after deep vein thrombosis and pulmonary embolism: A population-based, cohort study. Arch Intern Med. 1999;159:445–453. doi: 10.1001/archinte.159.5.445. [DOI] [PubMed] [Google Scholar]
- 29.Saphner T, Tormey DC, Gray R. Venous and arterial thrombosis in patients who received adjuvant therapy for breast cancer. J Clin Oncol. 1991;9:286–294. doi: 10.1200/JCO.1991.9.2.286. [DOI] [PubMed] [Google Scholar]
- 30.Goldhaber SZ. Tamoxifen: Preventing breast cancer and placing the risk of deep vein thrombosis in perspective. Circulation. 2005;111:539–541. doi: 10.1161/01.CIR.0000156099.83394.A7. [DOI] [PubMed] [Google Scholar]
- 31.Seddighzadeh A, Shetty R, Goldhaber SZ. Venous thromboembolism in patients with active cancer. Thromb Haemost. 2007;98:656–661. [PubMed] [Google Scholar]
- 32.Decousus H, Quere I, Presles E, et al. Superficial venous thrombosis and venous thromboembolism: A large, prospective epidemiologic study. Ann Intern Med. 2010;152:218–224. doi: 10.7326/0003-4819-152-4-201002160-00006. [DOI] [PubMed] [Google Scholar]
- 33.Sakon M, Maehara Y, Yoshikawa H, Akaza H. Incidence of venous thromboembolism following major abdominal surgery: a multi-center, prospective epidemiological study in Japan. J Thromb Haemo. 2006;4:581–586. doi: 10.1111/j.1538-7836.2006.01786.x. [DOI] [PubMed] [Google Scholar]
- 34.Pannucci CJ, Osborne NH, Jaber RM, Cederna PS, Wahl WL. Early fasciotomy in electrically injured patients as a marker for injury severity and deep venous thrombosis risk: An analysis of the national burn repository. J Burn Care Res. 2010;31:882–887. doi: 10.1097/BCR.0b013e3181f93597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pannucci CJ, Osborne NH, Wahl WL. Venous thromboembolism in thermally injured patients: Analysis of the national burn repository. J Burn Care Res. 2011;32:6–12. doi: 10.1097/BCR.0b013e318204b2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henke PK, Pannucci CJ. Venous thromboembolism risk factor assessment and prophylaxis. Phlebology. 2010;25:219–223. doi: 10.1258/phleb.2010.010018. [DOI] [PMC free article] [PubMed] [Google Scholar]