Abstract
Aims/hypothesis
SLC30A8 encodes a zinc transporter in the beta cell; individuals with a common missense variant (rs13266634; R325W) in SLC30A8 demonstrate a lower early insulin response to glucose and an increased risk of type 2 diabetes. We hypothesised that zinc supplementation may improve insulin secretion in a genotype-dependent manner.
Methods
We evaluated the early insulin response to glucose (using frequently sampled intravenous glucose tolerance testing) by R325W genotype before and after 14 days of supplementation with oral zinc acetate (50 mg elemental zinc) twice daily in healthy non-diabetic Amish individuals (N=55).
Results
Individuals with RW/WW genotypes (n=32) had the lowest insulin response to glucose at 5 and 10 min at baseline (vs RR homozygotes [n=23]). After zinc supplementation, the RW/WW group experienced 15% and 14% increases in the insulin response to glucose at 5 and 10 min, respectively (p≤0.04), and, compared with RR homozygotes, experienced a 26% (p=0.04) increase in insulin at 5 min. We observed reciprocal decreases in proinsulin:insulin in the RW/WW (p=0.002) vs RR group (p=0.048), suggesting a genotype-specific improvement in insulin processing.
Conclusions/interpretation
Zinc supplementation appears to affect the early insulin response to glucose differentially by rs13266634 genotype and could be beneficial for diabetes prevention and/or treatment for some individuals based on SLC30A8 variation.
Keywords: Insulin secretion, Pharmacogenetic, SLC30A8, Type 2 diabetes, Zinc
Introduction
There is great inter-individual variation in clinical and metabolic responses to commonly prescribed interventions such as lifestyle and medications for the prevention and treatment of type 2 diabetes. The sources of this variation are multifactorial and include clinical factors (e.g. age, sex, BMI [1]) as well as genetic factors [2]. The discovery of genetic variants that predict responses to diet (nutrigenomics) and medications (pharmacogenomics) for the prevention and treatment of type 2 diabetes offers great promise to allow treatment to be tailored to the individual based on genetic variation [2-7].
More than 50 genetic variants are associated with type 2 diabetes risk, but the effects of these variants are modest and provide little clinically useful information for patients [8-10]. However, these variants are excellent candidate genes for interaction with treatment interventions. The single nucleotide polymorphism (SNP), rs13266634, of the SLC30A8 gene, initially identified in genome-wide association studies, has been consistently associated with the risk of type 2 diabetes in European and Asian populations (OR approximately 1.15 associated with each copy of the R risk allele) [11-16]. The SLC30A8 gene encodes a zinc transporter specific to the beta cell of the pancreas, zinc transporter protein member 8 (ZnT8), which transports zinc into the insulin secretory vesicles of the beta cell [17]. This zinc facilitates the formation and stabilisation of insulin hexamers, which makes insulin less susceptible to degradation [18]. This packaged insulin in insulin secretory vesicles is then available for immediate release upon glucose stimulation [19]. Abnormalities in this process would therefore be anticipated to affect the acute insulin response to glucose.
rs13266634 is a non-synonymous SNP (single base change C→T) which encodes a nonconservative amino acid change from arginine (R) to tryptophan (W) at position 325 (R325W) [13]. Based on its function in pancreatic islets, the mechanism whereby this variant (R allele) increases the risk of type 2 diabetes is likely due to decreased insulin processing and/or secretion. In a study of individuals with a family history of type 2 diabetes, those with the RR genotype of rs13266634 had a decreased first-phase insulin response to an intravenous glucose load during a frequently sampled IVGTT (FS-IVGTT) compared with those with the WW genotype [20]. Other studies have identified differences in fasting proinsulin [21] and proinsulin:insulin ratio (measures of insulin processing) [22]; post-load insulin and proinsulin [22-24]; oral disposition index [22] on an OGTT; and C-peptide:insulin ratio (a measure of insulin clearance) by rs13266634 genotype, with the R allele associated with less favourable results [25].
Given the established association between rs13266634 and diabetes risk [11, 12], the function of the gene product of SLC30A8, ZnT8, as a zinc transporter in insulin secretory vesicles [17], and prior studies suggesting differential insulin secretion by rs13266634 genotype [20-23], we conducted a clinical trial to evaluate the effect of zinc supplementation on insulin secretion by rs13266634 genotype. We hypothesised that: (1) at baseline, participants with increasing copies of the diabetes risk R allele would have a less robust acute insulin response to intravenous glucose; and (2) zinc supplementation for 14 days would result in an improvement in this insulin response to glucose stimulation in a genotype-dependent manner.
Methods
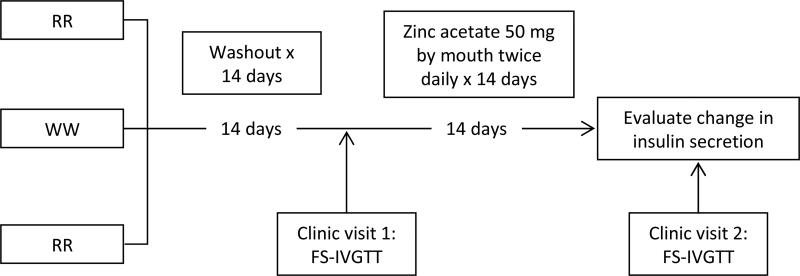
We recruited 57 healthy non-diabetic individuals by rs13266634 genotype (32 RW/WW and 23 RR) and performed an FS-IVGTT before and after 14 days of zinc supplementation in a nonrandomised clinical trial (NCT00981448; Fig. 1).
Fig. 1.
Design of the Zinc Insulin Pharmacogenetics study
Study population
To minimise genetic and environmental heterogeneity, all participants were Old Order Amish from Lancaster, PA, USA, aged 21-70 years and without known diabetes (no diagnosed diabetes and random glucose less than 11.10 mmol/l [200 mg/dl]). We excluded screened persons reporting significant gastrointestinal disease, rheumatoid arthritis, liver disease, haemochromatosis, kidney disease, a cancer diagnosis in the past 2 years, other serious disease, pregnancy or current breastfeeding. We also excluded persons with a serum albumin less than 0.035 g/l, abnormal thyroid stimulating hormone, estimated glomerular filtration rate less than 60 ml/min per 1.73 m2 by the Modification of Diet in Renal Disease equation [26], a haematocrit less than 34%, or those who had received chelation therapy in the past month. Persons on supplements, zinc-containing preparations and medications which affect glucose homeostasis (e.g. systemic corticosteroids, thiazide diuretics, antiretroviral therapy, antipsychotic medications, quinolone antibiotics) who were deemed unable or unwilling to stop these medications for the duration of the study were also excluded. We also excluded those using a zinc-containing denture adhesive. We accounted for relatedness in our study design by excluding first-degree relatives from being in the same genotype group. However, because of difficulty recruiting WW homozygotes, we were forced to relax this criterion and enrolled three pairs of participants who were first-degree relatives with the same genotype. We enriched for individuals with the less common RW and WW genotypes based upon genotyping of stored samples and then re-contacted potential research participants by in-person visits as in previous studies in this Amish population [27].
Washout
After initial screening, individuals were enrolled and then participated in a 14 day washout period during which they stopped all supplements and avoided oysters and beef shank, foods known to have a particularly high zinc content [28].
Intervention
After the 14 day washout period, each participant underwent a first in-person clinic visit for study measures (see below). After their initial clinic visit, all participants took one zinc acetate capsule (containing 50 mg of elemental zinc) by mouth twice daily (Galzin; Teva Pharmaceuticals, Sellersville, PA, USA) for 14 days. Participants were asked to maintain a medication diary and were instructed to resume taking the zinc on the regular schedule if a dose was missed. After 14 days of zinc supplementation, participants returned to the clinic for a second in-person study visit during which study measures were again taken.
Outcomes
The primary outcome was change in the acute insulin response to intravenous glucose estimated by the AUC for insulin during an FS-IVGTT at 5 min comparing measurements taken before and after zinc supplementation for 14 days. Secondary outcomes measured by FS-IVGTT included the change in AUC for insulin at 10 min; proinsulin:insulin at 5 and 10 min; C-peptide at 5 and 10 min; and C-peptide:insulin at 5 and 10 min. We asked participants about gastrointestinal symptoms and symptoms of anaemia at the second visit and also recorded any additional patient-reported side effects for all participants who began the intervention (n=57).
Study measures
Demographic and medical history information was provided by self-report at the in-home screening visit, and screening laboratory tests (basic metabolic profile and complete blood count) were performed by Quest Diagnostics (Horsham, PA, USA). Anthropometric measures were performed during the two clinic visits using standardised protocols for height and weight. At each clinic visit, serum and urine zinc (atomic absorption flame spectroscopy; Hartlab, Bolingbrook, IL, USA), serum glucose (YSI Glucose Analyzer; YSI Life Sciences, Yellow Springs, OH, USA), insulin (radioimmunoassay; EMD Millipore, Billerica, MA, USA), proinsulin (radioimmunoassay; EMD Millipore) and C-peptide (radioimmunoassay; EMD Millipore) were measured in the fasting state in the morning at the beginning of each FS-IVGTT.
FS-IVGTTs were performed using a standard protocol. Briefly, dextrose (0.3 g/kg body weight, 50% dextrose solution) was administered as a bolus at time 0, and blood samples for glucose and insulin were taken at the following time points: -15, -10, -5, -1, 0, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 20, 22, 25, 30, 40, 50, 70, 100, 120, 160 and 180 min. C-peptide and proinsulin were measured at 0, 5, 10, 14 and 20 min.
Genotyping
We performed genotyping of rs13266634 using the TaqMan Allelic Discrimination Assay (Applied Biosystems, Foster City, CA, USA) and tested for Hardy–Weinberg equilibrium (p=0.15). The genotyping call rate was 98% and concordance was 99%.
Analysis
Given the small number of WW homozygotes, we combined RW and WW genotypes and compared this group with RR homozygotes (results for all three genotypes are provided in electronic supplementary material [ESM] Tables 1 and 2). We compared baseline characteristics of participants by genotype group using analysis of variance to compare means, Kruskal–Wallis tests to compare medians, and Fisher's exact tests to compare proportions.
We computed the AUC for insulin using the trapezoidal method and regressed the AUC (natural log-transformed) of insulin on genotype assuming a dominant model. Proinsulin:insulin and C-peptide:insulin ratios were also natural log-transformed for analyses. For each outcome, we computed the relative change from baseline within genotype groups (RW/WW and RR). To compare across groups, we used the RR genotype as the reference and compared the relative change of AUC for insulin, proinsulin:insulin, C-peptide and C-peptide:insulin at 5, 10, 20 and 180 min. Covariates considered in the adjusted linear models included age, sex, baseline BMI, fasting insulin, proinsulin:insulin and HOMA of insulin resistance (HOMA-IR) [29]. HOMA-IR was calculated as fasting glucose (mmol/l) × fasting insulin (mmol/l)/22.5 and was natural log-transformed for analyses. β coefficients for genotype from the linear models were exponentiated to provide a ratio of relative change in the dependent variable.
To account for relatedness of participants: (1) we repeated analyses after excluding one individual (selected at random) from each of the three pairs of first-degree relatives with the same genotype; and (2) we conducted an analysis to account for family structure by including a heritability term in the regression model for the analyses of AUC of insulin at 5 and 10 min.
Participants provided written informed consent, and the study was approved by the relevant institutional review boards.
Results
Fifty-five participants completed the intervention study and were included in the analysis (ESM Fig. 1). Weight did not change significantly over the 14 days of zinc supplementation overall or by genotype. Baseline and fasting characteristics were generally similar across genotype groups with the exception of fasting proinsulin:insulin and fasting C-peptide:insulin, which were marginally lower for the RR genotype group compared with the RW/WW group (Table 1). Baseline FS-IVGTT showed that AUCs for insulin, proinsulin:insulin and C-peptide:insulin at 5 and 10 min were significantly different across genotypes: the AUC for insulin was highest and proinsulin:insulin and C-peptide:insulin were lowest for the RR genotype (Table 1).
Table 1.
Baseline characteristics of participants
| Characteristic | RR (n=23) | RW/WW (n=32) | p value |
|---|---|---|---|
| Age, years | 52 (10) | 52 (9.9) | 0.997 |
| Female, n (%) | 15 (65) | 16 (50) | 0.29 |
| BMI, kg/m2 | 29.8 (25.9, 33.2) | 26.2 (24.2, 30.7) | 0.14 |
| Fasting glucose, mmol/l | 5.51 (0.47) | 5.42 (0.56) | 0.56 |
| Fasting insulin, pmol/l | 96.54 (61.81, 120.84) | 71.14 (47.2, 100.00) | 0.17 |
| Loge HOMA-IR | 1.13 (0.78, 1.47) | 0.89 (0.48, 1.30) | 0.15 |
| Fasting proinsulin, pmol/l | 9.7 (6.9, 13.2) | 9.3 (5.9, 14.6) | 0.79 |
| Fasting C-peptide, nmol/l | 0.460 (0.373, 0.603) | 0.496 (0.293, 0.736) | 0.66 |
| Fasting proinsulin:insulin | 0.79 (0.59, 1.01) | 1.02 (0.74, 1.28) | 0.08 |
| Fasting C-peptide:insulin, nmol/pmol | 0.11 (0.08, 0.13) | 0.14 (0.11, 0.18) | 0.03 |
| Insulin AUC5min, pmol/l × min | 1,583.46 (1,150.79, 1,934.88) | 940.35 (654.91, 1,362.91) | 0.006 |
| Insulin AUC10min, pmol/l × min | 3,816.97 (2,639.79, 4,359.38) | 2,282.13 (1,783.48, 3,229.43) | 0.01 |
| Proinsulin:insulin5min | 0.22 (0.18 to 0.29) | 0.30 (0.23 to 0.42) | 0.02 |
| Proinsulin:insulin10min | 0.40 (0.30 to 0.48) | 0.47 (0.42 to 0.67) | 0.006 |
| C-peptide5min, nmol/l | 1.442 (0.932, 1.648) | 1.192 (0.912, 1.605) | 0.44 |
| C-peptide10min, nmol/l | 1.245 (0.819, 1.462) | 1.136 (0.756, 1.598) | 0.96 |
| C-peptide:insulin5min, nmol/pmol | 0.054 (0.045, 0.067) | 0.066 (0.057, 0.086) | 0.009 |
| C-peptide:insulin10min, nmol/pmol | 0.079 (0.062, 0.10) | 0.097 (0.093, 0.15) | 0.004 |
| Serum zinc, μmol/l | 10.6 (9.6, 12.1) | 10.7 (9.6, 11.3) | 0.60 |
Median (p25, p75) BMI, insulin, ln HOMA-IR, proinsulin, C-peptide, proinsulin:insulin, C-peptide:insulin and zinc shown; mean (SD) glucose shown
Medians compared using Kruskal–Wallis tests, means compared using analysis of variance, and proportions compared using Fisher's exact test
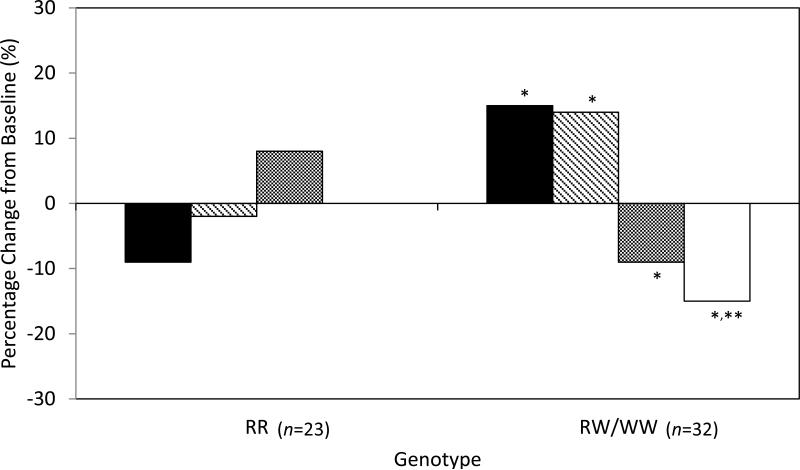
After 14 days of zinc supplementation, serum zinc levels increased by 23% and 33% in the RR and RW/WW groups, respectively (p=0.33). Insulin values over the first 5 min of the FSIVGTT (AUC5min) increased by 15% in the RW/WW genotype group (p=0.04) and did not change significantly in the RR group compared with baseline (Table 2; Fig. 2). The adjusted between-group difference in the change in AUC5min for insulin was 26% higher in the RW/WW genotype group compared with the RR group (p=0.04; Table 2). Results were similar for the analysis of AUC10min for insulin although the comparison with RR homozygotes did not quite reach statistical significance (p=0.058; Table 2 and Fig. 2). Results from sensitivity analyses were also similar.
Table 2.
Zinc, insulin, proinsulin and C-peptide after zinc supplementation
| Variable | RR (n=23) | RW/WW (n=32) |
|---|---|---|
| Fasting serum zinc, μmol/l | 13.2 (12.6, 14.5) | 13.5 (11.9, 15.0) |
| Insulin AUC5min, pmol/l × min | 1,309.83 (945.21, 2,200.18) | 1,263.99 (741.73, 1,895.99) |
| Relative change from baseline, ratio (95% CI) | 0.91 (0.78, 1.07); p=0.26 | 1.15 (1.01, 1.31); p=0.04 |
| Relative change vs RR, ratio (95% CI) | Reference | 1.26 (1.02, 1.56); p=0.036 |
| Insulin AUC10min, pmol/l × min | 3,173.87 (2,277.27, 4,864.28) | 3,273.87 (2,029.33, 4,123.94) |
| Relative change from baseline, ratio (95% CI) | 0.98 (0.86, 1.09); p=0.67 | 1.14 (1.01, 1.24); p=0.01 |
| Relative change vs RR, ratio (95% CI) | Reference | 1.17 (0.99, 1.38); p=0.058 |
| Proinsulin:insulin5min | 0.24 (0.20, 0.29) | 0.27 (0.20, 0.37) |
| Relative change from baseline, ratio (95% CI) | 1.08 (0.96, 1.19); p=0.17 | 0.91 (0.84, 1.00); p=0.056 |
| Relative change vs RR, ratio (95% CI) | Reference | 0.85 (0.74, 0.98); p=0.03 |
| Proinsulin:insulin10min | 0.38 (0.33, 0.45) | 0.44 (0.32, 0.63) |
| Relative change from baseline, ratio (95% CI) | 1.00 (0.89, 1.11); p=0.98 | 0.85 (0.77, 0.94); p=0.002 |
| Relative change vs RR, ratio (95% CI) | Reference | 0.85 (0.72, 1.00); p=0.048 |
| C-peptide5min, nmol/l | 1.242 (1.042, 1.782) | 1.342 (0.859, 1.895) |
| Relative change from baseline, ratio (95% CI) | 0.97 (0.88, 1.06); p=0.49 | 1.03 (0.96, 1.11); p=0.42 |
| Relative change vs RR, ratio (95% CI) | Reference | 1.07 (0.94, 1.21); p=0.32 |
| C-peptide10min, nmol/l | 1.895 (0.909, 1.515) | 1.119 (0.816, 1.472) |
| Relative change from baseline, ratio (95% CI) | 1.01 (0.90, 1.15); p=0.82 | 1.02 (0.91, 1.13); p=0.75 |
| Relative change vs RR, ratio (95% CI) | Reference | 1.00 (0.84, 1.20); p=0.97 |
| C-peptide:insulin5min, nmol/pmol | 0.057 (0.047, 0.072) | 0.062 (0.051, 0.073) |
| Change from baseline, ratio (95% CI) | 1.06 (0.98, 1.16); p=0.15 | 0.92 (0.86, 0.99); p=0.018 |
| Relative change vs RR, ratio (95% CI) | Reference | 0.86 (0.77, 0.97); p=0.015 |
| C-peptide :insulin10min, nmol/pmol | 0.078 (0.06, 0.11) | 0.090 (0.074, 0.12) |
| Change from baseline, ratio (95% CI) | 1.01 (0.88, 1.14); p=0.90 | 0.90 (0.80, 1.01); p=0.07 |
| Relative change vs RR, ratio (95% CI) | Reference | 0.89 (0.74, 1.08); p=0.23 |
Median (p25, p75) zinc, insulin AUC, proinsulin:insulin, C-peptide and C-peptide:insulin shown Relative change in insulin AUC, proinsulin:insulin adjusted for age, BMI and fasting ln HOMA-IR Relative change in C-peptide and C-peptide:insulin adjusted for age, BMI, fasting insulin and fasting C-peptide
Fig. 2.
Per cent change in insulin AUC and proinsulin:insulin ratio from baseline across genotypes. Black bars, insulin AUC5min; striped bars, insulin AUC10min; grey bars, proinsulin:insulin at 5 min; white bars, proinsulin:insulin at 10 min. *p<0.05 for change from baseline for insulin AUC5min and for insulin AUC10min; for change from baseline for insulin AUC5min for RW/WW vs RR; and for change in proinsulin:insulin at 5 and 10 min for RW/WW vs RR. **p<0.01 for change from baseline for proinsulin:insulin at 10 min
Compared with baseline, proinsulin:insulin at 5 min decreased non-significantly by 9% in the RW/WW genotype group (p=0.056) after zinc supplementation and did not change significantly in the RR group (Table 2). The adjusted relative change in proinsulin:insulin at 5 min was 15% lower in the RW/WW vs RR group (p=0.03; Table 2). Proinsulin:insulin decreased by 15% at 10 min for the RW/WW group (p=0.002); compared with the RR group, the adjusted relative change in proinsulin:insulin was also 15% lower (p=0.048).
Compared with baseline, C-peptide did not change significantly at 5 or 10 min after zinc supplementation in any of the genotype groups, but point estimates for the RW/WW group were generally consistent with findings for insulin and proinsulin:insulin (Table 2). Compared with baseline, C-peptide:insulin at 5 min decreased by 8% in the RW/WW group (p=0.018) and by 14% relative to RR homozygotes (p=0.015); results were similar at 10 min but did not reach statistical significance (Table 2). Analyses at 20 min and over the entire FS-IVGTT did not demonstrate significant differences in the response to zinc supplementation across the genotype groups for insulin AUC (data not shown).
There were no serious adverse events during the study. Active ascertainment revealed that 11 of 57 participants (19%) experienced a gastrointestinal symptom, mainly mild nausea (ESM Table 3). Five participants (9%) experienced this when not taking the zinc supplement with a meal. All symptoms were self-limited, and no participant withdrew from the study because of side effects. Side effects did not vary by genotype.
Discussion
In this study, we observed a differential response by rs13266634 genotype in the insulin response at 5 and 10 min after an intravenous glucose load before and after 14 days of zinc supplementation. Despite serum zinc concentrations increasing similarly among the genotype groups, our results show a consistent improvement in insulin indices for individuals in the RW/WW genotype group compared with those in the RR genotype group. After zinc supplementation, participants with the RW/WW genotype experienced 15% and 14% increases in the insulin response to intravenous glucose at 5 and 10 min, respectively, compared with baseline, and a 26% relative increase in the insulin response at 5 min compared with the change seen in the RR genotype group. Participants with the RW/WW genotype also had lower values of proinsulin:insulin and C-peptide:insulin compared with baseline and with the corresponding change in the RR group, suggesting improvements in insulin processing and decreased insulin clearance in the RW/WW group relative to RR homozygotes.
Epidemiological studies have demonstrated an association between zinc and glucose homeostasis: cross-sectional studies show that urine zinc levels are higher [30] and blood zinc levels are slightly lower in patients with diabetes compared with those without diabetes [30, 31], and a prospective study of 82,297 women found that higher self-reported zinc intake was associated with a decreased risk of diabetes over 24 years [32]. Finally, studies of zinc supplementation have demonstrated improvements in glucose and HbA1c in patients with and without diabetes [33, 34].
While our results suggest a differential insulin response of the rs13266634 genotype to zinc supplementation, they were contrary to what we had originally hypothesised. Based on prior studies demonstrating less favourable insulin and proinsulin:insulin indices for the RR genotype on FS-IVGTT and OGTTs, we anticipated observing the same in our population at baseline and expected an improvement in the insulin response after zinc supplementation for the RR genotype. However, at baseline, we observed higher insulin AUC and lower proinsulin:insulin at 5 and 10 min in the RR vs the RW/WW genotype group, and we found no significant change in the selected FS-IVGTT outcomes in the RR group after zinc supplementation. Importantly, our results for the RW/WW group were internally consistent across outcomes (i.e. insulin AUC and proinsulin:insulin at 5 and 10 min), suggesting that our findings are in fact true for our study population. Also, a recent cross-sectional study in Chinese participants demonstrated that the inverse association between plasma zinc and diabetes was attenuated in those with the RR genotype [35]; these results are consistent with our finding that zinc may be most beneficial in those with the W allele. Our WW genotype group was too small to make conclusions regarding its response. Thus, from our study, one could conclude that zinc supplementation improves the early insulin response and proinsulin:insulin ratio in those with at least one copy of the W allele.
An important factor in interpreting our findings is the baseline serum zinc status of our Amish study population. The normal range for serum zinc is 10.1-16.8 μmol/l [36], and baseline serum zinc levels were in the lower part of this range in our study population. Therefore, our study population may have lower zinc levels compared with those of other populations [37, 38], and the effect of rs13266634 on glucose-stimulated insulin response at baseline and after zinc supplementation could be different in a more ‘zinc replete’ population. In fact, the sensitivity of the effect of rs13266634 to nutrient levels was highlighted by a recent analysis of National Health and Nutrition Examination Survey data in which the authors reported a significant interaction between rs13266634 and serum levels of trans-β-carotene on diabetes risk (p=5×10−5): the R allele was associated with a significantly increased risk of type 2 diabetes (OR 1.8) among those with low trans-β-carotene levels but was associated with a decreased diabetes risk (OR 0.65) in those with high trans-β-carotene levels [39].
In support of a potential interaction with zinc intake, a cross-sectional meta-analysis in 34,150 participants without diabetes reported a modest but statistically significant interaction between the SLC30A8 rs11558471 SNP and total zinc intake for fasting glucose [40]. rs11558471 was in strong linkage disequilibrium with rs13266634 (r2=0.96) in that study [40].
Strengths of our study are that the Amish represent a homogeneous population with respect to lifestyle and genetics, and we avoided the potentially confounding influence of glucose intolerance/diabetes by studying non-diabetic individuals. However, there were also limitations. First, the study sample size was small, which increases the chance of both false-positive and false-negative findings. By chance, the baseline insulin indices (e.g. AUC5min for insulin) may not have reflected what would be seen across genotypes in a larger population. We observed the highest baseline AUC5min for insulin in the RR group, and this could have affected between-group changes in response to zinc supplementation; we might expect less of an increase in AUC5min for insulin for the group with the highest baseline AUC5min, which was the RR group in this study. Second, it is not clear that our findings are generalisable to other populations and to those with glucose intolerance/diabetes, which necessitates further studies.
While studies of the association between rs13266634 and diabetes and glucose homeostasis traits have been consistent in humans, animal and in vitro studies have been less definitive. Studies of Slc30a8−/− null mouse islets [41-43] and ZnT8 downregulated rat insulinoma cells [44] have consistently shown a significant decrease in the number of dense core granules (zinc insulin crystals) compared with wild-type islets or control cells. However, in vitro studies of the effects of downregulation [44] and overexpression [41, 45] of ZnT8 on glucose-stimulated insulin secretion have reported mixed results [41-43, 46]. Similarly, in vivo studies of intraperitoneal glucose tolerance tests [41, 42, 47] and OGTTs [43] in Slc30a8−/− and wild-type mice have demonstrated inconsistent effects of knocking out SLC30A8 on glucose tolerance. The inconsistencies across preclinical studies highlight that the role of ZnT8 in zinc transport and islet function is complex and that further studies will be required to better understand its biology and role in health and disease. A recent report found that 12 rare loss-of-function mutations in SLC30A8 were actually associated with a 65% reduced risk of diabetes (p=1.7×10−6) [48].
Regarding the functional consequences of the R325W variant in rs13266634, a single study of INS-1E cells expressing either the R325 or W325 variant demonstrated lower glucose-stimulated insulin secretion for the R325 vs the W325 cells [49]. Finally, two studies have used homology modelling (with the Escherichia coli zinc transporter YiiP) to deduce the impact of the rs13266634 SNP on ZnT8 structure with conflicting conclusions [41, 50]. Both studies determined that the site of the SNP (position 325 of the peptide) is cytoplasmic and near the interface of the monomers [41, 50]; however, one study postulated that the presence of the arginine (R) residue in place of tryptophan (W) places a positive charge in an area which could affect dimerisation of the protein and binding of zinc [41], while the other suggests that the 325 residue points away from the monomer interface and is too far from the zinc binding sites to impact them [50].
Results from a recent study suggest that a primary defect caused by rs13266634 is the dysregulation of hepatic insulin clearance related to the zinc co-secreted with insulin (from insulin secretory vesicles) into the portal circulation [25]. In this study, peripheral blood insulin levels were lower in the Slc30a8 knockout vs control mice on intraperitoneal glucose tolerance testing [25], and perfusion studies revealed that zinc inhibited hepatic clearance of insulin but did not affect peripheral C-peptide or proinsulin [25]. Correspondingly, C-peptide:insulin levels were higher in the Slc30a8 knockout mice, indicating relatively increased insulin clearance compared with control mice [25]. In humans, there was no significant difference in early peripheral insulin secretion on OGTT for the RR (n=12) vs RW/WW (n=42) genotypes, but C-peptide:insulin ratios were lower for the RW/WW group [25]. In our study, we did find that C-peptide:insulin decreased in the RW/WW genotype group after zinc supplementation, indicating a decrease in hepatic insulin clearance in this group. The relative contribution of rs13266634 on beta cell insulin secretion and hepatic insulin clearance to overall peripheral insulin remains unclear at this time, but further understanding of this may help to explain our unexpected findings.
In summary, we report a differential, significant and consistent response to brief zinc supplementation by rs13266634 genotype regarding the acute insulin, proinsulin:insulin and C-peptide:insulin response to intravenous glucose. While our findings for the RR genotype group were unexpected, consistency in the direction of effect of changes in acute insulin release and proinsulin:insulin as well as recent studies of rs13266634 [25, 35] lend support to our overall findings. Moreover, the complexity of SLC30A8 variation is highlighted by a recent study demonstrating that some rare loss-of-function mutations in SLC30A8 actually decrease diabetes risk [48] and that nutrient interactions with rs13266634 may be sensitive to baseline nutrition status [39]. Future studies should evaluate the benefit of zinc supplementation on glucose tolerance in individuals by rs13266634 genotype and explore the importance of baseline zinc status and hepatic insulin clearance to this interaction. In providing the first experimental data in humans on the effect of zinc supplementation on insulin response by SLC30A8 genotype, we show that, ultimately, zinc supplementation may prove to be beneficial for diabetes prevention and/or treatment in some individuals based on SLC30A8 variation.
Supplementary Material
Acknowledgements
The authors thank G. Brewer (University of Michigan, Ann Arbor, MI, USA) for his advice on the measurement of zinc in this study. The authors thank B. Mitchell and K. Ryan (University of Maryland School of Medicine, Baltimore, MD, USA) for their assistance with analyses. The authors are grateful to the Zinc Insulin Pharmacogenetics study participants and staff at the Amish Research Clinic in Lancaster, PA, USA (University of Maryland School of Medicine).
Funding
This research was supported in part by the Mid-Atlantic Nutrition Obesity Research Center, P30 DK072488, from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases and by The Johns Hopkins Clinical Research Scholars Program (1KL2 RR025006).
Abbreviations
- FS-IVGTT
Frequently sampled IVGTT
- HOMA-IR
HOMA of insulin resistance
- SNP
Single nucleotide polymorphism
- ZnT8
Zinc transporter protein member 8
Footnotes
Trial registration: ClinicalTrials.gov NCT00981448
Duality of interest
The authors have no conflicts of interest to disclose. Adeona Pharmaceuticals (Ann Arbor, MI, USA) provided measurement of serum zinc free of charge.
Contribution statement
NMM refined the study design, collected and interpreted the data, and drafted and revised the manuscript. MF collected and interpreted the data, contributed to the methods of the manuscript and reviewed/edited the manuscript. JMC refined the study design, interpreted the data and reviewed/edited the manuscript. ARS refined the study design, collected and interpreted the data and reviewed/edited the manuscript. WHLK interpreted the data and reviewed/edited the manuscript. All authors approved the final version of the paper except for WHLK, who died before she was able to do so. NMM takes responsibility for the contents of this article.
References
- 1.Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England Journal of Medicine. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jablonski KA, McAteer JB, de Bakker PIW, et al. Common variants in 40 genes assessed for diabetes incidence and response to metformin and lifestyle intervention in the Diabetes Prevention Program. Diabetes. 2010;59:2672–2681. doi: 10.2337/db10-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrulionyte L, Zacharova J, Chiasson JL, Laakso M. Common polymorphisms of the PPAR-gamma2 (Pro12Ala) and PGC-1alpha (Gly482Ser) genes are associated with the conversion from impaired glucose tolerance to type 2 diabetes in the STOP-NIDDM trial. Diabetologia. 2004;47:2176–2184. doi: 10.1007/s00125-004-1577-2. [DOI] [PubMed] [Google Scholar]
- 4.Zacharova J, Todorova BR, Chiasson JL, Laakso M. The G-250A substitution in the promoter region of the hepatic lipase gene is associated with the conversion from impaired glucose tolerance to type 2 diabetes: the STOP-NIDDM trial. J Intern Med. 2005;257:185–193. doi: 10.1111/j.1365-2796.2004.01435.x. [DOI] [PubMed] [Google Scholar]
- 5.Pearson ER, Donnelly LA, Kimber C, et al. Variation in TCF7L2 influences therapeutic response to sulfonylureas: a GoDARTS study. Diabetes. 2007;56:2178–2182. doi: 10.2337/db07-0440. [DOI] [PubMed] [Google Scholar]
- 6.Zhou K, Bellenguez C, Spencer CC, et al. Common variants near ATM are associated with glycemic response to metformin in type 2 diabetes. Nat Genet. 2011;43:117–120. doi: 10.1038/ng.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou K, Donnelly LA, Kimber CH, et al. Reduced-function SLC22A1 polymorphisms encoding organic cation transporter 1 and glycemic response to metformin: a GoDARTS study. Diabetes. 2009;58:1434–1439. doi: 10.2337/db08-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meigs JB, Shrader P, Sullivan LM, et al. Genotype score in addition to common risk factors for prediction of type 2 diabetes. The New England Journal of Medicine. 2008;359:2208–2219. doi: 10.1056/NEJMoa0804742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyssenko V, Jonsson A, Almgren P, et al. Clinical risk factors, DNA variants, and the development of type 2 diabetes. The New England Journal of Medicine. 2008;359:2220–2232. doi: 10.1056/NEJMoa0801869. [DOI] [PubMed] [Google Scholar]
- 10.Schmid R, Vollenweider P, Bastardot F, Vaucher J, Waeber G, Marques-Vidal P. Current genetic data do not improve the prediction of type 2 diabetes mellitus: the Colaus Study. Journal of Clinical Endocrinology & Metabolism. 2012;97:E1338–E1341. doi: 10.1210/jc.2011-3412. [DOI] [PubMed] [Google Scholar]
- 11.Jing YL, Sun QM, Bi Y, Shen SM, Zhu DL. SLC30A8 polymorphism and type 2 diabetes risk: evidence from 27 study groups. Nutr Metab Cardiovasc Dis. 2011;21:398–405. doi: 10.1016/j.numecd.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Cauchi S, del Guerra S, Choquet H, et al. Meta-analysis and functional effects of the SlC30A8 rs13266634 polymorphism on isolated human pancreatic islets. Mol Genet Metab. 2010;100:77–82. doi: 10.1016/j.ymgme.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 14.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science (New York, NY) 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saxena R, Voight BF, Lyssenko V, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi F, Serizawa M, Yamamoto K, et al. Confirmation of multiple risk loci and genetic impacts by a genome-wide association study of type 2 diabetes in the Japanese population. Diabetes. 2009;58:1690–1699. doi: 10.2337/db08-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chimienti F, Devergnas S, Favier A, Seve M. Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes. 2004;53:2330–2337. doi: 10.2337/diabetes.53.9.2330. [DOI] [PubMed] [Google Scholar]
- 18.Dunn MF. Zinc-ligand interactions modulate assembly and stability of the insulin hexamer—a review. Biometals. 2005;18:295–303. doi: 10.1007/s10534-005-3685-y. [DOI] [PubMed] [Google Scholar]
- 19.Hou JC, Min L, Pessin JE. Insulin granule biogenesis, trafficking and exocytosis. Vitam Horm. 2009;80:473–506. doi: 10.1016/S0083-6729(08)00616-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boesgaard TW, Zilinskaite J, Vanttinen M, et al. The common SLC30A8 Arg325Trp variant is associated with reduced first-phase insulin release in 846 non-diabetic offspring of type 2 diabetes patients—the EUGENE2 study. Diabetologia. 2008;51:816–820. doi: 10.1007/s00125-008-0955-6. [DOI] [PubMed] [Google Scholar]
- 21.Majithia A, Jablonski K, McAteer J, et al. Association of the SLC30A8 missense polymorphism R325W with proinsulin levels at baseline and after lifestyle, metformin or troglitazone intervention in the Diabetes Prevention Program. Diabetologia. 2011;54:2570–2574. doi: 10.1007/s00125-011-2234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stancakova A, Kuulasmaa T, Paananen J, et al. Association of 18 confirmed susceptibility loci for type 2 diabetes with indices of insulin release, proinsulin conversion, and insulin sensitivity in 5,327 nondiabetic Finnish men. Diabetes. 2009;58:2129–2136. doi: 10.2337/db09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirchhoff K, Machicao F, Haupt A, et al. Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia. 2008;51:597–601. doi: 10.1007/s00125-008-0926-y. [DOI] [PubMed] [Google Scholar]
- 24.Steinthorsdottir V, Thorleifsson G, Reynisdottir I, et al. A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet. 2007;39:770–775. doi: 10.1038/ng2043. [DOI] [PubMed] [Google Scholar]
- 25.Tamaki M, Fujitani Y, Hara A, et al. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J Clin Invest. 2013;123:4513–4524. doi: 10.1172/JCI68807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 27.Hsueh WC, Mitchell BD, Aburomia R, et al. Diabetes in the Old Order Amish: characterization and heritability analysis of the Amish Family Diabetes Study. Diabetes Care. 2000;23:595–601. doi: 10.2337/diacare.23.5.595. [DOI] [PubMed] [Google Scholar]
- 28.Office of Dietary Supplements of the National Institutes of Health [7 July 2014];Dietary supplement fact sheet: zinc. 2013 Available from http://ods.od.nih.gov/FactSheets/Zinc_pf.asp.
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 30.Kazi TG, Afridi HI, Kazi N, et al. Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biological Trace Element Research. 2008;122:1–18. doi: 10.1007/s12011-007-8062-y. [DOI] [PubMed] [Google Scholar]
- 31.Yerlikaya FH, Toker A, Aribas A. Serum trace elements in obese women with or without diabetes. The Indian Journal of Medical Research. 2013;137:339–345. [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Q, van Dam RM, Willett WC, Hu FB. Prospective study of zinc intake and risk of type 2 diabetes in women. Diabetes Care. 2009;32:629–634. doi: 10.2337/dc08-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Capdor J, Foster M, Petocz P, Samman S. Zinc and glycemic control: a meta-analysis of randomised placebo controlled supplementation trials in humans. Journal of Trace Elements in Medicine and Biology : Organ of the Society for Minerals and Trace Elements. 2013;27:137–142. doi: 10.1016/j.jtemb.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Jayawardena R, Ranasinghe P, Galappatthy P, Malkanthi R, Constantine G, Katulanda P. Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr. 2012;4:13. doi: 10.1186/1758-5996-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shan Z, Bao W, Zhang Y, et al. Interactions between zinc transporter-8 gene (SLC30A8) and plasma zinc concentrations for impaired glucose regulation and type 2 diabetes. Diabetes. 2014;63:1796–1803. doi: 10.2337/db13-0606. [DOI] [PubMed] [Google Scholar]
- 36.Porter RS, editor. The Merck manual of diagnosis and therapy. Merck Sharp & Dohme; Whitehouse Station, NJ: 2011. [Google Scholar]
- 37.Xu J, Zhou Q, Liu G, Tan Y, Cai L. Analysis of serum and urinal copper and zinc in Chinese northeast population with the prediabetes or diabetes with and without complications. Oxidative Medicine and Cellular Longevity. 2013;2013:635214. doi: 10.1155/2013/635214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hotz C, Peerson JM, Brown KH. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the Second National Health and Nutrition Examination Survey data (1976-1980). Am J Clin Nutr. 2003;78:756–764. doi: 10.1093/ajcn/78.4.756. [DOI] [PubMed] [Google Scholar]
- 39.Patel C, Chen R, Kodama K, Ioannidis JA, Butte A. Systematic identification of interaction effects between genome- and environment-wide associations in type 2 diabetes mellitus. Human Genetics. 2013;132:495–508. doi: 10.1007/s00439-012-1258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanoni S, Nettleton JA, Hivert M-F, et al. Total zinc intake may modify the glucose-raising effect of a zinc transporter (SLC30A8) variant. Diabetes. 2011;60:2407–2416. doi: 10.2337/db11-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicolson TJ, Bellomo EA, Wijesekara N, et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes. 2009;58:2070–2083. doi: 10.2337/db09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lemaire K, Ravier MA, Schraenen A, et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14872–14877. doi: 10.1073/pnas.0906587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wijesekara N, Dai FF, Hardy AB, et al. Beta cell-specific ZnT8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia. 2010;53:1656–1668. doi: 10.1007/s00125-010-1733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu Y, Tian W, Pratt EB, et al. Down-regulation of ZnT8 expression in INS-1 rat pancreatic beta cells reduces insulin content and glucose-inducible insulin secretion. PloS one. 2009;4:e5679. doi: 10.1371/journal.pone.0005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chimienti F, Devergnas S, Pattou F, et al. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci. 2006;119:4199–4206. doi: 10.1242/jcs.03164. [DOI] [PubMed] [Google Scholar]
- 46.Pound LD, Sarkar SA, Benninger RK, et al. Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. The Biochemical Journal. 2009;421:371–376. doi: 10.1042/BJ20090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pound LD, Sarkar SA, Cauchi S, et al. Characterization of the human SLC30A8 promoter and intronic enhancer. Journal of Molecular Endocrinology. 2011;47:251–259. doi: 10.1530/JME-11-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flannick J, Thorleifsson G, Beer NL, et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat Genet. 2014;46:357–363. doi: 10.1038/ng.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim I, Kang ES, Yim YS, et al. A low-risk ZnT-8 allele (W325) for post-transplantation diabetes mellitus is protective against cyclosporin A-induced impairment of insulin secretion. The Pharmacogenomics Journal. 2011;11:191–198. doi: 10.1038/tpj.2010.22. [DOI] [PubMed] [Google Scholar]
- 50.Weijers RN. Three-dimensional structure of beta-cell-specific zinc transporter, ZnT-8, predicted from the type 2 diabetes-associated gene variant SLC30A8 R325W. Diabetol Metab Syndr. 2010;2:33. doi: 10.1186/1758-5996-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.