Abstract
Background
The limited characterization of equine skin, eye and hoof epithelial stem cell (ESC) and differentiation markers impedes the investigation of the physiology and pathophysiology of these tissues.
Hypothesis/Objectives
To characterize ESC and differentiation marker expression in epithelial tissues of the equine eye, haired skin and hoof capsule.
Methods
Indirect immunofluorescence microscopy and immunoblotting were utilized to detect expression and tissue localization of keratin (K) isoforms K3, K10, K14, and K124, the transcription factor p63 (a marker of ESCs) and phosphorylated p63 (pp63, a marker of ESC to transit-amplifying (TA) cell transition) in epithelial tissues of the foot (haired skin, hoof coronet and hoof lamellae) and the eye (limbus and cornea).
Results
K14 expression was restricted to the basal layer of epidermal lamellae, and to basal and adjacent suprabasal layers of the haired skin, coronet and corneal limbus. Coronary and lamellar epidermis was negative for both K3 and K10, which were expressed in the cornea/limbus epithelium and haired skin epidermis, respectively. Variable expression of p63 with relatively low to high levels of phosphorylation was detected in individual basal and suprabasal cells of all epithelial tissues examined.
Conclusions
This is the first report of the characterization of tissue-specific keratin marker expression and the localization of putative epithelial progenitor cell populations, including ESCs (high p63 expression with low pp63 levels) and TA cells (high expression of both p63 and pp63), in the horse. These results will aid further investigation of epidermal and corneal epithelial biology and regenerative therapies in horses.
Introduction
Several aspects of equine anatomy predispose horses to epidermal and corneal injury and disease. The elongation of the distal limb and lateral placement of prominent eyes are associated with frequent and difficult to treat skin wounds and corneal ulcers.1-4 The equine digital integumentary accessory organ has evolved to form a hoof capsule that is anatomically and functionally integrated with the musculoskeletal system.5 The hoof capsule allows locomotion across hard surfaces, but is also the single most common source of lameness in horses.6 In particular, laminitis is a common and debilitating disease associated with chronic pain and lameness that frequently necessitates euthanasia.7 In contrast to the anatomy of equine haired skin and cornea, which are similar to those of other mammalian species, the equine hoof capsule has undergone extensive modification and specialization that is unique to the equidae. The hoof capsule is lined with 550–600 parallel cornified primary epidermal lamellae (PELs), each of which has 150–200 secondary epidermal lamellae (SELs), giving an estimated total surface area for lamellar attachment to the interdigitating secondary dermal lamellae (SDLs) and primary dermal lamellae (PDLs) of approximately 0.8 m2 per foot (see Figure S1 in Supporting Information).5,8,9 This epidermal-dermal lamellar attachment and dermal connective tissue suspend the distal phalanx within the hoof capsule.8 Laminitis-associated lamellar lesions include necrosis, inflammation and aberrant proliferation, with marked distortion of both epidermal and dermal components that often progress to biomechanical failure of the suspensory apparatus of the distal phalanx.10,11
The epidermis, SELs and the corneal epithelium are stratified epithelia, consisting of a single cell-thick basal layer that rests on the basement membrane, a variable number of suprabasal cell layers and a superficial cell layer that is continually shed (skin, cornea), or mechanically exfoliated (hoof wall and PELs).12,13 In contrast to skin and cornea, the interdigitated arrays of inner hoof capsule lamellae (e.g. stratum internum) comprise a single layer of columnar basal cells and a 1-2 cell thick layer of fusiform suprabasal cells that transitions abruptly to the central keratinized axis of each PEL, which abaxially merges with the hard keratinized tissues of the hoof wall (e.g. stratum medium) (Figure S1, B-E). However, in spite of the clinical significance of these vital structures, the basic molecular biology and differentiation of equine epithelial tissues are poorly defined.
Some explanation of the anatomical nomenclature for equine hoof capsule structures is warranted due to controversy in the literature over the use of “epidermal” to describe these structures. The current extant nomenclature, both in anatomy references and the laminitis literature, includes the use of “epidermal” and “dermal” as adjectives to describe the interdigitating lamellae of the inner hoof capsule and adjacent underlying corium, respectively.5,8,14 Similarly, “epidermal” is frequently used in reference to integumentary modifications in other species such as the claw of the dog,15,16 epidermal scales of fish and reptiles,17 epidermal scutes of the turtle shell,18 and the feathers of birds.5,16 In all cases, the intention of this nomenclature is to recognize the evolutionary and developmental origin of both skin and the adnexal structures from a common fetal epidermis, which is itself derived from the embryonic surface ectoderm,19,20 and to clearly differentiate the epidermal lamellae of the inner hoof capsule from the underlying dermal lamellae. However, some restrict the use of “epidermal” to the epidermis of haired skin exclusively and exclude its use in reference to adnexal structures. It is not disputed that nail or hoof are formed by the proliferation of epidermal cells, rather that the resulting structures differ enough from skin at the histological and biochemical levels to require specific descriptors.21,22 In particular, keratin isoform expression may differ between the two tissues23,24 and normal adult epidermal lamellae, in contrast to skin epidermis, do not demonstrate a granular cell layer, although a granular cell layer is present during fetal hoof development.25 We have chosen to use the term, “epidermal lamella,” because 1) it recognizes the developmental origin of the tissue, 2) it recognizes the shared histological and biochemical features of skin and lamellar stratified, cornifying epithelia, 3) it does not equate the tissue with skin epidermis because “epidermal” is used as an adjective to describe a specific anatomical structure, the lamella of the inner hoof capsule, 4) it facilitates the distinction of epidermal and dermal lamellae and the importance of dermo-epidermal integrity in the maintenance of the suspensory apparatus of the distal phalanx and 5) it is consistent with the majority of the laminitis literature.
The unique and divergent differentiation of equine cornea, skin and lamellae is partially determined by the keratin composition of these tissues. Keratin proteins belong to the intermediate filament family of cytoskeletal proteins and participate in the maintenance of cell structure, intercellular adhesion and cellular responses to extracellular forces.26,27 Although keratins comprise approximately 80% of the total protein content in the cells of stratified epithelia,13 few studies have characterized equine corneal and epidermal tissue-specific keratin expression.23,24,28,29 Moreover, changes in keratin isoform expression are associated with the response to wounding, disease states and regeneration of epithelial tissues, consistent with their essential role in epithelial structure and function.30-32 A previous quantitative proteomic analysis of equine lamellar tissue identified two novel keratins, K42 and K124, as the most abundant cytoskeletal proteins.24 The genes encoding K42 and K124 are pseudogenes in humans.33 K42 expression is restricted to the nail bed and matrix in mice34 and K124 is conceptually translated from cDNA libraries in mice and rats35 and from the draft genomic sequence of the opossum.36 If K42 and K124 expression is similarly restricted to the hoof capsule, these keratin isoforms could serve as tissue-specific differentiation markers for lamellar epithelial cells. K10 is a major keratin isoform in the postmitotic keratinizing cells in the suprabasal layers of the skin epidermis and could serve as a useful tissue-specific differentiation marker for postmitotic equine skin epidermal cells.13,37 K3 has been used as a differentiation marker for postmitotic corneal epithelial cells in multiple species, including the horse.28,38 The basal cell layer of all stratified epithelia, which includes proliferative epithelial stem cells (ESCs) and transit-amplifying (TA) cells, express K14.13 K14 is, therefore, frequently used as an epithelial basal cell marker, although K14 expression is not restricted to the basal layer in all stratified epithelia and is not exclusively expressed in ESCs and TA cells, necessitating the use of additional markers to identify these cell types.39 K14 is expressed in equine haired skin and lamellae,23,24,40 and could serve as a useful, but not exclusive, marker of equine stratified epithelial basal cells and ESCs.
Maintenance and repair of stratified epithelial tissues rely on the presence of epithelial progenitor cells, including both ESCs and TA cells, and the appropriate differentiation of TA cells to terminally differentiating cells.12 A previous study demonstrated that the dysplastic epidermal lamellae in horses with chronic laminitis have significantly decreased expression of p63, a marker of ESC proliferative capacity, consistent with the altered growth, differentiation and regenerative capacity of this tissue.40,41 One study has demonstrated suppression of the canonical Wnt signalling pathway in lamellar tissue from horses with laminitis and associated changes in lamellar epidermal expression of cell-cell and cell-matrix adhesion proteins, which is also consistent with altered cell and tissue differentiation.42 Although there is histopathological evidence of altered epidermal differentiation associated with laminitis, few studies have investigated the effect of laminitis on keratin isoform expression.30,43 Moreover, those studies did not investigate the expression of the major lamellar keratins, K42 and K124.24
The objective of this study was to characterize the expression of tissue-specific differentiation markers (K3, K10 and K124) and general markers for basal cells (K14), ESCs (p63) and TA cells (pp63) in equine stratified epithelia of the haired skin, cornea and hoof capsule.
Materials and methods
Subjects
All subjects were chosen as control horses for a laminitis tissue repository44 and had no clinical history, gross or histopathological evidence of laminitis, dermatological conditions or corneal disease. Six horses (Table 1) were donated for euthanasia and tissue retrieval due to diverse clinical conditions that could not be improved with standard veterinary interventions at the New Bolton Center.44 Horses were subjected to euthanasia by pentobarbital and phenytoin overdose, according to procedures approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Table 1.
Age, sex and breed of the horses used in the study
| Horse ID | Age (years) | Sex | Breed |
|---|---|---|---|
| 50 | 7 | Gelding | TB |
| 52 | 17 | Gelding | TB |
| 57 | 6 | Gelding | WB |
| 91 | 14 | Mare | Paso Fino |
| 92 | 18 | Mare | WB |
| 93 | 13 | Gelding | Welsh/TB |
TB = thoroughbred; WB = warmblood
Tissue retrieval
The anatomical locations of tissues dissected from the foot are illustrated in Figure S1A. Haired skin (1-10 cm proximal to the hoof capsule), coronet (coronary region of the hoof, including epidermal and supporting dermal tissue at the proximal edge of the hoof capsule from which the hoof wall grows, often including a portion of the periople) and lamellar tissues (the innermost layer of the hoof capsule, including PELs and SELs, corresponding primary and secondary dermal lamellae and adjacent dermal corium) were collected as described elsewhere.40,45 Briefly, after the digit was disarticulated at the metacarpophalangeal joint, two approximately 1.5 cm thick parasagittal sections were made on either side of the dorsal midline of the foot by bandsaw. Tissue blocks measuring approximately 5×5 mm that included epidermal and dermal tissues from the haired skin, coronary and lamellar regions were dissected from these parasagittal sections. Lamellar tissue blocks include approximately 1 mm of the adjacent hoof wall and the dermal corium up to the surface of the distal phalanx. Lamellar tissue sections were oriented in a transverse plane, as shown in Figure S1: B-E. Cornea and limbus were dissected by scalpel immediately postmortem. Tissue samples were immediately either snap frozen in liquid nitrogen and stored until sample processing for immunoblotting, or fixed in 10% neutral-buffered formalin for 24 h and stored in 70% ethanol until paraffin embedding for histological studies.
Antibodies and Immunoblotting
Proteins were extracted from pulverized frozen tissues (Bio-pulverizer, BioSpec Products, Bartlesville, OK, USA) with SDS buffer (pH 6.8) composed of 4% SDS, 120 mM Tris base, 20% glycerol, protease inhibitors (Complete Protease Inhibitor Cocktail Tablets and Pefabloc SC (4-(2-Aminoethyl)-benzenesulfonyl fluoride, hypochloride), Roche Diagnostics, Indianapolis, IN, USA) and separated by SDS-PAGE followed by electrophoretic transfer to polyvinylidene difluoride membrane as previously described.24,40 Immunoblots were blocked with 5% fish gelatin (Sigma-Aldrich, St. Louis, MO, USA) in 0.1% Tween/Tris-buffered saline and incubated with the following antibodies: mouse monoclonal anti-mouse p63 (1:500; clone 4A4, Sigma-Aldrich), rabbit polyclonal anti-human Ser 160/162 phosphorylated-p63, (pp63, 1:250; Ser 160/162, Cell Signaling Technology, Danvers, MA, USA), mouse monoclonal anti-human keratin (K)14 (1:500; clone LL002, Abcam, Cambridge, MA, USA), rabbit polyclonal anti-human integrin α6 (H-87, previously shown to react with the major type II keratin in lamellar tissue, K124,24 1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse monoclonal anti-rabbit K3/K76 antibody (1:500; clone AE5, Millipore, Billerica, MA, USA), and mouse monoclonal anti-human K10 (1:1000; clone DE-K10, Thermo Scientific, Waltham, MA, USA), followed by incubation with horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG antibodies (1:5000), or with mouse monoclonal anti-β-actin-peroxidase antibody (1:50,000; clone AC-15, Sigma-Aldrich) and the signals were visualized with enhanced chemiluminescence (Amersham ECL kit, GE Healthcare, Little Chalfont, UK) as detected by radiographic film exposure and processing. Gel-Pro 4.5 imaging software (Media Cybernetics, Rockville, MD, USA) was used to analyze scanned images. All experiments were performed at least three times with tissue samples from different horses.
Indirect immunofluorescence
Indirect immunofluorescence was performed on formalin-fixed, paraffin-embedded tissue sections (6 μm) as described elsewhere.24,40 Antigen retrieval was performed either by incubation with trypsin solution and 1% calcium chloride at 37°C for 20 min (for K3/K76) or steaming in sodium citrate (Antigen Unmasking Buffer, Vector Laboratories, Burlingame, CA, USA) for 45 min (for K14, integrin α6/K124 and pp63). K10 and p63 antibodies did not require antigen retrieval. Tissues were incubated with primary antibodies overnight at 4°C followed by incubation with Alexa Fluor® fluorochrome-conjugated secondary antibodies at room temperature for 1 h. The same primary antibodies listed for immunoblotting were used at the following dilutions: anti-K10 (1:100), anti-K14 (1:100), anti-integrin α6/K124 (1:100), anti-K3/K76 (1:500), anti-p63 (1:500) and anti-pp63 (1:150). The following secondary antibodies were used: Alexa Fluor 488 goat anti-mouse IgG (1:500, Invitrogen/Life Technologies Molecular Probes, Grand Island, NY, USA), Alexa Fluor 488 goat anti-rabbit IgG (1:500, Invitrogen/Life Technologies), Alexa Fluor 546 goat anti-mouse IgG (1:500, Invitrogen/Life Technologies), or Alexa Fluor 594 rabbit anti-mouse IgG (1:500, Invitrogen/Life Technologies). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole, dilactate (DAPI, 0.5 μg/ml, Invitrogen/Life Technologies) for 5 min at room temperature. Sections were mounted using VectaShield™ mounting medium (Vector Laboratories) for light microscopy with epifluorescence illumination (model DM5000B, Leica, Bannockburn, IL, USA). Images were acquired with a digital camera (CoolSNAP fx Photometrics, Tucson, AZ, USA) and processed with Image-Pro Plus, v.7.0 software, (Media Cybernetics). All experiments were performed at least three times with tissue samples from different horses.
Results
Expression of keratin markers in epithelial cells of equine eye, skin and hoof lamellae
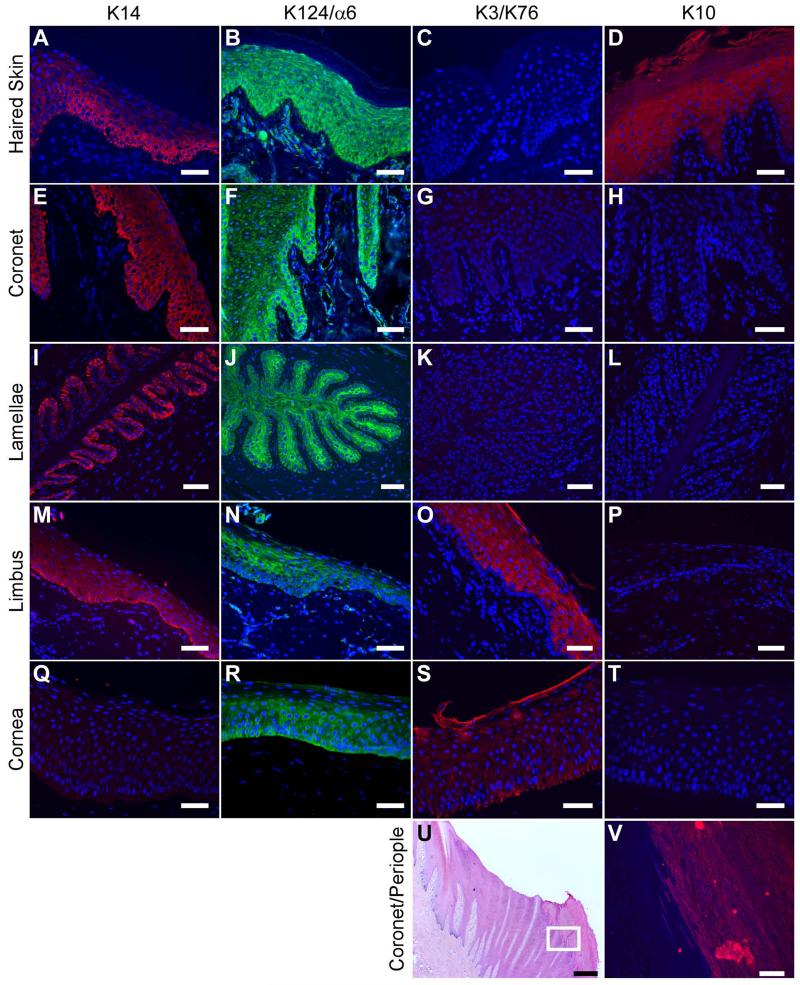
The expression of keratin markers in epithelial cells of the haired skin, coronary and lamellar regions of the foot, and the limbal and central corneal regions of the eye, was investigated via indirect immunofluorescence (Figure 1 and Table 2). The basal cell marker K14 was expressed in the basal layer of the haired skin, coronet and limbus with diminishing intensity in the suprabasal layers (Figure 1: A, E, M) while it was absent in the central area of the cornea (Figure 1: Q). Specifically, K14 expression was high in the basal layer, moderate in the spinous layer, low in the granular layer and undetectable in the cornified layer of equine haired skin (Figure 1: A). In the epidermal lamellae, K14 expression was restricted to the basal layer (Figure 1: I).
Figure 1.
Expression of epithelial cell markers K14, K124, K3, and K10 in equine skin, hoof coronet and lamellae, and limbus and central cornea of the eye. Representative indirect immunofluorescence images of formalin-fixed/paraffin-embedded tissue sections for keratin antibodies: K14 (red; A, E, I, M, Q), K124/anti-integrin α6 (green; B, F, J, N, R), K3/K76 (red; C, G, K, O, S), and K10 (red; D, H, L, P, T, V), counterstained with DAPI (blue). Shown are equine haired skin (A-D), coronet (E-H; V), mid-dorsal lamellar (I-L), limbal (M-P) and central corneal (Q-T) epithelia at 200× magnification. Panel U shows haematoxylin and eosin (H&E) staining of hoof coronet with adjacent external periople or stratum externum at 20× magnification. Panel V corresponds to the boxed area in U, showing positive staining for K10 in the periople epidermis but not in the adjacent coronary epidermis at 200× magnification. Scale bars = 50 μm except U (500 μm).
Table 2.
Summary of immunohistochemistry with stem cell and differentiation markers of epithelial layers of the skin, inner hoof capsule, ocular limbus and central cornea of the horse
| Epithelial layer | K14 | K124 | K3 | K10 | p63 | pp63 |
|---|---|---|---|---|---|---|
| Haired Skin | ||||||
| Basal | ++ | ++ | − | − | ++a | ++a |
| Spinous | ++ | ++ | − | + | +a | ++a |
| Granular | +/− | ++ | − | ++ | − | − |
| Cornified | − | − | − | + | − | − |
| Hoof - Coronet | ||||||
| Basal | ++ | ++ | − | − | ++a | ++a |
| Suprabasal | + | + | − | − | +a | ++a |
| Hoof – Lamellae | ||||||
| Basal | ++ | + | − | − | ++a | ++a |
| Suprabasal | − | ++ | − | − | +a | ++a |
| PEL KAb | − | + | − | − | − | − |
| Corneal Limbus | ||||||
| Basal | ++ | +/− | − | − | ++a | ++a |
| Suprabasal | + | ++ | ++ | − | +a | ++a |
| Surface | +/− | +/− | ++ | − | − | − |
| Central Cornea | ||||||
| Basal | − | ++ | + | − | ++a | ++a |
| Suprabasal | − | + | + | − | +a | ++a |
| Surface | − | +/− | + | − | − | − |
Relative expression in each epithelial layer is indicated by high positive (++), moderate positive (+), low positive (+/−) and negative (−).
Overall expression with variable levels among individual cells.
PEL KA: Primary Epidermal Lamellar Keratinized Axis
K3 localized to the cytoplasmic compartment of epithelial cells in the suprabasal layers of equine limbus (Figure 1: O) and basal and suprabasal layers of the cornea (Figure 1S), but not to the other epidermal tissues (Figure 1: C, G, K). Expression of K10 was detected in the spinous, granular and cornified layers of equine haired skin (Figure 1: D) and the periople, the outermost layer of the hoof capsule (Figure 1: U, V); while coronary, lamellar, limbal and corneal tissues were negative for this marker (Figure 1: H, L, P, T). The H-87 anti-α6 integrin antibody, shown previously to cross-react with K124, displayed a broad staining in all layers of epithelial tissues examined except for the cornified layer of the haired skin (Figure 1: B, F, J, N, R). Secondary antibodies alone revealed no specific signals (data not shown).
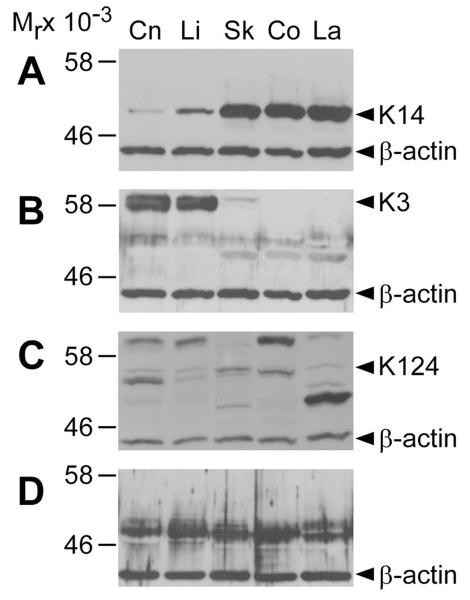
As shown in Figure 2, the utility of the same anti-keratin antibodies for immunoblotting was investigated. The K14 and K3/K76 antibodies detected specific bands corresponding to the predicted and reported molecular mass of K14 (52 kDa) and K3 (58 kDa), respectively (Figure 2: A, B).24 The H-87 integrin α6 antibody detected the band corresponding to K124 (58 kDa),24 but was also cross-reactive with multiple protein products in the 46-60 kDa range (Figure 2C). The anti-K10 (DE-K10) antibody displayed non-specific immunoreactivity to all epithelial tissues (Figure 2D). Control immunoblots with secondary antibody alone revealed no specific signals (data not shown).
Figure 2.
Immunoblot analysis of equine epithelial tissues with anti-keratin antibodies. Immunoblot analysis with antibodies to (A) K14, (B) K3/K76, (C) K124/integrin-α6, and (D) K10 in corneal (Cn), limbal (Li), haired skin (Sk), coronary (Co), and mid-dorsal lamellar (La) equine tissues as indicated. Anti-β-actin antibody was used as a loading control. Numbers to the left represent molecular mass standards (Mr × 10−3). Arrowheads on the right indicate the positions corresponding to the predicted molecular weights of K14 (52 kDa), K3 (60 kDa) and K124 (54 kDa). No bands corresponding to the predicted molecular weight of K10 (61 kDa) were detected.
Expression of epithelial progenitor cell markers in the equine eye, skin and hoof lamellae
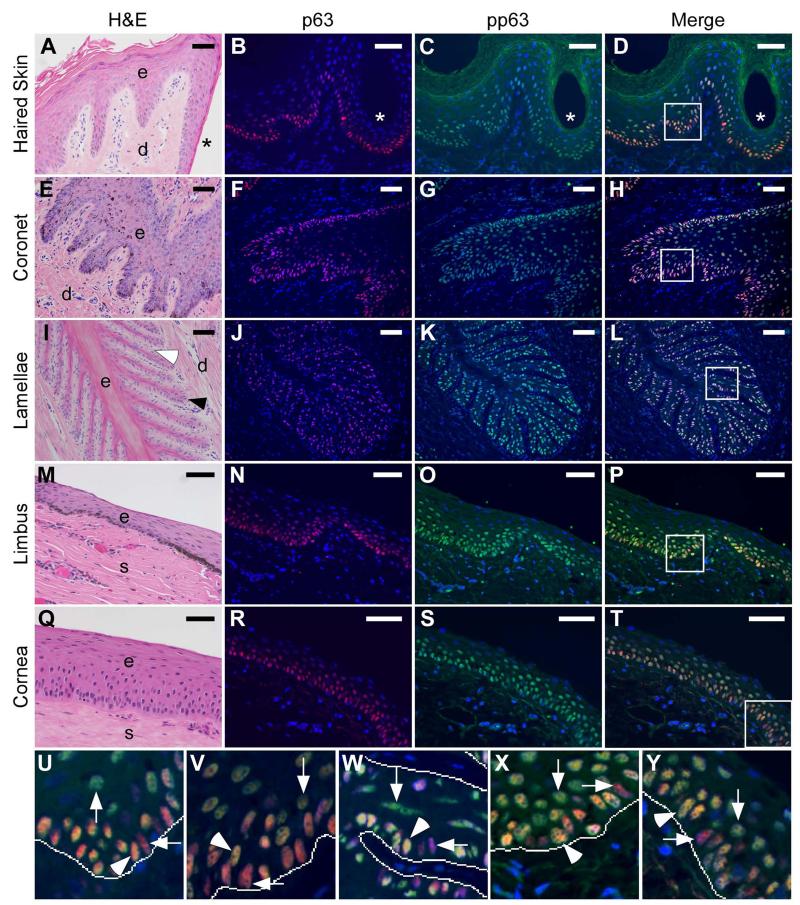
Localization of the transcription factor p63 and its phosphorylated form (pp63) in equine epithelial tissues is shown in Figure 3 and Table 2. The lamellar tissue images (Figure 3: I-L; W) are also presented in a larger format in Figure S2 in Supporting Information. Total p63 level was high in the nuclei of a subset of basal and adjacent suprabasal cells with overall decreasing intensity in the suprabasal cells of the haired skin, coronary and lamellar epidermis of the foot, and limbus and cornea of the eye (Figure 3: B, F, J, N, R). Specifically, p63 expression in the haired skin was high in cell nuclei in the basal layer, decreasing in the spinous layer with increased distance from the basal layer, and undetectable in the granular and cornified layers (Figure 3B). Localization of pp63 was also apparent within the basal and suprabasal layers of all epithelial tissues, with cell-to-cell variability within these layers, and was undetectable in the cell nuclei of the granular and cornified layers of haired skin (Figure 3: C, G, K, O, S).
Figure 3.
Expression of p63 and phosphorylated p63 (pp63) in equine skin, hoof coronet and lamellae, and limbus and central cornea of the eye. Representative images of tissue sections from equine haired skin (A-D, U), coronet (E-H, V), middle lamellae (I-L, W), limbus (M-P, X) and cornea (Q-T, Y) stained with H&E (A, E, I, M, Q) and antibodies against p63 (red; B, F, J, N, R) and pp63 (green; C, G, K, O, S), counterstained with DAPI (blue) at 200× magnification.
Labels indicate the lumen of a hair follicle (*, A-D), epidermal (e) and dermal (d) tissues of the haired skin (A), coronet (E) and lamellae (I); the epithelial (e) and stromal (s) tissues of the corneal limbus (M) and central cornea (Q); a secondary dermal lamella (white arrowhead, I) and a secondary epidermal lamella (black arrowhead, I). Panels in D, H, L, P and T show merged images of p63 and pp63 staining and nuclear counterstaining with DAPI.
Panels U-Y show enlarged views of the boxed areas in D, H, L, P and T, respectively. Lines indicate the approximate location of the basement membrane zone at the basal epidermal/epithelial-dermal/stromal tissue interface. Horizontal arrows and vertical arrows indicate representative examples of p63++/pp63+/− cells (pink) and p63+/pp63++ cells (yellow-green), respectively. Arrowheads indicate representative examples of p63++/pp63++ cells (orange-yellow). Dermal (U, V, W) and stromal (X, Y) cell nuclei are only positive for DAPI (blue). Scale bars = 50 μm.
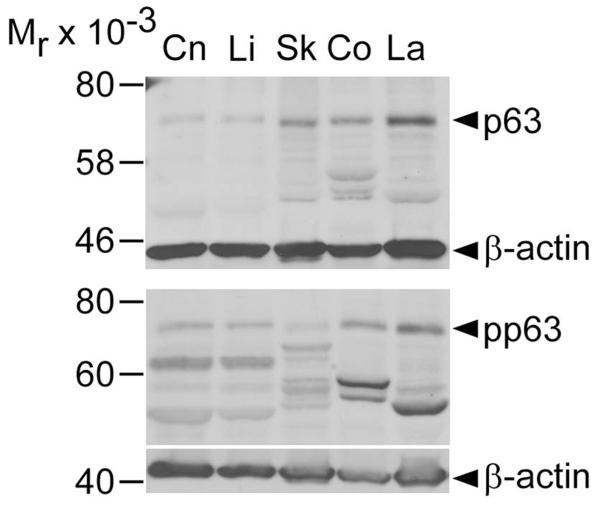
The keratinized axes of PELs display negligible or undetectable p63 and pp63 localization (Figure 3: J, K). Merged images demonstrate the overlap between p63 and pp63 expression in subsets of basal and suprabasal cells with variable relative staining intensity (Figure 3: D, H, L, P, T, U-Y). Specifically, these merged images show the presence of basal cells with high p63 staining intensity and relatively very low to non-detectable pp63 levels (p63++/pp63+/−), basal and suprabasal cells with high staining intensity for both p63 and pp63 (p63++/pp63++), or suprabasal cells with low levels of p63 and relatively high pp63 staining intensity (p63+/pp63++). Overall, as distance from the basement membrane increases, an increase in the pp63/p63 ratio and shift toward predominantly yellow-green staining is observed. As shown in Figure 4, immunoblotting with anti-p63 and anti-pp63 antibodies detected single bands within the 70-75 kDa range that approximate previously reported molecular weights of p63 and pp63.40,46
Figure 4.
Immunoblot analysis of equine epithelial tissues with anti-p63 and anti-phosphorylated p63 antibodies. Immunoblot analysis with antibodies against p63 (top panel) and pp63 (bottom panel) in equine corneal (Cn), limbal (Li), haired skin (Sk), coronary (Co) and mid-dorsal lamellar (La) tissues. Anti-β-actin antibody was used as a loading control. Numbers on the left indicate molecular mass standards (Mr × 10−3) while arrowheads on the right point to immunoreactive bands at the predicted molecular weight for p63 and pp63 (70-75 kDa).
Discussion
Epithelial stem cells (ESCs) reside in the basal layer of stratified epithelia and are essential for tissue homeostasis, regeneration following injury, and successful therapeutic engraftment.12,47-49 ESCs are endowed with a high capacity for self-renewal and continuous differentiation into TA cells, which in turn generate terminally-differentiated cells,12 but not all basal cells are considered ESCs. Far from being passive by-standers, basal cells in epithelia are active participants in pathological processes in horses40,42,50 and other species.51-53 Corneal epithelial cells are similarly active in wound healing and innate immunity, in part mediated by growth factor receptors and Toll-like receptors expressed by corneal basal cells.54-57 In spite of their clinical relevance to diseases of the hoof including laminitis, corneal injuries and disease, and dermatological wounds and disorders, equine basal cells, particularly ESCs and TA cells, have not been well characterized. The present study reports expression and localization of stem cell and differentiation markers of epithelial basal cells, progenitor cells and more highly differentiated cells in the haired skin, hoof coronet and lamellae, and ocular cornea and limbus in horses. We report here specific subsets of equine epithelial basal cells, including putative ESCs and TA cells, and tissue-specific suprabasal cell differentiation, by marker expression.
K14 was used as a specific marker for basal keratinocytes in stratified epithelia.13 Although all basal cells in stratified epithelia are K14 positive, not all basal cells represent ESCs and additional markers are needed to identify ESCs.27,39,58 In agreement with other reports,23,40 K14 was expressed in the basal layer of the SELs and basal and suprabasal layers of the haired skin and coronet. The restriction of K14 to the basal layer of the SELs of the hoof is striking, in contrast to its broader expression in basal and adjacent suprabasal layers in the haired skin and coronet. This unique K14 localization in the lamellae may reflect the uniquely abrupt transition from the basal cells to the keratinized cells within the primary epidermal lamellae.24 We also detected K14 expression in the equine ocular limbus, as previously reported,28 but not in the central cornea. In contrast, K14 is expressed in the basal layer of both limbus and the cornea in bovine eyes, suggesting a species difference in corneal epithelial cell differentiation.39 This notion is supported by studies of clonogenic cultures of cells isolated from central corneal epithelium which exhibited progenitor-like proliferative capacity in multiple species,38 but perhaps not the horse.28 The results of the present study suggest that K14 can serve as an equine basal epithelial cell marker and may be necessary, but not sufficient, for the identification of equine ESCs.
The data presented here show that K10, a specific marker for cornifying and cornified cells of stratified epithelia,13,23,24 was expressed in the cornifying (stratum granulosum) and cornified (stratum corneum) layers of the interfollicular epidermis as reported23 and in the periople (stratum externum) of the hoof capsule. The periople is composed of soft keratinized cornified epithelium13 and is homologous to the human eponychium and cuticle of the nail unit.21 In agreement with previous studies of bovine,39 rabbit,59 and equine28 cornea, the dual-specific AE5 monoclonal antibody, which detects K3 the major keratin of cornea and limbus, and K76, a keratin expressed in hard palate and gingiva,59,60 was immunoreactive with cells in all layers of equine cornea and limbus, with the exception of the basal layer of the limbus. The AE5 antibody did not detect any signal in other epidermal tissues examined. The absence of K3/K76 expression in equine lamellar tissues is also supported by a previous proteomic analysis, which failed to detect K3/K76 proteins in equine lamellar tissues.24 As summarized in Table 2, we conclude that K10 and K3/K76 can be used to distinguish differentiating/differentiated suprabasal cells in equine haired skin, cornea and lamellae.
A prior quantitative proteomic analysis identified a novel keratin, K124, as one of the most abundant cytoskeletal proteins in equine hoof lamellae.24 Although there are currently no K124-specific antibodies available, a previous study showed that the H-87 anti-integrin α6 antibody cross reacts with K124 on immunoblots, which was identified by mass spectroscopy of co-migrating bands on one- and two-dimensional SDS-PAGE gels.24 However, the present study demonstrates that this antibody detects multiple bands in the molecular weight range of keratins on immunoblots and is immunoreactive with cytoplasmic proteins of basal and suprabasal cells in multiple epithelial tissues. Therefore, this antibody may serve as a pan-epithelial cell marker in horse tissues. Since K124 accounts for more than 50% of type II keratins in lamellar tissue and the K124 gene expression is restricted to lamellar tissue (data not shown), the generation of a specific antibody against either K124 or its type I keratin partner, K42, will be useful for the future characterization of equine epidermal hoof lamellae.
The transcription factor p63, a marker of ESC proliferative capacity, is expressed in the nuclei of a subset of the basal and suprabasal epithelial cells of many types of stratified epithelia, including breast, skin, urothelia and ocular tissues of different species.38,41,47,61 A previous study validated the specificity of the 4A4 monoclonal antibody against p63 for use in equine tissues.40 That study identified p63-positive cells in basal and suprabasal layers of equine haired skin, coronary and lamellar tissues, and reported decreased p63 expression in the epidermal lamellae of horses with chronic laminitis and lamellar wedge formation representing altered differentiation and impaired epidermal lamellar regenerative capacity.40 The present study confirmed the localization of p63 in basal and suprabasal cells of the haired skin, coronet and lamellae, and further demonstrated a similar localization in equine ocular cornea and limbus. Another study has reported a similar pattern of p63 expression in the equine limbus, but did not include data on central cornea.28 A second study reported p63 expression in isolated cells from equine skin using a different antibody.62
As previously reported, assessing p63 level alone is not sufficient to identify ESCs.46,63 To circumvent this problem, a previous study introduced a second marker, pp63, and showed that relative pp63 levels to total p63 expression can distinguish ESCs from TA cells, which have more limited proliferative capacity. Expression of a band at the predicted molecular weight of pp63 was detected on immunoblots of protein extracts from all tissues examined (Figure 4).46 It was noted that variable lower molecular weight bands were also present on pp63 immunoblots, most likely due to the presence of pp63 protein degradation products. We are confident, however, that the pp63 antibody has been adequately validated according to established guidelines, specifically by the detection of a band at the predicted molecular weight as well as cell compartment (nuclei) and tissue (epithelia) immunolocalization that are consistent with published results.46,64 It was noted that the ESC to TA cell transition is marked by a relative increase in pp63 levels while total p63 expression is maintained high.46 In more highly differentiated cells both p63 and pp63 levels decrease due to the degradation of p63. In this study, variable levels of pp63 were detected within p63-positive cells and overall pp63/p63 ratios increased from the basal to the suprabasal layers of all equine epithelial tissues examined. As in the other species studied, such as human and mouse, we predict that ESCs in horses are included in the p63++/pp63+/− cell population, while TA cells and more highly differentiated cells exhibit a p63++/pp63++ and a p63+/pp63++ phenotype, respectively.46,63 If ESCs reside in the epithelial basal layer,12,49 then pp63 levels relative to total p63 expression could be used in combination with basal and suprabasal keratin isoform markers to identify ESCs. For example, ESCs in equine haired skin and limbus could be defined as K14++/K10−/p63++/pp63+/− cells and K14++/K3−/p63++/pp63+/− cells, respectively. Functional assessment of such predictions requires further investigations of the proliferative potential of each cell population.
A long term goal of this and ongoing studies is to establish markers for the characterization of altered epidermal differentiation associated with diseases of the equine hoof capsule, haired skin and cornea. Additionally, we propose that the combined use of ESC/TA cell markers and keratin differentiation markers would facilitate the establishment and characterization of equine ESC-based epidermal cell and organotypic culture models for dermatology research and regenerative medicine in horses. Specifically, ESC-based organotypic culture has been used for human epidermal tissue studies of epidermal physiology, pathophysiology and pharmacodynamics.65,66 Moreover, ESC-based regenerative medicine has been used in the treatment of severe corneal and skin burns in humans and might be applicable to equine corneal ulcers, skin wounds and to promote hoof wall regeneration in laminitic horses.67-69
In conclusion, this study characterized the expression and localization of progenitor cell and differentiation markers in multiple equine epithelial tissues. Defining markers for ESCs, TA cells and tissue-specific epithelial differentiation coupled with analysis of the proliferative potential will facilitate the investigation of the biology and pathology of equine epithelial tissues aimed at developing regenerative therapies in horses.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Robert K. Clark and Julie B. Engiles for critical review of the manuscript and editing assistance.
Sources of Funding
This study was supported by the Bernice Barbour Foundation, Inc. (0048.42008), the University of Pennsylvania Fund for Laminitis Research, the Raymond Firestone Trust, and the United States Department of Agriculture (USDA) 1433 Formula Funds 2010/2011 to HLG-H and the National Institute of Health (R01AR066755) to MS. RLL was supported by the USDA-National Institute of Food and Agriculture (NIFA) postdoctoral fellowship (#2012-67012-19994). SRM was supported by a fellowship from the National Institutes of Health (T35 RR07065).
Footnotes
Conflict of Interest The authors state no conflict of interest.
Parts of this study were presented at the Annual Conference of the International Society for Stem Cell Research, 2013 and the 6th International Equine Conference of Laminitis and Diseases of the Foot, 2011. An abstract for the latter presentation was published: J Eq Vet Sci 2011; 31: 582-583.
Additional Supporting Information may be found in the online version of this article.
Figure S1. Gross anatomy of the equine foot and microscopic anatomy of the hoof lamellae.
Figure S2. Expression of p63 and phosphorylated p63 (pp63) in equine hoof lamellae
References
- 1.Caston SS. Wound care in horses. Vet Clin North Am Equine Pract. 2012;28:83–100. doi: 10.1016/j.cveq.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Hanson RR. Complications of equine wound management and dermatologic surgery. Vet Clin North Am Equine Pract. 2008;24:663–696. doi: 10.1016/j.cveq.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Wada S, Hobo S, Niwa H. Ulcerative keratitis in thoroughbred racehorses in Japan from 1997 to 2008. Vet Ophthalmol. 2010;13:99–105. doi: 10.1111/j.1463-5224.2010.00767.x. [DOI] [PubMed] [Google Scholar]
- 4.Clode AB, Matthews A. Diseases and Surgery of the Cornea. In: Gilger BC, editor. Equine Ophthalmology. 2nd ed. Elsevier Saunders; St. Louis, MO: 2011. pp. 181–266. [Google Scholar]
- 5.Bragulla H, Hirschberg RM. Horse hooves and bird feathers: Two model systems for studying the structure and development of highly adapted integumentary accessory organs -the role of the dermo-epidermal interface for the micro-architecture of complex epidermal structures. J Exp Zool. 2003;298B:140–151. doi: 10.1002/jez.b.31. [DOI] [PubMed] [Google Scholar]
- 6.NAHMS . Lameness and Laminitis in U.S. Horses. #N318.0400. 2000. USDA:APHIS:VS, CEAH, National Animal Health Monitoring System; Fort Collins, CO: [Google Scholar]
- 7.Hood DM. Laminitis in the horse. Vet Clin North Am Equine Pract. 1999;15:287–294. doi: 10.1016/s0749-0739(17)30145-1. [DOI] [PubMed] [Google Scholar]
- 8.Pollitt CC. The anatomy and physiology of the suspensory apparatus of the distal phalanx. Vet Clin North Am Equine Pract. 2010;26:29–49. doi: 10.1016/j.cveq.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Leach DH, Oliphant LW. Ultrastructure of the equine hoof wall secondary epidermal lamellae. Am J Vet Res. 1983;44:1561–1570. [PubMed] [Google Scholar]
- 10.Engiles JB. Diseases of the Skeletal System. In: Buergelt CD, Del Piero F, editors. Color Atlas of Equine Pathology. John Wiley & Sons, Inc.; Ames, IA: 2014. pp. 301–343. [Google Scholar]
- 11.Collins SN, Van Eps AW, Kuwano A, et al. The lamellar wedge. Vet Clin North Am Equine Pract. 2010;26:179–195. doi: 10.1016/j.cveq.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Blanpain C, Fuchs E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nature Reviews. 2009;10:207–218. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bragulla HH, Homberger DG. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J Anatomy. 2009;214:516–559. doi: 10.1111/j.1469-7580.2009.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Association of Veterinary Anatomists ICoVGAN . Nomina Anatomica Veterinaria. Hannover (Germany), Columbia, MO (U.S.A.), Ghent (Belgium), Sapporo (Japan): 2012. [Google Scholar]
- 15.Muller GH, Kirk RW, Scott DW. Small Animal Dermatology. W.B. Saunders Company, Harcourt Brace Jovanovich, Inc.; Philadelphia, PA: 1989. Diseases of ears, eyelids, nails, and anal sacs; pp. 807–827. [Google Scholar]
- 16.Samuelson DA. Textbook of Veterinary Histology. Saunders Elsevier; St. Louis, Missouri: 2007. Integument; pp. 271–302. [Google Scholar]
- 17.Alibardi L, Segalla A, Dalla Valle L. Distribution of specific keratin-associated beta-proteins (beta-keratins) in the epidermis of the lizard Anolis carolinensis helps to clarify the process of cornification in lepidosaurians. J Exp Zool B Mol Dev Evol. 2012;318:388–403. doi: 10.1002/jez.b.22454. [DOI] [PubMed] [Google Scholar]
- 18.Moustakas-Verho JE, Zimm R, Cebra-Thomas J, et al. The origin and loss of periodic patterning in the turtle shell. Development. 2014;141:3033–3039. doi: 10.1242/dev.109041. [DOI] [PubMed] [Google Scholar]
- 19.Knottenbelt DC. Introduction. In: Knottenbelt DC, editor. Pascoe’s Principles & Practice of Equine Dermatology. Saunders Elsevier; Philadelphia, PA: 2009. pp. 1–24. [Google Scholar]
- 20.Bragulla H. Fetal development of the segment-specific papillary body in the equine hoof. J Morphology. 2003;258:207–224. doi: 10.1002/jmor.10142. [DOI] [PubMed] [Google Scholar]
- 21.Perrin C. Expression of follicular sheath keratins in the normal nail with special reference to the morphological analysis of the disatl nail unit. Am J Dermatopathol. 2007;29:543–550. doi: 10.1097/DAD.0b013e318158d741. [DOI] [PubMed] [Google Scholar]
- 22.Hargis AM, Ginn PE. The Integument. In: McGavin MD, Zachary JF, editors. Pathologic Basis of Veterinary Disease. Mosby Elsevier; St. Louis, MO: 2007. pp. 1107–1261. [Google Scholar]
- 23.Wattle O. Cytokeratins of the equine hoof wall, chestnut and skin: bio- and immunohistochemistry. Equine Vet J. 1998;26(Suppl.):66–80. doi: 10.1111/j.2042-3306.1998.tb05124.x. [DOI] [PubMed] [Google Scholar]
- 24.Carter RA, Shekk V, de Laat MA, et al. Novel keratins identified by quantitative proteomic analysis as the major cytoskeletal proteins of equine (Equus caballus) hoof lamellar tissue. J Anim Sci. 2010;88:3843–3855. doi: 10.2527/jas.2010-2964. [DOI] [PubMed] [Google Scholar]
- 25.Bragulla HH, Homberger DG. The role of the specific, profilaggrin-containing keratohyalin granules in the developing epidermis of the fetal horse hoof. Pferdeheilkunde. 2007;23:5–20. [Google Scholar]
- 26.Magin TM, Vijayaraj P, Leube RE. Structural and regulatory functions of keratins. Exp Cell Res. 2007;313:2021–2032. doi: 10.1016/j.yexcr.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochem Cell Biol. 2008;129:705–733. doi: 10.1007/s00418-008-0435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moriyama H, Kasashima Y, Kuwano A, et al. Anatomical location and culture of equine corneal epithelial stem cells. Vet Ophthalmol. 2014;17:106–112. doi: 10.1111/vop.12050. [DOI] [PubMed] [Google Scholar]
- 29.Grosenbaugh DA, Hood DM. Keratin and associated proteins of the equine hoof wall. Am J Vet Res. 1992;53:1859–1863. [PubMed] [Google Scholar]
- 30.Wattle O. Cytokeratins of the stratum medium and stratum internum of the equine hoof wall in acute laminitis. Acta Vet Scand. 2000;41:363–379. doi: 10.1186/BF03549627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geburek F, Ohnesorge B, Deegen E, et al. Alterations of epidermal proliferation and cytokeratin expression in skin biopsies from heavy draught horses with chronic pastern dermatitis. Vet Dermatol. 2005;16:373–384. doi: 10.1111/j.1365-3164.2005.00482.x. [DOI] [PubMed] [Google Scholar]
- 32.Kurokawa I, Mizutani H, Kusumoto K, et al. Cytokeratin, filaggrin, and p63 expression in reepithelialization during human cutaneous wound healing. Wound Repair Regen. 2006;14:38–45. doi: 10.1111/j.1743-6109.2005.00086.x. [DOI] [PubMed] [Google Scholar]
- 33.Schweizer J, Bowden PE, Coulombe PA, et al. New consensus nomenclature for mammalian keratins. J Cell Biol. 2006;174:169–174. doi: 10.1083/jcb.200603161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong X, Coulombe PA. A novel mouse type I intermediate filament gene, keratin 17n (K17n), exhibits preferred expression in nail tissue. J Invest Dermatol. 2010;122:965–970. doi: 10.1111/j.0022-202X.2004.22422.x. [DOI] [PubMed] [Google Scholar]
- 35.Hesse M, Zimek A, Weber K, et al. Comprehensive analysis of keratin gene clusters in humans and rodents. Eur J Cell Biol. 2004;83:19–26. doi: 10.1078/0171-9335-00354. [DOI] [PubMed] [Google Scholar]
- 36.Zimek A, Weber K. The organization of the keratin I and II gene clusters in placental mammals and marsupials show a striking similarity. Eur J Cell Biol. 2006;85:83–89. doi: 10.1016/j.ejcb.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Cerrato S, Ramió-Lluch L, Brazís P, et al. Development and characterization of an equine skin-equivalent model. Vet Dermatol. 2014;25:475–e77. doi: 10.1111/vde.12134. [DOI] [PubMed] [Google Scholar]
- 38.Majo F, Rochat A, Nicolas M, et al. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature. 2008;456:250–254. doi: 10.1038/nature07406. [DOI] [PubMed] [Google Scholar]
- 39.Chen B, Mi S, Wright B, et al. Investigation of K14/K5 as a stem cell marker in the limbal region of the bovine cornea. PLoS One. 2010;5:e13192. doi: 10.1371/journal.pone.0013192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carter RA, Engiles JB, Megee SO, et al. Decreased expression of p63, a regulator of epidermal stem cells, in the chronic laminitic equine hoof. Equine Vet J. 2011;43:543–551. doi: 10.1111/j.2042-3306.2010.00325.x. [DOI] [PubMed] [Google Scholar]
- 41.Senoo M, Pinto F, Crum CP, et al. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Pawlak EA, Johnson PJ, et al. Impact of laminitis on the canonical Wnt signaling pathway in basal epithelial cells of the equine digital laminae. PLoS One. 2013;8:e56025. doi: 10.1371/journal.pone.0056025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wattle OS. Cytokeratins of the matrices of the chestnut (torus carpeus) and periople in horses with acute laminitis. American Journal of Veterinary Research. 2001;62:425–432. doi: 10.2460/ajvr.2001.62.425. [DOI] [PubMed] [Google Scholar]
- 44.Galantino-Homer H, Carter R, Megee S, et al. The laminitis discovery database. J Equine Vet Sci. 2010;30:101. [Google Scholar]
- 45.Pollitt CC. Basement membrane pathology: a feature of acute equine laminitis. Equine Vet J. 1996;28:38–46. doi: 10.1111/j.2042-3306.1996.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki D, Senoo M. Increased p63 phosphorylation marks early transition of epidermal stem cells to progenitors. J Invest Dermatol. 2012;132:2461–2464. doi: 10.1038/jid.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang A, Schweitzer R, Sun D, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 48.Pellegrini G, Rama P, Mavilio F, et al. Epithelial stem cells in corneal regeneration and epidermal gene therapy. J Pathol. 2009;217:217–228. doi: 10.1002/path.2441. [DOI] [PubMed] [Google Scholar]
- 49.Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445–458. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leise BS, Watts M, Roy S, et al. Use of laser capture microdissection for the assessment of equine lamellar basal epithelial cell signalling in the early stages of laminitis. Equine Vet J. 2014 doi: 10.1111/evj.12283. Epub ahead of print, DOI: 10.1111/evj.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freedberg IM, Tomic-Canic M, Komine M, et al. Keratins and the keratinocyte activation cycle. J Invest Dermatol. 2001;116:633–640. doi: 10.1046/j.1523-1747.2001.01327.x. [DOI] [PubMed] [Google Scholar]
- 52.Yano S, Banno T, Walsh R, et al. Transcriptional responses of human epidermal keratinocytes to cytokine interleukin-1. J Cell Physiol. 2008;214:1–13. doi: 10.1002/jcp.21300. [DOI] [PubMed] [Google Scholar]
- 53.Murphy J-E, Robert C, Kupper TS. Interleukin-1 and cutaneous inflammation: a crucial link between innate and acquired immunity. J Invest Dermatol. 2000;114:602–608. doi: 10.1046/j.1523-1747.2000.00917.x. [DOI] [PubMed] [Google Scholar]
- 54.Saika S. TGF-beta signal transduction in corneal wound healing as a therapeutic target. Cornea. 2004;23:S25–S30. doi: 10.1097/01.ico.0000136668.41000.73. [DOI] [PubMed] [Google Scholar]
- 55.Susarla R, Liu L, Walker EA, et al. Cortisol biosynthesis in the human ocular surface innate immune response. PLoS One. 2014;9:e94913. doi: 10.1371/journal.pone.0094913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zieske JD, Hutcheon AE, Guo X, et al. TGF-beta receptor types I and II are differentially expressed during corneal epithelial wound repair. Invest Ophthalmol Vis Sci. 2001;42:1465–1471. [PubMed] [Google Scholar]
- 57.Hozono Y, Ueta M, Hamuro J, et al. Human corneal epithelial cells respond to ocular-pathogenic, but not to nonpathogenic-flagellin. Biochem Biophys Res Commun. 2006;347:238–247. doi: 10.1016/j.bbrc.2006.06.088. [DOI] [PubMed] [Google Scholar]
- 58.Fuchs E, Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980;19:1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- 59.Schermer A, Galvin S, Sun T-T. Differentiation-related expression of a major 64k corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun T-T, Green H. Immunofluorescent staining of keratin fibers in cultured cells. Cell. 1978;14:469–476. doi: 10.1016/0092-8674(78)90233-7. [DOI] [PubMed] [Google Scholar]
- 61.Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Broeckx SY, Maes S, Martinello T, et al. Equine epidermis: a source of epithelial-like stem/progenitor cells with in vitro and in vivo regenerative capacities. Stem Cells Dev. 2014;23:1134–1148. doi: 10.1089/scd.2013.0203. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki D, Senoo M. Expansion of epidermal progenitors with high p63 phosphorylation during wound healing of mouse epidermis. Exp Dermatol. 2013;22:374–376. doi: 10.1111/exd.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bordeaux J, Welsh AW, Agarwal S, et al. Antibody validation. Biotechniques. 2010;48:197–209. doi: 10.2144/000113382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pasonen-Seppänen S, Suhonen TM, Kirjavainen M, et al. Formation of permeability barrier in epidermal organotypic culture for studies on drug transport. J Invest Dermatol. 2001;117:1322–1324. doi: 10.1046/j.0022-202x.2001.01529.x. [DOI] [PubMed] [Google Scholar]
- 66.Bernerd F, Asselineau D. An organotypic model of skin to study photodamage and photoprotection in vitro. J Am Acad Dermatol. 2008;58:S155–S159. doi: 10.1016/j.jaad.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 67.Pellegrini G, Ranno R, Stracuzzi G, et al. The control of epidermal stem cells (Holoclones) in he treatment of massive full-thickness burns autologous keratinocytes cultured on fibrin. Transplantation. 1999;68:868–879. doi: 10.1097/00007890-199909270-00021. [DOI] [PubMed] [Google Scholar]
- 68.Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 69.De Luca M, Pellegrini G, Green H. Regeneration of squamous epithelia from stem cells of cultured grafts. Regen Med. 2006;1:45–57. doi: 10.2217/17460751.1.1.45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.