Abstract
While sexual partner age disparity is frequently considered as a potential risk factor for HIV amongst young women in Africa, no research has addressed this question amongst older women. Our aim was thus to determine whether sex partner age disparity was associated with subsequent HIV acquisition in women aged over 30.
Methods
To achieve this aim we conducted a quantitative analysis of a population-based, open cohort of women in rural KwaZulu-Natal, South Africa (n=1,737) using Cox proportional hazards models.
Results
As partner age rose, HIV acquisition risk fell significantly: compared to a same-aged partner, a five-year older partner was associated with a one-third reduction (hazard ratio [HR]: 0.63, 95%CI: 0.52–0.76) and a ten-year older partner with a one-half reduction (HR: 0.48, 95%CI: 0.35–0.67). This result was neither confounded nor effect-modified by women’s age or socio-demographic factors.
Conclusions
These findings suggest that existing HIV risk-reduction campaigns warning young women about partnering with older men may be inappropriate for older women. HIV prevention strategies interventions specifically tailored to older women are needed.
Keywords: HIV acquisition, age disparities, South Africa, women
Introduction
Despite the preventive effects of antiretroviral treatment (ART) and a range of biomedical and behavioral interventions, there were still an estimated 2.3 million new HIV infections worldwide in 2012 [1]. This high level of ongoing transmission has motivated a search for better understanding of risk factors for infection, and thus new prevention interventions. One area of recent investigation has been sexual relationship age disparities [2–4]. Interest in relationship age disparities has arisen from at least two sources. First, mathematical models highlight the potential role of age-disparate relationships in propagating the epidemic across generations [5]. Second, relationships in which the man is considerably older than the woman are believed to be more likely than similarly-aged partnerships to exhibit power imbalances, due to older men having more economic resources and social standing [6–9], providing more opportunity for their partners to pressure the women into riskier sexual behaviors [10,11]. Such risky behaviors in age disparate relationships are particularly troubling since rising HIV prevalence with age [1] implies that older male partners are more likely to be HIV infected than men the women’s own age.
Policy interest in age disparities as a target for prevention arises both because individuals are often well-able to identify their partner’s age, and because age disparities appear modifiable through interventions involving the provision of information or instrumental support to women [12,13]. Quantitative research on relationship age disparities and HIV amongst women aged under 30 has found a positive cross-sectional evidence in Kenya, Uganda, South Africa and Zimbabwe [14–17], but no longitudinal association [15,18]. Despite this mixed evidence, social media interventions in many sub-Saharan Africa countries have focused on reducing the number of relationships between young women and older men [18–20].
However, to date there has not been any investigation of how relationship age disparities might affect HIV risk for women aged over 30. This is despite the fact that HIV incidence remains a considerable concern in this age-range: in high-prevalence settings it been measured to be as high as 2% per year for women past their fortieth birthdays [21,22]. Indeed, female HIV incidence at older ages may even be increasing, as older men living with HIV are living and sexually active for longer due to increased ART availability [23], or older individuals remaining in the active sexual network for longer [24]. This latter factor may be particularly strong in rural South Africa, where individuals typically remain unmarried well into middle-age [25,26]. Late marriage in South Africa arises both from high levels of geographic mobility for men and for women due to labor migration [27–29], and from the common requirement that men must pay considerable bride prices which can take many years to raise [30].
It is not clear to what extent findings regarding age disparities and HIV amongst younger women might be expected to be replicated in older populations; neither of the key arguments for relationship age disparities conferring risk – higher HIV prevalence and greater power differentials – necessarily translates to older ages. First, since HIV prevalence declines for men after age 35 [21,31,32], older men are likely to be less, rather than more, infectious than similarly-aged ones for women over 30. Second, since older women are more likely than teenagers to have an income and to have accumulated some wealth, economic gradients are likely to matter less for partner choice in this age group.
On the one hand, age-disparate relationships may continue to be risky into middle-age. Even amongst older women, power within relationships is likely to be more skewed in age-disparate than in relationships of similar age, since men also continue to accrue wealth with age. Furthermore, as HIV prevalence is currently rising amongst older men due to decreased HIV-related mortality, infection risk may increase in age-disparate relationships for older women – at least relative to similarly age-disparate relationships in the past.
On the other hand, age disparities may become less important with age if economic differentials are the key metric for relationship bargaining power. Increased female migration and economic empowerment in the post-Apartheid era, and associated marital status choices, mean that women are often able to independently support themselves economically [27,28]. This economic independence should lead to a levelling of economic differentials in age-disparate relationships as age rises; indeed, the ratio of male-to-female employment in rural South Africa within cohorts falls steeply with age for relationships in which the man is five years older, but remains almost constant for same-age relationships (see Figure S1 [33]). As a result, while men continue to be economically advantaged overall at older ages, the difference in disparity between similarly-aged and older male partners disappears. It may even be that, in the absence of strong economic incentives, the decline in male HIV prevalence with age leads to age disparities being protective for older women.
Our objective in this study was to quantify the association between partner age disparity and HIV acquisition in a cohort of women aged between 30 and 57, in a rural community in KwaZulu-Natal, South Africa. The community in which we carry out this study has high HIV incidence into middle-age, and both low marriage rates and increasing ART provision. Our rationale for this study was that: (i) HIV incidence is considerable in this community and age-range; (ii) evidence on the effect of relationship age disparities on HIV acquisition amongst older women had not previously been studied; (iii) the plausible causal mechanisms outlined above leave us with ambiguity regarding the direction of any association between relationship age disparity and HIV in this population; and (iv) this community displays key risk factors relating to these putative causal mechanisms, such as low marriage rates and increasing ART provision. We next present the Methods used in the study, followed by the Results, a Discussion of our findings and finally policy Conclusions.
Methods
We conducted a quantitative, empirical analysis using data from a population-based, longitudinal surveillance system, carried out by the Africa Centre for Health and Population Studies (Africa Centre) in a predominantly rural community in the uMkhanyakude district of KwaZulu-Natal. The Africa Centre has been collecting household demographic data since 2000 [34]. Since 2003 all adults (≥15 years) in the community have also been invited each year to answer questions relating to their sexual history and to participate in HIV testing. These questions are asked face-to-face by fieldworkers recruited from the local community [35]. For this analysis we used data collected between January 2003 and June 2012.
Our sample comprised all individuals who were resident within the geographically bounded demographic surveillance area over the study observation period, and who met the inclusion criteria described below. In this simple random sample everyone living within the geographically bounded area is eligible for participation (i.e. the probability of selection for participation equals one). The individuals in this open cohort entered or exited the sample as they aged into or out of the study age-range, or entered or left the surveillance area. The cohort structure allowed temporal ordering in the direction of the hypothesized cause-effect relationship, i.e. that HIV infection occurred after each partnership had begun, which was important since past analyses only provided cross-sectional evidence of an association between age disparities and prevalent HIV [14–17]. The open structure maximized our power to see effects, since individuals did not have to be present during the entire follow-up period in order to contribute to the analysis.
The inclusion criteria for our analysis were that respondents: (i) were female; (ii) were aged between 30 and 50 years old at baseline (prior to 2007, only women aged under 50 were invited to participate in HIV testing or report on their sexual behavior); (iii) were HIV seronegative at first participation in the HIV surveillance and had at least one more valid HIV test result recorded; and (iv) participated at least once in the sexual behavior questionnaire. Individuals entered the cohort at the date of their first report of a sexual partnership, or at their thirtieth birthday if they had reported sexual partners prior to that date. If they did not acquire HIV over the study observation period, individuals were right-censored at the date of their most recent seronegative HIV test prior to 30 June 2012. Person-time subsequent to sexual debut, but during which the respondent indicated no sexual partners, was not included in the study under the assumption that she could not have been at risk of sexually-transmitted HIV infection during such time.
The primary outcome was HIV seroconversion as measured by antibody testing of dried blood spot samples [34]. HIV status is measured using sequential antibody testing with two HIV-1/HIV-2 ELISA assays – the first a broad-based assay (Vironostika HIV-1Microelisa System; Biomérieux, Durham, NC, USA), the second a confirmatory assay (Wellcozyme HIV 1+2 GACELISA; Murex Diagnostics Benelux B.V., Breukelen, The Netherlands) – as recommended by World Health Organization and UNAIDS guidelines for HIV surveillance [36]. We assumed the date of HIV seroconversion to be midway between the date of an individual’s last negative and first positive HIV test.
Our exposure of interest was the time-varying age disparity in the respondent’s most recently reported sexual partnership at each interview round; when the age disparity of the most recent partner changed, the value was updated. Individuals’ own ages were collected by asking respondents to self-report their date of birth, based on the date printed in their national identity document. Partner age, and thus relationship age disparity, was also reported by the respondent. We have recently validated this partner age report in the subset of relationships in the surveillance area that are conjugal (marital or marriage-like), finding good concordance between age as reported by a partner and self-reported age [37].
Given the absence of previous studies of age disparities and HIV acquisition amongst older women, we did not have strong prior beliefs about the functional form of age or age disparities that would best capture HIV acquisition risk. We therefore used a flexible form: age and age disparity as continuous measures with linear and higher-order polynomials terms. In a bivariate model, we started with linear age and age disparity terms, and then consecutively added higher-order polynomial terms. We kept all polynomial terms up to the point where the next order term did not improve model fit (see Table S1). Our final model included linear, quadratic and cubic terms in age, and linear and quadratic terms in age disparity.
We controlled for potential socio-demographic time-varying confounders: current completed education (categories: none or primary, 0–7 years; secondary, 8–12 years; tertiary, >12 years); household wealth (quintiles of the first component identified by a principal-components analysis of 28 household assets and sources of water, electricity, and energy); and marital status (never married, engaged, married, previously married). We further controlled for potential behavioral confounders: age at sexual debut; and three time-varying measures of sexual behavior in the past 12 months – multiple partners (yes vs. no), any casual partner (yes vs. no), and lowest level of condom use with any partner (never, sometimes, always).
Since the outcome of interest consisted of time-to-event data, we used Cox proportional hazards models, verifying the proportional-hazards assumption using the Schoenfeld residuals from each regression. The primary model included the woman’s age (centered at age 30) and the age disparity of their most recent relationship.
After conducting the primary regression, we evaluated whether any effect of age disparity varied by women’s age using four age strata (30–34, 35–39, 40–44, 45 or more years old). We then added socio-demographic and sexual behavior covariates to the model. Finally, we tested whether the primary effect of age disparity was effect-modified by any of the socio-demographic variables. Additionally, we reran our analyses after multiply imputing any missing data using chained equations (see Table S2 for details), and we reran our primary analysis using binary measures of age disparity at cut points of male partners being 5 and 10 years older. These cut points reflect common definitions of age-disparate relationships. All models also included indicator variables for the year of observation. All analyses were conducted in Stata version 13 (Statacorp, College Station, TX).
Ethical approval for the Africa Centre population surveillance was granted by the Biomedical Research Ethics Committee, University of KwaZulu-Natal. In the surveillance, informed consent is required separately for HIV serotesting and for the demographic and sexual behavior questionnaires. This analysis was exempted from additional ethical review by the Harvard School of Public Health Institutional Review Board due to its use of secondary data.
Results
Between January 2003 and June 2012, 1,734 women met the inclusion criteria and had full covariate information. These women contributed 5,714 person-years of observation time. Each woman was tested for HIV between two and nine times (24.4% twice, 17.2% three times; 18.5% four times; 17.5% five times; 23.5% 6–9 times). The median gap between tests was 363 days (interquartile range 339–385 days).
Descriptive statistics
Respondents’ baseline characteristics are shown in Table 1, divided into five-year age cohorts. The mean woman’s most recent sexual partner was four years older than her (interquartile range (IQR): 2–9 years older), a distribution that held steady as women aged (full distribution in Figure 1). Educational attainment was lower in older cohorts, although half the sample had attended secondary school or higher, and the sample’s household wealth was below the local average. Marital status was mixed: at baseline just over half the sample were married or separated, and a fifth of the sample was engaged. Marriage rates rose sharply from age 35. Very few women reported either more than one partner in the past year or a casual partner in the past year (both <1%). Condom use was rare in this population – over 80% of women reported not having used one with their most recent partner in the past year.
Table 1.
Baseline characteristics of women in analytic sample
| All
|
30–34
|
35–39
|
40–44
|
45–50
|
|
|---|---|---|---|---|---|
| Sample size | 1,734 | 491 | 368 | 424 | 451 |
| Number of subsequent seroconversions | 116 | 48 | 22 | 27 | 19 |
| Age at baseline | 40 (34 to 45) | 31 (30 to 33) | 37 (36 to 38) | 42 (41 to 43) | 47 (46 to 49) |
| Partner age disparity in most recent relationship | 4 (2 to 9) | 5 (2 to 8) | 5 (2 to 9) | 4 (2 to 9) | 4 (2 to 8) |
| Highest educational attainment | |||||
| None or Primary (0–7 years) | 50.0 | 22.4 | 48.9 | 60.8 | 70.7 |
| Secondary (8–12 years) | 40.6 | 63.5 | 41.8 | 31.6 | 23.1 |
| Tertiary | 9.4 | 14.1 | 9.2 | 7.5 | 6.2 |
| Household wealth quintile | |||||
| Lowest | 20.0 | 16.9 | 19.3 | 20.5 | 23.3 |
| 2nd lowest | 27.1 | 27.9 | 23.1 | 28.5 | 28.2 |
| Middle | 26.4 | 26.7 | 29.3 | 26.4 | 23.7 |
| 2nd highest | 15.7 | 15.3 | 18.2 | 15.8 | 14.2 |
| Highest | 10.8 | 13.2 | 10.1 | 8.7 | 10.6 |
| Marital status | |||||
| Never Married | 28.0 | 45.6 | 29.9 | 19.3 | 15.3 |
| Engaged | 20.4 | 25.3 | 18.2 | 20.8 | 16.4 |
| Married | 48.1 | 28.7 | 49.7 | 55.4 | 61.0 |
| Divorced/Separated/Widowed | 3.6 | 0.4 | 2.2 | 4.5 | 7.3 |
| Age at sexual debut | 18 (16 to 19) | 18 (16 to 19) | 17 (16 to 18) | 17 (16 to 19) | 18 (16 to 19) |
| Multiple partners in past 12 months | 0.9 | 1.0 | 0.5 | 1.4 | 0.7 |
| Casual partner in past 12 months | 0.9 | 1.2 | 0.5 | 0.9 | 0.7 |
| Lowest condom use level in relationships in past 12 months | |||||
| Never | 81.3 | 69.2 | 84.0 | 85.4 | 88.5 |
| Sometimes | 14.0 | 23.2 | 11.1 | 10.4 | 9.5 |
| Always | 4.7 | 7.5 | 4.9 | 4.2 | 2.0 |
Figures for categorical data are percentages; figures for continuous data are medians and (Interquartile ranges).
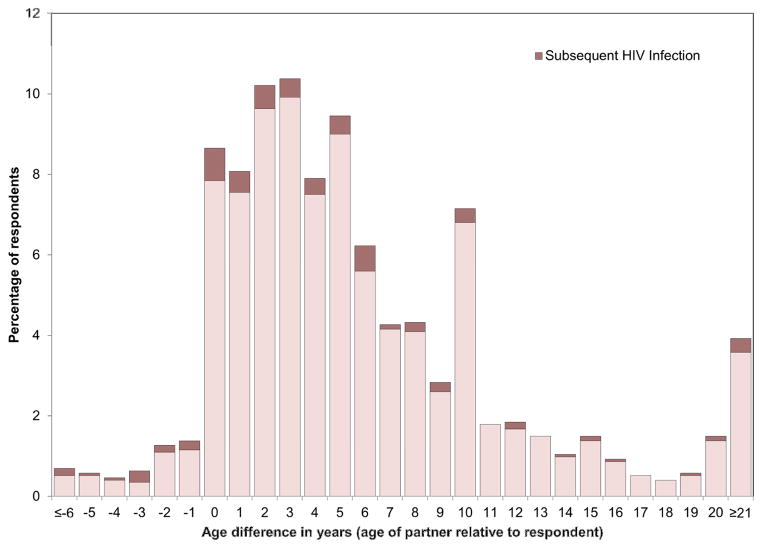
Figure 1.
Distribution of age disparity between female respondent and most recent male sexual partner
During follow-up 116 HIV seroconversions were observed (see Kaplan-Meier curve in Figure S2), at an incidence rate of 2.03 per 100 person-years (95% confidence interval (CI): 1.69–2.44). Incidence was highest for women aged 30–34 (3.87, 95%CI: 2.84–5.28) and lowest for those aged over 44 (1.23, 95%CI: 0.83–1.82). The crude correlation between partner age disparity at baseline and subsequent risk of seroconversion (Figure 1) suggested that women with partners closer to their own age were more likely to become infected.
Multivariable models
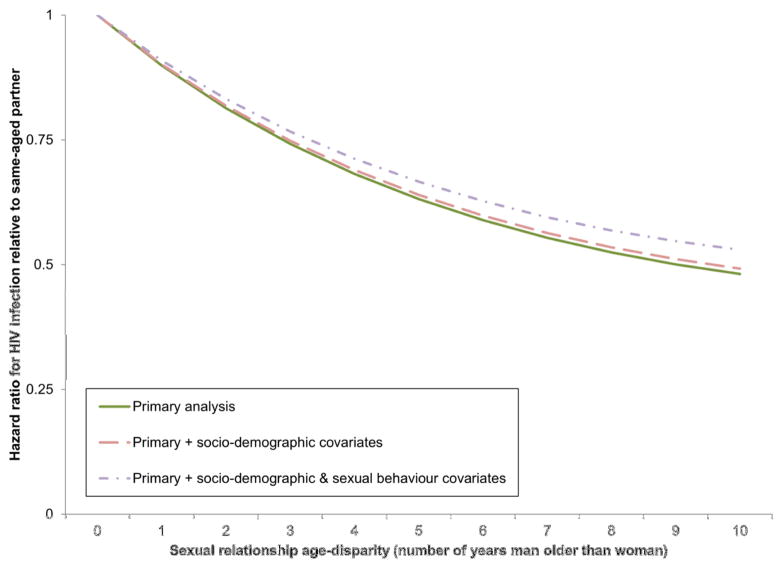
In survival analyses containing only respondent’s age and relational age disparity (Model 1, Table 2), having an older partner was associated with a significantly reduced risk of HIV acquisition (Hazard ratio (HR) comparing a woman with a same-aged partner to one with a partner five years her senior: 0.63, 95%CI: 0.52–0.76; and HR comparing a woman with a same-aged partner to one ten years her senior: 0.48, 95%CI: 0.35–0.67). This result did not vary significantly by age of the respondent (Model 2) and was unaffected by the addition of socioeconomic (Model 3) or behavioral (Model 4) covariates. The shape of the relationship between partner age disparity and HIV risk is shown in Figure 2 (Figure S3 displays the relationship stratified by women’s age).
Table 2.
Multivariable models of HIV acquisition due to continuous relationship age disparities
| Model 1 | Model 2a | Model 3 | Model 4 | |
|---|---|---|---|---|
|
|
||||
| Age-gap (one year increase in partner’s age) | 0.90 (0.86–0.93) | 0.90 (0.86–0.94) | 0.91 (0.87–0.95) | |
| Age disparity squared | 1.04 (1.03–1.05) | 1.04 (1.03–1.05) | 1.04 (1.02–1.05) | |
| 30–34 years old | ||||
| Age-gap | 0.91 (0.82–1.02) | |||
| Age disparity squared | 1.03 (0.99–1.07) | |||
| 35–39 years old | ||||
| Age-gap | 0.89 (0.83–0.96) | |||
| Age disparity squared | 1.04 (1.02–1.06) | |||
| 40–44 years old | ||||
| Age-gap | 0.88 (0.80–0.96) | |||
| Age disparity squared | 1.04 (1.01–1.07) | |||
| 45–57 years old | ||||
| Age-gap | 0.89 (0.83–0.96) | |||
| Age disparity squared | 1.05 (1.03–1.08) | |||
| Age of respondent (centered at 15 years old) | ||||
| Age | 0.77 (0.60–0.97) | 0.76 (0.58–0.99) | 0.78 (0.62–1.00) | 0.79 (0.62–1.01) |
| Age squared | 1.24 (0.94–1.64) | 1.27 (0.93–1.73) | 1.22 (0.92–1.62) | 1.22 (0.92–1.63) |
| Age cubed | 0.94 (0.86–1.03) | 0.93 (0.85–1.03) | 0.94 (0.86–1.03) | 0.94 (0.86–1.03) |
| Highest educational achievement | ||||
| None or Primary (0–7 years) | 0.99 (0.64–1.52) | 1.00 (0.65–1.56) | ||
| Secondary (8–12 years) | 1.00 | 1.00 | ||
| Tertiary | 0.35 (0.14–0.88) | 0.33 (0.13–0.84) | ||
| Household asset quintile | ||||
| Lowest | 1.22 (0.54–2.76) | 1.24 (0.55–2.82) | ||
| 2nd lowest | 1.20 (0.57–2.55) | 1.27 (0.60–2.71) | ||
| Middle | 1.96 (0.96–3.99) | 1.98 (0.97–4.03) | ||
| 2nd highest | 1.58 (0.74–3.38) | 1.63 (0.76–3.50) | ||
| Highest | 1.00 | 1.00 | ||
| Marital status | ||||
| Never Married | 1.00 | 1.00 | ||
| Engaged | 0.75 (0.45–1.25) | 0.77 (0.46–1.28) | ||
| Married | 0.56 (0.35–0.90) | 0.59 (0.37–0.94) | ||
| Divorced/Separated/Widowed | 1.15 (0.40–3.33) | 1.15 (0.40–3.33) | ||
| Lowest condom use level in relationships in past 12 months | ||||
| Never | 1.00 | |||
| Sometimes | 1.78 (1.12–2.81) | |||
| Always | 1.72 (0.89–3.30) | |||
| Age at sexual debut (one-year increment) | 0.93 (0.86–1.01) | |||
| Any casual partner in past 12 months | 2.82 (0.96–8.34) | |||
| Multiple partners in past 12 months | 1.99 (0.44–9.02) | |||
| Akaike Information Criterion | 1603.2 | 1598.8 | 1595.2 | 1611.6 |
For all models, n=1,734, time at risk=5,714 person-years and there were 116 seroconversions. All models are Cox proportional hazards models and contain indicator variables for year of observation (not shown). Values are hazard ratios and 95% confidence intervals. Coefficients for squared and cubic terms represent 10 and 100 unit change in the respective variables.
A joint test for equality on the four pairs of age disparity terms was not statistically significant comparing either a woman with a five-year older partner to one with a same aged partner (χ2(3): 0.42, p-value: 0.94), or a ten-year older partner to a same aged one (χ2(3): 0.54, p-value: 0.91).
Figure 2. Modelled association between relationship age disparity and HIV infection.
The modelled curve shown here is based the age disparity terms in Model 1, Table 2. This is a Cox proportional hazards model containing linear and quadratic terms in partner’s age disparity, as well as linear, quadratic and cubic terms in respondent’s age and indicator variables for year of observation.
No significant effect-modification of the relationship between age disparities and HIV infection was seen for marital status, educational attainment or wealth (Table 3). However, the protective effect of an older partner was notably stronger for women with any tertiary education, compared to the other groups. Women with tertiary education also had the smallest average relationship age disparity (baseline mean 4.8 years vs. 5.7 years for secondary and 6.5 years for primary education; Kruskall-Wallis equality test χ2(2) = 8.8, p=0.01).
Table 3.
Multivariable models of HIV acquisition due to relationship age disparity, stratified by socio-demographic variables
| Age disparity | Age disparity squared | χ2 test | p-value | |
|---|---|---|---|---|
|
|
||||
| Current marital status | ||||
| Never married | 0.90 (0.84–0.97) | 1.05 (1.03–1.07) | ||
| Engaged | 0.90 (0.79–1.02) | 1.05 (1.01–1.10) | ||
| Married | 0.88 (0.83–0.94) | 1.04 (1.02–1.05) | ||
| Divorced/Separated/Widowed | 0.89 (0.67–1.18) | 1.03 (0.94–1.13) | ||
| Age disparity: 5 years vs. none | 0.41 | 0.94 | ||
| Age disparity: 10 years vs. none | 0.91 | 0.82 | ||
|
|
||||
| Highest educational attainment | ||||
| None or Primary (0–7 years) | 0.89 (0.84–0.95) | 1.04 (1.02–1.06) | ||
| Secondary (8–12 years) | 0.92 (0.87–0.98) | 1.03 (1.01–1.05) | ||
| Tertiary | 0.82 (0.60–1.11) | 0.90 (0.53–1.51) | ||
| Age disparity: 5 years vs. none | 1.53 | 0.46 | ||
| Age disparity: 10 years vs. none | 1.32 | 0.52 | ||
|
|
||||
| Household wealth quintile | ||||
| Lowest | 0.92 (0.83–1.01) | 1.03 (0.98–1.08) | ||
| 2nd lowest | 0.85 (0.77–0.95) | 1.06 (1.02–1.10) | ||
| Middle | 0.95 (0.87–1.03) | 1.02 (1.00–1.05) | ||
| 2nd highest | 0.90 (0.82–0.98) | 1.04 (1.02–1.06) | ||
| Highest | 0.93 (0.73–1.18) | 1.00 (0.87–1.14) | ||
| Age disparity: 5 years vs. none | 2.37 | 0.67 | ||
| Age disparity: 10 years vs. none | 2.21 | 0.70 | ||
This table contains summary results for 3 separate regression models. For all models, n=1,734, time at risk=5,714 person-years and there were 116 seroconversions. Values are hazard ratios and 95% confidence intervals from Cox proportional hazards models. All models contain indicator variables for year of observation, age of respondent (linear, quadratic and cubic terms centered at age 15) and all socio-demographic and behavioral covariates from the main analysis. The χ2 tests are for equality of the pairs of age disparity terms (linear and quadratic), comparing women with partners of the stated age disparities, and thus have k-1 degrees of freedom, where k is the number of categories being compared.
Rerunning the analyses using the multiply imputed dataset did not qualitatively change the results (Table S3). Analyses using dichotomous measures of age disparity found similar effects to the continuous age disparity models, although with lower power to detect effects (Table S4).
Discussion
In this study, we quantify for the first time the relationship between age disparity and HIV acquisition risk in Africa amongst women over the age of 30. We found that HIV incidence in this population group was high overall and that risk fell sharply as partner age increased. Having a five-year older partner was associated with a one-third reduction in acquisition risk compared to having a same-aged partner, and having a ten-year older partner halved this risk.
There are at least three possible explanations for the observed decline in risk with increasing partner age disparity. First, in this age-group, younger individuals are on average more likely to be infectious. As our data show for women (see Figure S4), and previous research has shown for both men and women [21], HIV incidence declines with age after 30 even though prevalence continues to rise. Since those with acute incident HIV are far more likely to transmit the disease per sex act [38], higher infection rates for younger partners may reflect this age-distribution of incidence.
Second, even for a given age, partners of a similar age may be more likely to be infectious if similarly-aged relationships are qualitatively different from age-disparate ones. Given past evidence that relationship age disparities for women aged 30–50 in this setting vary by relationship type [39], decreasing risk with increasing age disparity might be thought to reflect different types of partnership at greater age disparities (in this cohort, casual partners are non-significantly younger than non-causal partners). However, this hypothesis is not supported by our evidence based on models containing interactions of age disparity and relationship type, which showed very similar associations between HIV risk and age disparity for all women (Table 3). A supplementary analysis including interactions of age disparity and the relationship type of each woman’s most recent partner (Figure S5), suggested that the association may be slightly different for casual partners compared to others, however these differences were not statistically significant and the analysis still supported a declining risk profile with increasing age disparity for all relationship types. We thus find no evidence that relationship type explains variation in HIV risk by age disparity.
Third, the age disparity itself may lead to differential power dynamics within relationships, which in turn lead to different sexual behaviors. The importance of power dynamics is supported by the literature relating to younger women with older partners [6–8], but these concerns may be less pressing amongst older women, who have both higher social and economic standing than younger women in their community. Empirically, it appears that allowing for sexual behaviors in our analysis does not affect the relationship between age disparities and HIV acquisition (model 4, table 2). However, this may reflect our inability to capture all aspects of sexual behavior in this sample.
While the association between age disparity and HIV acquisition risk was not significantly modified by any socio-demographic variables, it was notable that those with tertiary education had the strongest decline in risk with increasing age disparity, but also had the smallest average age disparity in their relationships and the lowest risk of HIV infection amongst those with similarly-aged partners. It is not clear from this small subsample of 163 women with tertiary education whether the steep decline seen is a function of very careful selection of older partners, or relatively high-risk similarly-aged partners – perhaps with men from outside the local area met while pursuing higher education.
Strengths and Limitations
This study has some notable strengths. Our dataset contained almost 6,000 person-years of time at risk and over 100 serologically confirmed incident HIV cases, providing considerable power to detect effects. Furthermore, the longitudinal nature of the data, collected over almost a decade, provided the opportunity to ensure temporality of association, and to move beyond a single snapshot of the HIV epidemic. The longitudinal data also allowed us to capture the exposure of partner age disparity in a time-varying manner, updating each woman’s age disparity information as her sexual relationships changed. Our dynamic analysis was only possible because of the unusual richness of the dataset, with multiple longitudinally linked waves of data collected over the observation period. Such a nuanced analysis is particularly important in this setting where relationships are likely to change more frequently than elsewhere due to frequent migration and the late age of marriage. The dataset also allowed us to control for time-varying socio-demographic and sexual behavior variables – marital status, educational attainment and household wealth – that may have confounded or effect-modified the relationship of interest.
We also note some limitations. As with any long-term community-based study, the cohort suffered from attrition and non-response. However, non-response was limited and our results did not change after accounting for data missingness through multiple imputation. Additionally, while our outcome is measured biologically, the primary exposure of partner’s age is self-reported. Self-report may introduce bias if social desirability leads to individuals systematically reporting age incorrectly. However, amongst women in the age range of this study it is unclear that there is strong social pressure to report partners of a particular age. More benignly, respondents may mistake their partner’s age, which could lead to non-differential misclassification and thus attenuation of any true association. As we have shown elsewhere [37], such reporting errors are limited in this setting. One potential reason for lower rates here compared to other African settings is the near-universal ownership of a national identity document which contains each person’s date of birth, meaning that general knowledge of ages is relatively high.
The extent to which our findings apply more widely will depend how much they arise from some unique features of our research setting. First, HIV prevalence and incidence in the age-range we study in rural KwaZulu-Natal are extremely high by international, and even national South African, standards. Since infection risk is likely to depend heavily on the prevalence of HIV in one’s partners’ cohort, settings in which the age distribution of both incident and prevalent HIV differs from that seen in this community may see different relationships between age disparity and HIV infection. Second, as we noted in our introduction, low and late marriage rates in this community mean that women over the age of 30 in this community are commonly not settled with a lifelong partner. If sexual behavior patterns (e.g. level of concurrency, frequency of partner change, rate of condom use) are strongly affected by marital status, the relationship between age disparity and HIV risk may be different in settings where marriage typically occurs at younger ages. Third, by the latter part of our period of observation ART was widely utilized in this community, leading to an increased number of older persons living with HIV [40], but a reduced number of such people with high HIV viral loads and thus infectiousness. The net result of these ART-related changes may be reduced risk of HIV acquisition by an uninfected woman with an older partner. In sum, care should be taken in generalizing our findings to settings with lower HIV prevalence or incidence, earlier marital ages or lower ART coverage. Comparative studies of age disparity and HIV infection in settings different in these regards is an important next step to further strengthen our understanding of HIV acquisition risk among older women.
Conclusions
In the initial decades of the HIV epidemic in sub-Saharan Africa the focus of research and prevention efforts has been on the young – especially young women – who have been at very high risk of HIV infection. Our results are a reminder that HIV infections continue to occur well into middle-age in rural South Africa, and that focusing prevention efforts exclusively on young women will miss a considerable segment of the epidemic. A more comprehensive strategy would broaden prevention efforts to the entire adult population, with careful consideration of the behaviors placing older men and women at risk of HIV infection.
Our study finds that in a rural South African setting, middle-aged women with similarly-aged partners are at increased risk of infection relative to those with older partners. While additional research is needed to confirm whether this pattern is seen more generally in high-prevalence settings, our findings suggest that there may be particular benefits to sensitizing middle-aged women to the potential for HIV infection when their partners are atypically young. Since our research does not investigate the decision-making process through which women select their partners, including the extent to which they hold agency in the process or consider HIV infection risk when making choice, the development of any such behavior-change intervention will require further qualitative research.
More broadly, our results – and their contrast to past findings for age-disparities and HIV amongst younger women – highlight the importance of careful targeting for prevention interventions in addressing the aging HIV epidemic in Africa. Sexual behaviors amongst middle-aged individuals are likely to differ from those in younger groups, and thus interventions targeting traditionally risky sexual behaviors may not be effective in this population. In our case, even where campaigns warning against older partners may be beneficial for young women, they may have the potential to cause harm for those in middle-age. Such findings promote the importance of evidence-based policy making as the HIV epidemic evolves.
Supplementary Material
Acknowledgments
This analysis is based on data collected by the Africa Centre Demographic Information System and would not have been possible without the kind contributions of all respondents and the support of many staff at the Africa Centre for Health and Population Studies, for which the authors are extremely grateful.
Footnotes
Authors’ contributions: GH and TB conceptualized the study. MLN and TB were involved in the data collection. GH conducted the analyses and wrote the first draft of the paper in collaboration with TB. GH summarized the results in tables and graphs. All authors contributed to the study design and data interpretation and to the revision of the final report.
Financial disclosure:
GH acknowledges support from the Harvard University Committee on African Studies for travel to the research site for this study. The Wellcome Trust, UK, provides core funding to the Africa Centre, including for the surveillance on which this work is based (grant 082384/Z/07/Z). TB and FT received financial support through grant 1R01-HD058482-01 from the National Institute of Child Health and Human Development, National Institutes of Health.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.UNAIDS. 2013 Report on the Global AIDS Epidemic. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2013. [Google Scholar]
- 2.Jennings JM, Luo RF, Lloyd LV, Gaydos C, Ellen JM, Rietmeijer CA. Age-bridging among young, urban, heterosexual males with asymptomatic Chlamydia trachomatis. Sex Transm Infect. 2007 Apr;83(2):136–41. doi: 10.1136/sti.2006.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spicknall IH, Aral SO, Holmes KK, Foxman B. Sexual networks are diverse and complex: prevalence of relationships bridging population subgroups in the Seattle Sex Survey. Sex Transm Dis. 2009 Aug;36(8):465–72. doi: 10.1097/OLQ.0b013e3181a31e4c. [DOI] [PubMed] [Google Scholar]
- 4.Hurt CB, Matthews DD, Calabria MS, et al. Sex With Older Partners Is Associated With Primary HIV Infection Among Men Who Have Sex With Men in North Carolina. J Acquir Immune Defic Syndr. 2010 Jun;54(2):185–90. doi: 10.1097/QAI.0b013e3181c99114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garnett GP, Anderson RM. Factors controlling the spread of HIV in heterosexual communities in developing countries: patterns of mixing between different age and sexual activity classes. Philos Trans R Soc Lond B Biol Sci. 1993;342(1300):137–59. doi: 10.1098/rstb.1993.0143. [DOI] [PubMed] [Google Scholar]
- 6.Hope R. Addressing Cross-Generational Sex: A desk review of research and programs. Washington, DC: Population Reference Bureau; 2007. [Google Scholar]
- 7.Leclerc-Madlala S. Age-disparate and intergenerational sex in southern Africa: the dynamics of hypervulnerability. AIDS. 2008;22(Suppl 4):S17–25. doi: 10.1097/01.aids.0000341774.86500.53. [DOI] [PubMed] [Google Scholar]
- 8.Luke N. Age and economic asymmetries in the sexual relationships of adolescent girls in sub-Saharan Africa. Stud Fam Plann. 2003;34(2):67–86. doi: 10.1111/j.1728-4465.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 9.Luke N. Economic Status, Informal Exchange, and Sexual Risk in Kisumu, Kenya. Econ Dev Cult Change. 2008;56(2):375–96. doi: 10.1086/522896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woolf SE, Maisto SA. Gender differences in condom use behavior? The role of power and partner-type. Sex Roles. 2008;9–10(58):689–701. [Google Scholar]
- 11.Wingood GM, DiClemente RJ. Application of the theory of gender and power to examine HIV-related exposures, risk factors, and effective interventions for women. Health Educ Behav. 2000 Oct;27(5):539–65. doi: 10.1177/109019810002700502. [DOI] [PubMed] [Google Scholar]
- 12.Baird SJ, Garfein RS, McIntosh CT, Ozler B. Effect of a cash transfer programme for schooling on prevalence of HIV and herpes simplex type 2 in Malawi: a cluster randomised trial. Lancet. 2012 Apr 7;379(9823):1320–9. doi: 10.1016/S0140-6736(11)61709-1. [DOI] [PubMed] [Google Scholar]
- 13.Dupas P. Do Teenagers Respond to HIV Risk Information? Evidence from a Field Experiment in Kenya. Am Econ J Appl Econ. 2011;3(1):1–34. [Google Scholar]
- 14.Gregson S, Nyamukapa CA, Garnett GP, et al. Sexual mixing patterns and sex-differentials in teenage exposure to HIV infection in rural Zimbabwe. Lancet. 2002;359(9321):1896–903. doi: 10.1016/S0140-6736(02)08780-9. [DOI] [PubMed] [Google Scholar]
- 15.Kelly RJ, Gray RH, Sewankambo NK, et al. Age differences in sexual partners and risk of HIV-1 infection in rural Uganda. J Acquir Immune Defic Syndr. 2003;32(4):446–51. doi: 10.1097/00126334-200304010-00016. [DOI] [PubMed] [Google Scholar]
- 16.Luke N. Confronting the ‘sugar daddy’ stereotype: age and economic asymmetries and risky sexual behavior in urban Kenya. Int Fam Plan Perspect. 2005;31(1):6–14. doi: 10.1363/3100605. [DOI] [PubMed] [Google Scholar]
- 17.Pettifor AE, Rees HV, Kleinschmidt I, et al. Young people’s sexual health in South Africa: HIV prevalence and sexual behaviors from a nationally representative household survey. AIDS. 2005 Sep 23;19(14):1525–34. doi: 10.1097/01.aids.0000183129.16830.06. [DOI] [PubMed] [Google Scholar]
- 18.Harling G, Newell M-L, Tanser F, Kawachi I, Subramanian SV, Bärnighausen T. Do Age-Disparate Relationships Drive HIV Incidence in Young Women? Evidence from a Population Cohort in Rural KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2014 Aug 1;66(4):443–51. doi: 10.1097/QAI.0000000000000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.KwaZulu-Natal Department of Health. [Accessed 2 Sept, 2014];Sugar Daddy Campaign. 2012 http://www.kznhealth.gov.za/sugardaddy.htm.
- 20.PSI. [Accessed 2 Sept, 2014];Cross-Generational Sex. http://www.psi.org/our-work/healthy-lives/interventions/cross-generational-sex.
- 21.Mossong J, Grapsa E, Tanser FC, Bärnighausen T, Newell M-L. Modelling HIV incidence and survival from age-specific seroprevalence after antiretroviral treatment scale-up in rural South Africa. AIDS. 2013 Jul 9;27(15):2471–9. doi: 10.1097/01.aids.0000432475.14992.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Olive FX, Angotti N, Houle B, et al. Prevalence of HIV among those 15 and older in rural South Africa. AIDS Care. 2013;25(9):1122–8. doi: 10.1080/09540121.2012.750710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bor J, Herbst AJ, Newell M-L, Bärnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013 Feb 22;339(6122):961–5. doi: 10.1126/science.1230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helleringer S, Kohler HP. Sexual network structure and the spread of HIV in Africa: evidence from Likoma Island, Malawi. AIDS. 2007 Nov 12;21(17):2323–32. doi: 10.1097/QAD.0b013e328285df98. [DOI] [PubMed] [Google Scholar]
- 25.Hosegood V, McGrath N, Moultrie T. Dispensing with marriage: Marital and partnership trends in rural KwaZulu-Natal, South Africa 2000–2006. Demogr Res. 2009;20:279–312. doi: 10.4054/DemRes.2009.20.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter M. The changing political economy of sex in South Africa: the significance of unemployment and inequalities to the scale of the AIDS pandemic. Soc Sci Med. 2007 Feb;64(3):689–700. doi: 10.1016/j.socscimed.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Seekings J. Race, Class, and Inequality in the South African City. In: Bridge G, Watson S, editors. The New Blackwell Companion to the City. Oxford, UK: Wiley-Blackwell; 2011. [Google Scholar]
- 28.Camlin CS, Snow RC, Hosegood V. Gendered Patterns of Migration in Rural South Africa. Population, Space and Place. 2013 Aug;20(6):528–51. doi: 10.1002/psp.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell MC. Are urban Black families nuclear? A comparative study of Black and White South African family norms. Soc Dyn. 2003;29(2):153–76. [Google Scholar]
- 30.Posel D, Rudwick S, Casale D. Is marriage a dying institution in South Africa? Exploring changes in marriage in the context of ilobolo payments. Agenda. 2011;25(1):102–11. [Google Scholar]
- 31.Shisana OR, Thomas Simbayi LC, Zuma K, et al. South African national HIV prevalence, incidence, behaviour and communication survey 2008: A turning tide among teenagers? Cape Town: HSRC Press; 2009. [Google Scholar]
- 32.ICF Macro. HIV Prevalence Estimates from the Demographic and Health Surveys. Calverton, MD: ICF Macro; 2010. [Google Scholar]
- 33.Ardington C, Case A, Hosegood V. Labor supply responses to large social transfers: longitudinal evidence from South Africa. Am Econ J Appl Econ. 2009;1:22–48. doi: 10.1257/app.1.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanser F, Hosegood V, Bärnighausen T, et al. Cohort profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. Int J Epidemiol. 2008;37(5):956–62. doi: 10.1093/ije/dym211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bärnighausen T, Tanser F, Newell M-L. Lack of a decline in HIV incidence in a rural community with high HIV prevalence in South Africa, 2003–2007. AIDS Res Hum Retroviruses. 2009;25(4):405–9. doi: 10.1089/aid.2008.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization, UNAIDS. Guidelines for using HIV testing technologies in surveillance: selection, evaluation and implementation – 2009 update. Geneva: World Health Organization; 2009. [PubMed] [Google Scholar]
- 37.Harling G, Tanser F, Mutevedzi T, Bärnighausen T. Assessing the validity of respondents’ reports of their partners’ ages in a rural South African population. BMJ Open. 2014 doi: 10.1136/bmjopen-2014-005638. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boily MC, Baggaley RF, Wang L, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009 Feb;9(2):118–29. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ott MQ, Bärnighausen T, Tanser F, Lurie MN, Newell M-L. Age-gaps in sexual partnerships: seeing beyond ‘sugar daddies’. AIDS. 2011;25(6):861–3. doi: 10.1097/QAD.0b013e32834344c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zaidi J, Grapsa E, Tanser F, Newell M-L, Bärnighausen T. Dramatic increase in HIV prevalence after scale-up of antiretroviral treatment. AIDS. 2013;27(10):2301–5. doi: 10.1097/QAD.0b013e328362e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.