Abstract
Background
Stereotactic radiosurgery is a well-accepted treatment for patients with intracranial metastases, but outcomes with volumetric modulated arc radiosurgery (VMAR) are poorly described.
Objective
To report our initial clinical experience applying a novel single-isocenter technique to frameless VMAR for simultaneous treatment of multiple intracranial metastases.
Methods
We performed a retrospective analysis of 15 patients undergoing frameless VMAR for multiple intracranial metastases using a single, centrally-located isocenter between 2009 and 2011. Among these, 3 patients were treated for progressive or recurrent intracranial disease. A total of 62 metastases (median 3 per patient, range 2-13) were treated to a median dose of 20 Gy (range, 15-30 Gy). 3 patients were treated with fractionated SRS. Follow-up including clinical examination and magnetic resonance imaging (MRI) occurred every 3 months.
Results
Median follow-up for all patients was 7.1 months (range, 1.1-24.3), with 11 patients (73.3%) followed until death. For the remaining 4 patients alive at the time of analysis, median follow-up was 19.6 months (range, 9.2-24.3). Local control at 6 and 12 months was 91.7 (95% Confidence Interval [C.I.], 84.6-100.0%) and 81.5 (95% C.I., 67.9-100.0%), respectively. Regional failure was observed in 9 patients (60.0%), and 7 patients (46.7%) received salvage therapy. Overall survival at 6 months was 60.0% (95% C.I., 40.3-88.2%). Grade 3 or greater treatment-related toxicity was not observed. Median total treatment time was 7.2 minutes (range, 2.8-13.2 minutes).
Conclusion
Single-isocenter, frameless VMAR for multiple intracranial metastases is a promising technique that may provide similar clinical outcomes compared to conventional radiosurgery.
Keywords: Brain metastasis, frameless, radiosurgery, surface imaging, volumetric modulated arc therapy, single isocenter
Introduction
Stereotactic radiosurgery (SRS) is a well-established therapy for the management of intracranial metastasis allowing highly conformal delivery of large doses to well-defined target volumes.1 Over half of cancer patients who develop brain metastasis present with more than one lesion,2 and SRS is routinely used for patients with multiple lesions. Importantly, randomized controlled trials have demonstrated the efficacy of SRS for the treatment of up to four metastases.3-5 Moreover, several retrospective studies have described a benefit of SRS for patients with more than four lesions.6-8
Several forms of SRS such as Gamma Knife (Elekta, Stockholm, Sweden), CyberKnife (Accuray Inc., Sunnyvale, California), and intensity modulated linear accelerator (linac) based radiosurgery (IMRS) are now widely used. Volumetric modulated arc therapy (VMAT) is a novel treatment paradigm utilizing conventional technology wherein radiation dose is continuously delivered as the treatment gantry rotates. Multileaf collimator (MLC) aperture, gantry rotation speed, and dose delivery rate are simultaneously adjusted with VMAT.9 As such, volumetric modulated arc radiosurgery (VMAR) is attractive for its ability to rapidly deliver highly conformal treatments.
SRS for multiple lesions typically employs multiple isocenters, requiring patient repositioning during a single session and sequential treatment of multiple lesions.10, 11 IMRS allows for treatment planning of multiple lesions using a single-isocenter, thereby eliminating the need for isocenter shifts, significantly reducing treatment times, and enhancing accuracy by reducing the potential for intrafraction patient motion.12 Such an approach is advantageous for both patients and clinicians in busy clinics. Importantly, static beam single-isocenter IMRS is associated with clinical outcomes similar to those of traditional SRS methods.13, 14 Although several dosimetric studies have evaluated the feasibility of VMAR compared to traditional SRS methods, the clinical outcomes associated with VMAR are not well known.15-19 Here, we describe our clinical experience using single-isocenter, frameless VMAR for patients with multiple intracranial metastases. To our knowledge, this is the first report of clinical outcomes associated with single-isocenter VMAR for multiple brain metastases.
Methods
Retrospective review of radiation oncology records was performed after obtaining institutional review board approval. We previously examined 100 patients with multiple intracranial metastases consecutively treated with single-isocenter, frameless SRS between March 2006 and March 2012 at a single institution.14 Patients with intracranial metastatic disease that was histologically confirmed at either the primary or metastatic site were eligible for treatment if they could lie still and tolerate simulation. This report evaluates a subgroup of those patients whose treatment was delivered using VMAT. In total, 15 patients received singleisocenter, frameless VMAR for multiple brain metastases between November 2009 and November 2011 (see Figure, Supplemental Digital Content 1). VMAR was cautiously adopted during this time, as it was considered investigational.
Patient characteristics are summarized in Table 1. Patients had a median of 3 intracranial metastases (range, 2-13). A total of 62 intracranial metastases were treated to a median dose of 20 Gy (range, 15-30 Gy). The maximum target diameter as determined by contrast enhanced T1-weighted magnetic resonance imaging (MRI) was less than 4.0 cm in all patients. Three patients (20%) were treated with more than one fraction. Of these 3 patients, 1 had previously been treated with WBRT, 1 had a brainstem lesion, and 1 had a 3.5 cm3 lesion with a composite PTV of 31.6 cm3. The remaining 12 patients (80%) were treated in a single fraction. In total, 3 patients were treated for progressive or recurrent intracranial disease after surgical resection, WBRT, or prior SRS. Two patients (13%) received WBRT prior to presentation, while the remaining 13 patients (87%) did not. Salvage therapy was offered to patients with recurrent local disease or new intracranial metastases and consisted of surgical resection, WBRT, or repeated frameless IMRS.
Table 1.
Patient characteristics.
| Characteristic | Value |
|---|---|
| Patients, n | 15 |
| Lesions, n | 62 |
| Lesions per patient | |
| Median (range) | 3 (2-13) |
| 2-4 | 10 |
| 5-8 | 4 |
| ≥9 | 1 |
| Sex, n | |
| Male | 5 |
| Female | 10 |
| Median age, years (range) | 63 (29-89) |
| Primary tumor | |
| Lung | 5 |
| Breast | 4 |
| Melanoma | 5 |
| Other | 1 |
| Median tumor size, mm (range) | 18 (10-35) |
| Recursive Partitioning Analysis class | |
| I | 1 |
| II | 8 |
| III | 6 |
| Median dose, Gy (range) | 20 (15-30) |
| Fractions | |
| Median (range) | 1 (1-5) |
| 1 | 12 |
| ≥2 | 3 |
| Treatment arcs | |
| 1-2 | 11 |
| 3-4 | 4 |
After obtaining informed consent, patients underwent stereotactic-protocol contrast enhanced T1-weighted brain MRI (26 cm field-of-view, 512 × 512 pixel size, 1.5 mm slice intervals) using a 3.0 Tesla Scanner (General Electric, Fairfield, Connecticut). A detailed description of our patient simulation and setup techniques has been previously published.13, 14 Notably, we immobilize patients without a bite block in favor of surface image guidance (SIG) in real-time with the AlignRT system (VisionRT Ltd, London, United Kingdom). As previously described, these patients also underwent simulation non-contrast CT for treatment planning purposes.20 During treatment, patient position was continuously monitored with SIG, with a beam hold initiated for deviation exceeding a predefined translational threshold of 1-2 mm or a rotational threshold of 1°.20
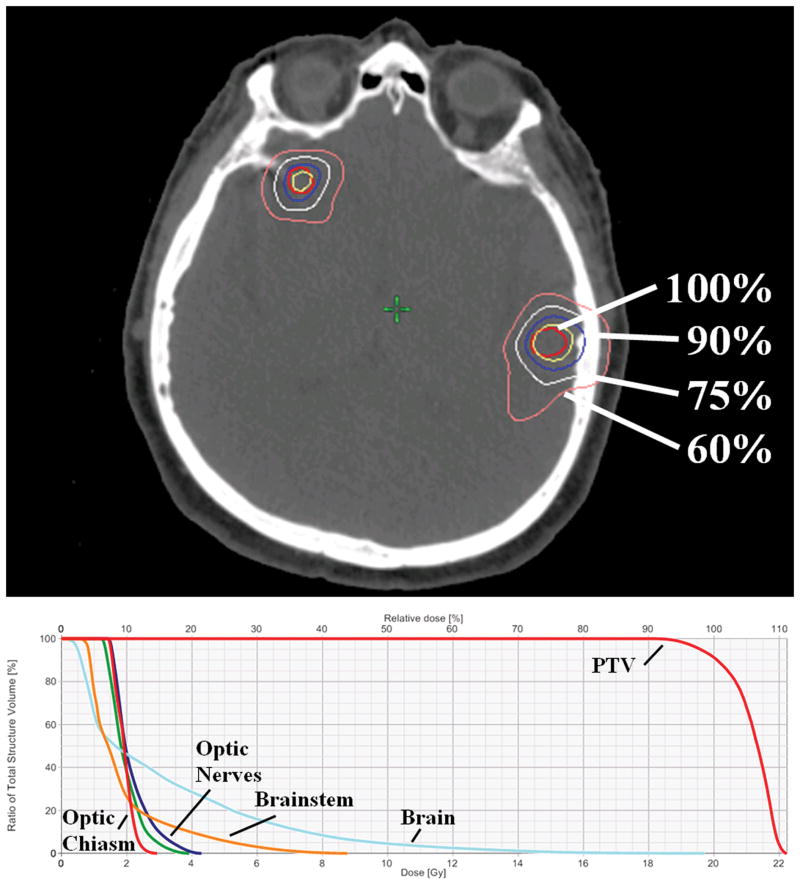
Gross tumor volume and clinical target volume (CTV) for each lesion were identical and defined as the contrast-enhancing volume on axial MRI. Planning target volume (PTV) for each lesion was generated typically by adding a 1 mm margin to each CTV. Subsequently, a composite PTV was established by adding each individual PTV. For 3 patients, no expansion margin was used. Dose was prescribed to the composite PTV such that each lesion for a given patient was prescribed the same dose. Plan optimization was performed using Eclipse software, version 8.9 (Varian Medical Systems, Palo Alto, California). VMAR planning provided uniform coverage of the PTV and minimal dose to critical structures (Figure 1). Median minimum and maximum PTV coverage was 92% and 112%, respectively. Treatment was delivered using 5 mm MLC leaves. Radiation Therapy Oncology Group (RTOG) Conformity Index (CI) was defined as the ratio of prescription isodose volume (PIV) to PTV. RTOG Heterogeneity Index (HI) was defined as the ratio of maximum PTV dose to prescription dose. Paddick CI was defined as the ratio of PTV receiving prescription dose squared to the product of PTV and PIV. These representations of conformity have been previously described.21, 22 Treatment plan characteristics are summarized in Table 2.
Figure 1.
Representative single-isocenter volumetric modulated arc radiosurgery plan for a patient with three intracranial metastases treated to a prescription dose of 20 Gy. Axial view (top) of the planning CT displaying PTVs and isodose curves. Dose-volume histogram (bottom) displaying doses in absolute (Gy, lower horizontal axis) and relative (%, upper horizontal axis) measures given composite PTV and normal structures.
Table 2.
Treatment plan characteristics by patient.
| Patient | Lesions | PTV (cm3) | RTOG CI | RTOG HI | Paddick CI | V12 Gy (cm3) | V4.5 Gy (cm3) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 5 | 31.6 | 1.09 | 1.16 | 0.83 | 346.2 | 766.2 |
| 2 | 2 | 2.0 | 1.43 | 1.08 | 0.67 | 18.6 | 106.1 |
| 3 | 2 | 8.3 | 1.15 | 1.10 | 0.83 | 46.3 | 266.9 |
| 4 | 13 | 23.6 | 1.42 | 1.12 | 0.68 | 432.0 | 1358.6 |
| 5 | 6 | 21.8 | 2.04 | 1.16 | 0.44 | 272.0 | 748.5 |
| 6 | 2 | 2.7 | 1.10 | 1.23 | 0.87 | 9.2 | 74.4 |
| 7 | 2 | 10.6 | 1.07 | 1.23 | 0.90 | 8.0 | 71.3 |
| 8 | 3 | 5.4 | 1.59 | 1.12 | 0.63 | 32.6 | 126.0 |
| 9 | 6 | 93.7 | 1.16 | 1.13 | 0.81 | 274.8 | 841.2 |
| 10 | 3 | 3.2 | 1.32 | 1.12 | 0.63 | 38.0 | 290.7 |
| 11 | 2 | 4.4 | 1.13 | 1.08 | 0.82 | 20.0 | 183.7 |
| 12 | 2 | 4.1 | 0.98 | 1.12 | 0.77 | 29.3 | 374.1 |
| 13 | 6 | 1.9 | 0.29 | 1.05 | 0.33 | 28.1 | 395.7 |
| 14 | 4 | 19.1 | 1.06 | 1.11 | 0.90 | 70.8 | 350.5 |
| 15 | 4 | 15.0 | 1.26 | 1.09 | 0.76 | 66.3 | 419.7 |
Abbreviations: PTV = composite Planning Target Volume; RTOG = Radiation Therapy Oncology Group; CI = Conformity Index; HI = Heterogeneity Index; V12 Gy = volume of brain receiving at least 12 Gy; V4.5 Gy = volume of brain receiving at least 4.5 Gy
All patients completed treatment as planned. Patients were routinely seen one week following VMAR for clinical examination. Thereafter, intracranial progression was assessed by physical evaluation and contrast enhanced MRI every 3 months. Treatment response was analyzed by local control, regional control, and overall survival. Local control was defined as the absence of disease progression, with progression radiographically defined as an increase of greater than 25% of the bi-dimensional product of the two largest diameters of the lesion. Regional control was defined as the absence of new intracranial metastatic disease occurring outside the treatment volume on radiographic examination. Intracranial status was deemed unknown if radiographic examination had not been obtained by the time of analysis. These patients were excluded from local and regional control analyses but were included in survival analysis. Local control and regional control from the date of treatment were estimated by the Kaplan-Meier method, as was overall survival from the date of diagnosis. Event times were censored at the time of last follow-up for patients without an event at the time of analysis. Toxicity was graded according to the RTOG scale.23 Statistical analyses were performed using GraphPad Prism version 6.05 (GraphPad Software, La Jolla, California).
Results
All patients were treated with single-isocenter, frameless VMAR. A median of 2 arcs (range, 1-4) were used. Arcs were coplanar except in the four patients treated with ≥3 arcs. Three patients were treated using flattening filter-free (FFF) mode. Median treatment time from initial beam on to final beam off was 7.2 minutes (range, 2.8-13.2). Increasing number of lesions (2-4, 5-8, and ≥9 metastases) was not associated with significantly longer treatment time (data not shown). Median total beam-on time was 5.5 minutes (range, 1.8-8.2). Dosimetric representations of conformity as described by RTOG CI, RTOG HI, and Paddick CI were generally within accepted limits (Table 2). Median RTOG CI was 1.15 (range, 0.29-2.04). Median RTOG HI was 1.12 (range, 1.05-1.23). Median Paddick CI was 0.77 (range, 0.33-0.90). The only patient with a “major violation” as defined by RTOG CI <0.9 or >2.5 had 6 very small lesions (cumulative PTV 1.9 cm3) treated with a single arc. No other major violations were observed.
Median follow-up for all patients was 7.1 months (range, 1.1-24.3) with 11 patients (73.3%) followed until death. For the remaining 4 patients alive at the time of analysis, median follow-up was 19.6 months (range, 9.2-24.3).
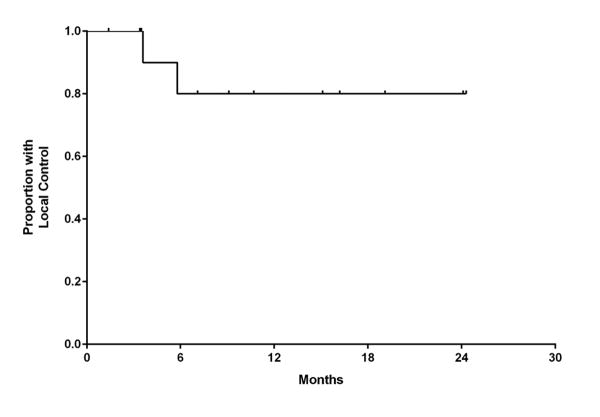
One patient was excluded from local control analysis owing to unknown intracranial status, and the remaining 14 patients (59 lesions) were evaluable for local control. Of this group, 12 patients (86%) and 57 lesions (97%) were without evidence of local progression at the time of analysis. Local control at 6 and 12 months was 91.7% (95% C.I., 84.6-100.0%) and 81.5% (95% C.I., 67.9-100.0%), respectively (Figure 2). For the 2 patients with local failure, salvage therapy consisted of repeated SRS (1 patient) or no further treatment (1 patient).
Figure 2.
Kaplan-Meier estimate of local control.
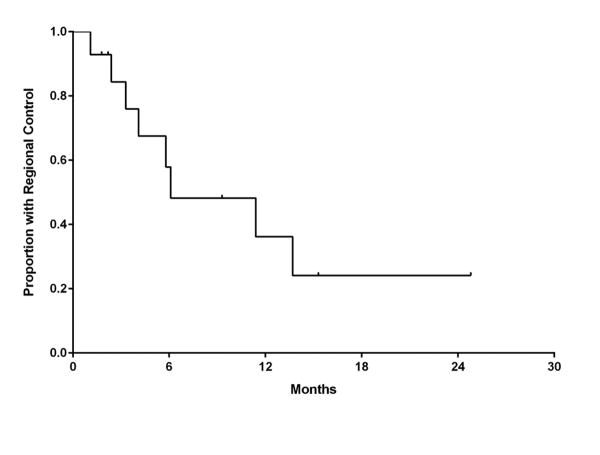
Intracranial failure outside the treatment volume was observed in 9 patients (60.0%). Regional control at 6 and 12 months was 57.9% (95% C.I., 35.8-89.8%) and 36.2% (95% C.I., 8.5-62.4%), respectively (Figure 3). For the 9 patients with a documented failure, the site of first failure was primarily outside the treatment volume (7 patients) rather than only local (1 patient) or simultaneously within and outside the treatment volume (1 patient). Salvage therapy for new intracranial metastases occurring outside of the treatment volume was received by 7 patients (47.0%) and consisted of repeated SRS (3 patients), WBRT (3 patients), and surgical resection (1 patient).
Figure 3.
Kaplan-Meier estimate of regional control.
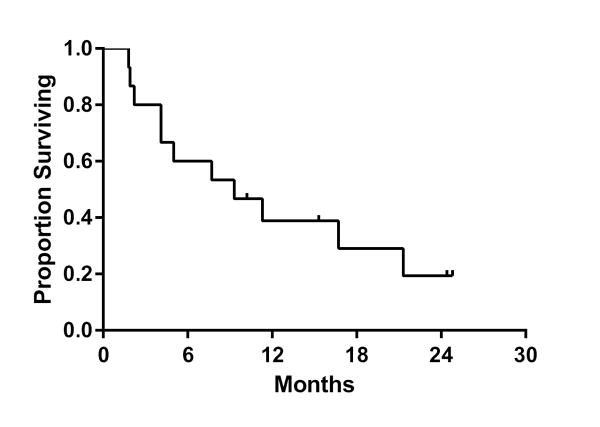
Overall survival at 6 and 12 months was 60.0% (95% C.I., 40.3-88.2%) and 38.9% (95% C.I., 15.6-62.5%), respectively (Figure 4). The number of patients by RTOG recursive partitioning analysis (RPA) class or who had previously received WBRT were too small to allow for meaningful comparisons stratified by these factors.
Figure 4.
Kaplan-Meier estimate of overall survival.
Acute treatment-related toxicity occurred in 1 patient (6.7%). This patient suffered a seizure attributed to cerebral edema responsive to steroids. Late treatment-related toxicity was not observed. Moreover, no grade 3 or greater acute or late treatment-related events were observed. Mean dose to normal brain was 4.2 Gy. Median volume of normal brain receiving ≥12 Gy (V12 Gy) was 38.0 cm3, and median volume of normal brain receiving ≥4.5 Gy (V4.5 Gy) was 350.5 cm3. No discernible relationship between dose to normal brain and toxicity was observed.
Discussion
SRS is a well-established therapy for the management of intracranial metastasis, with discrete lesions traditionally treated sequentially and treatment duration proportional to the number of isocenters used. We previously reported that static beam single-isocenter IMRS is associated with clinical outcomes comparable to traditional SRS methods.13, 14, 20 Whereas IMRS is delivered in step-and-shoot fashion using several fixed-position beams, VMAT is delivered continuously as the gantry rotates (see Figure, Supplemental Digital Content 2). VMAT is an attractive technique for rapid, conformal IMRS due to its simultaneous adjustments in MLC aperture, gantry rotation speed, and dose delivery rate. However, the clinical outcomes of this novel technique are not well described. Here, we report, to our knowledge, the first clinical series of single-isocenter, frameless VMAR for multiple intracranial metastases.
Key Results
In this series, local control at 12 months was 82%, with 97% of lesions without progression at the time of analysis. Seven patients (47%) received salvage therapy for new brain metastases outside of the treatment volume. Overall survival at 12 months was 39%. No grade ≥3 toxicity was observed.
Interpretation
The clinical outcomes of VMAR for intracranial metastasis are unknown. Local control of 82% at 12 months in this study is comparable to both frame-based and frameless SRS series.6, 14, 24, 25 Unfortunately, the prognosis for patients with intracranial metastasis remains poor, and overall survival at 12 months ranges from 28%-45% with SRS alone in randomized trials.4, 5 Survival at 12 months of 39% in the present series compares favorably, particularly when taking into account that 40% of these patients were RPA class III.
Mayo and colleagues reported a series of 12 patients with a total of 14 intracranial metastases undergoing fractionated VMAR with all but 1 patient receiving 21 Gy in 3 fractions.15 The two patients with two brain metastases each in their series were sequentially treated using separate isocenters. At a median follow-up of 3 months, no local failures or adverse events were observed.15 In comparison, all 15 patients in the present series had multiple brain metastases treated with a single isocenter, and 12 patients (80%) were treated in a single fraction. Thus, the patient population and treatment technique in this report are significantly different compared to the previously described study. Moreover, the radiobiology of single fraction treatment and fractionated treatment may be dissimilar.26 All together, single-isocenter, frameless VMAR for multiple brain metastases does not appear to compromise clinical outcomes when compared to traditional SRS techniques.
The feasibility and dosimetric implications of VMAR have been described. First, compared to single-isocenter static beam IMRS, single-isocenter VMAR is associated with reduced monitor units (MUs) and reduced treatment time.18 Next, a single-isocenter approach to VMAR rather than using multiple isocenters reduces both MUs and treatment time without compromising conformality.16 Finally, the significance of FFF mode in VMAR remains an open question. The dosimetric characteristics of VMAR with or without FFF mode for multiple brain metastases are comparable, although FFF mode provides even greater reductions in treatment time.19, 27 Taken together, the dosimetric profile of single-isocenter VMAR compares favorably to both conventional frame-based SRS techniques and static beam IMRS. In the present study, dosimetric characteristics of VMAR were generally within accepted standards in concordance with prior feasibility studies.18, 19
Traditional radiosurgery uses separate isocenters for each lesion which are then treated sequentially, whereas single-isocenter technique allows simultaneous treatment of multiple lesions. Dosimetric characteristics for single-isocenter compared to multiple-isocenter techniques have been described. Single-isocenter strategies have similar conformity with slightly increased dose to non-target tissue compared to multiple-isocenter techniques.16, 28 Reported clinical outcomes including local control and toxicity are comparable.13, 14, 20 With traditional multiple-isocenter technique, a given patient is typically repositioned multiple times during the treatment session. Alternatively, a patient may not require repositioning with singleisocenter technique, but accurate setup is critical since both translational and rotational error degrade conformality.29, 30 At our institution, intrafraction motion after initial setup is minimized by continuous monitoring in real-time with SIG.20
One consequence of VMAT is the increased volume of non-target tissue receiving low dose as a result of beam angle modulation through gantry rotation. Single-isocenter static beam IMRS is associated with low toxicity.13, 14 When compared to IMRS, VMAR is associated with a trend for slightly increased low isodose “spill” to surrounding tissue.18 Mean dose to normal brain in this series is similar to prior studies of linac-based SRS,13, 14, 18 but both V12 Gy and V4.5 Gy are higher, as expected, than those published for traditional SRS.31 Notably, most patients in this study were treated using one or two arcs, and increasing arc number is associated with superior dosimetric characteristics.31 Interestingly, the low isodose spill of four-arc VMAR is similar to traditional frame-based SRS methods using many fixed beam angles.31 Increased low isodose spill to non-target brain theoretically could increase the risk of adverse effects. Importantly, minimal toxicity was observed in the present series. Although radionecrosis may be underestimated with standard imaging techniques,32 no evidence of symptomatic radionecrosis was detected. While the long-term toxicity of low isodose spill associated with VMAT remains an open question, its clinical significance in patients with multiple intracranial metastases and poor prognosis is unclear.
A significant advantage of our technique is reduced treatment time, and VMAR is one of the most efficient forms of SRS to date. Treatment of multiple intracranial lesions with frame-based SRS techniques may last several hours.13 Treatment times for multiple-isocenter linac-based SRS often last over 30 minutes, although this can be reduced using a single-isocenter technique.12-14 In comparison, patients in this series completed treatment with VMAR for multiple metastases in just over 7 minutes. Reduced treatment time enhances both patient tolerance and provider efficiency, but also reduces intrafraction patient motion. The latter is particularly important for maximizing accuracy and minimizing risk to non-target tissue, even more so with frameless techniques.33, 34 Given its efficiency, single-isocenter, frameless VMAR as presented here is a noninvasive treatment strategy for multiple intracranial lesions that appeals to both patients and providers.
Limitations and Generalizability
As a retrospective analysis study from a single institution, this series is susceptible to all the inherent biases and shortcomings of such analyses. Ultimately, a prospective study would best assess the role of single-isocenter, frameless VMAR for multiple intracranial metastases. At our institution, we gradually implemented single-isocenter VMAR with attention to coverage of PTV and dose to critical structures. Until rigorous studies on this technique are available, it is best performed at a center with expertise using it.
Conclusion
Results of this study suggest that single-isocenter, frameless SIG VMAR for the simultaneous treatment of multiple intracranial metastases may produce clinical outcomes comparable to both conventional frame-based and other frameless SRS techniques while enhancing patient tolerance and reducing treatment time. Long-term outcomes for VMAR will continue to mature, but this series provides encouraging initial clinical outcomes such that this technique will be used for all intracranial radiosurgery treatments at our institution.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health grant PHSGM007198 (UCSD Medical Scientist Training Program). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosure: K. T. M. has received honoraria from Varian Medical Systems for giving lectures. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Suh JH. Stereotactic radiosurgery for the management of brain metastases. The New England journal of medicine. 2010 Mar 25;362(12):1119–1127. doi: 10.1056/NEJMct0806951. [DOI] [PubMed] [Google Scholar]
- 2.Delattre JY, Krol G, Thaler HT, Posner JB. Distribution of brain metastases. Archives of neurology. 1988 Jul;45(7):741–744. doi: 10.1001/archneur.1988.00520310047016. [DOI] [PubMed] [Google Scholar]
- 3.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004 May 22;363(9422):1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 4.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2006 Jun 7;295(21):2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 5.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011 Jan 10;29(2):134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatnagar AK, Flickinger JC, Kondziolka D, Lunsford LD. Stereotactic radiosurgery for four or more intracranial metastases. International journal of radiation oncology, biology, physics. 2006 Mar 1;64(3):898–903. doi: 10.1016/j.ijrobp.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 7.Hunter GK, Suh JH, Reuther AM, et al. Treatment of five or more brain metastases with stereotactic radiosurgery. International journal of radiation oncology, biology, physics. 2012 Aug 1;83(5):1394–1398. doi: 10.1016/j.ijrobp.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Kim CH, Im YS, Nam DH, Park K, Kim JH, Lee JI. Gamma knife radiosurgery for ten or more brain metastases. Journal of Korean Neurosurgical Society. 2008 Dec;44(6):358–363. doi: 10.3340/jkns.2008.44.6.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Medical physics. 2008 Jan;35(1):310–317. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 10.Cardinale RM, Benedict SH, Wu Q, Zwicker RD, Gaballa HE, Mohan R. A comparison of three stereotactic radiotherapy techniques; ARCS vs. noncoplanar fixed fields vs. intensity modulation. International journal of radiation oncology, biology, physics. 1998 Sep 1;42(2):431–436. doi: 10.1016/s0360-3016(98)00206-5. [DOI] [PubMed] [Google Scholar]
- 11.Sankaranarayanan V, Ganesan S, Oommen S, Padmanaban TK, Stumpf J, Ayyangar KM. Study on dosimetric parameters for stereotactic radiosurgery and intensity-modulated radiotherapy. Medical dosimetry : official journal of the American Association of Medical Dosimetrists. 2003 Summer;28(2):85–90. doi: 10.1016/S0958-3947(02)00238-8. [DOI] [PubMed] [Google Scholar]
- 12.Lawson JD, Fox T, Waller AF, Davis L, Crocker I. Multileaf collimator-based linear accelerator radiosurgery: five-year efficiency analysis. J Am Coll Radiol. 2009 Mar;6(3):190–193. doi: 10.1016/j.jacr.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Nath SK, Lawson JD, Simpson DR, et al. Single-isocenter frameless intensity-modulated stereotactic radiosurgery for simultaneous treatment of multiple brain metastases: clinical experience. International journal of radiation oncology, biology, physics. 2010 Sep 1;78(1):91–97. doi: 10.1016/j.ijrobp.2009.07.1726. [DOI] [PubMed] [Google Scholar]
- 14.Lau SK, Zhao X, Carmona R, et al. Frameless single-isocenter intensity modulated stereotactic radiosurgery for simultaneous treatment of multiple intracranial metastases. Transl Cancer Res. 2014 Aug;3(4):383–390. doi: 10.3978/j.issn.2218-676X.2014.07.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayo CS, Ding L, Addesa A, Kadish S, Fitzgerald TJ, Moser R. Initial experience with volumetric IMRT (RapidArc) for intracranial stereotactic radiosurgery. International journal of radiation oncology, biology, physics. 2010 Dec 1;78(5):1457–1466. doi: 10.1016/j.ijrobp.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Clark GM, Popple RA, Young PE, Fiveash JB. Feasibility of single-isocenter volumetric modulated arc radiosurgery for treatment of multiple brain metastases. International journal of radiation oncology, biology, physics. 2010 Jan 1;76(1):296–302. doi: 10.1016/j.ijrobp.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Clark GM, Popple RA, Prendergast BM, et al. Plan quality and treatment planning technique for single isocenter cranial radiosurgery with volumetric modulated arc therapy. Practical radiation oncology. 2012 Oct-Dec;2(4):306–313. doi: 10.1016/j.prro.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Wang JZ, Pawlicki T, Rice R, et al. Intensity-modulated radiosurgery with rapidarc for multiple brain metastases and comparison with static approach. Medical dosimetry : official journal of the American Association of Medical Dosimetrists. 2012 Spring;37(1):31–36. doi: 10.1016/j.meddos.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Wang JZ, Rice R, Mundt AJ, Sandhu A, Murphy KT. Feasibility and advantages of using flattening filter-free mode for radiosurgery of multiple brain lesions. Practical radiation oncology. 2012 Oct-Dec;2(4):e165–171. doi: 10.1016/j.prro.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Pan H, Cervino LI, Pawlicki T, et al. Frameless, real-time, surface imaging-guided radiosurgery: clinical outcomes for brain metastases. Neurosurgery. 2012 Oct;71(4):844–851. doi: 10.1227/NEU.0b013e3182647ad5. [DOI] [PubMed] [Google Scholar]
- 21.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. International journal of radiation oncology, biology, physics. 2000 May 1;47(2):291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 22.Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg. 2000 Dec;93(Suppl 3):219–222. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 23.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) International journal of radiation oncology, biology, physics. 1995 Mar 30;31(5):1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 24.Sneed PK, Suh JH, Goetsch SJ, et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. International journal of radiation oncology, biology, physics. 2002 Jul 1;53(3):519–526. doi: 10.1016/s0360-3016(02)02770-0. [DOI] [PubMed] [Google Scholar]
- 25.Chitapanarux I, Goss B, Vongtama R, et al. Prospective study of stereotactic radiosurgery without whole brain radiotherapy in patients with four or less brain metastases: incidence of intracranial progression and salvage radiotherapy. J Neurooncol. 2003 Jan;61(2):143–149. doi: 10.1023/a:1022173922312. [DOI] [PubMed] [Google Scholar]
- 26.Oermann EK, Kress MA, Todd JV, et al. The impact of radiosurgery fractionation and tumor radiobiology on the local control of brain metastases. J Neurosurg. 2013 Nov;119(5):1131–1138. doi: 10.3171/2013.8.JNS122177. [DOI] [PubMed] [Google Scholar]
- 27.Stieler F, Fleckenstein J, Simeonova A, Wenz F, Lohr F. Intensity modulated radiosurgery of brain metastases with flattening filter-free beams. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2013 Dec;109(3):448–451. doi: 10.1016/j.radonc.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Vanderspek L, Wang J, Alksne J, Murphy KT. Single fraction, single isocenter intensity modulated radiosurgery (IMRS) for multiple brain metastases: Dosimetric and early clinical experience. International journal of radiation oncology, biology, physics. 2007;69(3 Suppl):S265. doi: 10.1016/j.ijrobp.2009.07.1726. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Shiu A, Wang C, et al. Dosimetric effect of translational and rotational errors for patients undergoing image-guided stereotactic body radiotherapy for spinal metastases. International journal of radiation oncology, biology, physics. 2008 Jul 15;71(4):1261–1271. doi: 10.1016/j.ijrobp.2008.02.074. [DOI] [PubMed] [Google Scholar]
- 30.Guckenberger M, Roesch J, Baier K, Sweeney RA, Flentje M. Dosimetric consequences of translational and rotational errors in frame-less image-guided radiosurgery. Radiation oncology. 2012;7:63. doi: 10.1186/1748-717X-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas EM, Popple RA, Wu X, et al. Comparison of plan quality and delivery time between volumetric arc therapy (RapidArc) and Gamma Knife radiosurgery for multiple cranial metastases. Neurosurgery. 2014 Oct;75(4):409–417. doi: 10.1227/NEU.0000000000000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stockham AL, Tievsky AL, Koyfman SA, et al. Conventional MRI does not reliably distinguish radiation necrosis from tumor recurrence after stereotactic radiosurgery. J Neurooncol. 2012 Aug;109(1):149–158. doi: 10.1007/s11060-012-0881-9. [DOI] [PubMed] [Google Scholar]
- 33.Cervino LI, Pawlicki T, Lawson JD, Jiang SB. Frame-less and mask-less cranial stereotactic radiosurgery: a feasibility study. Phys Med Biol. 2010 Apr 7;55(7):1863–1873. doi: 10.1088/0031-9155/55/7/005. [DOI] [PubMed] [Google Scholar]
- 34.Cervino LI, Detorie N, Taylor M, et al. Initial clinical experience with a frameless and maskless stereotactic radiosurgery treatment. Practical radiation oncology. 2012;2(1):54–62. doi: 10.1016/j.prro.2011.04.005. 2012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.