Abstract
Background
Minor ginsenosides, those having low content in ginseng, have higher pharmacological activities. To obtain minor ginsenosides, the biotransformation of American ginseng protopanaxadiol (PPD)-ginsenoside was studied using special ginsenosidase type-I from Aspergillus niger g.848.
Methods
DEAE (diethylaminoethyl)-cellulose and polyacrylamide gel electrophoresis were used in enzyme purification, thin-layer chromatography and high performance liquid chromatography (HPLC) were used in enzyme hydrolysis and kinetics; crude enzyme was used in minor ginsenoside preparation from PPD-ginsenoside; the products were separated with silica-gel-column, and recognized by HPLC and NMR (Nuclear Magnetic Resonance).
Results
The enzyme molecular weight was 75 kDa; the enzyme firstly hydrolyzed the C-20 position 20-O-β-D-Glc of ginsenoside Rb1, then the C-3 position 3-O-β-D-Glc with the pathway Rb1→Rd→F2→C-K. However, the enzyme firstly hydrolyzed C-3 position 3-O-β-D-Glc of ginsenoside Rb2 and Rc, finally hydrolyzed 20-O-L-Ara with the pathway Rb2→C-O→C-Y→C-K, and Rc→C-Mc1→C-Mc→C-K. According to enzyme kinetics, Km and Vmax of Michaelis–Menten equation, the enzyme reaction velocities on ginsenosides were Rb1 > Rb2 > Rc > Rd. However, the pure enzyme yield was only 3.1%, so crude enzyme was used for minor ginsenoside preparation. When the crude enzyme was reacted in 3% American ginseng PPD-ginsenoside (containing Rb1, Rb2, Rc, and Rd) at 45°C and pH 5.0 for 18 h, the main products were minor ginsenosides C-Mc, C-Y, F2, and C-K; average molar yields were 43.7% for C-Mc from Rc, 42.4% for C-Y from Rb2, and 69.5% for F2 and C-K from Rb1 and Rd.
Conclusion
Four monomer minor ginsenosides were successfully produced (at low-cost) from the PPD-ginsenosides using crude enzyme.
Keywords: Aspergillus niger g.848, Panax quinquefolius, ginsenosidase type-I, minor ginsenosides, protopanaxadiol-type ginsenosides
1. Introduction
Ginseng, an important traditional medicinal herb, has been widely used for thousands of years in Asia, and has been popularized in many Western countries during recent decades. Ginseng refers to species within the genus Panax (Araliaceae family) that comprise approximately 14 species of slow-growing perennial plants with fleshy roots; the most widely used (high-yielding ginseng and commercialized ginseng) Panax species are Panax ginseng (Korean or Asian ginseng), Panax quinquefolius (American ginseng), and Panax notoginseng (Notoginseng or Sanchi ginseng) [1].
The major physiological activity compositions of ginseng are ginsenosides, which are triterpenoid saponin groups that can be classified into two groups by the skeleton of their aglycones, namely dammarane- and oleanane-type. The dammarane-type ginsenosides can be classified into the protopanaxadiol (PPD)-type ginsenoside (such as Rb1, Rb2, Rc, and Rd) and protopanaxatriol-type ginsenoside (such as Re, Rf, and Rg1) [2,3].
So far, >150 naturally occurring ginsenosides have been isolated from roots, leaves/stems, fruits, and/or flower heads of ginseng [3]. However, >80–90% ginsenosides in Korean ginseng are Rb1, Rb2 , Rc, Rd, Re, Rg1, and Rf; >80–90% of ginsenosides in American ginseng are Rb1, Rb2, Rc, Rd, Re, and Rg1; and >80–90% ginsenosides in Notoginseng are ginsenosides Rb1, Rg1, Rd, Re, and R1 [4].
These major ginsenosides have multiple sugar moieties, have low activities and are hardly absorbed by the human body [5]. After oral intake of ginseng, the ginsenosides are hydrolyzed by digestive enzymes and/or intestinal bacteria into minor ginsenosides, which are absorbed slowly in the gastrointestinal tract to exhibit physiological activity [6]; but, these conversions are low, and the absorption of major ginsenosides, including Rb1, Rb2 , Rc, Rd, Re and Rg1, by the gastrointestinal tract is quite poor [7].
The minor ginsenosides, such as F2, Compound-K (C-K), Compound-C-Mc (C-Mc), Compound-Y (C-Y), Rg3, Rg2, Rh2, Rh1, and F1, which are present at a low concentration in red ginseng and wild ginseng, can be produced by hydrolyzing the sugar moieties of major ginsenosides, including Rb1, Rb2 , Rc, Rd, Rf, Re, and Rg1 [8–10]. Many studies show that the minor ginsenosides have good pharmacological activities [11], such as anticarcinogenic [12–14], immunomodulatory, anti-inflammatory, antiallergic [15], antiatherosclerotic, antihypertensive, antiaging, and antidiabetic [16] effects as well as antistress activity and effects on the central nervous system [11,17,18]. Therefore, ginsenoside sugar chains are closely related to their biological activity, and the modification of their sugar chains may markedly change their pharmacological activity. The pharmacological activity of ginsenoside increases with the decrease of the number of the sugar moieties [10,19,20].
To obtain the minor ginsenosides, which have higher pharmacological activities, and are easily absorbed by the body, the methods of microbial or enzymatic transformation, and cloned ginsenosidase have been reported [10]: for example, ginsenoside Re can be hydrolyzed into Rg1 and Rh1, and ginsenoside Rb1 can be hydrolyzed to F2 and C-K by intestinal bacteria [5,6]; cloned ginsenosidase can convert ginsenoside Rc to C-Mc1 and C-Mc, and convert ginsenoside Rb2 to C-O and C-Y [21,22], etc.
In our laboratory, four kinds of ginsenosidase, named Type I [23,24], Type II [25], Type III [22], and Type IV [23], have been discovered. Ginsenosidase Type I can hydrolyze the 3-O- and 20-O-glycosides of PPD type ginsenoside; Type II can hydrolyze the 20-O-glycosides of PPD type ginsenoside; Type III can hydrolyze the 3-O-glycosides of PPD type ginsenoside; and Type IV can hydrolyze the 6-O- and 20-O-glycosides of protopanaxatriol type ginsenoside including Re and Rg1 to obtain wide variety of minor ginsenosides.
However, many studies on the preparation of minor ginsenosides used the high-cost monomer ginsenosides or high-cost pure enzyme. It is therefore necessary to produce the minor ginsenoside C-Y, C-Mc, F2, and C-K from PPD ginsenoside using the crude enzyme of low cost.
In this paper, a special ginsenosidase type-I from Aspergillus niger g.848 strain, the enzyme reaction pathway was different from ginsenosidase type-I from A. niger g.48 strain [24], was purified and characterized; and the enzyme reaction kinetics of the ginsenosides Rb1, Rb2, Rc, and Rd were studied. In the enzyme purification, the pure enzyme yield was very low, losing >95% enzyme [23–26]. Therefore, to obtain minor ginsenosides at a low cost, the production of minor ginsenosides such as C-Mc, C-Y, F2, and C-K was studied from the American ginseng PPD-ginsenoside containing Rb1, Rb2, Rc, and Rd using the non–gene-cloning crude enzyme from A. niger g.848 strain; and the product mixture of minor ginsenosides C-Mc, C-Y, F2, and C-K was separated to monomers with a silica-gel column.
2. Materials and methods
2.1. Materials
The standard ginsenosides Rb1, Rb2, Rc, Rd, F2, C-K, C-Mc, C-Mc1, C-Y, C-O were obtained from Dalian Green Bio Co Ltd, China. PPD-type ginsenoside of American ginseng was purchased from Tianle Ltd, Shenyang, China; the weight content ratio of Rb1, Rb2, Rc, and Rd in PPD-ginsenosides was 39.7% for Rb1, 3.96% for Rb2, 21.3% for Rc, and 35.0% for Rd (i.e., the molar content in 100 g of PPD-ginsenoside was 35.8 mmol for Rb1, 3.67 mmol for Rb2, 19.7 mmol for Rc, and 37.0 mmol for Rd). The A. niger g.848 strain obtained from Culture Collection of Biotechnology Engineering of Dalian Polytechnic University (Dalian, Liaoning Province, P.R. China) was isolated from traditional Chinese koji (Daqu in Chinese) [23,25]. Plates of Silica gel (60-F254; Merck, Darmstadt, Germany) were used for thin-layer chromatography (TLC) analysis. High performance liquid chromatography (HPLC) grade acetonitrile was obtained from Merck. The other chemicals used in this study were a minimum of analytical reagent grade. Standard proteins: phosphorylase b (97.2 kDa), serum albumin (66.4 kDa), ovalbumin (44.3 kDa), carbonic anhydrase (29.0 kDa), trypsin inhibitor (20.1 kDa), and lysozyme (14.3 kDa) were purchased from Takara Bio Inc, (Japan).
2.2. Enzyme production, purification, and molecular weight
The A. niger g.848 strain was cultured in the medium (200 mL in 1000 mL Erlenmeyer flask) containing 1% ginseng extract and 5% wheat bran extraction at 30°C for 5–6 days. After removing the cells by centrifugation, the culture was treated with (NH4)2SO4 40% saturation at 4°C for 8 h to remove the protein precipitate by centrifuging (RCF=22470* g); then (NH4)2SO4 powder was added to 70% saturation and stored at 4°C overnight to collect the protein precipitate by centrifuging; and dialyzed against 0.01M and pH 5.0 acetate buffer, diluted to 1/10 volume of culture with 0.02M and pH 5.0 acetate buffer. Nondissolved material was removed by centrifuging to obtain crude enzyme solution.
Enzyme purification was carried out by anion exchange chromatography and performed using WH-500 USB Protein Chromatographic Working Station (HDL UV detector, and BSZ-160 fraction collector; Shanghai Kingdom Biochemical Instrument Co. Ltd., China). A 10-mL sample of the crude enzyme solution was eluted on a DEAE-cellulose DE-52 column (φ 2.0 cm × 10 cm, Whatman), and the proteins were fractionated stepwise with 0.06M, 0.12M, 0.18M, 0.24M, 0.3M, 0.40M, 0.50M, and 0.60 M KCl in 0.02M acetate buffer (pH 5.0; fraction, 3.0 mL/tube), and the fractions were examined for enzyme activity hydrolyzing ginsenoside Rb1. The fractions hydrolyzing ginsenoside Rb1 were pooled and freeze-dried, and dissolved in 1/10 (w/v) distilled water. Then, further purification of the ginsenosidase was carried out using vertical slab polyacrylamide gel electrophoresis (PAGE) [27]. After electrophoresis, the enzyme band of the gel was cut, and dissolved in acetate buffer to remove nondissolved material by centrifugation to obtain purified enzyme solution. The purity of enzyme protein was also examined by the method of HPLC using a TOSOH TSK-Gel-2000 SW chromatographic column, and by the method of sodium dodecyl sulfate (SDS)-PAGE. The purified enzyme solution was used to evaluate the molecular weight and enzyme kinetic parameters.
The molecular weight of the enzyme was determined with SDS-PAGE [28], using 5% (w/v) stacking polyacrylamide gel and 12% (w/v) separating gel. The calibration curve was done using standard proteins: lysozyme (14.3 kDa), trypsin inhibitor (20.1 kDa), carbonic anhydrase (29.0 kDa), ovalbumin (44.3 kDa), serum albumin (66.4 kDa), and phosphorylase b (97.2 kDa). Protein bands were visualized with Coomassie brilliant blue R-250. The enzyme protein concentration with the Folin phenol reagent [29].
2.3. Enzyme analysis and kinetics
A 0.2 mL sample of enzyme from A. niger g.848 strain was mixed with the same volume of 25mM Rb1, 25mM Rb2, 25mM Rc, 2.5mM Rd, and PPD-type ginsenosides (substrate) in 0.02M acetate buffer (pH 5.0) and allowed to react with shaking at 45°C for 3 h (Rb1, Rb2, and Rc) or 0.5 h (Rd). Thereafter, 0.4 mL of water-saturated n-butanol was added to the reaction mixture to stop the enzyme reaction. The reaction product in the n-butanol layer was analyzed by TLC and HPLC. A 20-mL sample of crude enzyme was mixed with the same volume of 6% of PPD-ginsenosides from American ginseng in 0.02M acetate buffer (pH 5.0; final concentration of PPD-ginsenoside, 3%) and allowed to react with shaking at 45°C for 12 h, 18 h, 24 h, or 30 h. Then the 0.2 mL of reaction mixture was extracted with 0.4 mL of water-saturated n-butanol, and analyzed by TLC and HPLC. The spots on the silica plate were scanned using a Shimadzu CS-930 spectrophotometer (Shimadzu Corp., Kyoto, Japan).
One unit of enzyme activity was defined as the amount of enzyme that hydrolyzed 1mM of the Rb1 substrate/h in the optimal enzyme reaction condition [24,26].
In determination of enzyme kinetics: the values of the Michaelis–Menten equation constant (Km) and the maximal reaction velocity (Vmax) for ginsenosidase type-I were determined by incubating in 0.02M acetate buffer (pH 5.0) at 45°C with ginsenoside Rb1, Rb2, and Rc at concentrations of 14.3mM, 16.7mM, 20.0mM, 25.0mM, 33.0mM, and 50.0mM (final concentration in reaction: 7.15mM, 8.35mM, 10mM, 12.5mM, 16.5mM, and 25mM, respectively), reacting for 5 min, 10 min, 20 min, 40 min, 60 min, 90 min, 120 min, and 180 min; with Rd at 0.83mM, 1.00mM, 1.25mM, 1.67mM, 2.50mM, and 5.00mM (final concentration: 0.42mM, 0.50mM, 0.63mM, 0.84mM, 1.25mM, and 2.5mM), reacting for 5 min, 10 min, 20 min, 40 min, 60 min, 90 min, 120 min, and 180 min. The reaction results were determined by TLC. The conversion of TLC was obtained using Bandscan software (Glyko Inc., 1998) to analyze the area and shade of the plots on the TLC silica gel [26]. Values for Km and Vmax were calculated from Lineweaver–Burk plots [30]. The transformation velocity of the hydrolysis on the PPD type ginsenosides was calculated from the Michaelis–Menten equation [28].
2.4. TLC and HPLC analysis
TLC was carried out using a silica gel G 60 F254 plate (Merck) with developing solvent consisting of chloroform, methanol, and water [7:2.5:0.5 (v/v/v)] (under layer), and the produced ginsenosides on the silica gel plate were determined by scanning the TLC spots using a Shimadzu CS-930 [22,26].
The ginsenoside products from the enzyme reaction and enzyme protein purity were both examined by HPLC (Waters 2695 Separations Module with Waters 2996 Photodiode Array Detector, Waters Corp., Milford, USA.). A Knauer C-18 chromatographic column (5 μm, φ 3 mm × 300 mm, Knauer Corp., Berlin, Germany) was used to analyze the enzymatic reaction products. The mobile phase was A (acetonitrile) and B (water): 0–20 min, 20% A; 20–31 min, A from 20% to 32%; 31–40 min, A from 32% to 43%; and 40–70 min, A from 43% to 100%. The detection wavelength, 203 nm; injected volume, 10 μL; flow rate, 0.6 mL/min; and column temperature, 35°C. All reagent used in HPLC were Chromatographic grade, and produced from Merck, USA.
The sample used for the HPLC was prepared as follows: 1–2 mL enzymatic reaction mixture was eluted on 10 mL column of AB-8 Diaion resin column (from Tianjin Chemical Plant of Nankai University, P. R. China). The resin column was first washed with 80 mL of a 0.2M phosphate buffer (pH 6.0) and 50 mL of 20% alcohol, and then eluted with 60 mL of 83% alcohol to separate and collect the reaction products. These products were dried by vacuum distillation, and dissolved in 1 mL of methanol prior to the HPLC analysis.
A TOSOH TSK-Gel-2000 SW chromatographic column (φ 7.8 mm × 300 mm, TOSOH ASA Corp., Yamaguchi, Japan) was used in the determination of the enzyme purity. The mobile phase was 0.02M pH 6.7 phosphate buffer containing 0.05% sodium azide; measuring wavelength was 280 nm; the injected volume was 100 μL; and flow rate, 1.0 mL/min.
2.5. Preparation of minor ginsenoside C-Mc, C-Y, F2, and C-K from American ginseng PPD ginsenosides using crude enzyme
The effects of PPD-ginsenoside substrate concentration, pH and temperature on crude enzyme reaction were examined with the ginsenoside Rb1 instead of PPD ginsenoisde, and the Rb1 decrement was determined. The substrate concentration (Rb1) was fixed to 2%, 4%, 6%, 8%, and 10%; the pH was fixed to 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, and 10.0; the temperature was fixed to 20°C, 25°C, 30°C, 35°C, 40°C, 45°C, 50°C, 55°C, 60°C, 65°C, and 70°C in the 3% substrate in reaction.
In the preparation of minor ginsenoside C-Mc, C-Y, F2, and C-K from American ginseng PPD ginsenosides, the substrate of 6% PPD ginsenoside in 0.02M and pH 5.0 acetate buffer was reacted with the same volume of crude enzyme from A. niger g.848 strain in the Bioreactor (RAT-5D, Shanghai Shenshun Ltd, China). The reaction was stopped by addition of 95% alcohol (3× volume of reaction solution), centrifuged, and the supernatant was concentrated by vacuum distillation to 10% of the original volume to obtain the reaction products solution containing C-Mc, C-Y, F2, and C-K. The reaction mixture was eluted on the AB-8 macroporous resin column (the volume was 20× dried ginsenoside weight) to absorb ginsenoside, the remove impurities of sugar with water, the volume of which was 5× that of the column; then the column was eluted with 4–5× the column volume of 80–84% alcohol; the alcohol solution was eluted on the anion exchange resin D280 (volume was same with AB-8 column) for discoloration; the discolored solution was concentrated and dried by vacuum distillation to get the product containing minor ginsenoside C-Mc, C-Y, F2, and C-K.
The enzyme reaction products were purified using a silica gel column. The products from the enzyme reaction for the purification were firstly pretreated by dissolving in methanol and chloroform, and mixed with the 80–100 mesh silica gels, which was 2.3× the sample weight; then the mixture was dried in a constant temperature bath and stirred constantly to form powder; and the mixture powder was put in a column (diameter: height = 1:15–20) containing 20× of sample weight for the 300–400 mesh sample–silica-gel; 2 cm cotton was put on top of the column. The column was firstly dredged by 100% chloroform, and then eluted with a solvent consisting of chloroform and methanol [9.5:0.5 (v/v)], the fractions were 80–300 mL. According to TLC of the fraction, the products with the same component were then collected and dried by vacuum distillation.
2.6. NMR analysis
The structures of enzymolysis products C-Mc, C-Y, C-K, and F2 from PPD-type ginsenosides were analyzed using NMR. The products were dissolved in pyridine-d5, and the NMR spectra were recorded by using the Bruke Avance 600 (1H: 600 MHz; 13C: 150 MHz) NMR spectrometer (Switzerland).
3. Results and discussion
3.1. Enzyme fermentation, purification and characterization
The A. niger g.848 strain was cultured in medium (200 mL in 1000 mL Erlenmeyer flask) containing 1% ginseng extract and 5% wheat bran extract; the best enzyme production was obtained with 30°C culturing for 120 h. The cell-free culture from A. niger g.848 strain was removed the precipitate by (NH4)2SO4 (40% saturation); and when (NH4)2SO4 concentration reached 70% saturation, most ginsenosidase type-I was precipitated. Then, the protein precipitate collected by centrifuging was dissolved and dialyzed at to obtain 1/10 volume of the culture, i.e., crude enzyme. The 10 mL of crude enzyme was eluted on a DEAE-Cellulose DE-52, and then fractionated stepwise with respectively 50 mL of 0.06M, 0.12M, 0.18M, 0.24M, 0.30M, 0.40M, 0.50M, and 0.60M KCl in 0.02M and pH 5.0 acetic buffer. The enzyme activities of the fractions were examined: the 32–36 fractions eluted by 0.12M and 0.18M KCl solution hydrolyzed the 20mM ginsenoside Rb1; and the 34 and 35 fractions enzyme activity exhibited the highest enzyme activity of hydrolyzing ginsenoside Rb1 and almost single band in the PAGE.
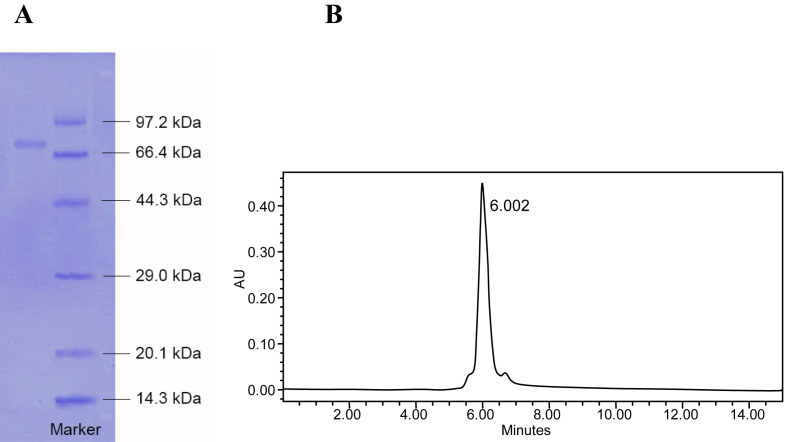
In order to be prudent, vertical slab SDS-PAGE was used for further purification of special ginsenosidase type-I. The result of SDS-PAGE examination is shown in Fig. 1A: the enzyme represents a single band on the gel. By calculating the mobility, the molecular weight (MW) of the ginsenosidase type-I was approximately 75 kDa, which was similar with the MW 80 kDa of ginsenosidase type-I from Aspergillus sp.g48p strain [23], and the MW 74 kDa of ginsenosidase type-I from A. niger g.48 strain [24]. The purity of the purified ginsenosidase type-I was examined by HPLC (Fig. 1B). Only one peak was shown on the HPLC spectrum at 6.002 min. All these results indicated that the ginsenosidase type-I was pure enzyme, and it was further used to evaluate the enzyme reaction velocity and kinetic parameters hydrolyzing the different glycosides of the PPD type ginsenosides.
Fig. 1.
Purified ginsenosidase Type-I in SDS-PAGE and HPLC. (A) Ginsenosidase Type-I SDS-PAGE; marker, marker protein: phosphorylase b (97.2 kDa), serum albumin (66.4 kDa), ovalbumin (44.3 kDa), carbonic anhydrase (29.0 kDa), trypsin inhibitor (20.1 kDa), and lysozyme (14.3 kDa). Protein quantity, 3 μg. (B) Ginsenosidase Type-I HPLC. HPLC, high performance liquid chromatography; PAGE, polyacrylamide gel electrophoresis; SDS, sodium dodecyl sulfate.
The optimum temperature of ginsenosidase type-I from A. niger g.848 strain was 45°C, and the optimum pH was 5.0 (data not shown). In the purification, the yield of the ginsenosidase was about 3.1%, and the specific activity of the enzyme increased 13 times (data not shown).
3.2. Pure enzyme hydrolysis of the monomer ginsenosides Rb1, Rb2, Rc, and Rd
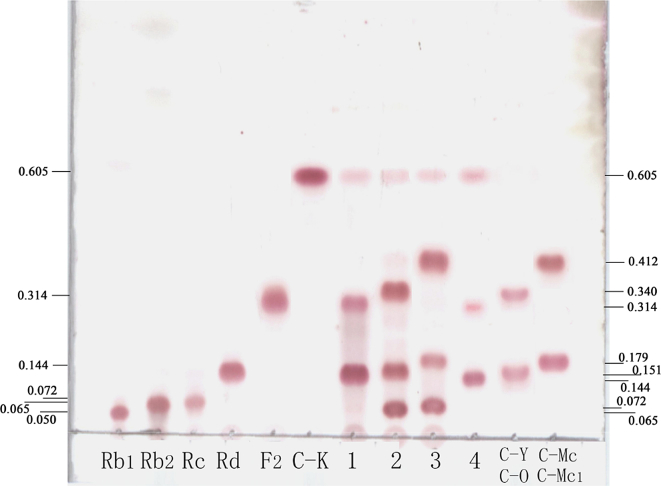
The enzyme from the A. niger g.848 strain reacted with 25mM monomer ginsenoside Rb1, Rb2 and Rc at 45°C for 3 h, respectively; and reacted with 2.5mM Rd at 45°C for 0.5 h. The enzyme reaction products were examined by TLC (Fig. 2).
Fig. 2.
The hydrolysis of the pure enzyme from the Aspergillus niger g.848 strain on the monoginsenoside Rb1, Rb2, Rc, and Rd. Rb1, Rb2, Rc, Rd, F2, C-K, C-Y, and C-O, C-Mc, and C-Mc1, standard ginsenosides. 1–3, enzyme reaction product from 25mM Rb1, 25mM Rb2, 25mM Rc reacted at 45°C for 3 h; 4, enzyme reaction product from 2.5mM Rd. Solvent, chloroform:methanol:water = 7.5:2.5:0.5; 10% H2SO4 as a chromogenic agent.
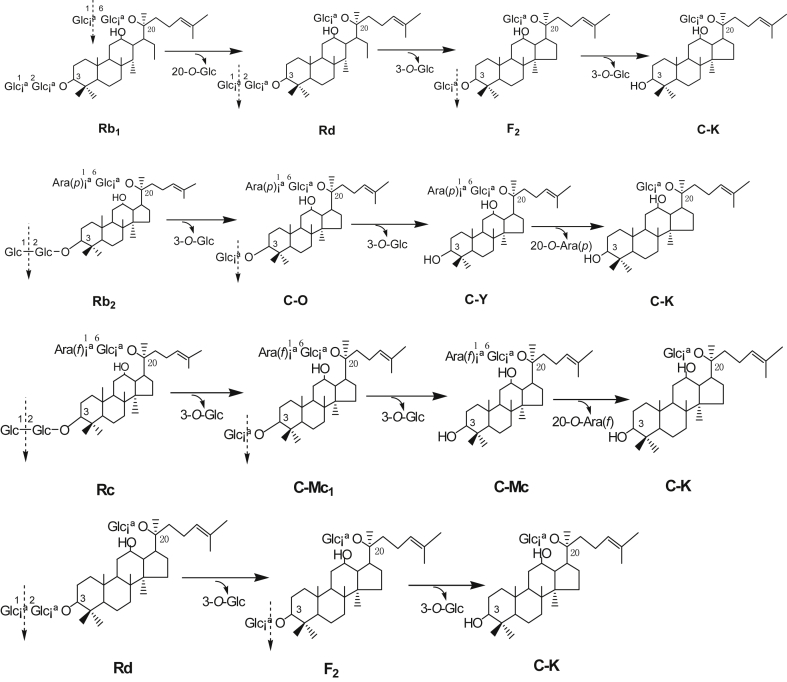
As shown in Fig. 2, the ginsenosidase produced by A. niger g.848 strain firstly hydrolyzed the 20-O-β-D-(1→6)-glucopyranoside of Rb1 into Rd, then hydrolyzed the 3-O-β-D-(1→2)-glucopyranoside of Rd to F2, further to C-K. However, the enzyme firstly hydrolyzed the 3-O-β-D-(1→2)-glucopyranoside of Rb2 into C-O, hydrolyzed 3-O-β-D-glucopyranoside of C-O into C-Y, further hydrolyzed the 20-O-α-L-(1→6)-arabinopyranoside (arap) of C-Y into C-K; the enzyme also firstly hydrolyzed the 3-O-β-D-(1→2)-glucopyranoside of Rc to C-Mc1, hydrolyzed the 3-O-β-D-glucopyranoside of C-Mc1 into C-Mc, and further hydrolyzed 20-O-α-L-(1→6)-arabinofuranoside (araf) of C-Mc into C-K. The biotransformation pathway of the ginsenosidase produced by A. niger g.848 strain is shown in Fig. 3.
Fig. 3.
Biotransformation pathway of ginsenosides Rb1, Rb2, Rc, and Rd by culture of Aspergillus niger g.848 strain.
Therefore, the enzyme from A. niger g.848 strain can hydrolyze the 3-C position (3-O-) and 20-C-position (20-O-) multiglycoside of PPD-type ginsenosides such as Rb1, Rb2, Rc, and Rd, and should be classified to ginsenosidase type-I produced by Aspergillus sp.48 strain [23] and A. niger g.48 strain [24]. However, the hydrolysis pathway of the special ginsenosidase type-I from A. niger g.848 strain in present study is different with that of ginsenosidase type-I from A. niger g.48 strain; the ginsenosidase type-I (molecular weight, 75 kDa) hydrolysis pathway from A. niger g.848 strain on ginsenoside Rb1 (in this study) is Rb1→Rd→F2→C-K; but the ginsenosidase type-I (molecular weight, 74 kDa) from A. niger g.48 strain hydrolyzes both 3-O- and 20-O-glucosides of Rb1 with two pathways: i.e., one pathway was Rb1→Rd→F2→C-K; another, Rb1→Gyp17→Gyp75→C-K [24]. Thus, the enzyme A. niger g.848 strain is a special ginsenosidase type-I differentiating with the ginsenosidase type-I from A. niger g.48 strain. The ginsenosidase type-I from A. niger g.848 strain is are suitable than that of A. niger g.48 strain, because the ginsenoside Rb1 is hydrolyzed by ginsenosidase type-I from A. niger g.848 strain with one pathway, and the enzyme from A. niger g.48 strain hydrolyzes Rb1 with two pathways [24].
3.3. Enzyme reaction kinetics
To determine the enzyme kinetic parameters for Rb1, the enzyme was reacted with Rb1 at different substrate concentrations (7.15mM, 8.35mM, 10.0mM, 12.5mM, 16.5mM, 25.0 mM) for 5 min, 10 min, 20 min, 40 min, 60 min, 90 min, 120 min, and 180 min at 45°C. The Bandscan software method [26] was used to determine the conversion rate of Rb1 (the substrate) from the TLC reaction results (the TLC is omitted). The Rb1 conversion or reduction can be identified as the amount of production of Rd and F2.
To examine the more true velocity data, the enzyme reaction velocity (V) was calculated from the amount of conversion of the substrate Rb1 using average data of triplicate measurements in the initial reaction time of 5–10 min; the average velocity (V) values of ginsenosidase type I in the hydrolysis for the concentrations 7.15mM, 8.35mM, 10mM, 12.5mM, 16.5mM, and 25mM of ginsenoside Rb1 to Rd were 25.3mM/h, 26.2mM/h, 30.0mM/h, 32,6mM/h, 40.9mM/h, and 49.7mM/h respectively. Using the Lineweaver–Burk plot of 1/V vs 1/[S] [30], the kinetic parameters of the ginsenosidase type I hydrolysis of the 20-O-Glc of ginsenoside Rb1→Rd in Michaelis–Menten equation were Km = 16.6 ± 1.6mM and Vmax = 79.6 ± 7.5mM/h. Using the same method as Rb1, the kinetic parameters of ginsenosidase type I for the ginsenosides Rb2 and Rc were estimated at the substrate concentrations of 7.15mM, 8.35mM, 10mM, 12.5mM, 16.5mM, and 25mM, the enzyme kinetic parameters for the hydrolysis of 3-O-Glc of ginsenosides Rb2 were Km = 20.4 ± 2.1mM and Vmax = 45.6 ± 4.6mM/h; the enzyme kinetic parameters for the hydrolysis of 3-O-Glc of ginsenosides Rc were Km = 5.46 ± 0.5mM and Vmax = 6.16 ± 0.6mM/h; the kinetic parameters for hydrolysis of 3-O-Glc of Rd at the substrate concentrations of 0.42mM, 0.50mM, 0.63mM, 0.84mM, 1.25mM, and 2.5mM were Km = 0.603 ± 0.04mM and Vmax = 1.19 ± 0.11mM/h. The above results used average data of triplicate measurements in the initial reaction time of 5–10 min (Table 1).
Table 1.
Kinetic parameters of ginsenosidase type-I
| Substrate | Pathway | Km (mM) | Vmax (mM/h) | V01) (mM/h) |
|---|---|---|---|---|
| Rb1 | Hydrolysis of 20-O-Glc of Rb1 to Rd | 16.6 ± 1.6 | 79.6 ± 7.5 | 29.9 |
| Rb2 | Hydrolysis of 3-O-Glc of Rb2 to C-O | 20.4 ± 2.1 | 45.6 ± 4.6 | 15.0 |
| Rc | Hydrolysis of 3-O-Glc of Rc to C-Mc1 | 5.46 ± 0.5 | 6.16 ± 0.6 | 3.98 |
| Rd | Hydrolysis of 3-O-Glc of Rd to F2 | 0.603 ± 0.04 | 1.19 ± 0.11 | 1.12 |
Velocities (V0), in 10mM substrate; using average data of triplicate measurements in the initial reaction time of 5–10 min.
The larger the Km value, the slower the hydrolysis velocity, and the larger the Vmax value, the faster the hydrolysis velocity. The above Km and Vmax values of the enzyme reaction (Table 1) cannot fully represent the enzyme reaction velocities. Therefore, using the Michaelis–Menten equation [28], the enzyme reaction velocities for the different ginsenosides (10mM) were calculated. The enzyme velocities were 29.9mM/h for Rb1, 15.0mM/h for Rb2, 3.98mM/h for Rc, and 1.12mM/h for Rd. According to V0 values in 10mM substrate (Table 1), the ginsenosidase type-I reaction velocities were 20-O-Glc of Rb1 > 3-O-Glc of Rb2 > 3-O-Glc of Rc > 3-O-Glc of Rd.
According to the above results, the minor ginsenosides C-Y, C-Mc, F2, and C-K, which have low sugar-moieties can be prepared from the monomer ginsenosides Rb2, Rc, Rb1, and Rd by controlling enzyme reaction conditions such as substrate concentration and reaction time.
However, the pure ginsenosidase type-I yield was only about 3.1% to lose over 95% enzyme in the enzyme purification [23,24,26], and the cost of monomer ginsenoside Rb1, Rb2, Rc, and Rd was higher. Therefore, it is necessary to produce the minor ginsenoside C-Y, C-Mc, F2, and C-K from PPD ginsenoside using the low-cost crude enzyme.
3.4. Crude enzyme hydrolysis on PPD-ginsenoside containing Rb1, Rb2, Rc, and Rd
To obtain the minor ginsenosides C-Y, C-Mc, F2, and C-K at low cost, the PPD-ginsenosides containing Rb1, Rb2, Rc, and Rd from American ginseng were reacted with the non–gene-cloning crude enzyme from A. niger g.848 strain; the non–gene-cloning enzyme using for ginseng products is more welcome for consumers.
When the crude enzyme from the A. niger g.848 strain was reacted with monomer ginsenoside Rb1, Rb2, Rc, and Rd, the results are similar to that of pure enzyme. When the crude enzyme was reacted the mixture of PPD-ginsenosides containing Rb1, Rb2, Rc, and Rd (weight content ratio was about 39.7% for Rb1, 3.96% for Rb2, 21.3% for Rc, and 35.0% for Rd; i.e., the molar content in 100 g of PPD-ginsenoside was 35.8 mmol for Rb1, 3.67 mmol for Rb2, 19.7 mmol for Rc, and 37.0 mmol for Rd); the main products were minor ginsenoside C-Y, C-Mc, F2, and C-K. In optimizing the crude enzyme reaction for the PPD ginsenoside, the effects of PPD-ginsenoside substrate concentration, pH, and temperature on crude enzyme reaction were examined with ginsenoside Rb1 instead of PPD-ginsenoside; good enzyme reaction was observed in the 3% of PPD-ginsenoside at 45°C and pH 5.0.
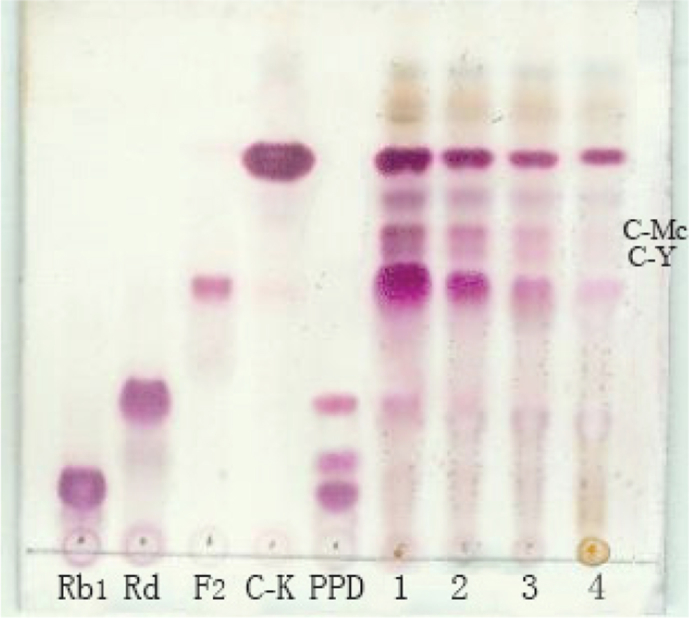
To obtain good enzyme reaction time, the 20 mL 6% PPD-ginsenoside was reacted with the same volume of the crude enzyme solution from A. niger g.848 strain (final 3% PPD-ginsenoside) at 45°C and pH 5.0 reacted for 12 h, 18 h, 24 h, and 30 h; the reaction products are shown in Fig. 4.
Fig. 4.
Crude enzyme hydrolysis on the PPD ginsenoside for different reaction time. Rb1, Rd, F2, C-K, standard ginsenosides. PPD, substrate of PPD-ginsenoside. C-Mc and C-Y were recognized with the standard C-Mc and C-Y. The 6% of PPD-ginsenoside with the same volume of enzyme (final 3% substrate of PPD-ginsenoside) was reacted at 45°C for 12 h, 18 h, 24 h, and 30 h; Sample 1 reacted for 12 h; Sample 2 reacted for 18 h; Sample 3 reacted for 24 h; and Sample 4 reacted for 30 h. Solvent, chloroform:methanol:water = 7.5:2.5:0.5 (v/v/v); 10% H2SO4 as a chromogenic agent.
Fig. 4 shows that, when the crude enzyme reacted with 6% PPD-ginsenoside (actual substrate concentration was 3%) at 45°C and pH 5.0 for 12 h, the transformation of ginsenoside Rd in PPD was not complete; when reacted for 18 h, the main enzyme reaction products were C-K, C-Mc, C-Y, and F2, and the substrate PPD was almost completely converted; when reacted over 24 h, the main enzyme reaction product was C-K. Thus, the good enzyme reaction time to obtain minor ginsenoside C-K, C-Mc, C-Y, and F2 was 18 h.
Therefore, when producing the minor ginsenoside C-K, C-Mc, C-Y, and F2 from PPD-ginsenoside of American ginseng, the good reaction conditions were as the enzyme was reacted at 45°C and pH 5.0 for 18 h in 3% substrate concentration of PPD-ginsenoside.
3.5. Preparation and separation of minor ginsenosides C-Mc, C-Y, F2, and C-K from PPD-ginsenosides
The mixture of minor ginsenosides C-Mc, C-Y, F2, and C-K was produced from the PPD type ginsenoside using the crude enzyme from A. niger g.848 strain. Under the above optimal reaction condition, the 80 g PPD ginsenosides were dissolved in 1300 mL of pH 5.0, 0.02M acetate buffer, and mixed with same volume of crude enzyme, reacted at 45°C for 18 h (final substrate concentration of PPD-ginsenoside in reaction solution was 3%); then the 3× volume of 95% alcohol was added to the reaction mixture to stop the enzyme reaction, stored overnight to precipitate enzyme protein, centrifuged to get supernatant, the supernatant was vacuum concentrated to 10% times of its original volume; then the product mixture was adsorbed on 1.5 L AB-8 resin column, eluted by water (5× volume of the column) to remove soluble impurity such as sugar; then the column was eluted with 5× volume 70–84% alcohol to elute minor ginsenoside from reaction; the eluted solution was discolored by 1.5 L column of D-280 resin, vacuum concentrated, dried to get 50 g of minor ginsenoside mixture; the weight yield was about 62%. With the same method, the 150 g mixture of minor ginsenosides was obtained for using in the next experiments.
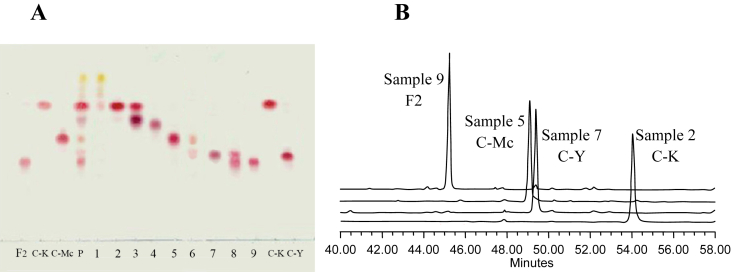
The 5 g of mixture product of minor ginsenosides (equivalent to product from 8 g of PPD-ginsenoside), was separated using a silica-gel column (φ 25 mm × 400 mm). The column was eluted with the solution mixing with the solvent consisting of chloroform and methanol [95:0.5 (v/v)], the fractions were 80 mL. The fraction ginsenosides were checked by TLC; the fractions of same ginsenoside were concentrated by vacuum, dried to get single ginsenosides such as F2, C-Mc, C-Y, and C-K as shown in Table 2 and Fig. 5A.
Table 2.
Separation of 5 g mixture of minor ginsenosides obtaining from 8 g PPD-ginsenoside of American ginseng with a silica gel column φ 25 mm × 400 mm. fractions, 80 mL
| Dried sample No. | Fractions | Purified ginsenosides | Weight yield (g) | Molar yield (%) |
|---|---|---|---|---|
| 1 | 1–2 | Impurity | 0.03 | – |
| 2 | 3–4 | C-K | 1.65 | 69.5 on C-K and F2 |
| 3 | 5 | C-K mixture | 0.31 | – |
| 4 | 6 | Single sport, but impurity | 0.12 | – |
| 5 | 7 | C-Mc | 0.50 | 43.7 |
| 6 | 8 | Mixture of C-Mc and C-Y | 0. 15 | – |
| 7 | 9 | C-Y | 0.09 | 42.4 |
| 8 | 10 | Mixture of C-Y and F2 | 0. 12 | – |
| 9 | 11–21 | F2 | 1.60 | 69.5 on C-K and F2 |
| 10 | 22–30 | Impurity | 0.42 | – |
–, not determined.
Fig. 5.
The separated minor ginsenoside products of Table I with the silica gel column in the TLC and HPLC. (A) TLC: F2, C-K, C-Mc, C-Y, standard ginsenosides. P, minor ginsenoside product from PPD substrate by the enzyme. Sample 1, product from fractions 1 and 2; Sample 2, C-K from fractions 3–4; Sample 3, from fraction 5; Sample 4, from fraction 6; Sample 5, C-Mc from fraction 7; Sample 6, from fraction 8; Sample 7, C-Y from fraction 9; Sample 8, from fraction 10; and Sample 9, F2 from fractions 10–20. Solvent, chloroform:methanol:water = 7.5:2.5:0.5; 10% H2SO4 as a chromogenic agent. (B) HPLC: The pure Samples 9, 7, 5, and 2 of minor ginsenosides from enzyme reaction in HPLC. Retention time: 45.228 min for Sample 9 (F2); 49.092 min for Sample 5 (C-Mc); 59.395 min for Sample 7 (C-Y); 54.079 min for Sample 2 (C-K). HPLC, high performance liquid chromatography.
Table 2 and Fig. 5A show that the separated pure Sample 2 is 1.65 g of C-K; Sample 5 is 0.50 g of C-Mc; Sample 7 is 0.09 g of C-Y; and Sample 9 is 1.60 g of F2 from 5 g product produced from 8 g PPD-ginsenoside by enzyme reaction. The purity of minor ginsenosides is 95% for C-K, 94% for C-Mc, 90% for C-Y, and 90% for F2 (Fig. 5B).
The 50 ± 2.0 g mixture of minor ginsenoside F2, C-Mc, C-Y, and C-K were produced from 80 g PPD-ginenoside from American ginseng, and was separated using the silica-gel column to obtain the monomer ginsenoside about 5.20 ± 0.50 g of C-Mc, 0.96 ± 0.17 g of C-Y, 16.3 ± 1.5 g of F2, and 16.9 ± 1.3 g of C-K (the result is the average data of 3 experiments). The minor ginsenoside C-Mc was only produced from Rc, so the theoretical yield (molar yield) of C-Mc was about 43.7%. C-Y was only produced from Rb2, so the molar yield of C-Y was about 42.4%. However, the minor ginsenoside F2 can be produced from ginsenoside Rb1 and Rd; the C-K can be produced from the ginsenoside Rb1, Rd, Rb2, and Rc; therefore, to obtain the calculation of theoretical yield of F2 and C-K, all the ginsenoside Rb1, Rd, Rb2, and Rc in the substrate must be considered. The molar yield of minor ginsenosides was approximately 43.7% for C-Mc from Rc, 42.4% for C-Y from Rb2, and rough estimate 69.5% for F2 and C-K from Rb1 and Rd (ignoring C-K from the Rb2 and Rc).
In the above experiment, four kinds of monomer ginsenoside were successfully produced from the low-cost PPD-ginsenosides containing ginsenoside Rb1, Rb2, Rc, and Rd using the low-cost crude enzyme from A. niger g.848 strain.
3.6. Structure of the reaction product C-Mc, C-Y, F2, and C-K by NMR
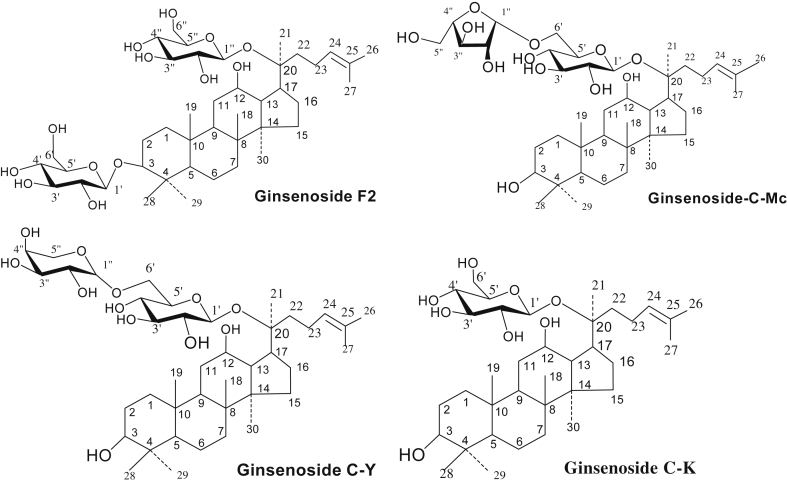
NMR was used to determine the structure of the enzyme reaction products. The 13C NMR (600 MHz, pyridine-d5) spectral data of minor ginsenoside products are shown in Table 3. These data correspond with previous report [31], and the enzyme reaction products were determined to be minor ginsenosides F2, C-Mc, C-Y, and C-K, as shown in Table 3.
Table 3.
The 13C NMR spectroscopic data of enzyme reaction products F2, C-Mc, C-Y, and C-K1)
| Carbon site | F2 | C-Mc | C-Y | C-K | Carbon site | F2 | C-Mc | C-Y | C-K |
|---|---|---|---|---|---|---|---|---|---|
| Aglycone moiety | 3-O-Glc | ||||||||
| C-1 | 23.39 | 39.60 | 39.75 | 39.72 | C-1′ | 107.10 | – | – | – |
| C-2 | 26.93 | 28.41 | 28.40 | 28.41 | C-2′ | 75.93 | – | – | – |
| C-3 | 88.98 | 79.40 | 79.45 | 79.43 | C-3′ | 79.43 | – | – | – |
| C-4 | 39.37 | 39.75 | 39.61 | 39.58 | C-4′ | 71.80 | – | – | – |
| C-5 | 56.56 | 56.57 | 56.58 | 56.53 | C-5′ | 78.50 | – | – | – |
| C-6 | 18.63 | 18.97 | 18.97 | 18.93 | C-6′ | 63.26 | – | – | – |
| C-7 | 35.30 | 35.37 | 35.38 | 35.34 | 20-O-Glc | ||||
| C-8 | 40.22 | 40..26 | 40.27 | 40.24 | C-1′ | 98.42 | 98.25 | 98.25 | 98.40 |
| C-9 | 50.37 | 50.49 | 50.52 | 50.47 | C-2′ | 75.28 | 75.19 | 75.03 | 75.27 |
| C-10 | 37.13 | 37.55 | 37.55 | 37.52 | C-3′ | 78.90 | 78.29 | 78.29 | 78.40 |
| C-11 | 31.06 | 31.00 | 31.04 | 31.10 | C-4′ | 72.05 | 72.28 | 71.25 | 71.80 |
| C-12 | 70.35 | 70.46 | 70.37 | 70.35 | C-5 | 78.43 | 76.69 | 77.04 | 78.22 |
| C-13 | 49.65 | 49.62 | 49.67 | 49.65 | C-6′ | 63.04 | 68.67 | 69.36 | 63.04 |
| C-14 | 51.60 | 51.62 | 51.61 | 51.59 | 20-Ara(p) | ||||
| C-15 | 30.95 | 60.94 | 30.97 | 30.95 | 1′′ | – | – | 105.95 | – |
| C-16 | 26.82 | 26.84 | 26.85 | 26.81 | 2″ | – | – | 71.68 | – |
| C-17 | 51.81 | 51.86 | 51.84 | 51.79 | 3″ | – | – | 74.28 | – |
| C-18 | 16.98 | 16.55 | 16.54 | 16.53 | 4″ | – | – | 68.76 | – |
| C-19 | 16.47 | 16.55 | 16.54 | 16.50 | 5″ | – | – | 67.13 | – |
| C-20 | 83.48 | 83.61 | 83.67 | 83.46 | 20-Ara(f) | ||||
| C-21 | 25.95 | 26.02 | 26.03 | 25.94 | 1″ | – | 110.28 | – | – |
| C-22 | 36.31 | 36.36 | 36.38 | 36.32 | 2″ | – | 83.47 | – | – |
| C-23 | 23.39 | 23.35 | 23.34 | 23.37 | 3″ | – | 79.01 | – | – |
| C-24 | 126.12 | 126.22 | 126.21 | 126.12 | 4″ | – | 86.19 | – | – |
| C-25 | 131.09 | 131.23 | 131.31 | 131.07 | 5″ | – | 62.84 | – | – |
| C-26 | 22.56 | 22.53 | 22.45 | 22.52 | |||||
| C-27 | 17.95 | 18.10 | 18.16 | 17.94 | |||||
| C-28 | 28.33 | 28.91 | 28.90 | 28.86 | |||||
| C-29 | 16.16 | 16.25 | 16.27 | 16.19 | |||||
| C-30 | 17.56 | 17.60 | 17.64 | 17.56 | |||||
In this work, assignments were based on 1H, 13C, DEPT, HSQC NMR experiments and compared with [31].
According to Table 3, the structures of minor ginsenosides should be F2, C-Mc, C-Y, and C-K as shown in Fig. 6.
Fig. 6.
The structure of minor ginsenosides F2, C-Mc, C-Y, and C-K.
In conclusion, four kinds of minor ginsenoside C-Mc, C-Y, F2, and C-K were produced from PPD-ginsenoside containing ginsenoside Rb1, Rb2, Rc, and Rd of American ginseng using a special ginsenosidase type-I from A. niger g.848 strain. The pure enzyme molecular weight is about 75 kDa, and firstly hydrolyzed the C-20 position 20-O-β-D-Glc of ginsenoside Rb1, then hydrolyzed C-3 position 3-O-β-D-Glc with the pathway Rb1→Rd→F2→C-K. However, the enzyme firstly hydrolyzed C-3 position 3-O-β-D-Glc of ginsenoside Rb2 and Rc, finally hydrolyzed 20-O-L-Ara with the pathway Rb2→C-O→C-Y→C-K and Rc→C-Mc1→C-Mc→C-K. The enzyme kinetic parameters were Km = 16.6 ± 1.6mM and Vmax = 79.6 ± 7.5mM/h for Rb1; Km = 20.4 ± 2.1mM and Vmax = 45.6 ± 4.6mM/h for Rb2; Km = 5.46 ± 0.5mM and Vmax = 6.16 ± 0.6mM/h for Rc; and Km = 0.603 ± 0.04mM and Vmax = 1.19 ± 0.11mM/h for Rd; reaction velocities on ginsenosides were Rb1 > Rb2 > Rc > Rd. However, the pure enzyme yield was only 3.1%, a loss of >95%, so crude enzyme was used for minor ginsenoside preparation. The crude enzyme hydrolysis pathways on Rb1, Rb2, Rc, and Rd were the same as that of pure enzyme. When the crude enzyme reacted in 3% American ginseng PPD-ginsenoside at 45°C and pH 5.0 for 18 h, the main products were minor ginsenosides C-Mc, C-Y, F2, and C-K. The 150 g mixture of minor ginsenoside producing from 240 g PPD ginsenoside was separated using the silica-gel column to obtain the monomer ginsenoside about 5.20 ± 0.50 g of C-Mc, 0.96 ± 0.17 g of C-Y, 16.3 ± 1.5 g of F2, and 16.9 ± 1.3 g of C-K. The theoretical yield was approximately 43.7% for C-Mc from Rc, 42.4% for C-Y from Rb2, and rough estimate 69.5% for F2 and C-K from Rb1 and Rd (ignoring C-K from the Rb2 and Rc).
Therefore, four monomer minor ginsenosides (C-Mc, C-Y, F2, and C-K) were successfully produced from the low-cost PPD-ginsenosides containing ginsenoside Rb1, Rb2, Rc, and Rd using the low-cost crude enzyme from A. niger g.848 strain. This is the first report on the production of four minor ginsenosides from American ginseng PPD-ginsenoside by enzyme.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This work was supported by Major Projects of China National Science and Technology Significant New Drugs Creation Project of China No. 2012ZX09503001-003; the Program for China Liaoning Innovative Research Teams in Universities (LNIRT: 2009T007, LT2010009).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jgr.2014.12.003.
Contributor Information
Hong-Shan Yu, Email: hongshan@dlpu.edu.cn.
Feng-Xie Jin, Email: fxjin@dlpu.edu.cn.
Supplementary data
The following is the supplementary data related to this article:
References
- 1.Jin F.X. Chemical Industry Press; Beijing: 2009. Biotransformation of natural products; pp. 74–113. [in Chinese] [Google Scholar]
- 2.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 3.Christensen L.P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 4.Liu C.Y., Song J.G., Li P.F., Yu H.S., Jin F.X. Ginsenoside contents in three different ginseng. J Dalian Polytechnic Univ. 2011;30:79–82. [Google Scholar]
- 5.Kobashi K. Glycosides are natural prodrugs—evidence using germ-free and gnotobiotic rats associated with a human intestinal bacterium. J Trad Med. 1998;15:1–13. [Google Scholar]
- 6.Bae E.A., Shin J.E., Kim D.H. Metabolism of ginsenoside Re by human intestinal microflora and its estrogenic effect. Bio Pharm Bull. 2005;28:1903–1908. doi: 10.1248/bpb.28.1903. [DOI] [PubMed] [Google Scholar]
- 7.Tawab M.A., Bahr U., Karas M., Wurglics M., Schubert-Zsilavecz M. Degradatin of ginsenosides in humans after administration. Drug Metab Dispos. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 8.Cui C.H., Kim S.C., Im W.T. Characterization of the ginsenoside transforming recombinant β-glucosidase from Actinosynnema mirum and bioconversion of major ginsenosides into minor ginsenosides. Appl Microbiol Biotech. 2013;97:649–659. doi: 10.1007/s00253-012-4324-5. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa H., Sung J.H., Matsumiya S., Uchiyama M. Main ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1996;62:453–457. doi: 10.1055/s-2006-957938. [DOI] [PubMed] [Google Scholar]
- 10.Park C.S., Yoo M.H., Noh K.H., Oh D.K. Biotransformation of ginsenosides by hydrolyzing sugar moieties of ginsenosides using microbial glycosidases. Appl Microbiol Biotechnol. 2010;87:9–19. doi: 10.1007/s00253-010-2567-6. [DOI] [PubMed] [Google Scholar]
- 11.Leung K.W., Wong A.S. Pharmacology of ginsenosides: a literature review. Chin Med. 2010;5:20–22. doi: 10.1186/1749-8546-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi S., Kim T.W., Singh S.V. Ginsenoside Rh2-mediated G1 phase cell cycle arrest in human breast cancer cells is caused by p15 Ink4B and p27 Kip1-dependent inhibition of cyclin-dependent kinases. Pharm Res. 2009;26:2280–2288. doi: 10.1007/s11095-009-9944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi S.H., Shin T.J., Hwang S.H., Lee B.H., Kang J., Kim H.J. Differential effects of ginsenoside metabolites on HERG k channel currents. J Ginseng Res. 2011;35:191–199. doi: 10.5142/jgr.2011.35.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y.J., Yamabe N., Choi P., Lee J.W., Ham J., Kang K.S. Efficient thermal deglycosylation of ginsenoside Rd and its contribution to the improved anticancer activity of ginseng. J Agric Food Chem. 2013;61:9185–9191. doi: 10.1021/jf402774d. [DOI] [PubMed] [Google Scholar]
- 15.Kim S.J., Kang B.Y., Cho S.Y., Sung D.S., Chang H.K., Yeom M.H., Kim D.H., Sim Y.C., Lee Y.S. Compound K induces expression of hyaluronan synthase 2 gene in transformed human keratinocytes and increases hyaluronan in hairless mouse skin. Biochem Biophys Res Commun. 2004;316:348–355. doi: 10.1016/j.bbrc.2004.02.046. [DOI] [PubMed] [Google Scholar]
- 16.Kim M., Ahn B.Y., Lee J.S., Chung S.S., Lim S., Park S.G., Jung H.S., Lee H.K., Park K.S. The ginsenoside Rg3 has a stimulatory effect on insulin signaling in L6 myotubes. Biochem Biophys Res Commun. 2009;389:70–73. doi: 10.1016/j.bbrc.2009.08.088. [DOI] [PubMed] [Google Scholar]
- 17.Lee J.H., Ahn J.Y., Shin T.J., Choi S.H., Lee B.H., Hwang S.H. Effects of minor ginsenosides, ginsenoside metabolites, and ginsenoside epimers on the growth of Caenorhabditis elegans. J Ginseng Res. 2011;35:375–383. doi: 10.5142/jgr.2011.35.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sala F., Mulet J., Choi S., Jung S.Y., Nah S.Y., Rhim H., Valor L.M., Criado M., Sala S. Effects of ginsenoside Rg2 on human neuronal nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2002;301:1052–1059. doi: 10.1124/jpet.301.3.1052. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa H. Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci. 2004;95:153–157. doi: 10.1254/jphs.fmj04001x4. [DOI] [PubMed] [Google Scholar]
- 20.Popovich D.G., Kitts D.D. Structure-function relationship exists for ginsenosides in reducing cell proliferation and inducing apoptosis in the human leukemia (THP-1) cell line. Arch Biochem Biophys. 2002;406:1–8. doi: 10.1016/s0003-9861(02)00398-3. [DOI] [PubMed] [Google Scholar]
- 21.Cui C.H., Liu Q.M., Kim J.K., Sung B.H., Kim S.G., Kim S.C., Im W.T. Identification and characterization of Mucilaginibacter sp. QM49 β-glucosidase and its use in producing the pharmaceutically active minor ginsenosides, Rh1(S) and Rg2(S) Appl Environ Microbiol. 2013;79:5788–5798. doi: 10.1128/AEM.01150-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin X.F., Yu H.S., Wang D.M., Liu T.Q., Liu C.Y., An D.S., Im W.T., Kim S.G., Jin F.X. Kinetics of a cloned special ginsenosidase hydrolyzing 3-O-glucoside of multi-protopanaxadiol-type ginsenosides, named ginsenosidase type III. J Microbiol Biotechnol. 2012;22:343–351. doi: 10.4014/jmb.1107.07066. [DOI] [PubMed] [Google Scholar]
- 23.Yu H.S., Zhang C.Z., Lu M.C., Sun F., Fu Y.Y., Jin F.X. Purification and characterization of new special ginsenosidase hydrolyzing multi-glycisides of protopanaxadiol ginsenosides, ginsenosidase type I. Chem Pharm Bull. 2007;55:231–235. doi: 10.1248/cpb.55.231. [DOI] [PubMed] [Google Scholar]
- 24.Liu C.Y., Jin Y.H., Yu H.S., Sun C.K., Gao P., Xiao Y.K., Zhang T.Y., Xu L.Q., Im W.T., Jin F.X. Biotransformation pathway and kinetics of ginsenosidase type I hydrolyzing 3-O- and 20-O-multi- glucosides of PPD type ginsenosides. Process Biochem. 2014;49:813–820. [Google Scholar]
- 25.Yu H.S., Liu Q.M., Zhang C.Z., Lu M.C., Fu Y.Y., Im W.T., Lee S.T., Jin F.X. A new ginsenosidase from Aspergillus strain hydrolyzing 20-O-multi-glycoside of PPD ginsenoside. Process Biochem. 2009;44:772–775. [Google Scholar]
- 26.Wang D.M., Yu H.S., Song J.G., Xu Y.F., Jin F.X. Enzyme kinetics of ginsenosidase type IV hydrolyzing 6-O-multi-glycosides of protopanaxatriol type ginsenosides. Process Biochem. 2012;47:133–138. [Google Scholar]
- 27.Li J.W. University Press; Beijing: 1997. Methods for biochemistry; pp. 189–196. [in Chinese] [Google Scholar]
- 28.Wang J.Y. Higher Education Press; Beijing: 2002. Biochemistry; pp. 356–361. [in Chinese] [Google Scholar]
- 29.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 30.Lineweaver H., Burk D. The determination of enzyme dissociation constants. J Am Chem Soc. 1934;56:658–666. [Google Scholar]
- 31.Tanaka I., Kasai R. vol. 46. Springer; Berlin: 1984. Saponins of ginseng and related plants; pp. 1–76. (Progress in the chemistry of organic natural products). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.