Abstract
Autism spectrum disorder (ASD) is characterized by reduced attention to social stimuli including the human face. This hypo-responsiveness to stimuli that are engaging to typically developing individuals may result from dysfunctioning motivation, reward, and attention systems in the brain. Here we review an emerging neuroimaging literature that emphasizes a shift from focusing on hypo-activation of isolated brain regions such as the fusiform gyrus, amygdala, and superior temporal sulcus in ASD to a more holistic approach to understanding face perception as a process supported by distributed cortical and subcortical brain networks. We summarize evidence for atypical activation patterns within brain networks that may contribute to social deficits characteristic of the disorder. We conclude by pointing to gaps in the literature and future directions that will continue to shed light on aspects of face processing in autism that are still under-examined. In particular, we highlight the need for more developmental studies and studies examining ecologically valid and naturalistic social stimuli.
Keywords: autism, faces, fMRI, connectivity, social cognition, eye gaze
1. Introduction
Faces offer a wealth of cues important for social interaction by rapidly conveying information such as identity, emotional expression, and gaze direction. Deficits in face perception abilities have a profound influence on social development. Improper face identification and emotion recognition can have significant implications for fluent social interaction. Some of the most pronounced social deficits characteristic of autism spectrum disorders (ASD) are diminished interest in and attention to the human face (Bird et al., 2006; Hobson et al., 1998). Over the past several decades, the brain systems supporting these social cognitive processes have been carefully mapped out using functional neuroimaging. Atypical neural responses to the human face in ASD are well documented in the functional magnetic resonance imaging (fMRI) literature. While the field of autism research has historically been dominated by theories positing malfunction of individual brain regions such as the fusiform gyrus (FG), amygdala, and superior temporal sulcus (STS) (Adolphs et al., 2001; Baron-Cohen et al., 2000; Pelphrey & Carter, 2008; Schultz, 2005), there is increasing awareness that autism is characterized by widespread abnormalities throughout the brain, and particularly in the patterns of connectivity across cortical and subcortical regions (Maximo et al., 2014; Müller et al., 2011).
The human brain is organized into large-scale networks that dynamically interact to produce cognition and behavior (Bressler & Menon, 2010; Mesulam, 1990). In cognitive and affective neuroscience, there is an increasing consensus that taking a network perspective to understanding brain function can provide more parsimonious explanations of the complex relationship between brain and behavior (Pessoa, 2014; Lindquist & Barrett, 2012) and in particular can elucidate key principals of functional brain organization underlying typical and atypical development (Uddin et al., 2010). This movement from focusing on brain regions to brain networks has also begun to critically inform social neuroscience. As recently reviewed by Kennedy and colleagues, individual brain regions that have been historically linked with social processing (including face perception) can now be considered in the context of larger networks in which they are embedded, such as the “amygdala network”, “mentalizing network”, “empathy network”, and “action-perception network” (Kennedy & Adolphs, 2012).
Here we review studies of face perception in ASD, shifting the focus from a model of deficits within individual brain regions to highlight atypicalities within face processing brain networks. We begin with a comprehensive summary of fMRI studies focusing on various aspects of face processing in autism, from perception to emotion recognition and gaze processing. We then review the emerging (but limited) literature examining functional connectivity of face processing networks in the disorder. We conclude by pointing to gaps in the current literature and outlining future directions for research aimed at further incorporating network approaches to the study of face processing in ASD. In particular, we note that while ASD is a heterogeneous condition, the majority of neuroimaging studies that have been conducted have focused on high-functioning adults with the disorder. This is largely due to the demands placed on participants, who must remain still and follow complex instructions in an MR environment—a task that proves difficult, if not impossible, for younger and low-functioning children with the disorder (Yerys, 2009). This focus on high-functioning individuals unfortunately limits the generalizability of neuroimaging findings to the larger ASD population. Still, the insights that have been gained from studies of this subset of individuals are critical for progress in understanding the neurobiology of social perception in autism.
2. Face processing networks in the neurotypical brain
Functional MRI investigations in neurotypical (NT) individuals have identified two pathways involved in face perception. A subcortical pathway involving the superior colliculus, pulvinar and amygdala, is proposed as an early face perception and expression recognition network in typically developing (TD) infants and small children (for a review, see Johnson, 2005). The subcortical pathway is also implicated in processing low-spatial frequency information (Vuillemier et al., 2003) and attention to emotional expressions in typical adults (Hariri et al., 2002; Zald, 2003). A cortical pathway involves the inferior occipital gyrus (IOG), FG, STS, and amygdala, together forming a network responsible for perceiving changeable and unchangeable face features (Haxby, Hoffman, & Gobbini, 2000). Unchangeable face features convey information such as gender and identity and are related to increased blood oxygen level dependent (BOLD) activity in the lateral inferior occipital lobes and the fusiform gyrus when viewing faces compared to other objects such as houses or cars (Kanwisher & Yovel, 2006). Changeable face features convey information such as emotional expression and eye gaze direction and are related to increased BOLD activity in the STS (Hoffman & Haxby, 2000; Puce et al., 1998; Wicker et al., 1998). The amygdala is also implicated in emotional expression perception (Wang et al., 2014; Whalen et al., 2013), including dynamic expression perception (Harris et al., 2014) and reflexive orienting to expressions (Gamer & Buchel, 2009; Gamer et al., 2013). Additionally, cortical areas such as the FG that are implicated in face discrimination and identity recognition are also activated during expression recognition (Pessoa et al., 2002a, 2002b; Winston et al., 2004). This is in accord with the idea from Haxby et al. (2000) that unchangeable face aspect mechanisms are necessary when discerning changeable face aspects. That is, in order to perceive a change of a facial expression, one must perceive that change within a ‘face’ framework.
The large body of work on cortical and subcortical pathways for face perception in the NT brain point to specific, dissociable neural substrates for different aspects of face processing, some of which may be intact and some impaired in ASD. We will return to examine this distinction and implications for understanding social deficits in ASD in Section 4.
3. Typical development of face processing networks
The developmental trajectories of face- and object-selective cortical areas within the ventral pathway have different maturation patterns. Behaviorally, children demonstrate similar face perception abilities as adults, albeit with longer reaction times and lower accuracy (de Heering et al., 2012). Cortical areas such as the FG and IOG are sensitive to objects but not faces in children aged 5-8, while children aged 11-14 show greater activation to faces compared to objects (Scherf et al., 2007). The volume of the FG increases around 11 years of age with, and this increase is correlated with enhanced recognition memory for faces (Golorai et al., 2007). Additionally, functional connectivity of face selective cortical areas increases with age (Cohen Kadosh, 2011). It is thought that development of the face pathways occurs later due to the fact that faces are typically processed on the individual level (eg. Bill Clinton, The Pope) because the social importance of identifying faces often leads most individuals to become experts in face-processing (Tanaka, 2001). On the other hand, objects are typically processed on the basic level (eg. house, car) (Carey & Diamond, 1977; Diamond and Carey, 1986; however see, McKone et al., 2012) unless expertise is involved (e.g., bird or car experts; Tanaka & Curran, 2001). Haist and colleagues (2013), found that the extended face network including the amygdala, inferior frontal gyrus (IFG), insula, and anterior cingulate is hyper-active in children compared with adults, while FG activation stays relatively constant across development. Finally, Cohen Kadosh et al. (2013) found that the IOG modulates relevant and irrelevant information of gaze and expression differently in children and adults, showing different patterns of specialization of the IOG as a function of age. Thus, the maturation of the face perception abilities involves changes across an entire network, rather than isolated brain areas (Cohen Kadosh, 2011).
4. Face identity and emotion processing in ASD
Behaviorally, individuals with ASD often, but not always, demonstrate deficits in identity (for a review see, Weigelt et al., 2012) and expression processing (for reviews see, Harms et al., 2010; Uljarevic & Hamilton, 2012) such as reduced accuracy and longer reaction times compared with TD individuals. Additionally, individuals with ASD often have more difficulties in face perception tasks when emotional expressions are involved, compared with tasks involving only neutral expressions (Braverman et al., 1989; Celani et al., 1999; Teunisse & de Gelder, 2001; however see: Ozonoff et al., 1990).
One proposed neural mechanism for behavioral deficits in face perception in ASD is that early visual deficits lead to downstream difficulties involving higher order processing of changeable and unchangeable aspects of face perception. Individuals with ASD are often superior in local visual search tasks where an item is identified when surrounded by similar distracters (e.g., identifying the letter u among o), but are impaired at global visual tasks such as motion identification (e.g., identifying the circular motion of random dots) compared with TD individuals (for reviews see, Dakin & Frith, 2005; Mottron et al., 2006; Simmons et al., 2009). Global and motion processing impairments are cited as evidence for the “weak central coherence” theory of ASD (Happe & Frith, 2006), leading to the idea that low-level motion deficits contribute to higher order face perception impairments. TD individuals often rely on ‘holistic’ processing where face features (eyes, nose, mouth) are perceived as a ‘whole’ (Tanaka & Farah, 1993), while expression and gaze processing rely on biological motion mechanisms due to their dynamic real world representation (Haxby et al., 2000). Thus, low-level global processing deficits in ASD may hinder higher order ‘holistic’ face perception, while low-level motion processing deficits may hinder higher order gaze and expression biological motion processing (Berhmann et al., 2006). Face perception studies have found both disruption (Bird et al., 2006; Kleinhans et al., 2011; Zürcher et al., 2013) and preservation (Coutanche et al., 2011; Jiang et al., 2013; Pelphrey et al., 2007; von dem Hagen et al., 2014) of early visual processing stream cortical areas in individuals with ASD. These studies are reviewed in more detail below.
In one of the few studies to focus on the subcortical face pathway in ASD, Kleinhans et al. (2011) presented adult ASD and NT groups with fearful faces for 23ms (masked by scrambled images) while participants identified randomly placed intermittent fixation crosses. Although the presentation time was in accord with previously used subliminal processing tasks, most participants in both groups reported being able to see the facial expressions, making this a supraliminal task. Group comparisons showed hypoactivation of the left amygdala, bilateral FG and superior colliculi, and the right pulvinar in individuals with ASD. This suggests that the subcortical face processing system may be disrupted in ASD (Table 1).
Table 1.
Cortical activation for ASD neutral face studies.
| Study | Demographics | ASD compared to TD | |||
|---|---|---|---|---|---|
| ASD | TD | Hyperactivity | Hyperactivity | Equal activity | |
| Subcortical Face Pathway | |||||
| Kleinhans et al., 2011 | n=28 23.57+/- 6.60 |
n=25 23.32+/-5.15 |
Amyg FG |
||
| Neutral Faces | |||||
| Pierce & Redcay, 2008 | n=11 9.9+/- 2.14 (6-12; 2F) |
n=11 9.8 +/-1.80 (6-13; 2F) |
Unfamiliar Faces: FG/Amyg | Mother: FG/Amyg | |
|
| |||||
| Scherf etal., 2010 | n=10 12.1 +/-1.1 (10-13; OF) |
n=10 12.4+/-1.35 (10-14; OF) |
OG FG STS |
||
|
| |||||
| Schultz et al., 2000 | n=14 23.8+/-12.4 (OF) |
Grp1: n=14 Grp 2:n=14 21.7+/-7.2 21.5+/-10.6 (OF) |
FG | ||
|
| |||||
| Pierce et al., 2001 | n=6 29.5+/-8 (21-41;0F) |
n=8 28.3 (20-42;0F) |
FG STS Amyg |
||
|
| |||||
| Hadjikhani etal., 2004 | n=11 36+/-12 (18-52; 0F) |
n=10 26+/-6 (20-43; OF) |
FG OG |
||
|
| |||||
| Hadjikhani et al., 2007 | n=10 34+/-11 (2F) 1 excluded |
n=7 35 +/-12 (3F) |
STS Amyg IFG |
FG OG |
|
|
| |||||
| Grelotti et al., 2005 | DD:11yrs/CC:17yrs (0F) |
TDC:10yrs (OF) |
Unfamiliar Faces: DD/CC FG |
Digimon DD: FG Amyg |
|
|
| |||||
| Humphreys et al., 2008 | n=13 27+/-10 (18-53; 0F) |
n=15 29 +/-10 (18-44; OF) |
FG STS OG |
||
| Ex.1 – n=8 Ex.2 – n=7 |
Ex.1 – n=7 Ex.2 – n=6 |
||||
|
| |||||
| Bookheimer et al., 2008 | n=12 11.3+/-4.0 (7.8-19.6; OF) |
n=12 11.9+/-2.4 (8.1-15.7; OF) |
IFG Amyg |
FG | |
|
| |||||
| Kleinhans et al., 2009 | n=19 21.9+/- 5.9 (18-44) |
n=20 24.7 +/- 7.9 (18-43) |
Amyg | FG | |
|
| |||||
| Koshino et al., 2009 | n=11 24.5 +/-10.2 (OF) |
n=11 28.7+/-10.9 (1F) |
IFG STG |
FG | |
|
| |||||
| Domes et al., 2013 | n=14 24.0 +/- 6.9 (OF) |
n=14 23.4+/-5.4 (OF) |
Placebo: FG Amyg OG |
Oxytocin: Amyg | |
FG: fusiform gyrus, Amyg: amygdala, STS: superior temporal sulcus, STG: superior temporal gyrus, OG: occipital gyrus, IFG: inferior frontal gyrus, ACC: anterior cingulate cortex.
5. Fusiform Gyrus, Superior Temporal Sulcus, and the Amygdala in ASD: Neutral Faces
The FG (Dawson et al., 2005), STS (Zilbovicius et al., 2006, 2013), and amygdala (Baren-Cohen et al., 2000) have been implicated in the aberrant neuropathology of ASD. The studies reviewed here examined the function of these areas within the context of the cortical face pathway described above (Schultz, 2005). These studies examined the perception of neutral expressions within the context of face discrimination (e.g., discriminating faces from other objects) and identity recognition (eg. discriminating a specific face from other faces) using passive and active viewing paradigms. It is important to note that FG activation in the context of face perception paradigms has generally been identified belonging to the ‘fusiform face area’ (FFA; Kanwisher et al., 1997). However, recent research has shown that there are up to three distinct clusters of face and object selective regions located within the traditionally defined FFA (Weiner & Grill-Spector, 2010; Cukur et al., 2013). Thus, we will use the more general term ‘F’ rather than the more specific ‘FFA’ terminology due to the ambiguity in precisely identifying the FFA within the brain.
In passive viewing paradigms, participants are instructed to view unfamiliar neutral faces and control stimuli such as scrambled faces (Hadjikhani et al., 2004, 2007), or objects and open fields (Humphreys et al., 2008; Scherf et al., 2010). These studies have found hypo-activation of the occipital face area (OFA; Humphreys et al., 2008; Scherf et al., 2010), FG (Humphreys et al., 2008; Scherf et al., 2010), amygdala (Hadjikhani et al., 2007), inferior frontal cortex (Hadjikhani et al., 2007), and STS (Hadjikhani et al., 2007; Humphreys et al., 2008; Scherf et al., 2010) for faces compared to control stimuli in individuals with ASD. Two of these studies found no differences in FG and IOG activation between ASD and TD groups (Hadjikhani et al., 2004, 2007).
In active viewing paradigms, participants are presented with unfamiliar neutral faces and control stimuli such as objects (Kleinhans et al., 2009; Schultz et al., 2000) and shapes (Bookheimer et al., 2008; Pierce et al., 2001) and are asked to judge if image pairs are identical/different (Schultz et al., 2000), identify female faces and circles (Pierce et al., 2001), match upright and inverted stimuli to one of two images presented below the target (Bookheimer et al., 2008), identify repeated stimuli (Kleinhans et al., 2009), or identify previously presented stimuli (Koshino et al., 2008). These studies generally show similar performance accuracy between ASD and TD groups (Kleinhans et al., 2009; Koshino et al., 2008; Pierce et al., 2001; Schultz et al., 2000) with one study finding 75% of participants with ASD matching TD accuracy (Bookheimer et al., 2008), one study finding slower reaction times in ASD (Kleinhans et al., 2009), and one study finding faster inverted stimuli matching in ASD (Bookheimer et al., 2008).
Overall, these studies generally demonstrate that individuals with ASD show no reaction time or accuracy differences in face perception tasks involving neutral expressions. However, despite similarities in behavioral performance, individuals with ASD exhibit significantly different brain activation patterns and patterns of functional connectivity compared with NT individuals. Overall, there is evidence for atypical patterns of brain activity in the form of hypoactivation of the FG (Humphreys et al., 2008; Pierce & Redcay, 2008; Scherf et al., 2010; Schultz et al., 2000), STS (Hadjikhani et al., 2007; Humphreys et al., 2004; Scherf et al., 2010; Pierce et al., 2001), amygdala (Domes et al., 2013; Hadjikhani et al., 2007; Pierce et al., 2001), and the occipital lobes (Humphreys et al., 2004;, Pierce et al., 2001; Scherf et al., 2010), alongside hypoconnectivity of the FG (Koshino et al., 2008) in individuals with ASD. Notably, most of these brain regions from the core face perception network, and tend to demonstrate general decreases in BOLD response in individuals with ASD. In addition, individuals with ASD also demonstrated hypoactivation of the IFG (Bookheimer et al., 2008; Hadjikhani et al., 2007; Koshini et al., 2008), ITG (Schultz et al., 2000) and hypoconnectivity of the IFG and MFG (Koshino et al., 2008), areas that are part of the extended face perception network. These results demonstrate that atypical brain activation during face perception is not restricted to the core face perception pathway, but also extends to other cortical areas related to executive functions such as attentional control and inhibition. Taken together, these findings suggest that atypical face perception in ASD is mediated by other factors in addition to pure visual perception.
Additionally, two studies demonstrated that individuals with ASD show activity in brain areas typically related to the object perception pathway in TD individuals (Koshino et al, Scherf et al., 2010), suggesting that individuals with ASD may be compensating for a lack of functionality in the core and extended face perception pathways by recruiting regions comprising more general object perception networks. This may explain the findings that although individuals with ASD show atypical brain responses to neutral faces, they are still able to perform reasonably well on the behavioral tasks, perhaps by adopting compensatory strategies.
Two studies utilized familiar stimuli that included family members and Digimon characters. Pierce and Redcay (2008) showed that children with ASD performed equally to TD children when identifying familiar faces and objects, but were slower and less accurate when identifying unfamiliar female faces. Additionally, both groups had comparable FG activation for familiar people such as their mother, while the ASD group showed FG hypoactivation for strangers. Grelotti and colleagues (2005) examined an 11-year-old child with ASD (DD) who was an expert in identifying Digimon cartoon characters, a 17-year-old adolescent with ASD (CC) unfamiliar with Digimon characters, and a 10 year old TD child (TDC) who was an expert in identifying Pokemon cartoon characters and occasionally watched Digimon. DD was faster at identifying Digimon than faces and objects, while TDC was equally fast for faces and Pokeman but slower for objects and Digimon. An fMRI task where the participants made same/different judgments for image pairs consisting of unfamiliar faces, objects, and cartoon characters was then administered. DD exhibited greater right FG and bilateral amygdala activation for Digimon characters than unfamiliar faces and objects compared with TDC and CC. Both of these studies demonstrate that familiarity of experimental stimuli and motivational factors can influence behavior and brain activity in ASD.
These two studies utilized stimuli with more intrinsic importance to individuals with ASD, such as images of mothers (Pierce & Redcay, 2008) or Digimon characters (Grelotti et al., 2005), and showed similar activation of the FG and amygdala to that seen in NT individuals. This suggests that the subjective importance of the stimuli may contribute to the atypical engagement of the FG and amygdala observed in individuals with ASD. Thus, disinterest or lack of motivation to engage with unfamiliar stimuli may contribute to the hypo-activation patterns often observed in fMRI studies. Further, this disinterest does not seem to be driven by exogenously directed attention, as atypical brain activation in ASD is found in both passive (Hadjikhani et al., 2004; Hadjikhani et al., 2007; Humphreys et al, 2008; Scherf et al., 2010; Schultz et al., 2000) and directed (Bookheimer et al., 2008; Grelotti et al., 2005; Kleinhans et al, 2009; Koshino et al., 2008; Pierce et al., 2001; Pierce & Redcay, 2008) viewing of neutral faces. This would also suggest that an endogenous lack of attention to faces is driving atypical brain responses in ASD when perceiving neutral faces. Additionally, the lack of an endogenous drive or motivation to attend to unfamiliar faces in ASD does not seem to be affected by developmental stage - both children and adults show a lack of typical brain activation patterns to unfamiliar faces, suggesting that the absence of such a mechanism may be a core feature of the disorder rather than being due to an immature face perception network.
Of interest, Domes et al. (2013) demonstrated that FG activity in individuals with ASD was increased when oxytocin was administered, further suggesting that the lack of interest in unfamiliar faces may be linked to neurotransmitter deficits. Oxytocin has been shown to increase positive social behavior and interaction in humans (Kemp & Guastella, 2011) and macaques (Simpson et al., 2014), providing for an intrinsic reward mechanism as a motivator for social interaction. The fact that oxytocin normalizes brain activity in ASD during face perception suggests that social disinterest may be related to an absence of intrinsic reward mechanisms that may contribute to atypical brain responses to faces in ASD.
6. Fusiform Gyrus, Superior Temporal Sulcus, and the Amygdala in ASD: Emotional Expressions
Emotional expression perception investigations in NT individuals have implicated the amygdala (Adolphs, 2008; Wang et al., 2014; Whalen et al., 2013) and STS (Hoffman & Haxby, 2000; Puce et al., 1998; Wicker et al., 1998) as important subcortical and cortical areas responsive to emotional expressions. These brain regions are thought to be important for changeable aspects of faces that include emotional expressions (reviewed here) and gaze information (reviewed in Section 7).
The use of expressions is somewhat varied throughout the literature, with studies using various combinations of angry, happy, fear, neutral, and sad expressions (See Table 2 for specific experiment information). The studies reviewed in this section have generally used active viewing paradigms that involve imitating expressions (Dapretto et al., 2006), gender judgments (Critchley et al., 2000; Deeley et al., 2011; Hubl et al., 2003; Weng et al., 2011), judging if images match (Bird et a., 2006; Corbett et al., 2009; Kleinhans et al., 2010), judging if images match linguistic labels (Piggot et al., 2004; Wang et al., 2004), expression identification (Critchley et al., 2000; Hubl et al., 2003; Malisza et al., 2011), or simply pressing a button in response to images (Ashwin et al., 2007; Pelphrey et al., 2007).
Table 2.
Cortical activation for ASD emotional expression studies.
| Expressions | |||||||
|---|---|---|---|---|---|---|---|
| Study | Demographics | ASD compared to TD | |||||
| ASD | TD | Viewing/Identifying Expressions | Matching Expressions | Matching words w/Expressions | Matching Identities | Identifying Gender | |
|
Piggot et al., 2004 Expressions A, F, S, |
n=14 13.1+/-2.5 (9-17; OF) |
n=10 14.4+/-3.3 (10-18; OF) |
FG Hypo | No Diff: FG | |||
|
| |||||||
|
Wang et al., 2004 Expressions A, H |
n=12 12.2+/-4.8 (8-23; OF) |
n=12 11.8+/-2.5 (8-16; OF) |
FG Hypo | No Diff: FG | |||
|
| |||||||
|
Dapretto et al., 2006 Expressions A, F, H, N, S |
n=10 12.05+/-2.50 (1F) |
n=10 12.38+/-2.22 (1F) |
Imitation: No Diff: FG/Amyg Hypo: Pars Opercularis Insula, Ventral Striatum Hyper: Anterior Parietal Occipital | ||||
|
| |||||||
|
Weng et al., 2011 Expressions F, H, N, S |
n=22 14.36+/-1.70 (11.17-16.75; 5F) |
n=20 14.97+/-1.95 (10.25-18; 1F) |
Hypo: Amyg VMPFC Striatum | ||||
|
| |||||||
|
Corbett et al., 2009 Expressions A, F, H, N S |
n=12 9.01+/-1.60 (8-12; OF) |
n=15 9.17+/-1.44 (8-12; 2F) |
Amyg Hypo | FG Hypo | |||
|
| |||||||
|
Malisza et al., 2011 Expressions A, H |
n=9 12.2+/-1.5 (9-14; 2F) |
n=9 12.1+/-2.0 (9-14; 2F) |
Hypo: FG/STS/OG STS/IFG | ||||
|
| |||||||
|
Critchley et al., 2000 Expressions A, H, N |
n=9 37+/-7 (26-47; OF) |
n=9 27+/-7 (OF) |
FG Hypo | Amyg Hypo | |||
|
| |||||||
|
Hubl et al., 2003 Expressions A, H, N, S |
n=7 25.3+/-6.9 (OF) |
n=7 27.7+/-7.8 (OF) |
FG Hypo OG Hyper |
FG Hypo OG Hyper |
|||
|
| |||||||
| Ashwin et al., 2006 Expressions F, N |
n=13 31.2+/-9.1 (OF) |
n=13 25.6+/-5.1 (OF) |
Hyper: STS/ACC No Amyg to High Fear | ||||
|
| |||||||
|
Bird et al., 2006 Expressions F, N |
n=16 33.3+/-11.5 (2F) |
n=16 35.3+/-12.1 (2F) |
Localizer Task: No Diff: FG/OG Main Task: FG/OG Hypo | ||||
|
| |||||||
|
Bolte et al., 2006 Expressions A, H, N, S |
n=5 29.4+/-5.9 (OF) |
N/A | Post Training: No Diff: FG SPL/OG Hyper | ||||
|
| |||||||
|
Pelphrey et al., 2007 Expressions A, F, N |
n=8 24.5+/-11.5 (17.9-50.3; 2F) |
n=8 24.1+/-5.6 (18.1-32.2;2F) |
Dynamic Images: FG/Amyg Hypo Dynamic vs. Static: STS Hypo | ||||
|
| |||||||
|
Kleinhans et al., 2010 Expressions A, F |
n=29 23.57+/-6.60 (2F) |
n=25 23.32+/-5.15 (2F) |
No Diff: FG/Amyg Anxiety: FG Hypo Amyg Hyper | ||||
|
| |||||||
|
Domes et al., 2014 Expressions A, D, H, F, S, Sur |
n=14 24.0+/-6.9 (OF) |
n=14 23.6+/-5.4 (OF) |
Amyg increase in ASD after Oxytocin | ||||
FG: fusiform gyrus, Amyg: amygdala, STS: superior temporal sulcus, OG: occipital gyrus, IFG: inferior frontal gyrus, ACC: anterior cingulate cortex, VMPFC: ventral medial pre-frontal cortex, Hypo: hypoactivation, Hyper: hyperactivation. A: angry, D: disgust, F: fear, H: happy, N: neutral, S: sad, Sur: surprise.
These studies collectively demonstrate that individuals with ASD have more difficulty than NT individuals when completing behavioral tasks involving emotional expressions. A few studies showed non-significant differences in behavioral performance between individuals with ASD and TD individuals (Bird et al., 2006; Dapretto et al., 2006; Deeley et al., 2011; Kleinhans et al., 2010; Weng et al., 2011; Wicker et al., 2008 -reviewed below). Several studies found that ASD groups showed deficits in identifying (Ashwin et al., 2007; Critchley et al., 2000; Hubl et al., 2003; Malisza et al., 2011) or matching expressions (Piggot et al., 2004; Wang et al., 2004) while some studies found slight or no between group differences when matching an expression to a word label (Piggot et al., 2004; Wang et al., 2004) or when making gender or identity judgments involving images with expressions (Critchley et al., 2000; Hubl et al., 2003). One study found that individuals with ASD performed worse than TD individuals when matching identities of images with expressions (Corbett et al., 2009). The findings in this section and the previous section on perception of neutral faces are in accord with previous behavioral research showing poorer performance for face perception tasks involving emotional expressions compared to tasks involving neutral expressions in individuals with ASD (Braverman et al., 1989; Celani et al., 1999; Teunisse & de Gelder, 2001; however see: Ozonoff et al., 1990).
These results of these studies also show that brain activation patterns in ASD vary according to the task demands and processing required. When matching emotional expression images, individuals with ASD showed hypo-activation of the FG (Piggot et al., 2004; Wang et al., 2004) and amygdala (Corbett et al., 2009) but no group differences when matching expressions with word labels (Piggot et al., 2004; Wang et al., 2004) or matching objects (Wang et al., 2004). When identifying or viewing emotional expressions, individuals with ASD showed hypo-activity of the FG (Critchley et al., 2000; Deeley et al., 2011; Hubl et al., 2003; Malisza et al., 2010; Pelphrey et al., 2007), STS (Ashwin et al., 2007; Malisza et al., 2010; Pelphrey et al., 2007) and OG (Deeley et al., 2011; Malisza et al., 2010). When matching expression images based on identity, individuals with ASD exhibited hypo-activation of the FG (Corbett et al., 2009). When identifying the gender of faces displaying emotional expressions, individuals with ASD showed FG hypo-activation (Critchley et al., 2000; Hubl et al., 2003) along with STS (Critchley et al., 2000) and amygdala (Weng et al., 2011) hyper-activation. No between-group differences in activation of the FG (Bird et al., 2006; Kleinhans et al., 2010) or amygdala (Domes et al., 2014; Kleinhans et al., 2010) were found when passively viewing expressions or imitating expressions (Dapretto et al., 2006) (Table 2).
The finding that task demands influence brain responses in ASD during viewing emotional expressions is in contrast to the general finding that task demands do not appear to influence brain responses during viewing neutral faces. When individuals with ASD are given explicit instructions to evaluate stimuli in the form of faces with emotional expressions, they exhibit atypical brain responses, whereas passive viewing of emotional expressions produces no brain activation differences compared with NT individuals. The data also show that it is the act of directing attention to the face itself, rather than the emotional expression per se, that disrupts brain activity in ASD, as atypical brain activation was found when focusing on gender and identity as well as the emotional expression. This suggests that “forcing” the processing of invariant face features (gender and identity) also activates the mechanisms related to variant face features (expression) in ASD. One possibility is that whereas NT individuals are able to seamlessly integrate the explicit use of invariant face perception mechanisms with implicit activation of variant face perception mechanisms, individual Is with ASD have interference from the implicit activation of variant face perception mechanisms when attempting explicit use of their invariant face perception mechanisms. This interference does not seem to be affected by development as children, adolescents, and adults with ASD all present with the same general pattern of findings.
On a network level, individuals with ASD generally showed hypo-activity in the FG (Corbett et al., 2009; Critchley et al., 2000; Deeley et al., 2011; Malisza et al., 2011; Pelphrey et al., 2007; Piggot et al., 2004; Wang et al., 2004) that has been tied to invariant aspects of face perception while showing hyper-activity in other areas such as the STS (Ashwin et al., 2007; Critchley et al., 2000) and the amygdala (Weng et al., 2011) that are related to variant aspects of face perception. Hyper-activity in frontal (Ashwin et al., 2007; Hubl et al., 2003; Weng et al., 2011), and parietal (Dapretto et al., 2006) areas related to the extended face perception network has also been reported. Additionally, the areas that demonstrate hyper-activity also demonstrate hypo-activity in some studies, while no studies demonstrate hyper-activity of the FG in ASD. This finding combined with the finding of general hypo-activation of the FG for neutral faces also implicates FG hypo-activity as a common pattern in ASD with regards to both neutral and emotional expressions, and suggests a general deficit in this node of the network related to invariant perception of face features regardless of task demands. The finding of hypo/hyper-activity in tasks where attention was directed to faces displaying emotional expressions suggest that directing attention to faces regardless of the expression and task type disrupts mechanisms in ASD related to processing of variant aspects of faces.
There was no identifiable pattern of responses across individuals with ASD during tasks where attention was explicitly directed. Hypo- and hyper-activation was found in both expression and gender identification tasks in ASD within different cortical areas. The reason for these differences is unclear. One possibility is that there was a wide variation in the types of emotional expressions that were presented, with some studies presenting only one type of expression and other studies presenting several different types of expression. Along these lines, the specific expression and perception of the expression's intensity may have also been contributing factors. Weng et al. (2011) found that hyper-activity of the amygdala, VMPFC, and the striatum in ASD was greatest in response to sad faces. Ashwin et al. (2007) found hyper-activation of the left amygdala and orbito-frontal cortex in response to high intensity versus low intensity fearful expressions in NT individuals, with no differences between high and low intensity fearful expressions in the ASD group. Finally, Deeley et al., 2011 found that positive and negative expressions varying in intensity modulated cortical activity in individuals with ASD. Taken together, these findings suggest that valence and arousal both contribute to brain activation differences observed between ASD and TD individuals.
Another possible explanation for the variation in brain activation is that individual differences in emotional expression perception in ASD are more heterogeneous than individual differences in perceiving neutral expressions in ASD. Along these lines, Bolte et al. (2006) showed that emotional expression perception can improve in ASD if individuals view expressions and receive feedback, and that this improvement correlates with increased activity in the extended face perception network in areas related to visual and attentional processing, rather than the core face perception network. Thus, differences in attention and/or experience with processing emotional expressions may have been driving some of the differences in brain responses across studies.
Just as with the improvement in perception of neutral faces, Domes et al. (2014) showed that oxytocin increased expression identification in the ASD group while also increasing brain activity in both the core and extended face perception network. This would further suggest that hormones such as oxytocin modulate the response to faces in ASD and that there are physiological factors that influence the ability to perceive faces that are not solely related to neuronal activation. However, it is currently unknown if long-term oxytocin administration is a viable treatment for deficits in face perception in ASD.
7. Gaze Processing in ASD
Eye gaze direction is an important social cue that often signals another person's attention is directed towards someone or something (for reviews see: Itier & Batty, 2009; Pfeiffer et al., 2013). For example, gazing in the direction of an object may signify one's intention to grasp or reach for that object. TD infants show sensitivity to direct vs. averted eye gaze direction before the age of one (Corkum & Moore, 1998; Hood et al., 1998), demonstrating early sensitivity to changes in another's attention. Although TD gaze processing was originally categorized alongside expression perception under the more general term ‘biological motion’ (Haxby et al., 2000), recent research has focused exclusively on cortical mechanisms of gaze direction centering around the STS (Itier & Batty, 2009; Nummenmaa & Calder, 2009; Pelphrey et al., 2003) and its connections to the FG, MT/V5, and the middle frontal gyrus (MFG; Nummenmaa et al., 2009b).
Studies investigating gaze processing in ASD have used both passive and active paradigms. Passive viewing paradigms have consisted of participants viewing faces displaying emotional expressions (angry, fearful, happy, neutral) with direct and averted gaze (Davies et al., 2011) and watching an animated video of a man appearing to walk past participants with a direct or averted gaze (Pitskel et al., 2011). Active viewing paradigms have consisted of having participants perform likeability ratings (Georgescu et a., 2013) or gender judgments (von dem Hagen et al., 2014) of animated videos consisting of faces displaying neutral expressions that shift between direct and averted gaze directions.
The active viewing studies show similar behavioral performance patterns in ASD and NT groups for gaze direction tasks (Wicker et al., 2008 - reviewed below; von dem Hagen et al., 2014) with ASD groups giving lower likeability ratings than TD groups for longer direct gazes and with both groups giving lower likeability ratings to averted gaze compared to direct gaze (Georgescu et al., 2013). Eye-gaze tracking in two studies demonstrated no group differences in gaze fixation or duration (Georgescu et al., 2013; von dem Hagen et al., 2014).
The overall pattern of activation suggests that individuals with ASD show no differences in processing invariant aspects of faces with gaze direction differences as evidenced by the lack of group differences in FG activation (see Table 3). The most apparent differences were from the fMRI data showing that ASD groups tend to produce greater activation for averted compared to direct gaze, with the opposite pattern in TD individuals. Direct compared to averted gaze in TD individuals was related to cross-study activity in the right insula (Georgescu et al., 2013; Pitskel et al., 2011), TPJ (Georgescu et al., 2013; von dem Hagen et al., 2014), IFG (Pitskel et al., 2011), and the superior temporal cortex (Georgescu et al., 2013; von dem Hagen et al., 2014). These areas are linked to the mirror system and mentalizing network (Molnar-Szakacs & Uddin, 2013) and suggests atypical gaze processing could also be related to difficulties in mentalizing that are often observed in the disorder (e.g., Baron-Cohen, 2001).
Table 3.
Cortical activation for ASD gaze processing studies.
| Eve Gaze | ||||
|---|---|---|---|---|
| Demographics | ASD compared to TD | |||
| Study | ASD | ID | Direct Gaze | Averted Gaze |
| Davies et al., 2011 | n=16 11.69+/-2.71 (2F) |
n=16 12.30+/-1.88 (8-17; 2F) |
Hypo: VLPFC | Hyper: Somatosensory |
|
| ||||
| Pitskel et al., 2011 | n=15 23.4+/-6.9 (14.8-37.8; OF) |
n=14 24.2+/-7.4 (16.1-42.4; 2F) |
Hypo: insula/IFG | Hyper: IOG/cerebellum |
|
| ||||
| Georgescu et al., 2013 | n=13 31.23+/-4.87 (24-39; 4F) |
n=13 30.23+/-3 (24-36; 4F) |
Hypo: MTG/STS/TPJ | |
|
| ||||
| Von dem Hagen et al., 2014 | n=21 29+/-7 (OF) 3 Excl |
n=25 26+/-6 (OF) 2 Excl |
Hypo: MTG/STS/TPJ/Amyg | |
MTG: middle temporal gyrus, STS: superior temporal sulcus, Amyg: amygdala, IFG: inferior frontal gyrus, IOG: inferior occipital gyrus, TPJ: temporal-parietal junction, Hypo: hypoactivation, Hyper: hyperactivation.
The insula has recently been implicated in salience detection and is thought to direct attention to important exogenous and endogenous stimuli (Seeley, 2007; Uddin, 2014). The fact that individuals with ASD show reduced insula activation (Georgescu et al., 2013; Pitskel et al., 2011) to direct gaze further suggests that they may not process direct eye gaze as an important salient visual cue. Accordingly, Gerogescu et al. (2013) found that longer gaze duration for TD individuals was related to activity in the mOFC, left insula, and dACC with no influence of gaze duration in individuals with ASD.
Of note, none of these studies investigated young children, so it is unknown how differences across development influence brain activation in ASD in response to gaze detection. Future studies will need to consider the role of development in gaze detection in ASD.
8. Gaze Cueing in ASD
In addition to the research reviewed in Section 7 examining how eye gaze direction influences brain activity in ASD, a related area of research focuses on how eye gaze direction facilitates or inhibits the identification of lateralized targets placed in congruence or incongruence with gaze direction. Studies in NT adults show that the facilitation of target identification that is congruent or incongruent with eye gaze direction activates the STS, DMPFC, and ACC (Mundy, 2003; Pfeiffer et al., 2013).
TD infants younger than one show gaze cueing facilitation by having faster saccades towards targets congruent opposed to targets incongruent with gaze direction (Farroni et al., 2000). This skill appears in ASD later from 2-5 years of physical age (Leekam et al., 2000; Mundy, 2003) and 48 months of mental age (Leekam et al., 1998). One popular explanation is ASD impacts the ability to draw mental inferences from gaze direction, rather than simple location detection (Baron-Cohen et al., 1995; for other explanations see Gillespie-Lynch et al., 2013). The results of behavioral gaze cueing studies in older children and adults have been mixed; some studies found no differences between ASD and TD (Chawarska et al., 2003; Kylliainen & Hietanen, 2004; Senju et al., 2004;) while other studies have found significant group differences (Goldberg et al., 2008; Ristic et al., 2005; Vlamings et al., 2005).
Paradigms investigating gaze-cueing in ASD typically focus on facilitation and interference effects using some combination of gaze direction of neutral faces (Dichter & Beiger, 2007; Hanson et al., 2013; Vaidya et al., 2011), line drawings of faces (Greene et al., 2011), directional arrows (Dichter & Beiger, 2007; Greene et al., 2011; Vaidya et al., 2011), or directional words (e.g., left or right; Vaidya et al., 2011) as cues in conjunction with targets placed on either side of those cues. One study used an animated character that directed gaze towards a checkerboard or an empty space on the side (Pelphrey et al., 2005). Facilitation effects occur when participants are faster to identify a target placed in congruence with the cue while interference effects occur when participants are slower to identify targets placed incongruently with the cue.
Taken together, the behavioral data show similar arrow and face facilitation (Greene et al., 2011; Murphey et al., 2012 - reviewed below; Vaidya et al., 2011) and arrow and face interference (Dichter & Beiger, 2007; Murphey et al., 2012; Vaidya et al., 2011) effects between ASD and TD, with Pelphrey et al. (2005) finding no group differences in accuracy or reaction time in response to gaze cueing a checkerboard or blank space. These behavioral results show largely similar ASD and TD group performance for gaze cueing facilitation and interference effects.
The fMRI results show that the inferior frontal cortex and the STS are important areas differentiating ASD and TD groups during gaze and arrow cueing (Table 4). The most obvious difference was related to general activation of these areas for faces in TD and general activation of these areas for arrows in ASD (Dichter & Beiger, 2007; Hanson et al., 2013; Pelphrey et al., 2005; Vaidya et al., 2011). This combined with the finding of generally similar behavioral performance suggests that although both groups are able to acquire directional information from eye gaze, there was more social relevance of gaze cueing in TD individuals. Accordingly, both groups showed cingulate cortex activation in response to gaze and arrow cueing (Dichter & Beiger, 2007; Hanson et al., 2003; Vaidya et al., 2011). The implication of the cingulate cortex in error monitoring for TD individuals (Carter et al., 1998) also suggests that individuals with ASD were sensitive to correct and incorrect directional information, but not to the social relevance of such information provided by eye gaze.
Table 4.
Cortical activation for ASD gaze-cueing studies.
| Gaze-Cueing | ||||||
|---|---|---|---|---|---|---|
| ASD > TD | ||||||
| Demographics | Arrows/Objects | Faces | ||||
| Study | ASD | TD | Facilitation | Interference | Facilitation | Interference |
| Vaidya et al., 2011 | n=15 10.78+/-1.29 (7.83-12.61; 4F) |
n=18 10.96+/-1.26 (4F) |
Hyper: STS/OG/IFG MFG/ACC | Hypo: ACC | Hypo: STS/OG/FG IFG/ACC | Hyper:ACC/MFG |
|
| ||||||
| Pelphrey et al., 2005 | n=10 23.2+/-9.9 (17.9-50.7; 1F) |
n=9 23.4+/-5.8 (15.5-32.4; 1F) |
N/A | N/A | Blank Space: Hypo: STS | |
|
| ||||||
| Dichter & Beiger, 2007 | n=14 22.9+/-5.2 (1F) |
n=15 24.6+/-6.5 (1F) |
No Diff: MFG IFG/ACC | Hypo:MFG IFG/ACC | ||
|
| ||||||
| Greene et al., 2011 | n=22 13.19+/-2.44 (10-17; 2F) |
n=21 12.95+/-2.46 (9-17; 2F) |
Facilitation & Interference: Hyper: IFG/STG/MTG | Faciliation & Interference: Hypo: IFG/STG/MTG No Diff: STS | ||
FG: fusiform gyrus, Amyg: amygdala, STS: superior temporal sulcus, OG: occipital gyrus, IFG: inferior frontal gyrus, ACC: anterior cingulate cortex, VMPFC: ventral medial pre-frontal cortex, MFG: middle frontal gyrus, Hypo: hypoactivation, Hyper: hyperactivation.
Finally, there were no differences across development, with both younger (Vaidya et al., 2011; Greene et al., 2011) and older groups (Dichter & Beiger, 2007; Pelphrey et al., 2005) showing similar behavioral and brain activation patterns across gaze cueing paradigms. This suggests that although both younger and older individuals with ASD can detect directional information from gaze and arrow cueing paradigms, both groups fail to appreciate the social significance of gaze cueing.
9. Face Perception and Eye Fixation in ASD
One explanation for the mixed hypo- and hyper-activation findings in the face literature focuses on ASD eye tracking patterns when perceiving faces (Hadjikhani, 2007; Tottenham et al., 2014; for other explanations such as methodological differences, see: Jemel et al., 2006). The amygdala is highly responsive to the eye region of faces displaying emotional expressions (Gamer & Buchel, 2009). Individuals with ASD show atypical eye tracking patterns when perceiving faces, with an obvious lack of eye fixation (Pelphrey et al., 2002). These two findings produced the proposal that fixation on the eye or mouth area could modulate amygdala activation in ASD (Kliemann et al., 2012; Perlman et al., 2011).
Two accounts of amygdala dysfunction underlying eye-gaze fixation in ASD that are related but not mutually exclusive have been the focus of fMRI research manipulating upper or lower face-feature fixation (Birmingham et al., 2011; Spezio et al., 2007; Tottenham et al., 2014; for more accounts see: Senju & Johnson, 2009; Neumann et al., 2006). One account proposes that ASD is characterized by active eye-area avoidance (Richer & Cross, 1976; Kylliainen & Hietanen, 2006) while another account proposes an indifference to the eye area from a lack of social interest (Birmingham et al., 2011). In the active avoidance theory, increased amygdala responses to eye-fixation would cause eye area avoidance, possibly due to amygdala activation signaling a threat. According to the indifference account, decreased amygdala response could be due to a lack of social reward mechanisms in ASD.
Paradigms exploring eye-fixation in ASD have utilized neutral (Zürcher et al., 2013) and emotional expressions (Dalton et al., 2005; Kliemann et al., 2012; Perlman et al., 2011; Tottenham et al., 2013) in tasks involving expression identification (Dalton et al., 2005; Kliemann et al., 2012), identity judgments of familiar family members (Dalton et al., 2005), identifying color changes of a fixation cross moving around the screen (Perlman et al., 2011), matching upright and inverted pairs of ‘Thacherized’ face stimuli (Zürcher et al., 2013), or identifying an object placed in the eye region of face stimuli (Tottenham et al., 2013). For example, Perlman et al. (2011) used a moving fixation cross to force eye-fixation over various regions of the face, Kliemann et al. (2012) moved the presentation of the face so that either the eye or mouth region randomly replaced the fixation cross on each trial, Zürcher et al. (2013) randomly inverted the eye or mouth region while telling participants where to focus on each trial, and Tottenham et al. (2013) randomly placed an opaque object in the left or right eye across trials.
Taken together, these eye-fixation investigations show that behavioral performance in ASD is generally impaired and task-dependent, much like the emotion expression studies reviewed above. Dalton et al. (2005) showed that although the ASD group was slower and less accurate for straightforward and forty-five degree angle expression identification, there were no differences for neutral expressions at either angle. The ASD group was also less accurate at identifying familiar family members, with no reaction time differences. Perlman et al. (2011) showed no group differences in the ability to identify two color changes of the moving fixation cross. Kliemann et al. (2012) found that the ASD group was marginally slower than NTs overall and also identified fewer expressions; however, both groups were fastest for happy expressions. Zürcher et al. (2013) found that the NT group was more accurate overall, while both groups made more inverted face errors; the NT group made more inverted mouth errors and the ASD group made fewer upright eye errors. The ASD group was also faster for inverted faces. Finally, Tottenham et al. (2013) found that both groups labeled angry expressions correctly in a behavioral task outside the scanner, with higher threat ratings than neutral and happy expressions; additionally, the ASD group was more likely to label neutral expressions as negative. Additionally, the three studies that acquired eye-tracking data during portions of the experiment all showed that individuals with ASD spent less time fixating on the eye region of the faces compared to TD individuals (Dalton et al., 2005; Kliemann et al., 2012; Tottenham et al., 2013).
The fMRI data demonstrate that forcing eye-region fixation in individuals with ASD tends to increase FG (Dalton et al., 2005; Perlman et al., 2011; Zürcher et al., 2013) and amygdala (Dalton et al., 2005; Kleinmann et al., 2012; Tottenham et al., 2013) activity, suggesting that face feature fixation manipulation can modulate and even normalize face sensitive cortical activity (Table 5). Although forced eye-fixation increases FG and amygdala activation in ASD, it is unclear if this provides support for either the active-avoidance or eye-area indifference theory. If the active-avoidance theory were correct, one would expect that hyper-activation of the amygdala would be necessary in order to signal a threat, not comparable to activation observed in NT individuals. On the other hand, if eye-area indifference were the reason for a lack of eye-fixation, one would expect a lack of amygdala activation no matter where an individual with ASD is forced to look. It could be that there is an unidentified mediator that modulates the relationship between amygdala activity and eye-indifference.
Table 5.
Cortical activation for eye-fixation and functional connectivity ASD studies.
| Eye-Fixation | ||||
|---|---|---|---|---|
| Demographics | Eye-Fixation vs. | |||
| Study | ASD | TO | Mouth-Fixation for ASD | |
|
Dalton et al., 2005 Expressions A, F, H, N |
n=14 15.9+/-4.71 (OF) |
n=12 17.1+/-2.78 (OF) |
Increased: Amyg | |
|
| ||||
|
Perlman et al., 2011 Expressions F |
n=12 25.5+/-7.47 (18-37; 1F) |
n=7 28.57+/-5.74 (22-37; OF) |
Increased: FG No Diff: Amyg |
|
|
| ||||
|
Kleimann et al., 2012 Expressions H, F, N |
n=16 30.44+/-6.34 (OF) |
n=7 30.47+/-6.24 (OF) |
Increased: Amyg | |
|
| ||||
|
Zürcher et al., 2013 Expressions N |
n=16 23.5+/-6.8 (3F) |
n=18 25.8+/-5.3 (2F) |
Increased: Amyg/IFG | |
|
| ||||
|
Tottenham et al., 2013 Expressions A, H, N |
n=42 17+/-8 (6-35; 12F) |
n=28 16+/-7 (6-34; 3F) |
Increased: Amyg | |
| Functional Connectivity | ||||
|---|---|---|---|---|
| Demographics | ASD compared to TD | |||
| Study | ASD | TO | Hypo-connected | Hyper-connected |
|
Wicker et al., 2008 Expressions A, H |
n=12 27+/-11 (18-53; 1F) |
n=14 23.4+/-10 (OF) |
OC & FG LPFC & FG VLPFC & STS |
|
|
| ||||
|
Kleinhans et al., 2008 Expressions N |
n=19 23.5+/-7.8 (18-44) |
n=21 25.1+/-7.6 (18-43) |
FG & Amyg | |
|
| ||||
|
Murphy et al., 2012 Expressions A, F |
n=12 10.42+/-1.28 (3F) |
n=13 11.05+/-1.33 (3F) |
STS & Amyg | Amyg & ACC |
FG: fusiform gyrus, Amyg: amygdala, STS: superior temporal sulcus, OG: occipital gyrus, IFG: inferior frontal gyrus, ACC: anterior cingulate cortex, VMPFC: ventral medial pre-frontal cortex, LPFC: lateral pre-frontal cortex. A: angry, F: fear, H: happy, N: neutral.
10. Functional Connectivity of the Face Network
Functional connectivity analysis identifies temporal BOLD signal correlations between areas that signify correlated activity patterns (Friston 1994). Task-related functional connectivity analysis focuses on task specific correlations, while resting-state functional connectivity analysis focuses on task-free correlations, i.e., when the brain is at “rest” (Biswal, 1995). Effective connectivity analysis identifies the direction of influence between brain areas (Büchel & Friston, 2000). Resting-state connectivity between face sensitive cortical areas such as the OFA, FG, STS, and amygdala is positively correlated with performance on face perception tasks (O'Neil et al., 2014; Zhang et al., 2009; Zhu et al., 2011) in NT individuals. Functional task-related correlations have also been found between face sensitive cortical areas (Miyahara et al., 2013) in the NT brain. There is a growing literature on brain connectivity in autism, pointing to patterns of both hyper- and hypo-connectivity during task and resting states (see Uddin et al., 2013; Kana & Just, 2011; Muller et al., 2011; Vissers et al., 2011; Hernandez et al., 2014 for review). Here we focus specifically on research describing functional and effective connectivity within networks relevant for face processing (Table 5).
The few paradigms investigating functional connectivity in ASD have utilized face-processing tasks where participants make expression and age judgments of dynamically changing expressions (Wicker et al., 2008) and identify repeated presentations of neutral faces and houses (Kleinhans et al., 2008) resulting in no group performance differences. One study utilized a gaze-cueing paradigm similar to the studies reviewed in the gaze-cueing section above that also found no group differences in behavioral performance (Murphy et al., 2012).
The fMRI results show that individuals with ASD can exhibit task-based hypo-activity alongside hypo-connectivity (Wicker et al., 2008), equal task-based intensity for ASD and TD in the FG alongside ASD hypo-connectivity (Kleinhans et al., 2008), and both hypo- and hyper-connectivity in gaze cueing paradigms (Murphy et al., 2012). More specifically, some studies found hypo-connectivity involving the FG (Kleinhans et al., 2008; Koshinio et al., 2008 - reviewed above; Wicker et al., 2008), STS (Wicker et al., 2008), and amygdala (Kleinhans et al., 2008) while other studies found hyper-connectivity involving the FG (Wicker et al., 2008) and amygdala (Murphy et al., 2012).
All three studies found hypo- and hyper-connectivity between areas within the core face perception network and also between the core and extended face perception networks, showing atypical communication between and within the face perception networks in ASD. This would suggest that individuals with ASD have difficulties not only with the initial perception of facial features, but that there is aberrant communication between the extended face perception network and the core network that may lead to atypical evaluation or mediation of the automatic reaction to initial face perception. While few functional connectivity studies currently exist, these findings also suggest that impaired interactions between brain regions can persist even in the presence of typical levels of signal intensity (Kleinhans et al., 2008).
11. Tying it together: Face Processing and Social Brain Networks in ASD
A general explanation accounting for the wide range of behavioral and neural differences between ASD and TD groups across various face perception tasks proposes atypical processing for social stimuli in ASD generally. Processing of social stimuli has been categorized into two main areas: ‘social cognition’ involves such processes as mentalizing (e.g., theory of mind, TOM: inferring another's internal state; Tager-Flusberg, 2007) while ‘social motivation’ involves processes such as orienting (directing attention towards socially relevant stimuli), motivation (pursuing and enjoying socially relevant stimuli) and maintaining/enjoyment (working to keep social bonds strong because of intrinsic enjoyment of social interaction; see Chevallier et al., 2012 for a contrast/integration of social cognition and social motivation in ASD).
Both social cognition and social motivation have relevance for the face perception literature in ASD. Deficits in social cognition related to TOM processing have been linked to atypical eye gaze processing (Baron-Cohen, 2001; Baron-Cohen et al., 1995; Baron-Cohen et al., 1999; Baron-Cohen et al., 2000, Mundy, 2003) and failure to grasp what causes another's emotional expression (Baron-Cohen, 1991). Deficits in social orienting are implicated in drawing attention to the face (Bal et al., 2010; Chevallier et al., 2012; Dawson et al., 2002; Kirchner et al., 2011; Klin et al., 2002; Nakano et al., 2010) while deficits in social motivation are implicated in the lack of sustained attention to relevant facial social cues such as expression (Dawson et al., 2005; Kleinhans et al, 2010; Nuske et al., 2013).
The ‘social network’ was first postulated from animal studies and included the amygdala, STS, and orbitofrontal cortex (Brothers, 1990) and was later extended to include human systems sub-serving reward mechanisms (ventral striatum: Adolphs, 2003), mentalizing MPFC, TPJ; (Gallagher et al., 2000; Frith & Frith, 2003; Kennedy & Adolphs, 2012; Van Overwalle & Baetens, 2009), and the mirror system (IFG, superior temporal cortex, and insula; Carr et al., 2003; Frith, 2007; Iacoboni & Dapretto, 2006). The amygdala is implicated in social orienting, where faces automatically capture attention by pulling attention to the eye area (Adolphs & Spezio, 2006) while the STS is implicated in perceiving the biological movement of gaze and expression changes (Haxby et al., 2000). The ventral striatum is implicated in social maintaining via the intrinsic reward that accompanies social interaction in TD individuals (Haber & Knutson, 2010) while the orbitofrontal cortex is implicated in connecting subjective value of external stimuli with internal states such as motivation (Padoa-Schioppa & Cai, 2011; Klein et al., 2009) that in turn guide behavior (Chevallier et al., 2012).
Mentalizing and mirror systems are thought to make important contributions to understanding the emotional state of another person and imitating expressions (Carr et al., 2006; Schulte-Ruther et al., 2007). Emotional contagion is thought to be a first step in mentalizing, whereby emotional expression perception initiates the spontaneous replication of that expression allowing for automatic replication of the same expression and emotional state of another (Frith, 2007). Imitation is thought to rely on action-perception encoding via the mirror neuron system where actions are encoded in the superior temporal and inferior frontal cortex via the insular cortex (Carr et al., 2006). Empathizing with the expression of another builds on spontaneous imitation whereby one contrasts internally represented imitated states with the perceived emotional state of another while keeping a separation of the self from the other person; this allows for an overall assessment of imitated and perceived emotional states (Decety & Lamm, 2006; Preston & de Waal, 2002). Empathizing with other individuals is mediated by activation in the insula (de Greek et al., 2012; Menon & Uddin, 2010), IFG (Chakrabarti et al., 2006; Liakakis et al., 2011), STG (Nomi et al., 2008), ACC (Lavin et al., 2013), and the MPFC (Frith, 2007).
Connectivity of the cortical areas that comprise the extended social network has been found to be dysfunctional in individuals with ASD (Iacoboni & Dapretto, 2006; Gotts, 2012), which in turn is thought to contribute to deficits in social behavior (Bachevalier & Loveland, 2006). Many areas implicated in the extended social network such as the ACC, IFG, MPFC, STG, and the insula have shown atypical activation throughout the studies reviewed in this paper (Figure 1), suggesting wider social cognition dysfunction that goes beyond face perception deficits in ASD. Recent meta-analyses investigating social dysfunction in ASD have implicated atypical activation patterns related to a broad range of social tasks. For example, Di Martino et al. (2008) found that the ACC and the right anterior insula showed hypo-activation in ASD across a number of studies of social processing, while Dickstein et al. (2013) found hypo-activation of the right STG across a number of social studies. Finally, a recent metaanalysis of the ASD face perception literature by Nickl-Jockschat et al. (2014) reported hypo-activation of the left FG that was functionally hypo-connected with the IFG and temporo-occipital cortex across a number of studies. These attempts at synthesis across studies are beginning to create a picture of a dysfunctional face processing networks in ASD that are part of larger social networks in the brain.
Figure 1.
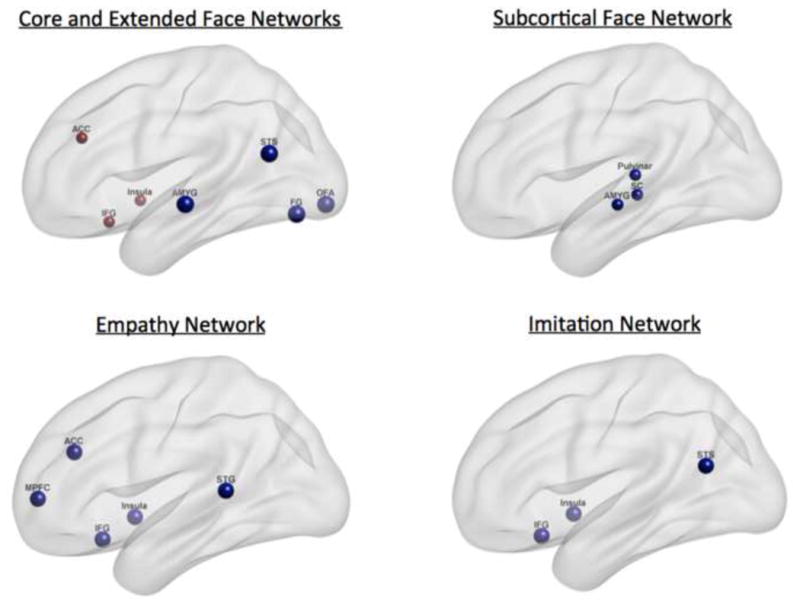
Neural networks involved in face processing. Nodes represent brain areas typically involved in each network. Core and extended face networks (upper left): red = extended face network, blue = core face network. STS: superior temporal sulcus, STG: superior temporal gyrus, AMYG: amygdala, FG: fusiform gyrus, OFA: occipital face area, ACC: anterior cingulate cortex, IFG: inferior frontal gyrus, SC superior colliculus, MPFC: medial pre-frontal cortex.
In accordance with this view of understanding atypical face perception in ASD as a more general dysfunction in social processing, additional literature suggests that these aberrant activation patterns are not strictly tied to face perception. Face perception deficits may certainly occur in concert with social dysfunction in ASD, but more likely arise from broader social dysfunction, as these areas also differ from TD individuals in tasks unrelated to face perception. For example, STS dysfunction in ASD has been implicated in atypical understanding of the intentions of actors and animated figures (see Pelphrey et al., 2011 for a review), and speech perception, (Redcay, 2008; Zilbovicius et al., 2006) while amygdala dysfunction is implicated in atypical mental state eye inferences (Baron-Cohen et al., 1999). This wider range of dysfunction involving the core face-processing network (STS, amygdala, and FG) in individuals with ASD suggests that atypical function of these areas may be related to social processing in general.
12. Conclusions and Future Directions
Here we highlight the importance of going beyond the traditional focus on the occipital gyrus, FG, and amygdala in characterizing face perception in ASD. Although each of these areas has been shown to exhibit atypical function in ASD, there are many studies that show that atypical face perception can be tied to many other brain areas in the extended face perception network, and other networks related to social processing such as TOM and mirror networks. This is especially evident in tasks that explore face perception involving expression and gaze information, as reviewed in the sections above. This makes it imperative that explorations attempting to characterize atypical brain processing related to face perception in ASD take into consideration the larger context of social processing and attention networks that face perception falls within.
The more obvious differences in the extended face perception network were related to emotional expression, eye gaze, and functional connectivity paradigms. In fact, differences in the extended face perception network were just as likely to be found as differences in the core face perception network. This is also likely due to the fact that these studies were more likely to use whole brain analysis approaches, but it also may be because emotional expression and gaze direction carry more complex social information such as emotional state and direction of social attention. Due to the fact that individuals with ASD show deficits in socio-emotive processing such as TOM and empathy, one would expect differences in higher-order processing of faces regarding more complex informational cues.
Another point to consider is the fact that many studies have found no difference in behavioral performance between ASD and TD groups despite finding differences in brain function. This was especially the case for face perception involving neutral expressions, where behavioral performance in ASD was equal to that of TD individuals, but brain function between the two groups were significantly different for a number of brain areas in the core face perception network. This highlights the importance of using neuroimaging to determine if individuals with ASD are using different types of cognitive processes in order to perceive faces compared with TD individuals, despite similar behavior performance in face processing tasks. Behavioral work using eye-tracking demonstrates that whereas TD individuals fixate on major features of the face such as the eyes a majority of the time, individuals with ASD tend to fixate on portions of the face that do not contain core facial features (Pelphrey et al., 2002). Accordingly, studies examining eye-fixation demonstrated that individuals with ASD spend less time fixated on the eye region (Dalton et al., 2005; Kliemann et al., 2012; Tottenham et al., 2013) and that manipulating fixation over the eye region can result in significant differences in brain activation in response to faces. Thus, neuroimaging paradigms such as these have the ability to provide information about how neural differences in face processing in ASD may emerge despite normal behavioral performance.
Additionally, the finding that individuals with ASD show similar behavioral performance to TD individuals in tasks utilizing neutral faces but show deficient behavioral performance when those faces display emotional expression suggest that it is not the face itself, but rather the socio-emotional information of faces that causes difficulty in face perception. This would suggest that the automatic fluent processing of emotional expression by TD individuals in such processes as imitation (Carr et al., 2006; Frith, 2007) are not functional in individuals with ASD and that automatic fluent imitation in TD is actually represented by interference in ASD. This is in accord with the idea that atypical face processing in ASD is related to a broader social dysfunction in social processing and that it is not tied to the face itself. Face processing deficits may instead results from atypical function of mechanisms that attempt to automatically extract social information.
The emergence of studies that find atypical functional connections between brain areas despite typical BOLD activation levels within the face perception network (e.g. Wicker et al., 2008) offers an explanation for some of the studies finding no activation differences of the core face perception network between ASD and TD individuals. These studies suggest that atypical face perception can be modulated by aberrant functional connections between brain areas rather than a univariate difference in activation within a single brain area. This again highlights the need for identifying differences in network activation and connectivity between cortical areas rather than simply exploring activation differences in individual cortical areas. Thus, it will be important for future studies to identify how differences in BOLD activation and functional connectivity individually contribute to atypical face perception in ASD.
Individual differences and task type are sure to play a role in differential findings throughout some of the studies showing hypo-, hyper-activation, or no differences of brain activation in ASD compared to TD. A recent study by Scherf and colleagues (2015) demonstrates a relationship for activation patterns of the FG with symptom severity in ASD, while amygdala activation was related to task type. Specifically, more severe symptoms were related to decreased right FG activity while amygdala activation in ASD was much greater in response to scrambled faces compared to TD adolescents. However, it is difficult to arrive at conclusions about individual differences from cross-study comparisons. Future studies will need to explore how individual differences and task type mediate other types of face perception such as expression recognition and gaze detection/cueing.
Interestingly, there did not seem to be any differences between neuroimaging studies exploring younger and older individuals with ASD, as most studies presented with the same general conclusions. This is in contrast to a body of behavioral literature that examines developmental differences in face perception between ASD and TD, and may be due to the fact that most of those studies have focused on behavioral differences between young children with ASD and TD children (<10 years old) (for a review see Golorai et al., 2006), finding that children with ASD do not have the face perception skills that TD children have. In this sense, developmental delays are quantified by the absence of skills. Thus, it would be difficult to find developmental differences between ASD and TD in the current review, as they would not be quantified in the same way. Additionally, while behavioral studies often include lower-functioning children, neuroimaging studies focus on high-functioning individuals who can comply with the demands of the scanner environment, confounding direct comparisons between these types of studies.
The way that developmental changes in the cited studies would be quantified is by changes in magnitude of the BOLD response. However, it is difficult to compare magnitudes of activation across studies utilizing different acquisition and task parameters. Thus, it is unclear if the magnitude of face perception deficits between ASD and TD would change over time as defined by the BOLD signal. Accordingly, it is unclear what types of face perception processes would change the most over time. Some evidence that emotional expression recognition can be improved through exposure and feedback (Bolte et al., 2006) suggests that at least the recognition of emotional expressions can be enhanced through such an approach, but it is unclear if other processes such as gaze detection and gaze cueing can be enhanced in a similar manner.
The question of developmental changes in face perception also ties into the proposal that atypical face perception in ASD is related to a broader social dysfunction and/or social disinterest. The few studies that have examined children generally demonstrate the same type of face processing deficits as observed in adults. However, it may be the case that these studies are conducted in children who have already been guided by a lack of social interest in faces to not develop their face perception skills. That is, these children may already be too old to disentangle the question of how social disinterest relates to face processing. Thus, it is still unknown if atypical face perception is part of the early pathology of ASD or if it is a result of disinterest in faces from birth that leads to poor face perception processing later in life.
There were a few studies that demonstrated how to manipulate brain activation with regards to face perception processes in ASD. Two studies used oxytocin to increase face perception (Domes et al., 2013, 2014), one study used a training program to increase expression recognition (Bolte et al., 2006), while a new area of research focused on forcing eye-fixation in individuals with ASD (e.g., Tottenham et al., 2013). These studies showed that there are several approaches to modifying brain activity in ASD with regards to face perception. Future studies should seek to determine if combining different approaches would further enhance perceptual processing of faces in ASD. For example, it is unclear how ASD eye-fixation is modulated by administration of oxytocin and if some of the cortical changes observed when eye-fixation is forced are still present long-term after oxytocin administration. Additionally, it is unclear if ASD training programs would be more effective if eye-fixation is forced during training or if oxytocin was administered before and during training.
While hypo- and hyper-activity of brain areas within face processing networks in ASD are the most common results reported in the literature, several outstanding questions regarding the generalizability of these findings remain. Autism is a developmental order, with early life onset and variable developmental trajectory (Picci & Scherf, 2014). At present, however, the vast majority of functional activation ASD face-processing studies have been conducted in adults, after a lifetime of compensatory strategies may have already occurred. Thus, there is an urgent need for studies of younger individuals and longitudinal studies of ASD to achieve a greater understanding of the atypical developmental trajectories underlying ASD face processing abnormalities (Amaral, 2011). The functional connectivity literature already recognizes that developmental changes in ASD are under-studied, and that results obtained from adults with the disorder often do not generalize to younger individuals (Uddin et al., 2013). The disorder is typically diagnosed by age three while most neuroimaging studies explore the neural correlates of face processing in children older than age seven. Obtaining data from younger individuals represents a significant challenge that must be addressed in future work and would help to disentangle if poor face perception in ASD is part of the early pathology of the disorder, or if it results from a general disinterest in social stimuli.
In addition to the scarcity of functional imaging studies examining young children with the disorder, there is a gap in our knowledge of gender differences in face processing. Due to the 4:1 ratio of males to females with ASD, most studies have focused disproportionately on males with the disorder; almost nothing is known about the neural representation of faces in females with ASD and whether it differs from that in males.
Despite the considerable progress in studying social cognition in autism as summarized here, another outstanding question remains with respect to the ecological validity of the stimuli used in many studies. More naturalistic social interactions should be investigated in order to assess to what extent the brain differences observed here impact real life social interactions (see for example: Gordon et al., 2013; Redcay et al., 2013, Zaki & Ochsner, 2009).
Finally, as more sophisticated functional brain imaging data analytic approaches are developed, it is increasingly apparent that where univariate analyses can sometimes not be sensitive enough to detect subtle group differences in activation levels, other approaches can prevail. Multivariate pattern analysis (MVPA) examines the pattern of activation across voxels rather than signal intensity levels (Norman et al., 2006). In two recent studies using MVPA, this method was shown to be more sensitive in identifying subtle differences in the patterns of brain activation between ASD and TD groups (Coutanche et al., 2011; Jiang et al., 2013). These newer network approaches will be important in better characterizing differences in face perception in ASD than traditional univariate techniques that focus on isolated brain areas.
In sum, although there has been a tremendous amount of progress in mapping the relationship between atypical face perception and brain function in ASD, more studies need to be done in order better characterize face perception deficits in this population. Additionally, it is imperative that future studies take into consideration that face perception in humans involves a number of overlapping networks related to processing of visual stimuli, social processing, and attention mechanisms rather than individual cortical areas such as the FG, amygdala, and STG. Future work utilizing different analytic techniques including functional connectivity and MVPA, alongside ecologically valid investigations exploring the influences of development and gender, will further elucidate the relationship between atypical face perception and atypical neural activation in ASD, and relationships with broader social deficits in this disorder.
Highlights.
Studies of face processing implicate both cortical and subcortical brain regions
Autism is characterized by atypical activation of several face processing areas
Network approaches to understanding face processing are emerging
Acknowledgments
This work was supported by a National Institute of Mental Health Career Development Award [K01MH092288], a Slifka/Ritvo Innovation in Autism Research Award from the International Society for Autism Research, and a NARSAD Young Investigator Award to LQU.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral DG. The promise and the pitfalls of autism research: an introductory note for new autism researchers. Brain Res. 2011;1380:3–9. doi: 10.1016/j.brainres.2010.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwin C, Baron-Cohen S, Wheelwright S, O'Riordan MO, Bullmore ET. Differential activation of the amygdala and the ‘social brain’ during fearful face-processing in asperger syndrome. Neuropsychologia. 2007;45(1):2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Cognitive neuroscience of human social behavior. Nature Rev Neurosci. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Fear, faces, and the human amygdala. Curr Opin Neurobiol. 2008;18(2):166–172. doi: 10.1016/j.conb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Spezio M. Role of the amygdala in processing visual social stimuli. Prog Brain Res. 2006;156:363–378. doi: 10.1016/S0079-6123(06)56020-0. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav Rev. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Bal E, Harden E, Lamb D, Van Hecke A, Denver J, Porges S. Emotion recognition in children with autism spectrum disorders: Relations to eye gaze and autonomic state. J Autism Dev Disord. 2010;40(3):358–370. doi: 10.1007/s10803-009-0884-3. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Do people with autism understand what causes emotion? Child Dev. 1991;62:385–395. [PubMed] [Google Scholar]
- Baron-Cohen S. Theory of mind and autism: A review. Int Rev Res Ment Ret. 2001;23:169–184. [Google Scholar]
- Baron-Cohen S, Campbell R, Karmiloff-Smith A, Grant J, Walker J. Are children with autism blind to the mentalistic significance of the eyes? Dev Psycho. 1995;12:379–398. [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SCR. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24:355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, William SCR. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Thomas C, Humphreys K. Seeing it differently: Visual processing in autism. Trends Cogn Sci. 2006;10(6):258–264. doi: 10.1016/j.tics.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Bird G, Catmur C, Silani G, Frith C, Frith U. Attention does not modulate neural response s to social stimuli in autism spectrum disorders. NeuroImage. 2006;31:1614–1624. doi: 10.1016/j.neuroimage.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Birmingham E, Cerf M, Adolphs R. Comparing social attention in autism and amygdala lesions: Effects of stimulus and task condition. Soc Neurosci. 2011;6(5-6):420–435. doi: 10.1080/17470919.2011.561547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bolte S, Hubl D, Feineis-Matthews S, Prvulovic D, Dierks T, Poustka F. Facial affect recognition training in autism: Can we animate the fusiform gyrus? Behav Neurosci. 2006;120(1):211–216. doi: 10.1037/0735-7044.120.1.211. [DOI] [PubMed] [Google Scholar]
- BookHeimer SY, Wang AT, Scott A, Sigman M, Dapretto M. Frontal contributions to face processing differences in autism: Evidence from fMRI of inverted face processing. J Int Neuropsycho Soc. 2008;14(6):922–932. doi: 10.1017/S135561770808140X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman M, Fein D, Lucci D, Waterhouse L. Affect comprehension in children with pervasive developmental disorders. J Autism Dev Disord. 1989;19:301–315. doi: 10.1007/BF02211848. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14(6):277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Brothers L. The social brain: A project for integrating primate behavior nad neurophysiology in a new domain. Concepts Neurosci. 1990;1:27–51. [Google Scholar]
- Büchel C, Friston K. Assessing interactions among neuronal systems using functional neuroimaging. Neural Networks. 2000;13:871–882. doi: 10.1016/s0893-6080(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Carey S, Diamon R. From piecemeal to configurational representation of faces. Science. 1977;195:312–314. doi: 10.1126/science.831281. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. P Natl Acad Sci USA. 2003;100(9):5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Celani G, Battachhi MW, Arcidiacono L. The understanding of the emotional meaning of facial expression in people with autism. J Autism Dev Disord. 1999;29(1):57–66. doi: 10.1023/a:1025970600181. [DOI] [PubMed] [Google Scholar]
- Chakrabarti B, Bullmore E, Baron-Cohen S. Empathizing with basic emotions: common and discrete neural substrates. Soc Neurosci. 2006;1:364–384. doi: 10.1080/17470910601041317. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Volkmar F. Automatic attention cueing through eye movement in 2-year-old children with autism. Child Dev. 2003;74:1108–1122. doi: 10.1111/1467-8624.00595. [DOI] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory in autism. Trends Cogn Sci. 2012;16(4):231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K. What can emerging cortical face networks tell us about mature brain organisation? Dev Cog Neurosci. 2011;1:246–255. doi: 10.1016/j.dcn.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K, Cohen Kadosh R, Dick F, Johnson MH. Developmental changes in effective connectivity in the emerging core face network. Cereb cortex. 2011;21:1389–1394. doi: 10.1093/cercor/bhq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K, Johnson MH, Dick F, Cohen Kadosh R, Blakemore SJ. Effects of age, task performance and structural brain development of face processing. Cereb cortex. 2013;23:1630–1642. doi: 10.1093/cercor/bhs150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Carmean V, Ravizza S, Wendelken C, Henry ML, Carter C, Rivera SM. A functional and structural study of emotion and face processing in children with autism. Psychiatry Res. 2009;173(3):196–205. doi: 10.1016/j.pscychresns.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkum V, Moore C. The origins of joint visual attention in infants. Dev Psychol. 1998;34:28–38. doi: 10.1037/0012-1649.34.1.28. [DOI] [PubMed] [Google Scholar]
- Coutanche MN, Thompson-Schill SL, Schultz RT. Multi-voxel pattern analysis of fMRI data predicts clinical symptom severity. NeuroImage. 2011;57(1):113–123. doi: 10.1016/j.neuroimage.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams SCR, Amelsvoort TV, Robertson DM, et al. The functional neuroanatomy of social behavior: Changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123:2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Cukur T, Huth AG, Nishimoto S, Gallant JL. Functional subdomains within human FFA. J Neurosci. 2013;33(42):16748–16766. doi: 10.1523/JNEUROSCI.1259-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin S, Frith U. Vagaries of visual perception in autism. Neuron. 2005;48:497–507. doi: 10.1016/j.neuron.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understading emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MS, Dapretto M, Sigman M, Sepeta L, Bookheimer SY. Neural basis of gaze and emotion processing in children with autism spectrum disorders. Brain Behav. 2011;1(1):1–11. doi: 10.1002/brb3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Carver L, Meltzoff AN, Panagiotides H, McPartland J, Webb SJ. Neural correlates of face and objects recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73(3):700–717. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev Neuropsychol. 2005;27(3):403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- de Greck M, Wang G, Yang X, Wang X, Northoff G, Han S. Neural Substrates underlying intentional empathy. Soc Cog Aff Neurosci. 2010;7(2):135–144. doi: 10.1093/scan/nsq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Lamm C. Human empathy through the lens of social neuroscience. Scientific World Journal. 2006;6:1146–1163. doi: 10.1100/tsw.2006.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeley Q, Daly EM, Surguladze S, Page L, Toal F, Robertson D, et al. An event related functional magnetic resonance imaging study of facial emotion processing in asperger syndrome. Biol Psychiatry. 2007;62:207–217. doi: 10.1016/j.biopsych.2006.09.037. [DOI] [PubMed] [Google Scholar]
- de Heering A, Rossion B, Maurer D. Developmental changes in face recognition during childhood: Evidence from upright and inverted faces. Cognitive Dev. 2012:17–27. [Google Scholar]
- Diamond R, Carey S. Why faces are and are not special: An effect of expertise. J Exper Psychol: Gen. 1986;115(2):107–117. doi: 10.1037//0096-3445.115.2.107. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Beiger A. Social stimuli interfere with cognitive control in autism. NeuroImage. 2007;35(3):1219–1230. doi: 10.1016/j.neuroimage.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Pescosolido MF, Reidy BL, Galvan T, Kim KL, Seymour KE. Developmental meta-analysis of the functional neural correlates of autism spectrum disorders. J Am Acad Child Psy. 2013 doi: 10.1016/j.jaac.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: An activation likelihood estimation meta-analysis. Biol Psychiatry. 2008;65(1):63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes D, Heinrichs M, Kumbier E, Grossman A, Hauenstein K, Herpetz SC. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol Psychiatry. 2013;74(3):164–171. doi: 10.1016/j.biopsych.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Domes D, Kumbier E, Heinrichs M, Herpertz SC. Oxytocin promotes facial recognition in amygdala reactivity in adults with asperger syndrome. Neuropsychopharmacol. 2014;39:698–706. doi: 10.1038/npp.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farroni T, Johhson MH, Brockbank M, Simion F. Infants' use of gaze direction to cue attention: The importance of perceived motion. Vis Cogn. 2000;7:705–718. [Google Scholar]
- Frith CD. The social brain? Philos Trans R Soc. 2007;362:671–678. doi: 10.1098/rstb.2006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358(1431):459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity in neuroimaging: A synthesis. Hum Brain Mapp. 1994;2:56–78. [Google Scholar]
- Gallahger HL, Jack AI, Roepstorff A, Frith C. Imaging the intentional stance in a competitive gaze. NeuroImage. 2002;16:814–821. doi: 10.1006/nimg.2002.1117. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Büchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. P Natl Acad Sci USA. 2010;107(20):9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M, Büchel C. Amygdala activation predicts gaze toward fearful eyes. J Neurosci. 2009;29(28):9123–9126. doi: 10.1523/JNEUROSCI.1883-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M, Schmitz AK, Tittgemeyer M, Schilback L. The human amygdala drives reflexive orienting towards facial features. Curr Biol. 2013;23(20):R917–R918. doi: 10.1016/j.cub.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Gillespie-Lynch K, Elias R, Escudero P, Hutman T, Johnson SP. Atypical gaze following in autism: A comparison of three potential mechanisms. J Autism Dev Disord. 2013;43:2779–2792. doi: 10.1007/s10803-013-1818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu AL, Kuzmanovic B, Schilback L, Tepest R, Kulbida R, Bente G, et al. Neural correlates of “social gaze” processing in high-functioning autism under systematic variation of gaze duration. NeuroImage: Clin. 2013;3:340–351. doi: 10.1016/j.nicl.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelotti DJ, Klin AJ, Gauthier I, Skudlarski P, Cohen DJ, Gore JC, et al. fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsycholgia. 2004 doi: 10.1016/j.neuropsychologia.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Greene DJ, Colich N, Iacoboni M, Zaidel E, Bookheimer SY, Dapretto M. Atypical neural networks for social orienting in autism spectrum disorders. NeuroImage. 2011;56(1):354–362. doi: 10.1016/j.neuroimage.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golorai G, Grill-Spector K, Reiss AL. Autism and the development of face processing. Clinical Neurosci Res. 2006;6:145–160. doi: 10.1016/j.cnr.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golorai G, Ghahremani DG, Whitfield-Gabriel S, Reiss A, Eberhardt JL, Gabrielei, et al. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nat Neurosci. 2007;10:512–522. doi: 10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MC, Mostow AJ, Vecera SP, Larson JC, Mostofsky SH, Mahone EM, et al. Evidence for impairments in using static line drawing of eye gaze cues to orient visual-spatial attention in children with high functioning autism. J Autism Dev Disord. 2008;38:1405–1413. doi: 10.1007/s10803-007-0506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Elibott JA, Feldman R, Pelphrey KA, Vander Wyk BC. Social, reward, and attention brain networks are involved when online bids for joint attention are met with congruent versus incongruent responses. Soc Neurosci. 2013;8(6):544–554. doi: 10.1080/17470919.2013.832374. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Simmons WK, Milbury LA, Wallace G, Cox RW, Martin A. Fractionation of social brain circuits in autism spectrum disorders. Brain. 2012;135(9):2711–2725. doi: 10.1093/brain/aws160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacol Rev. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Chabris CF, Joseph RM, Clark J, McGrath L, Aharon I, Feczk, et al. Early visual cortex organization in autism: an fMRI study. NeuroReport. 2004;2(15):267–270. doi: 10.1097/00001756-200402090-00011. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Chabris CF, Clark J, Steele S, et al. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. NeuroImage. 2004;22:1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex. 2006;16:1276–1282. doi: 10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Abnormal activation of the social brain during face perception in autism. Hum Brain Mapp. 2007;28:441–449. doi: 10.1002/hbm.20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haist F, Adamo M, Han, Lee K, Stiles J. The functional architecture for face-processing expertise: fMRI evidence of the developmental trajectory of the core and the extended face systems. Neuropsychologia. 2013;51(13):2893–2908. doi: 10.1016/j.neuropsychologia.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson C, Hanson SJ, Ramsey J, Glymour C. Atypical effective connectivity of social brain networks in individuals with autism. Brain Connect. 2013;3(6):578–589. doi: 10.1089/brain.2013.0161. [DOI] [PubMed] [Google Scholar]
- Happe F, Frith U. The weak coherence account: Detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. 2006 doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage. 2002;17(1):317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Harms MB, Martin A, Wallace GL. Facial emotion recognition in autism spectrum disorders: A review of behavioral and neuroimaging studies. Neuropsychol Rev. 2010;20:290–322. doi: 10.1007/s11065-010-9138-6. [DOI] [PubMed] [Google Scholar]
- Harris RJ, Young AW, Andrews TJ. Dynamic stimuli demonstrate categorical representation of facial expression in the amygdala. Neurospychologia. 2014;56(100):47–52. doi: 10.1016/j.neuropsychologia.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hernandez LM, Rudie JD, Green SA, Bookheimer S, Dapretto M. Neuropsychopharmacol. 2014 doi: 10.1038/npp.2014.172. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson RP, Ouston J, Lee A. What's in a face? The case of autism. Br J Psychol. 1988;79(4):441–453. doi: 10.1111/j.2044-8295.1988.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nature Neurosci. 2000;3:80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- Hood B, Willen D, Driver J. Adult's eye trigger shifts of visual attention in human infants. Psychol Sci. 1998;9:131–134. [Google Scholar]
- Hubl D, Boelte S, Feineis-Matthews S, Lanfermann H, Federspiel A, Strik W, Poustka F, Dierks T. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61(9):1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- Humphreys K, Hasson U, Avidan G, Minshew N, Behrmann M. Cortical patterns of category-selective activation for faces, places and objects in adults in autism. Autism Res. 2008;1(1):52–63. doi: 10.1002/aur.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, et al. Oxytocin enchances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30(14):4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde KL, Samson F, Evans AC, Mottron L. Neuroanatomical differences in brain areas implicated in perceptual and other core features of autism revealed by cortical thickness analysis and voxel-based morphometry. Hum Brain Mapp. 2009;31(4):556–566. doi: 10.1002/hbm.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7(12):942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Batty M. Neural basis of eye and gaze processing: the core of social cognition. Neurosci Biobehav Rev. 2009;33(6):843–863. doi: 10.1016/j.neubiorev.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian X, Bollich A, Cox P, Hyder E, James J, Gowani SA, et al. A quantitative link between face discrimination deficits and neuronal selectivity for faces in autism. NeuroImage: Clin. 2013;(2):320–331. doi: 10.1016/j.nicl.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemel B, Mottron L, Dawson M. Impaired face processing in autism: Fact or artifact? J Autism Dev Disord. 2006;36(1):91–106. doi: 10.1007/s10803-005-0050-5. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Subcortical face processing. Nat Rev Neurosci. 2005;6(10):766–774. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Kadosh KC, Johnson MH, Henson RNA, Dick F, Blakemore SJ. Diffferential face-network adaptation in children, adolescence and adults. NeuroImage. 2013;69:11–20. doi: 10.1016/j.neuroimage.2012.11.060. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Guastella AJ. The role of oxytocin in human affect: A novel hypothesis. Psychological Science. 2011;20(4):222–231. [Google Scholar]
- Kana RK, Just MA. Autism as a disorder of functional brain connectivity. In: Amaral DG, Geschwind D, Dawson G, editors. Handbook of Autism Spectrum Disorders. New York: Oxford University Press; 2011. pp. 981–9. [Google Scholar]
- Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Phil Trans R Soc B. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J of Neurosci. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn Sci. 2012;16(11):559–572. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy L, Wallace GL, Birn R, Milleville SC, Case LK, Bandettini PA, et al. Aberrant neural mediation of verbal fluency in autism spectrum disorders. Brain Cognition. 2013;83(2):218–226. doi: 10.1016/j.bandc.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner J, Hatri A, Heekeren H, Dziobek I. Autistic symptomatology, face processing abilities, and eye fixation patterns. J Autism Dev Disord. 2011;41(2):158–167. doi: 10.1007/s10803-010-1032-9. [DOI] [PubMed] [Google Scholar]
- Klein JT, Shepherd SV, Patt ML. Social attention and the brain. Curr Biol. 2009;19(20):1–11. doi: 10.1016/j.cub.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(4):1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Johnson LC, Richards T, Mahurin R, Greenson J, Dawson G, Aylward E. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am J Psychiatry. 2009;166:467–475. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Weaver K, Johnson LC, Greenson Dawson G, et al. Association between amygdala resonse to emotional faces and social anxiety in autism spectrum disorders. Neuropsychologia. 2010;48(12):3665–3670. doi: 10.1016/j.neuropsychologia.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Johnson LC, Weaver KE, Greenson J, Dawson G, Aylward E. fMRI evidence of neural abnormalities in the subcortical face processing system in ASD. NeuroImage. 2011;54(1):697–704. doi: 10.1016/j.neuroimage.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleimann F, Dziobek I, Hatri A, Steimke R, Heekeren HR. Atypical reflexive gaze patterns on emotional faces in autism spectrum disorders. J Neurosci. 2012;30(37):12281–12287. doi: 10.1523/JNEUROSCI.0688-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Koshino H, Kana R, Keller TA, Cherkasky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: Visual coding and underconnectivity with frontal areas. Cereb Cortex. 2008;18:289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kylliänen A, Hietanen JK. Attention orienting by another's gaze direction in children with autism. J Child Psychol Psychiat. 2004;45:435–444. doi: 10.1111/j.1469-7610.2004.00235.x. [DOI] [PubMed] [Google Scholar]
- Kylliänen A, Hietanen JK. Skin conductance responses to another person's gaze in children with autism. J Autism Dev Disord. 2006;36:517–525. doi: 10.1007/s10803-006-0091-4. [DOI] [PubMed] [Google Scholar]
- Lavin C, Melis C, Mikulan E, Gelormini C, Huepe D, Ibanez A. The anterior cingulate cortex: An integrative hub for socially-driven interactions. Front Neurosci. 2013;7(64):1–4. doi: 10.3389/fnins.2013.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leekam SR, Hunnisett E, Moore C. Tagets and Cues: Gaze-following in children with autism. J Child Psychol Psychiat. 1998;39(7):951–962. [PubMed] [Google Scholar]
- Leekam SR, Lopez B, Moore C. Attention and joint attention in preschool children with autism. Dev Psychol. 2000;36(2):261–273. doi: 10.1037//0012-1649.36.2.261. [DOI] [PubMed] [Google Scholar]
- Liakakis G, Nickel J, Sietz RJ. Diversity of the inferior frontal gyrus - A meta-analysis of neuroimaging studies. Behav Brain Res. 2011;225:341–347. doi: 10.1016/j.bbr.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Linquist KA, Barrett LF. A functional architecture of the human brain: emerging insights from the science of emotion. Trends Cogn Sci. 2012;16(11) doi: 10.1016/j.tics.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malisza KL, Clancy C, Shiloff D, Holden J, Jones C, Paulson K, et al. Functional magnetic resonance imaging of facial information processing in children iwith autistic disorder, attention deficit hyperactivity disorder and typically developing controls. Int J Adolescent Medicine and Health. 2011;23(3):269–277. doi: 10.1515/ijamh.2011.055. [DOI] [PubMed] [Google Scholar]
- Maximo JO, Cadena EJ, Kana RK. The implications of brain connectivity in the neuropsychology of autism. Neuropsychol Rev. 2014;24(1):16–31. doi: 10.1007/s11065-014-9250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKone E, Crookes K, Jeffery L, Dilks D. A critical review of the development of face recognition: Experiences is less important than previously believed. Cogn Neuropsychol. 2012;1-2:174–212. doi: 10.1080/02643294.2012.660138. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28(5):597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Miyahara M, Harada T, Ruffman T, Sadato N, Iidaka T. Functional connectivity between amygdala and facial regions involved in recognition of facial threat. Soc Cog Aff Neurosci. 2011;8:181–190. doi: 10.1093/scan/nsr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, and attention and control: A network model of insula function. Brain Struct Funct. 2010;214(5-6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar-Szakacs I, Uddin LQ. Self-processing and the default mode network: Interactions with the mirror neuron system. Front Hum Neurosci. 2013;7(571) doi: 10.3389/fnhum.2013.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Weng SJ, Wiggins JL, Kurapati N, Louro HMC, Carrasco M, et al. Neural circuitry of emotional face processing in autism spectrum disorders. J Psychiatry Neurosci. 2010;35(2):105–114. doi: 10.1503/jpn.090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulieres I, Hubert B, Burack J. Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. J Autism Dev Disord. 2006;36(1):27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Müller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. 2011;21(10):2233–2243. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P. Annotation: The neural basis of social impairments in autism: the role of the dorsal medial-frontal cortex and anterior cingulate system. J Child Psychol Psyc. 2003;44(6):793–809. doi: 10.1111/1469-7610.00165. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Foss-Feig J, Kenworthy L, Gaillard W, Vaidya CJ. Atypical functional connectivity of the amygdala in childhood autism spectrum disorders during spontaneous attention to eye-gaze. Autism Res Treat. 2012;2012:1–12. doi: 10.1155/2012/652408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Tanaka K, Endo Y, Yamnae Y, Tamamoto T, Nakano Y, et al. Atypical gaze patterns in children and adults with autism spectrum disorders dissociated from developmental changes in gaze behavior. Poc R Soc B. 2010;277(1696):2935–2943. doi: 10.1098/rspb.2010.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D, Spezio ML, Piven J, Adolphs R. Looking you in the mouth: Abnormal gaze in autism resulting from impaired top-down attentional modulations of visual attention. Soc Cog Affective Neurosci. 2006;1(3):194–202. doi: 10.1093/scan/nsl030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickl-Jockschat T, Rottschy C, Thommes J, Schneider F, Laird AR, Fox PT, Eickhoff SB. Neural networks related to dysfunctional face processing in autism spectrum disorder. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0791-z. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi JS, Scherfeld D, Friederichs S, Schäfer R, Franz M, Wittsack J, Azari NP, et al. On the neural networks of empathy: A principal component analysis of an fMRI study. Behav Brain Funct. 2008;4(41):1–13. doi: 10.1186/1744-9081-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn Sci. 2006;10(9):424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Calder AJ. Neural mechanisms of social attention. Trends Cogn Sci. 2009;13:135–143. doi: 10.1016/j.tics.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Nuske HJ, Vivanti G, Dissanayake C. Are emotion impairments unique to, universal, or specific in autism spectrum disorder? A comprehensive review. Cognition & Emotion. 2013;27(6) doi: 10.1080/02699931.2012.762900. [DOI] [PubMed] [Google Scholar]
- O'Neil EB, Hutchinson RM, McLean DA, Köhler S. Resting-state fMRI reveals functional connectivity between face-selective perirhinal cortex and the fusiform face area related to face inversion. NeuroImage. 2014;15(92):349–355. doi: 10.1016/j.neuroimage.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Pennington BF, Rogers SJ. Are there emotional perception deficits in young autistic children? J Child Psychol Psyc. 1990;31:343–361. doi: 10.1111/j.1469-7610.1990.tb01574.x. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Cai X. Orbitofrontal cortex and the computation of subjective value: Consolidated concepts and new perspectives. Ann N Y Acad Sci. 2011;1239:130–137. doi: 10.1111/j.1749-6632.2011.06262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Carter EJ. Brain mechanisms for social perception: lessons from autism and typical development. Ann N Y Acad Sci. 2008;1145:283–299. doi: 10.1196/annals.1416.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G, LaBar KS. Perception of dynamic changes in facial affect and identity in autism. Soc Cog Aff Neu. 2007;2:140–149. doi: 10.1093/scan/nsm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. J Autism Dev Disord. 2002;32(4):249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Singerman JF, Allison T, McCarthy G. Brain activation evoked by perception of gaze shifts: the influence of context. Neuropsychologia. 2003;41:156–170. doi: 10.1016/s0028-3932(02)00146-x. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, Vander Wyk BC. Research review: constraining heterogeneity: The social brain and its development in autism spectrum disorder. J Child Psychol Psyc. 2011;52(6):631–644. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Hudac CM, Pegors T, Minshew NJ, Pelphrey KA. Experimental manipulation of face-evoked activity in the fusiform gyrus in individuals with autism. Soc Neurosci. 2011;6(1):22–30. doi: 10.1080/17470911003683185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. Understanding brain networks and brain organization. Physics of Life Reviews. doi: 10.1016/j.plrev.2014.03.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. P Natl Acad Sci USA. 2002a;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider G. Attentional control of the processing of neutral and emotional stimuli. Cogn Brain Res. 2002b;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- Pfeiffer UJ, Vogeley K, Schilbach L. From gaze cueing to dual eye-tracking: Novel approaches to investigate the neural correlates of gaze in social interaction. Neurosci Biobehav Rev. 2013;37(10 pt 2):2516–2518. doi: 10.1016/j.neubiorev.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Picci G, Scherf KS. A two-hit model of autism: Adolescence as the second hit. Clinical Psychol Sci. 2014:1–23. doi: 10.1177/2167702614540646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the ‘fusiform face area’ in autism: evidence from functional MRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Pierce K, Redcay E. Fusiform function in children is a matter of “who”. Biol Psychiatry. 2008;64(7):552–560. doi: 10.1016/j.biopsych.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot J, Kwon H, Mobbs D, Blasey C, Lotspeich L, Menon V, et al. Emotional attribution in high-functioning individuals with autistic spectrum disorder: A functional imaging study. J Am Acad Child Adolesc Psychiatry. 2004;43(4):473–480. doi: 10.1097/00004583-200404000-00014. [DOI] [PubMed] [Google Scholar]
- Pitskel NB, Boiling DZ, Hudac CM, Lantz SD, Minshew NJ, Vander Wyk BC, et al. Brain mechanisms for processing direct and averted gaze in individuals with autism. J Autism Dev Disord. 2011;41(12):1686–1693. doi: 10.1007/s10803-011-1197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, de Waal FB. Empathy: Its ultimate and proximate bases. Behav Brain Sci. 2002;25(1):1–120. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Bentin S, Gore J, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. J Neurosci. 1998;18:2188–2199. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E. The superior temporal sulcus performs a common function for social and speech perception: implications for the emergence of autism. Neurosci Biobehav Rev. 2008;32(1):123–142. doi: 10.1016/j.neubiorev.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Redcay E, Dodell-Feder D, Mavros PL, Mavros PL, Kleiner M, Pearrow MJ, Triantafyllou C, et al. Atypical brain activation patterns during a face-to-face joint attention game in adults with autism spectrum disorder. Hum Brain Mapp. 2013;34(10):2511–2523. doi: 10.1002/hbm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richer JM, Cross RG. Gaze aversion in autistic and normal children. Acta Psychiatr Scand. 1976;53:193–210. doi: 10.1111/j.1600-0447.1976.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Ristic J, Mottron L, Friesen CK, Iarocci G, Burack JA, Kingstone A. Eyes are special but not for everyone: the case of autism. Cogn Brain Res. 2005;24:715–718. doi: 10.1016/j.cogbrainres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Behrmann M, Humphreys K, Luna B. Visual category-selectivity for faces, places and objects emerges along different developmental trajectories. Devi Sci. 2007;10(4):15–30. doi: 10.1111/j.1467-7687.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Elbich D, Minshew N, Behrmann M. Indiivduals differences in symptom severity and behavior predict neural activation during face processing in adolescents with autism. NeuroImage: Clinical. 2015;7:53–67. doi: 10.1016/j.nicl.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Luna B, Minshew N, Behrmann M. Location, location, location: alterations in the functional topography of face- but not object- or place-related cortex in adolescents with autism. Front Hum Neurosci. 2010;22:1–16. doi: 10.3389/fnhum.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Rüther M, Greimel E, Markowitsch HJ, Kamp-Becker I, Remschmidt H, Fink GR, et al. Dysfunctions in brain networks supporting empathy: an fMRI study in adults with autism spectrum disorders. Soc Neurosci. 2011;6(1):1–21. doi: 10.1080/17470911003708032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, Skudlarski P, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and asperger syndrome. Arch Gen Psyc. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Devl Neuroscience. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schultz R, Grelotti DJ, Klin A, Kleinman J, Van de Gaag C, Marois R, Skudlarski P. The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philos Trans R Soc London, Se B Biol Sci. 2003;358:415–427. doi: 10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A, Johnson MH. Atypical eye contact in autism: models, mechanism and development. Neurosci Biobehav Rev. 2009;33:1204–1214. doi: 10.1016/j.neubiorev.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Senju A, Tojo Y, Dairoku H, Hasegawa T. Reflexive orienting in response to eye gaze and arrow in children with and without autism. J Child Psychol Psyc. 2004;45:445–458. doi: 10.1111/j.1469-7610.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- Simmons DR, Robertson AE, McKay LS, Toal E, McAleer Ph, Pollick FE. Vision in autism spectrum disorders. Vision Res. 2009;49:2705–2739. doi: 10.1016/j.visres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Simpson EA, Sclafani V, Paukner A, Hamel AF, Noval MA, Meyer JS, et al. Inhaled oxytocin increases positive social behaviors in newborn macaques. P Natl Acad Sci USA. 2014;111(19):6922–6927. doi: 10.1073/pnas.1402471111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spezio ML, Huang PY, Castelli F, Adolphs R. Amygdala damage impairs eye contact during conversations with real people. J Neurosci. 2007;27:3994–3997. doi: 10.1523/JNEUROSCI.3789-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka JW. The entry point of face recognition: evidence for face expertise. J Exp Psychol Gen. 2001;130(3):534–543. doi: 10.1037//0096-3445.130.3.534. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Curran T. A neural basis for expert object recognition. Psychological Science. 2001;12(1):43–47. doi: 10.1111/1467-9280.00308. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Farah MJ. Parts and wholes in face recognition. Q J Exp Psychol. 1993;46A(2):225–245. doi: 10.1080/14640749308401045. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H. Evaluating the theory-of-mind hypothesis of autism. Curr Dir Psychol Sci. 2007;16(6):311–315. [Google Scholar]
- Tottenham N, Hertzig ME, Gillespie-Lynch K, Gilhooly T, Millner AJ, Casey BJ. Elevated amygdala response to faces and face aversion in autism spectrum disorder. Soc Cog Aff Neurosci. 2013;9(1):106–117. doi: 10.1093/scan/nst050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunisse JP, De Gelder B. Impaired categorical perception of facial expressions in high-functioning adolescents with autism. Child Neuropsychol. 2001;7(1):1–14. doi: 10.1076/chin.7.1.1.3150. [DOI] [PubMed] [Google Scholar]
- Uljarevic M, Hamilton A. Recognition of emotions in autism: A formal metaanalysis. J Autism Dev Disord. 2012;43(7):1517–1526. doi: 10.1007/s10803-012-1695-5. [DOI] [PubMed] [Google Scholar]
- Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2014 doi: 10.1038/nrn3857. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Front Syst Neurosci. 2010;4(21) doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Superkar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci. 2013;7(458) doi: 10.3389/fnhum.2013.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Foss-Feig J, Shook D, Kaplan L, Kenworthy L, Gaillard W. Controlling attention to gaze and arrows in childhood: an fMRI study of typical development and autism spectrum disorders. Developmental Sci. 2011;14(4):911–924. doi: 10.1111/j.1467-7687.2011.01041.x. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others' actions and ogals my mirror and mentalizing systems: A meta-analysis. NeuroImage. 2009;48:564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Vissers ME, Cohen MX, Geurts HM. Brain connectivity and high functioning autism: A promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci Biobehav Rev. 2012;36(1):604–625. doi: 10.1016/j.neubiorev.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Vlamings PH, Stauder JE, van Son IA, Mottron L. Atypical visual orienting to gaze- and arrow-cues in adults with high functioning autism. J Autism Dev Disord. 2005;35:267–277. doi: 10.1007/s10803-005-3289-y. [DOI] [PubMed] [Google Scholar]
- von dem Hagen EAH, Stoyanova RS, Rowe JB, Baron-Cohen S, Calder AJ. Direct gaze elicits atypical activation of the theory-of-mind network in autism spectrum conditions. Cereb Cortex. 2014;4(24):1485–1492. doi: 10.1093/cercor/bht003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat Neurosci. 2003;6:624–631. doi: 10.1038/nn1057. [DOI] [PubMed] [Google Scholar]
- Wang AT, Dapretto M, Hariri AR, Sigman M, Bookheimer SY. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Psy. 2004;43(4):481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Wang S, Tudusciuc O, Mamelak AN, Ross LB, Adolphs R, Rutishauser U. Neurons in the human amygdala selective for perceived emotion. P Natl Acad Sci USA. 2014:1–11. doi: 10.1073/pnas.1323342111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt S, Koldewyn K, Kanwisher N. Face identity recognition in autism spectrum disorder: A review of behavioral studies. Neurosci Biobehav Rev. 2012;36:1060–1084. doi: 10.1016/j.neubiorev.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Weiner KS, Grill-Spector K. Sparsely-distributed organization of face and limb activations in human ventral temporal cortex. NeuroImage. 2010;52(4):1559–1573. doi: 10.1016/j.neuroimage.2010.04.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng SJ, Carrasco M, Swartz JR, Wiggins JL, Kurapati N, Liberzon I, et al. Neural activation to emotional faces in adolescents with autism spectrum disorders. J Child Psychol Psychiatry. 2011;52(3):296–305. doi: 10.1111/j.1469-7610.2010.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Raila H, Bennett R, Mattek A, Brown A, Taylor J, et al. Neuroscience and facial expressions of emotion: The role of amygdala-prefrontal interactions. Emotion Rev. 2013;5(1):78–83. [Google Scholar]
- Wicker B, Michel F, Henaff MA, Decety J. Brain regions involved in the perception of gaze: A PET study. NeuroImage. 1998;8:221–227. doi: 10.1006/nimg.1998.0357. [DOI] [PubMed] [Google Scholar]
- Wicker B, Fonlupt P, Hubert B, Tardif C, Gepner B, Deruelle Abnormal cerebral effective connectivity during explicit emotional processing in audits with autism spectrum disorder. Soc Cog Aff Neurosci. 2008;3:135–143. doi: 10.1093/scan/nsn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JS, Henson RN, Fine-Goulen MR, Dolan RJ. fMRI-adaptation reveals dissociable neural representations of identity and expression in face perception. J Neurophysiol. 2004;92(3):1830–1839. doi: 10.1152/jn.00155.2004. [DOI] [PubMed] [Google Scholar]
- Zaki J, Ochsner K. The need for a cognitive neuroscience of naturalistic social cognition. Ann N Y Acad Sci. 2009;1167:16–30. doi: 10.1111/j.1749-6632.2009.04601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Res Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- Zhang H, Tian J, Liu J, Li J, Lee K. Intrinsically organized network for face perception during the resting state. Neurosci Lett. 2009;454(1):1–5. doi: 10.1016/j.neulet.2009.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Zhang J, Luo YLL, Dilks DD, Liu J. Resting-state neural activity across face-selective cortical regions is behaviorally relevant. J of Neurosci. 2011;31(28):10323–10330. doi: 10.1523/JNEUROSCI.0873-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, Boddaert N. Autism, the superior temporal sulcus and social perception. Trends in Neurosci. 2006;29(7):359–366. doi: 10.1016/j.tins.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Zürcher NR, Donnelly N, Rogier O, Russo B, Hippolyte L, Hadwin J, et al. It's all in the eyes: Subcortical and cortical activation during grotesqueness perception in autism. PLOS. 2013;8(1) doi: 10.1371/journal.pone.0054313. [DOI] [PMC free article] [PubMed] [Google Scholar]


