ABSTRACT
In the filamentous cyanobacterium Anabaena, patS and hetN encode peptide-derived signals with many of the properties of morphogens. These signals regulate the formation of a periodic pattern of heterocysts by lateral inhibition of differentiation. Here we show that intercellular transfer of the patS- and hetN-dependent developmental signals from heterocysts to vegetative cells requires HetC, a predicted ATP-binding cassette transporter (ABC transporter). Relative to the wild type, in a hetC mutant differentiation resulted in a reduced number of heterocysts that were incapable of nitrogen fixation, but deletion of patS or hetN restored heterocyst number and function in a hetC background. These epistasis results suggest that HetC is necessary for conferring self-immunity to the inhibitors on differentiating cells. Nine hours after induction of differentiation, HetC was required for neither induction of transcription of patS nor intercellular transfer of the patS-encoded signal to neighboring cells. Conversely, in strains lacking HetC, the patS- and hetN-encoded signals were not transferred from heterocyst cells to adjacent vegetative cells. The results support a model in which the patS-dependent signal is initially transferred between vegetative cells in a HetC-independent fashion, but some time before morphological differentiation of heterocysts is complete, transfer of both signals transitions to a HetC-dependent process.
IMPORTANCE How chemical cues that regulate pattern formation in multicellular organisms move from one cell to another is a central question in developmental biology. In this study, we show that an ABC transporter, HetC, is necessary for transport of two developmental signals between different types of cells in a filamentous cyanobacterium. ABC transporters are found in organisms as diverse as bacteria and humans and, as the name implies, are often involved in the transport of molecules across a cellular membrane. The activity of HetC was shown to be required for signaling between heterocysts, which supply fixed nitrogen to the organism, and other cells, as well as for conferring immunity to self-signaling on developing heterocysts.
INTRODUCTION
Differentiation of heterocysts by filamentous cyanobacteria represents a simple but elegant model of biological patterning. In response to a shortage of combined nitrogen, linear filaments of Anabaena sp. strain PCC 7120 (here, Anabaena) form a periodic pattern of single, terminally differentiated, nitrogen-fixing heterocysts separated by approximately 10 totipotent vegetative cells. The spacing between heterocysts reflects the metabolic interdependence of the two cell types; fixed nitrogen is supplied to vegetative cells from heterocysts, and in return, heterocysts receive a source of carbon and reductant to compensate for their lack of photosystem II and the Calvin cycle (for a review of heterocyst differentiation, see reference 1). As filaments lengthen by growth and division of vegetative cells, subsequent rounds of heterocyst differentiation place new heterocysts between existing ones to preserve the pattern and vegetative cell-to-heterocyst ratio during prolonged diazotrophic growth.
Between 9 and 13 h after induction of differentiation by removal of combined nitrogen, cells that have been designated to differentiate irreversibly commit to a heterocyst fate; removing the signal for induction after this time by supplying filaments with fixed nitrogen does not reverse or stop the differentiation process (2). Morphological differentiation of functional heterocysts is complete after about 24 h. There are two prominent theories on how the cells that differentiate are specified. In the first, a small diffusible peptide, PatS, acts by lateral inhibition in a Turing-like reaction-diffusion fashion to differentially regulate the activity of HetR, an activator of differentiation, to specify individual cells that will differentiate (3–7). Once the initial pattern is specified for the initial round of differentiation, it has been proposed that a second diffusible inhibitor, HetN, participates in the placement of heterocysts in subsequent rounds of differentiation (8, 9). Recent experimental evidence, however, questions an underlying assumption of this first theory, that at the time of induction of differentiation each cell of a filament has the same potential to become a heterocyst. In the second theory of patterning, biased inheritance of PatN, an integral membrane protein, specifies small groups of contiguous cells with increased competency for differentiation prior to induction of differentiation (10, 11). These groups of cells are arranged in a periodic pattern, and upon induction by nitrogen starvation, differentiation is limited to a single cell from each competent group by lateral inhibition initially by PatS and later by HetN.
The patS and hetN genes encode developmental signals that have many of the characteristics of morphogens (3, 7, 12). These signals move from source cells to neighboring vegetative cells to prevent their differentiation (3, 7, 13, 14). Both patS and hetN encode putative peptides that contain the sequence RGSGR, which is necessary for inhibitory activity and represents the C terminus of the putative product of patS (3, 7, 13). The RGSGR pentapeptide binds to HetR, preventing it from binding to DNA targets and promoting its degradation (5, 15, 16). HetR is a transcription factor that promotes differentiation. In addition to regulating the transcription of a large number of morphogenesis and metabolism genes, HetR positively autoregulates its own production and is necessary for induction of transcription of patS and hetN (16–18). The full-length 17-amino-acid (17-aa) patS-encoded peptides and 287-aa hetN-encoded peptides do not appear to move from cell to cell, and the presumed fragments that do are unknown (3, 12). However, experimental evidence suggests a C-terminal 5-, 6-, or 8-aa peptide cleavage product in the case of patS (3, 13, 19).
HetC from Anabaena is a predicted ATP-binding cassette transporter (ABC transporter) that is a potential facilitator of intercellular movement of the patS- and/or hetN-encoded signal. Its architecture, an ATP-binding domain, a peptidase domain, and an inner membrane domain in a single protein, suggests that it functions as an exporter (20). Mutants with insertions in hetC do not make mature heterocysts, but they do show patterned expression of hetR and hetC (21, 22). Expression of hetR in groups of contiguous cells, rather than in individual cells, has led to the suggestion that HetC may be involved in the transition of heterocysts to a nondividing state (22). In addition, expression of patS and hetN at 24 and 48 h postinduction is apparent in a subset of the undifferentiated cells of a hetC insertion mutant (23). It has been hypothesized that the phenotype of the mutant is a consequence of a lack of inhibitor export from source cells. A lack of inhibitor export could result in inhibition of differentiation of these source cells rather than inhibitor export to, and inhibition of, neighboring cells, as occurs in the wild type (24). The findings of a recent epistasis analysis with hetC and patS null mutants were consistent with this hypothesis (25).
Two approaches for detection of the patS- and hetN-encoded signals have recently been described; an antibody directed against the RGSGR peptide motif has been used to visualize the patS-encoded signal in cells adjacent to heterocysts (3), and patS/hetN-dependent degradation of HetR has been used to examine signal activity in cells adjacent to source cells producing the patS- and/or hetN-encoded signal (5, 12). In the latter approach, source cells can be genetically engineered to produce one of the signals or can be vegetative cells or heterocysts in which transcription of patS or hetN or both has been induced by removal of combined nitrogen. Source cells are typically tagged with a fluorophore to aid in their identification. A fluorescent form of HetR produced in all cells acts as an indicator of patS- and/or hetN-encoded signal activity adjacent to source cells because each of the signals independently promotes degradation of HetR with a concomitant decrease in fluorescence. This system was recently used to show that the number of vegetative cells affected by the hetN paracrine signal, the signal range, was reduced in strains lacking SepJ, a channel-forming protein that facilitates intercytoplasmic communication between adjacent cells (12). Channels connecting the cells of Anabaena filaments were recently visualized by electron tomography, but the composition of these channels is unknown (26). Here we provide genetic and cytologic evidence for the involvement of HetC in the transfer of patS- and hetN-encoded inhibitors from mature heterocysts to adjacent vegetative cells.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The growth of Escherichia coli and wild-type Anabaena sp. strain PCC 7120 and its derivatives, concentrations of antibiotics, and the induction of heterocysts in media lacking a source of combined nitrogen were as previously described (27, 28). Growth medium containing 6 mM ammonia as a nitrogen source was prepared as previously described and used to grow strains prior to induction of differentiation of heterocysts (29). To assess diazotrophic growth, single colonies of the wild type, all mutant strains, and a ΔhetR mutant (UHM103) as a negative control were streaked onto three independent plates containing BG-11 medium lacking a combined nitrogen source and incubated for 2 weeks. Plates were assessed visually, and strains were considered positive for diazotrophic growth if any growth was visible; the ΔhetR mutant did not display visible growth under these conditions. To determine heterocyst percentages, 500 cells were counted, and only those that appeared morphologically distinct and stained with alcian blue were recorded as heterocysts. The frequencies of single, double, and higher numbers of contiguous heterocysts were determined by counting 300 heterocyst occurrences. All results are expressed as the average of three replicates, and error bars represent one standard deviation. The mean vegetative cell interval between heterocysts was determined by counting 300 intervals. All results are expressed as the mean and the standard deviation of the mean. Plasmids were introduced into Anabaena strains by conjugation from E. coli as previously described (30). Expression from the copper-inducible petE promoter was achieved with the addition of copper to a final concentration of 2 μM (31).
Plasmid construction.
Plasmids and oligonucleotide primers used in this study are listed in Table S4 in the supplemental material. The integrity of all PCR-derived products was verified by sequencing. Plasmid pPJAV247 is a mobilizable shuttle vector containing PpetE-hetR(H69Y)-CFP transcribed divergently from PpatS-YFP. The coding region of cyan fluorescent protein (CFP) was amplified by PCR using pUC57-PS12-cfp (32) as a template with the primers CCFP2-PpetE-OEX-F and CCFP2-SacI-R. The petE promoter was amplified from chromosomal DNA with the primers PpetE-XhoI-F and CCFP2-PpetE-OEX-R. The petE promoter was fused to CFP by overlap extension PCR (33), cloned into the SmaI site in pBlueScript SK(+) (Stratagene), and subsequently moved as a XhoI-SacI fragment into the SalI-SacI sites of pPJAV123 (34) to create pPJAV243. The coding region of hetR(H69Y) was amplified from pDR306 (5) by using the primers HetR-F-NdeI-express and hetR-tln-BamHI-R, cloned into the SmaI site in pBlueScript SK(+), and subsequently moved as a NdeI-BamHI fragment into pPJAV243. The patS promoter region was amplified by PCR from pAM1951 with the primers PpatS-MunI-F and PpatS-OEX-R. The coding region of yellow fluorescent protein (YFP) was amplified using pUC57-PS12-yfp (32) as a template with the primers Turbo-OEX-F and YFP-MunI-R. The patS promoter and YFP were fused via overlap extension PCR and cloned into the SmaI site in pBlueScript SK(+). The PpatS-YFP fragment was moved as a MunI fragment into the EcoRI site of pPJAV243 containing hetR(H69Y) and screened by PCR to determine directionality to create pPJAV247.
Plasmid pKH206 is a suicide plasmid based on pRL277 that was used to cleanly delete the entire coding region of the hetC gene (alr2817). Regions upstream and downstream of hetC were amplified by PCR from chromosomal DNA with the primer sets del-hetC-up-F/del-hetC-up-R and del-hetC-dn-F/del-hetC-dn-R, respectively. The up- and downstream products were fused together by overlap extension PCR and cloned as a BglII-SacI fragment into the same sites of pRL277 to create pKH206.
Strain creation.
All Anabaena strains used in this study are listed in Table S4 of the supplemental material. Clean deletion of the hetC coding region and replacement of the coding region of hetN or patS with an Ω interposon that conferred resistance to spectinomycin and streptomycin via the plasmid pKH206, pSMC164, or pSMC182, respectively, was accomplished by allelic exchange as previously described (8, 27, 35). Strains with mutations in the hetC, hetN, or patS gene were verified by PCR with the primer sets del-hetC-dn-out/del-hetC-up-out, patSfor/patSrev, and up-hetN-F/down-hetN-R, respectively, which anneal outside the region used to make the deletion. Chromosomal alterations were made in the order indicated in Table S4.
Microscopy, alcian blue staining, and acetylene reduction assays.
Cells were routinely viewed and imaged as previously described (27). Confocal microscopy and determination of the signal ranges of patS- and hetN-dependent activities using ImageJ were conducted as previously described (12), and EcFbFP was imaged as described previously (36). Heterocyst-specific exopolysaccharides were stained with alcian blue as previously described (28). Aerobic and anaerobic acetylene reduction assays were performed as previously described (18, 27, 37).
RESULTS
A hetC mutant forms morphologically distinct heterocysts.
Strains of Anabaena with an otherwise wild-type genetic background that have hetC inactivated with the incorporation of foreign DNA, either a transposon or an omega interposon, do not make heterocysts (21, 25). However, a strain with the predicted peptidase domain cleanly deleted differentiates a reduced number of heterocysts that are inactive (25). In the former case, the mutation has the potential to be polar, and in the latter, only one domain of the protein is removed. To determine the hetC-null phenotype, a mutant with the entire hetC coding region cleanly deleted was created (strain UHM232), and the phenotype was assessed. Twenty-four hours after induction of differentiation by removal of combined nitrogen, strain UHM232 was similar to previously described insertion mutants; it did not produce morphologically distinct proheterocysts or mature heterocysts. However, at 48 h after induction, a small number of morphologically distinct heterocysts were observed. At 48 h, about 3% of cells in filaments were heterocysts, and by 72 h and thereafter 4 to 5% of cells were heterocysts (Fig. 1; see also Table S1 in the supplemental material). In contrast, the wild type had about 9% heterocysts 24 h after induction and thereafter. Heterocysts from the hetC mutant were distinctly larger than vegetative cells, were visibly less pigmented, had thicker cell envelopes, and were readily stained with alcian blue, which preferentially binds to heterocyst polysaccharides. However, filaments of strain UHM232 were incapable of diazotrophic growth and did not fix nitrogen under oxic or anoxic conditions, as indicated by an acetylene reduction assay (see Fig. S1 in the supplemental material). The phenotype of strain UHM232 is similar to that of the recently reported peptidase domain mutant and, unlike the early developmental role speculated for hetC based on mutants that have been reported to make small dividing proheterocyst-like cells (22), no small cells were observed in this or the previous work (25). The nonpolar nature of the mutations in these two strains suggests that they represent the true hetC-null phenotype. Therefore, HetC is not absolutely required for many of the morphological changes that distinguish heterocysts, but in its absence the number of cells that differentiate is limited and the timing of morphogenesis is delayed and incomplete.
FIG 1.
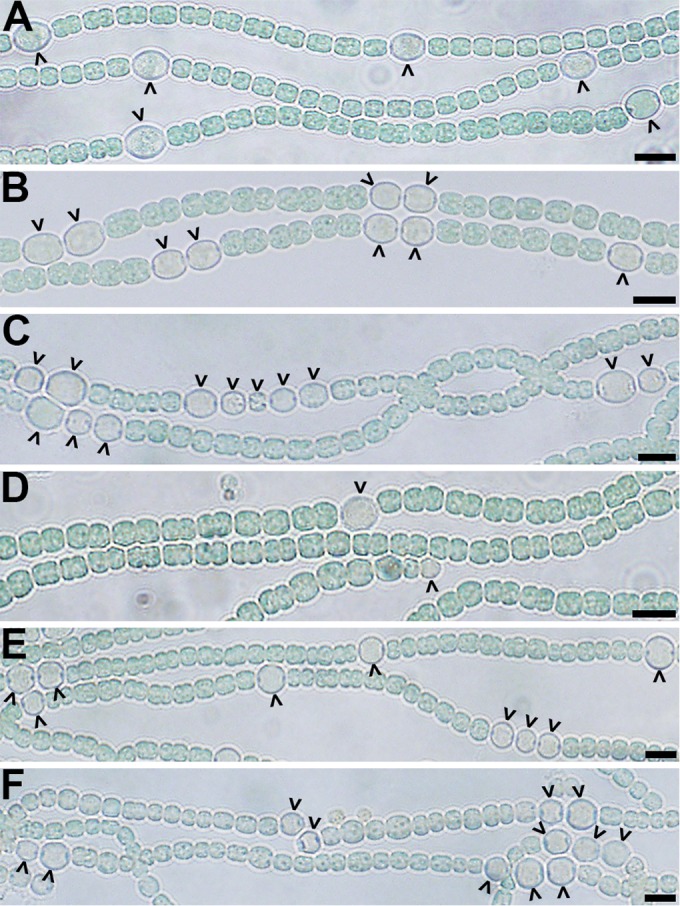
Phenotypes of mutant strains. Representative bright-field micrographs of the wild-type (A), ΔpatS (B), ΔhetN (C), ΔhetC (D), ΔhetC ΔpatS (E), and ΔhetC ΔhetN (F) strains at 24 h (A, B, and E) or 72 h (C, D, and F) after the removal of combined nitrogen. Carets indicate heterocysts. Bar, 10 μm.
Deletion of patS or hetN is epistatic to deletion of hetC.
A prediction of the hypothesis that credits lack of export of one or both of the inhibitors from source cells for the reduced level of differentiation of those source cells into heterocysts (24) is epistasis of null mutations in the inhibitor gene patS or hetN to a null mutation in hetC. In other words, inactivation of patS or hetN would be expected to restore heterocyst function in a hetC background and yield a pattern of heterocysts typical of the patS or hetN single mutants. A similar epistasis analysis was recently reported (25). However, our epistasis results differ substantially, probably as a consequence of the nature of the mutations in hetC, and so are reported here.
Strains UHM344 and UHM224 have patS inactivated in a wild-type and hetC background, respectively. The phenotype of the patS hetC double mutant, UHM224, was more similar to that of the patS mutant than the hetC mutant; 16.27% ± 1.7% of cells were heterocysts in the hetC patS double mutant, compared to 24.9% ± 2.7% of cells in the patS single mutant and 0% of cells in the hetC single mutant 24 h postinduction (Fig. 1; see also Table S1 in the supplemental material). In addition, unlike the hetC single mutant, both the patS single and patS hetC double mutants were capable of diazotrophic growth and nitrogen fixation (see Fig. S1 in the supplemental material), indicating that the heterocysts that formed were functional. The heterocyst percentage reached and the presence of multiple contiguous heterocysts were in agreement with published results (25); however, the heterocysts reported here were functional. The patS-null mutant was clearly epistatic to the hetC-null mutant.
The hetN gene was deleted from UHM232 to create the hetN hetC double mutant, strain UHM225, and the phenotype was comparable to that of a hetN single mutant, strain UHM115, which was created from the wild type in a similar manner (27). In this case, deletion of hetN was not clearly epistatic to that of hetC; the phenotype of the double mutant had characteristics of both the hetN and hetC single mutants. Heterocyst formation was delayed until 48 h postinduction in both the double mutant and the hetC single mutant, which differed from the hetN single mutant, but those heterocysts were functional in the double mutant, as they are in the hetN single mutant (see Fig. S1 in the supplemental material). The number of heterocysts produced by the double mutant was, however, between that of the two single mutants, which is consistent with recently published results (Fig. 1; see also Table S1 in the supplemental material). The number and functionality of heterocysts produced by the hetN hetC double mutant was higher than those reported in a previous study (25). In sum, inactivation of patS or hetN from a hetC-null strain restored heterocyst function and increased the number of cells that differentiated, whereas inactivation of patS alone from a hetC-null strain restored the timing of differentiation.
The timing of induction of transcription of hetN, but not that of patS, is affected by mutation of hetC.
The ultimate objective of this study was to determine whether mutation of hetC affects intercellular movement of the patS- and hetN-dependent signals, so it was first important to determine when patS and hetN are transcribed in the hetC mutant. Transcription of patS in the wild type is elevated in single cells arranged in a periodic pattern prior to morphological differentiation of cells into heterocysts (2, 7). To determine if transcription of patS was similar in the wild-type and hetC mutant strains, a transcriptional fusion of the patS promoter with green fluorescent protein (GFP) on plasmid pAM1951 was introduced to both strains. At 9 h postinduction, GFP-dependent fluorescence from the wild-type strain was observed primarily in single cells arranged in a periodic pattern (Fig. 2). A similar pattern of GFP fluorescence was also observed in the hetC mutant containing the same reporter at the same time, suggesting that at 9 h postinduction a lack of HetC does not affect the production of PatS (Fig. 2).
FIG 2.
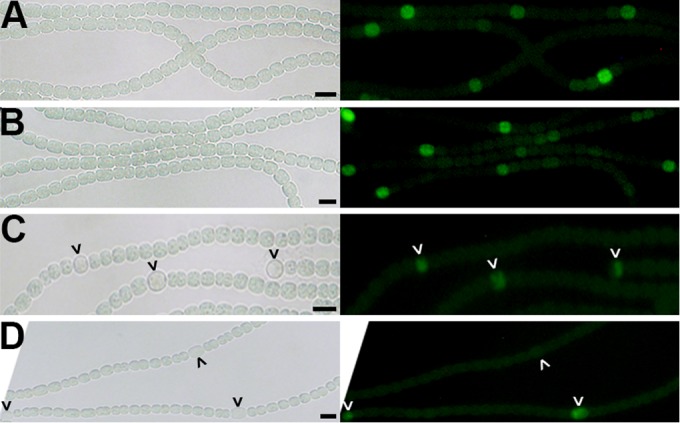
Transcription of hetN but not patS is delayed in a ΔhetC mutant. Bright-field (left panel) and fluorescence (right panel) micrographs for detection of transcription from the patS (A and B) or hetN (C and D) promoters via fusions to gfp present in pAM1951 or pSMC126, respectively, in the wild type (A and C) and a ΔhetC mutant (B and D). Following growth on ammonia, cultures were analyzed at 9 h (A and B), 18 h (C), and 40 h (D) after the removal of combined nitrogen. Carets indicate (pro)heterocysts. Bar, 10 μm.
In contrast to expression of patS, induction of transcription of hetN in the wild-type strain occurs in proheterocysts arranged in a periodic pattern several hours later (8). To compare the timing and patterning of transcription of hetN in the wild-type and hetC mutant strains, a transcriptional fusion of the hetN promoter with GFP on plasmid pSMC126 was introduced to both strains. In the wild type, a periodic pattern of GFP fluorescence was first observed in proheterocysts 18 h after induction of differentiation (Fig. 2). Conversely, GFP fluorescence from the hetC mutant was not apparent until 40 h after induction, and a periodic pattern of expression was not apparent. GFP fluorescence from the hetC mutant was delayed relative to that with the same reporter in the wild type and was observed in very few cells, primarily those that showed signs of morphological differentiation. These results suggest that the production of HetN is delayed in the hetC mutant.
HetC is not required for intercellular transfer of a patS-encoded inhibitor between vegetative cells.
Intercellular transfer of patS- and/or hetN-dependent inhibitor signals from source cells to target cells can be detected by degradation of HetR in target cells (5, 12). The signal range of the inhibitor signals adjacent to source cells is indicated by a decrease in fluorescence from HetR tagged with a fluorophore. Here, the effect of a null mutation in hetC on the presence or absence of a signal range adjacent to naturally occurring source cells was assessed. A form of HetR with reduced activity that has been used previously to report inhibitor transfer between cells, HetR(H69Y) (5), was fused to CFP and used as a reporter of PatS and/or HetN activity. Absence of CFP fluorescence acted as a reporter of patS- and hetN-encoded inhibitor activity in target cells at single-cell resolution. To identify source cells producing PatS, the promoter of patS was fused to YFP. Both the HetR(H69Y)-CFP and PpatS-YFP constructs were introduced on a replicating plasmid that was selected for in all cells of filaments. Six strains, the wild-type as well as patS, hetN, and hetC single mutants and hetC patS and hetC hetN double mutants, containing the plasmid pPJAV247 were examined for transfer of the inhibitors from source cells to adjacent target cells at 0 and 9 h after induction of differentiation. Nine hours postinduction was chosen, based on the observation of similar transcription profiles of patS at this time in both the wild-type and hetC mutant strains and on previous observations of resolution of transcription of patS in single cells at about this time (2).
At 0 h after induction, YFP fluorescence was not detected in any of the six strains, consistent with the absence of source cells (see Fig. S2 in the supplemental material). In addition, fluorescence from CFP was similar in all cells of filaments, suggesting that cell-to-cell variability in HetR(H69Y) levels was minimal in the absence of source cells. In contrast, at 9 h postinduction, a pattern of cells marked by YFP was observed in all six strains, indicative of source cells expressing patS (Fig. 3). Because transcription of patS but not that of hetN was developmentally upregulated at 9 h postinduction, source cells would be expected to only produce PatS, making the following analysis specific for transfer of the patS-encoded inhibitor. In four of the six strains, the wild type, hetN and hetC single mutants, and the hetN hetC double mutant, groups of contiguous cells adjacent to yellow source cells lacked CFP fluorescence, indicative of degradation of HetR-CFP in cells adjacent to source cells (Fig. 3). This lack of CFP fluorescence was seen in cells adjacent to all YFP-tagged source cells observed in each of the four strains. For all but the hetC single mutant, CFP fluorescence was, in some cases, absent from all vegetative cells between two cells expressing patS, presumably because the effect of the patS-dependent signal from these cells overlapped in the cells between them (see Fig. S3 in the supplemental material). In other cases where a sufficient number of vegetative cells were present between two PatS source cells, the number of cells affected by the patS-dependent signal was evident based on the presence of CFP fluorescence beyond that number. It is these instances that are pictured in Fig. 3 and that were used for further analysis. Signal ranges, defined as the largest number of contiguous cells adjacent to a source cell that fell below a threshold value (twice the value measured in control cells without the fluorophore [12]), were calculated from a small subset of the observed source cells (Table 1). In each case the average signal range was about 7 cells, and none varied significantly from that of the wild type (see Table S2 in the supplemental material). Two of the four strains in which inhibitor transfer was observed lacked hetC, indicating that hetC was not necessary for intercellular transfer of the patS-encoded inhibitor between source and target vegetative cells at 9 h postinduction.
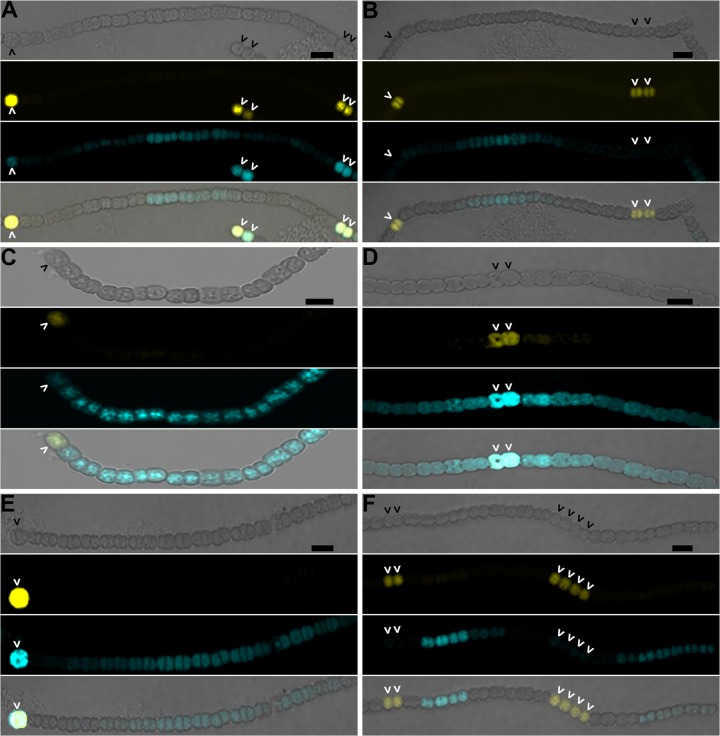
FIG 3.
The hetC gene product is not involved in movement of inhibitors of differentiation from proheterocysts. The wild type (A), ΔhetC (B), ΔpatS (C), ΔhetC ΔpatS (D), ΔhetN (E), and ΔhetC ΔhetN (F) strains harboring pPJAV247, which encodes YFP expressed from the patS promoter to mark proheterocysts and HetR(H69Y)-CFP expressed from the copper-inducible petE promoter, were imaged 9 h after the removal of combined nitrogen. From top to bottom: bright field, yellow fluorescence from PpatS-YFP, blue fluorescence from HetR(H69Y)-CFP, and the composite image. Carets indicate proheterocysts. Bar, 10 μm.
TABLE 1.
Signal ranges from various strains at 9, 24, or 48 h following the removal of combined nitrogen
| Strain | Signal rangea ± SD (no. of events) |
|
|---|---|---|
| 9 h postinduction | 24 or 48 h postinduction | |
| Wild type | 7.8 ± 1.2 (6) | 7.6 ± 1.5 (5; 24 h) |
| UHM115 (ΔhetN::Ω Spr Smr) | 6.8 ± 1 (4) | 7.5 ± 1.3 (4; 24 h) |
| UHM334 (ΔpatS::Ω Spr Smr) | 0 (6) | NA (6; 24 h) |
| UHM232 (ΔhetC) | 7.4 ± 1.9 (11) | 0 (8; 48 h) |
| UHM224 (ΔhetC ΔpatS::Ω Spr Smr) | 0 (4) | 0.1 ± 0.3 (13; 24 h) |
| UHM225 (ΔhetC ΔhetN::Ω Spr Smr) | 6 ± 3 (6) | 0 (5; 48 h) |
Signal ranges are expressed as the number of cells away from a proheterocyst that lacked fluorescence, measured using ImageJ. NA, not available.
In contrast, two strains had an average signal range of zero; CFP fluorescence was observed in cells adjacent to yellow source cells as well as in the source cells themselves (Fig. 3 and Table 1). These two strains were the patS single mutant and the patS hetC double mutant, indicating that patS is necessary for, and likely encodes, the sole inhibitor that was necessary for loss of CFP fluorescence in the other four strains. As mentioned previously, this was not surprising, given that induction of expression of hetN in source cells does not occur until later in the differentiation process (8, 38, 39).
HetC is required for intercellular transfer of patS- and hetN-encoded inhibitors between heterocysts and vegetative cells.
Because the hetC mutant strains described in this study all differentiated morphologically into distinct heterocysts, it was possible to examine the effect of a null mutation in hetC on transfer of patS- and hetN-encoded inhibitors between mature heterocysts and adjacent vegetative cells. The genetic backgrounds of strains and fluorescent reporters used were the same as those used in the previous section, but the presence or absence of HetR-CFP fluorescence adjacent to heterocysts, which in this case were identified by cellular morphology as well as yellow fluorescence from YFP, was observed at the earliest appearance of morphologically distinct heterocysts, which varied between strains (see Table S1 in the supplemental material). Three of the six strains had signal ranges consistently greater than zero; groups of contiguous cells adjacent to heterocysts lacked CFP fluorescence, indicative of degradation of HetR-CFP in cells adjacent to heterocysts (Fig. 4). These three strains were the wild type and the hetN and patS single mutants. For these strains, CFP fluorescence was, as in the previous section, absent in some cases from all vegetative cells between two heterocysts, presumably because the signal ranges of heterocysts overlapped (see Fig. S4 in the supplemental material). For the wild-type and hetN single mutant strains, it was possible to find heterocysts spaced sufficiently far apart to allow the determination of signal ranges, and for each strain the average signal range was about 7 cells (Table 1). The average signal ranges of these two strains were not significantly different (see Table S3 in the supplemental material). For the patS single mutant strain, it was not possible to calculate the signal ranges of individual heterocysts, presumably because the distance between heterocysts was less than the signal range in all cases.
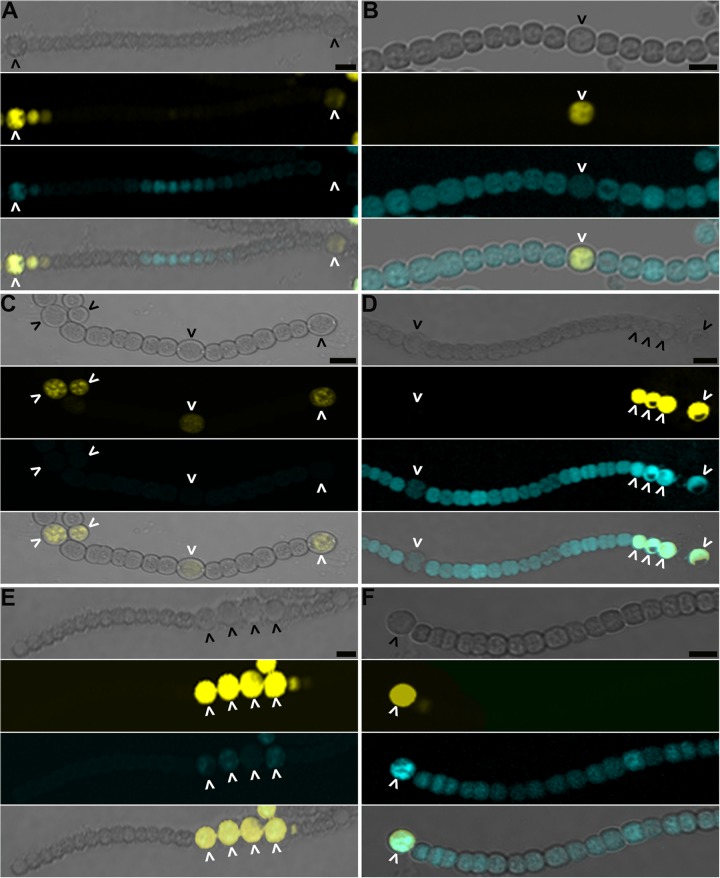
FIG 4.
The hetC gene product is necessary for movement of the inhibitors of differentiation from heterocysts. The wild type (A), ΔhetC (B), ΔpatS (C), ΔhetC ΔpatS (D), ΔhetN (E), and ΔhetC ΔhetN (F) strains harboring pPJAV247, which encodes YFP expressed from the patS promoter to mark heterocysts and HetR(H69Y)-CFP expressed from the copper-inducible petE promoter, were imaged 24 h (A, C, and D) or 48 h (B, E, and F) after the removal of combined nitrogen. From top to bottom: bright field, yellow fluorescence from PpatS-YFP, blue fluorescence from HetR(H69Y)-CFP, and the composite image. Carets indicate heterocysts. Bar, 10 μm.
In contrast to the signal ranges observed in the wild type and the hetN and patS single mutants, signal ranges in the other three strains were almost always zero; CFP fluorescence was observed in cells immediately adjacent to heterocysts (Fig. 4 and Table 1). These three strains were the hetC single mutant and the patS hetC and hetN hetC double mutants. Signal ranges were 0 adjacent to all heterocysts observed in the hetC single mutant and the hetN hetC double mutant, but in one instance, a single cell adjacent to a heterocyst had lost fluorescence in the patS hetC double mutant. In the latter case, the average signal range was calculated as 0.1 cells. Average signal ranges in these three strains were significantly different from the signal ranges of the other strains with nonzero signal ranges (see Table S3 in the supplemental material). Note that in each of the double mutants described above, the gene for only one of the inhibitors was intact, allowing assessment of each inhibitor independently. These three strains with greatly diminished signal ranges all lacked a functional hetC gene, indicating that hetC is necessary for patS- and hetN-dependent signal ranges emanating from heterocysts.
Robust YFP fluorescence from heterocysts of all strains indicated that expression of patS was not affected by a lack of HetC in the two strains that had hetC deleted and patS intact. To determine if mutation of hetC affected expression of hetN in mature heterocysts, the promoter of hetN was fused to the FMN-dependent fluorophore EcFbFP and introduced on plasmid pPJAV341 into the wild-type, hetC single, and hetC patS double mutant strains. Fluorescence from each of the fluorophores was observed in the heterocysts of each strain (see Fig. S5 in the supplemental material), indicating that downregulation of expression in the hetC strains did not account for the apparent lack of transfer of the hetN-dependent inhibitor.
In addition to lack of intercellular transport of patS- and hetN-dependent signals, the lack of a signal range in the cells adjacent to heterocysts could be explained by an inability of cells lacking HetC to respond to the signals. In other words, the developmental signals were present in cells with CFP fluorescence, but the system that responds to them by degradation of HetR-CFP was not active. To test this alternate explanation, the pentapeptide RGSGR, which has been shown to promote degradation of HetR with or without an attached fluorophore (5), was added to each of the six strains, and CFP fluorescence was monitored over time. For each of the six strains used here, fluorescence from HetR-CFP was no longer detected 6 h after the addition of RGSGR peptide to cultures, indicating that the HetR degradation pathway that responds to patS- and hetN-dependent inhibitors was intact (see Fig. S6 in the supplemental material). Taken together, these results strongly suggest that HetC is required for transfer of patS- and hetN-encoded inhibitors from heterocysts to vegetative cells.
DISCUSSION
ATP-binding cassette systems couple the hydrolysis of ATP with a biological function, often transport of a molecule across a cell membrane. They are ubiquitous among organisms of the three kingdoms of life and have been implicated in eukaryotic developmental programs. For example, there is evidence for their involvement in polar auxin transport in plants (40), cell type specification in Dictyostelium (41), larval development in Caenorhabditis elegans (42), and transport of lipid-modified peptides that act as germ cell attractants in Drosophila melanogaster (43). Our results indicate that the predicted ABC transporter HetC was necessary for intercellular transfer of the patS- and hetN-encoded developmental signals from heterocysts to vegetative cells. In contrast, HetC was not required for transfer of the patS-encoded signal between vegetative cells at 9 h postinduction, consistent with the formation of an initial wild-type periodic pattern by the hetC mutant at this time. The hetN-encoded signal was not produced at this time, so the effect of mutation of hetC could not be assessed. Greater temporal resolution will be necessary to determine if the hetN-encoded signal undergoes a similar change in HetC dependency or if transfer is HetC dependent when the HetN signal first becomes active. At some time between 9 h postinduction, as cells are committing to a heterocyst fate, and the production of functional heterocysts at 24 h, the requirements for intercellular transfer of the patS-encoded signal change to a requirement for HetC.
The hypothesis driving the work described herein was based on the phenotype of the hetC mutant. It was envisioned that in strains lacking HetC, confinement of PatS, and later in development HetN, to proheterocyst source cells would short-circuit the differentiation process as the concentrations of inhibitor increased in cells that had induced the differentiation pathway and become source cells (24). Although putative unprocessed forms of PatS are less effective at interacting with HetR and preventing differentiation (14, 19), unnaturally high concentrations of the RGSGR PatS-5 motif out of context in other peptides has been shown to prevent differentiation (14). Results here and those recently presented elsewhere demonstrating epistasis of mutation of patS to that of hetC are consistent with the hypothesis above. While both noted the ability of a patS mutation to rescue heterocyst differentiation in a hetC mutant, only those results presented here demonstrate the formation of functional heterocysts from both the patS hetC and hetN hetC double mutants. Additionally, the hetN hetC double mutant produced 13.5% heterocysts at 48 h postinduction, compared to 2.1%, as presented in previous work. In the absence of patS, inhibitor concentrations would not increase in proheterocysts and short-circuit the differentiation process, permitting heterocyst formation. Interpretation of the hetN epistasis results is more complicated but can also be understood in the context of the hypothesis above. Because patS is intact in the hetN hetC double mutant, differentiation of heterocysts is delayed approximately 24 h until after cells transition to expression of hetN as the primary inhibitor of differentiation, some time around 24 h postinduction (7, 8).
Each of the hetC mutants produced cells displaying many of the morphological characteristics of heterocysts in response to induction of differentiation. Formation of heterocysts by the hetC single mutant was consistent with an indirect role for HetC in promoting differentiation. The patS hetC and hetN hetC double mutants appeared to have fully functional heterocysts, suggesting that HetC promotes differentiation indirectly by preventing self-suppression of cells that upregulate the production of PatS and HetN. The inactive form of heterocysts in a hetC single mutant is likely explained by patS- and hetN-dependent arrest of development at a late stage, preventing function of the few cells that do show signs of differentiation in a hetC mutant.
In the strains used here, hetC was necessary for intercellular transfer of both inhibitors from heterocysts to adjacent vegetative cells. The most direct interpretation of these results envisions the transfer of patS- and hetN-encoded inhibitors by the ABC transporter activity of HetC from a heterocyst to an adjacent vegetative cell, activity that is interrupted upon mutation of hetC. If true, then two routes of transfer seem likely. In the first, HetC would transfer the inhibitors across the cytoplasmic membrane to the periplasm of a heterocyst, which is contiguous with that of adjacent vegetative cells (44). The inhibitors would then move to and between vegetative cells along the filament within the periplasm and subsequently be transported by an unknown mechanism across the cytoplasmic membrane of vegetative cells, where they can interact with their target, HetR. Importation of the inhibitors into the cytoplasm of vegetative cells could potentially drive the formation of a concentration gradient of inhibitor in the periplasm. Inhibition of differentiation by addition of the RGSGR peptide to cultures is consistent with the existence of a mechanism for transport of the inhibitors from the periplasm to the cytoplasm (7). This route of transfer was first proposed by Yoon and Golden with the discovery of patS (7). In the second scenario, transfer would be from the cytoplasm of a heterocyst, across the heterocyst cytoplasmic membrane, the peptidoglycan, and the vegetative cell cytoplasmic membrane to the cytoplasm of the adjacent vegetative cell. In the latter case, HetC would likely work with additional proteins in a manner similar to other ABC transporters that comprise the inner membrane translocator of type 1 secretion systems in Gram-negative bacteria (20). Recent localization of HetC to cell septa of proheterocysts and heterocysts with a fluorescent reporter fusion is consistent with the latter cytoplasm-to-cytoplasm exchange (25). In either case, the need for an intact predicted peptidase domain for proper HetC function (25) suggests concomitant export and processing of the inhibitors.
Because proheterocysts are sources of the patS- and hetN-encoded signals, it has long been speculated that a mechanism that confers insensitivity of these cells to the signals prevents autoinhibition of development (7). The epistasis results presented here and elsewhere suggest that HetC is necessary for insensitivity of developing cells to both of the inhibitors. This immunity to self-signaling likely relies on the putative export activity of HetC and perhaps on peptidase activity. It may also indicate more than one role for HetC during development. Because hetC is upregulated 3 to 6 h following the removal of combined nitrogen (45), it is possible that HetC does more than contribute to the movement of inhibitory signals. Though we did not describe a role for HetC early in the differentiation process, this possibility is not precluded by our results. Whether HetC confers immunity of cells exclusively to self-signaling or also from inhibitors produced in neighboring cells remains to be seen.
Supplementary Material
ACKNOWLEDGMENTS
We thank Loralyn Cozy for insightful discussions.
This work was supported by National Science Foundation grant MCB-1121346.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00304-15.
REFERENCES
- 1.Kumar K, Mella-Herrera RA, Golden JW. 2010. Cyanobacterial heterocysts. Cold Spring Harb Perspect Biol 2:a000315. doi: 10.1101/cshperspect.a000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoon H-S, Golden JW. 2001. PatS and products of nitrogen fixation control heterocyst pattern. J Bacteriol 183:2605–2613. doi: 10.1128/JB.183.8.2605-2613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corrales-Guerrero L, Mariscal V, Flores E, Herrero A. 2013. Functional dissection and evidence for intercellular transfer of the heterocyst-differentiation PatS morphogen. Mol Microbiol 88:1093–1105. doi: 10.1111/mmi.12244. [DOI] [PubMed] [Google Scholar]
- 4.Meinhardt H. 2008. Models of biological pattern formation: from elementary steps to the organization of embryonic axes. Curr Top Dev Biol 81:1–63. doi: 10.1016/S0070-2153(07)81001-5. [DOI] [PubMed] [Google Scholar]
- 5.Risser DD, Callahan SM. 2009. Genetic and cytological evidence that heterocyst patterning is regulated by inhibitor gradients that promote activator decay. Proc Natl Acad Sci U S A 106:19884–19888. doi: 10.1073/pnas.0909152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolk CP. 1967. Physiological basis of the pattern of vegetative growth of a blue-green alga. Proc Natl Acad Sci U S A 57:1246–1251. doi: 10.1073/pnas.57.5.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon H-S, Golden JW. 1998. Heterocyst pattern formation controlled by a diffusible peptide. Science 282:935–938. doi: 10.1126/science.282.5390.935. [DOI] [PubMed] [Google Scholar]
- 8.Callahan SM, Buikema WJ. 2001. The role of HetN in maintenance of the heterocyst pattern in Anabaena sp. PCC 7120. Mol Microbiol 40:941–950. doi: 10.1046/j.1365-2958.2001.02437.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhu M, Callahan SM, Allen JA. 2010. Maintenance of heterocyst patterning in a filamentous cyanobacterium. J Biol Dyn 4:621–633. doi: 10.1080/17513751003777507. [DOI] [PubMed] [Google Scholar]
- 10.Meeks JC, Elhai J. 2002. Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiol Mol Biol Rev 66:94–121. doi: 10.1128/MMBR.66.1.94-121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Risser DD, Wong FCY, Meeks JC. 2012. Biased inheritance of the protein PatN frees vegetative cells to initiate patterned heterocyst differentiation. Proc Natl Acad Sci U S A 109:15342–15347. doi: 10.1073/pnas.1207530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivers OS, Videau P, Callahan SM. 2014. Mutation of sepJ reduces the intercellular signal range of a hetN-dependent paracrine signal, but not of a patS-dependent signal, in the filamentous cyanobacterium Anabaena sp. strain PCC 7120. Mol Microbiol 94:1260–1271. doi: 10.1111/mmi.12836. [DOI] [PubMed] [Google Scholar]
- 13.Higa KC, Rajagopalan R, Risser DD, Rivers OS, Tom SK, Videau P, Callahan SM. 2012. The RGSGR amino acid motif of the intercellular signaling protein, HetN, is required for patterning of heterocysts in Anabaena sp. strain PCC 7120. Mol Microbiol 83:682–693. doi: 10.1111/j.1365-2958.2011.07949.x. [DOI] [PubMed] [Google Scholar]
- 14.Wu X, Liu D, Lee MH, Golden JW. 2004. patS minigenes inhibit heterocyst development of Anabaena sp. strain PCC 7120. J Bacteriol 186:6422–6429. doi: 10.1128/JB.186.19.6422-6429.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldmann EA, Ni S, Sahu ID, Mishler CH, Risser DD, Murakami JL, Tom SK, McCarrick RM, Lorigan GA, Tolbert BS, Callahan SM, Kennedy MA. 2011. Evidence for direct binding between HetR from Anabaena sp. PCC 7120 and PatS-5. Biochemistry 50:9212–9224. doi: 10.1021/bi201226e. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Dong Y, Zhao J. 2004. HetR homodimer is a DNA-binding protein required for heterocyst differentiation, and the DNA-binding activity is inhibited by PatS. Proc Natl Acad Sci U S A 101:4848–4853. doi: 10.1073/pnas.0400429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y, Yeb Z, Joachimiak G, Videau P, Young J, Hurd K, Callahan SM, Gornicki P, Zhao Z, Haselkorn R, Joachimiak A. 2013. Structures of complexes comprised of Fischerella transcription factor HetR with Anabaena DNA targets. Proc Natl Acad Sci U S A 110:E1716–E1723. doi: 10.1073/pnas.1305971110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Videau P, Ni S, Rivers OS, Ushijima B, Feldman EA, Cozy LM, Kennedy MA, Callahan SM. 2014. Expanding the direct HetR regulon in Anabaena sp. strain PCC 7120. J Bacteriol 196:1113–1121. doi: 10.1128/JB.01372-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldmann EA, Ni S, Sahu ID, Mishler CH, Levengood JD, Kushnir Y, McCarrick RM, Lorigan GA, Tolbert BS, Callahan SM, Kennedy MA. 2012. Differential binding between PatS C-terminal peptide fragments and HetR from Anabaena sp. PCC 7120. Biochemistry 51:2436–2442. doi: 10.1021/bi300228n. [DOI] [PubMed] [Google Scholar]
- 20.Davidson AL, Dassa E, Orelle C, Chen J. 2008. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol Mol Biol Rev 72:317–364. doi: 10.1128/MMBR.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khudyakov I, Wolk CP. 1997. hetC, a gene coding for a protein similar to bacterial ABC protein exporters, is involved in early regulation of heterocyst differentiation in Anabaena sp. strain PCC 7120. J Bacteriol 179:6971–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X, Wolk CP. 2001. Role for hetC in the transition to a nondividing state during heterocyst differentiation in Anabaena sp. J Bacteriol 183:393–396. doi: 10.1128/JB.183.1.393-396.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Xu X. 2005. Regulation by hetC of genes required for heterocyst differentiation and cell division in Anabaena sp. strain PCC 7120. J Bacteriol 187:8489–8493. doi: 10.1128/JB.187.24.8489-8493.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolk CP, Zarka K. 1998. Genetic dissection of heterocyst differentiation, p 191–196. In Subramanian G, Kaushik BD, Venkataraman GS (ed), Cyanobacterial biotechnology: proceedings of the international symposium, Sept. 18–21, 1996. Oxford & IBH Publishing Co., New Delhi, India. [Google Scholar]
- 25.Corrales-Guerrero L, Flores E, Herrero A. 2014. Relationships between the ABC-exporter HetC and peptides that regulate the spatiotemporal pattern of heterocyst distribution in Anabaena. PLoS One 9:e104571. doi: 10.1371/journal.pone.0104571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omairi-Nassar A, Haselkorn R, Austin J II. 2014. Visualization of channels connecting cells in filamentous nitrogen-fixing cyanobacteria. FASEB J 28:3016–3022. doi: 10.1096/fj.14-252007. [DOI] [PubMed] [Google Scholar]
- 27.Borthakur PB, Orozco CC, Young-Robbins SS, Haselkorn R, Callahan SM. 2005. Inactivation of patS and hetN causes lethal levels of heterocyst differentiation in the filamentous cyanobacterium Anabaena sp. PCC 7120. Mol Microbiol 57:111–123. doi: 10.1111/j.1365-2958.2005.04678.x. [DOI] [PubMed] [Google Scholar]
- 28.Higa KC, Callahan SM. 2010. Ectopic expression of hetP can partially bypass the need for hetR in heterocyst differentiation by Anabaena sp. strain PCC 7120. Mol Microbiol 77:562–574. doi: 10.1111/j.1365-2958.2010.07257.x. [DOI] [PubMed] [Google Scholar]
- 29.Mitschke J, Vioque A, Haas F, Hess WR, Muro-Pastor AM. 2011. Dynamics of transcriptional start site selection during nitrogen stress-induced cell differentiation in Anabaena sp. PCC7120. Proc Natl Acad Sci U S A 108:20130–20135. doi: 10.1073/pnas.1112724108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elhai J, Wolk CP. 1988. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol 167:747–754. [DOI] [PubMed] [Google Scholar]
- 31.Buikema WJ, Haselkorn R. 2001. Expression of the Anabaena hetR gene from a copper-regulated promoter leads to heterocyst differentiation under repressing conditions. Proc Natl Acad Sci U S A 98:2729–2734. doi: 10.1073/pnas.051624898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norris MH, Kang Y, Wilcox B, Hoang TT. 2010. Stable site-specific fluorescent tagging constructs optimized for Burkholderia species. Appl Environ Microbiol 76:7635–7640. doi: 10.1128/AEM.01188-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higuchi R, Krummel B, Saiki RK. 1988. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res 16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Videau P, Cozy LM, Young JE, Ushijima B, Oshiro RT, Rivers OS, Burger AH, Callahan SM. 2015. The trpE gene negatively regulates differentiation of heterocysts at the level of induction in Anabaena sp. strain PCC 7120. J Bacteriol 197:362–370. doi: 10.1128/JB.02145-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orozco CC, Risser DD, Callahan SM. 2006. Epistasis analysis of four genes from Anabaena sp. strain PCC 7120 suggests a connection between PatA and PatS in heterocyst pattern formation. J Bacteriol 188:1808–1816. doi: 10.1128/JB.188.5.1808-1816.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Videau P, Oshiro RT, Cozy LM, Callahan SM. 2014. Transcriptional dynamics of developmental genes assessed with an FMN-dependent fluorophore in mature heterocysts of Anabaena sp. strain PCC 7120. Microbiology 160:1874–1881. doi: 10.1099/mic.0.078352-0. [DOI] [PubMed] [Google Scholar]
- 37.Ernst A, Black T, Cai Y, Panoff J-M, Tiwari DN, Wolk CP. 1992. Synthesis of nitrogenase in mutants of the cyanobacterium Anabaena sp. strain PCC 7120 affected in heterocyst development or metabolism. J Bacteriol 174:6025–6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bauer CC, Ramaswamy KS, Endley S, Scappino LA, Golden JW, Haselkorn R. 1997. Suppression of heterocyst differentiation in Anabaena sp. strain PCC 7120 by a cosmid carrying wild-type genes encoding enzymes for fatty acid synthesis. FEMS Microbiol Lett 151:23–30. [DOI] [PubMed] [Google Scholar]
- 39.Li B, Huang X, Zhao J. 2002. Expression of hetN during heterocyst differentiation and its inhibition of hetR up-regulation in the cyanobacterium Anabaena sp. PCC 7120. FEBS Lett 517:87–91. doi: 10.1016/S0014-5793(02)02582-6. [DOI] [PubMed] [Google Scholar]
- 40.Noh B, Bandyopadhyay A, Peer WA, Spalding EP, Murphy AS. 2003. Enhanced gravi- and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature 423:999–1002. doi: 10.1038/nature01716. [DOI] [PubMed] [Google Scholar]
- 41.Good JR, Cabral M, Sharma S, Yang J, Van Driessche N, Shaw CA, Shaulsky G, Kuspa A. 2003. TagA, a putative serine protease/ABC transporter of Dictyostelium that is required for cell fate determination at the onset of development. Development 130:2953–2965. doi: 10.1242/dev.00523. [DOI] [PubMed] [Google Scholar]
- 42.Yabe T, Suzuki N, Furukawa T, Ishihara T, Katsura I. 2005. Multidrug resistance-associated protein MRP-1 regulates dauer diapause by its export activity in Caenorhabditis elegans. Development 132:3197–3207. doi: 10.1242/dev.01909. [DOI] [PubMed] [Google Scholar]
- 43.Ricardo S, Lehman. 2009. An ABC transporter controls export of a Drosophila germ cell attractant. Science 323:943–946. doi: 10.1126/science.1166239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flores E, Herrero A, Wolk CP, Maldener I. 2006. Is the periplasm continuous in filamentous multicellular cyanobacteria? Trends Microbiol 14:439–443. doi: 10.1016/j.tim.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Muro-Pastor AM, Flores E, Herrero A. 2009. NtcA-regulated heterocyst differentiation genes hetC and devB from Anabaena sp. strain PCC 7120 exhibit a similar tandem promoter arrangement. J Bacteriol 191:5765–5774. doi: 10.1128/JB.00616-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.