Abstract
Alzheimer's disease (AD) and Parkinson's disease (PD) are devastating, frequent, and still incurable neurodegenerative diseases that manifest as cognitive and motor disorders. Epidemiological data support an inverse relationship between the amount of physical activity (PA) undertaken and the risk of developing these two diseases. Beyond this preventive role, exercise may also slow down their progression. Several mechanisms have been suggested for explaining the benefits of PA in the prevention of AD. Aerobic physical exercise (PE) activates the release of neurotrophic factors and promotes angiogenesis, thereby facilitating neurogenesis and synaptogenesis, which in turn improve memory and cognitive functions. Research has shown that the neuroprotective mechanisms induced by PE are linked to an increased production of superoxide dismutase, endothelial nitric oxide synthase, brain-derived neurotrophic factor, nerve growth factor, insulin-like growth factor, and vascular endothelial growth factor, and a reduction in the production of free radicals in brain areas such as the hippocampus, which is particularly involved in memory. Other mechanisms have also been reported in the prevention of PD. Exercise limits the alteration in dopaminergic neurons in the substantia nigra and contributes to optimal functioning of the basal ganglia involved in motor commands and control by adaptive mechanisms involving dopamine and glutamate neurotransmission. AD and PD are expansive throughout our ageing society, and so even a small impact of nonpharmacological interventions, such as PA and exercise, may have a major impact on public health.
Keywords: Alzheimer's disease, Parkinson's disease, exercise, prevention, slowing-down effects, physical activity
INTRODUCTION
The prevalence of neurodegenerative diseases such as Alzheimer's disease (AD) and Parkinson's disease (PD) increases markedly with age.1 Genetic and environmental factors contribute to the development of both of these diseases,2,3,4,5 which are progressive and irreversible and result in cognitive and/or motor disorders. AD is the more frequent of the two dementia types, while PD, which mainly affects motor functioning, is the second most common neurodegenerative disease.6 AD is characterized by neuronal and synaptic impairments in the cerebral cortex, as well as in certain subcortical regions, which affect cognitive function and psychobehavioral function.7 PD affects neurons located at the level of the basal ganglia and, more specifically, those of the substantia nigra, which secrete dopamine. The lack of dopamine induces postural and motor deficits and functional incapacities.8
It has been repeatedly reported during the past 10 years that physical exercise (PE) constitutes an effective intervention in neurodegenerative diseases, attenuating or limiting their progression.9,10,11 Acute PE increases cardiac output, leading to increased cerebral blood flow, which triggers various neurobiological mechanisms in the brain tissues. The regular (repeated) increases in cerebral blood flow associated with regular PE probably contribute to increases in angiogenesis, neurogenesis, synaptogenesis, and neurotransmitter synthesis in the different cerebral areas involved in cognition (e.g., memorization) and mobility.11,12 Experimental data support the theory that PE is likely to maintain and even improve cognitive and motor functions in healthy subjects.13 It is also suggested that overall physical activity (PA) can protect against the onset of AD and PD,14,15,16,17 and that PE can slow down the progression of these pathologies.18,19
Nevertheless, most data on the protective roles of PA and PE on the neurologic system are based on animal studies, and so the mechanisms through which PA and PE exert their neuroprotective effects and thus counteract the onset and progression of neurodegenerative pathologies in humans remain to be established. Hence, the aim of this narrative review was to report the current state of the scientific knowledge relating to the preventive and attenuating effects of PA and PE against AD and PD.
ALZHEIMER'S DISEASE
Preventive effects of physical activity against Alzheimer's disease
Most of the longitudinal epidemiological studies that have investigated the association between PA and the risk of cognitive decline support the idea that PA delays the onset of AD and dementia in older people. A meta-analysis conducted in 2009 concluded that PA reduced the risk of developing AD by 45% [0.55, 95% confidence interval (CI)=0.36-0.84, p=0.006].20 This protective role of PA against AD is defined, at least partially, by a dose-response relationship.15,21 In a recent study examining the population-attributable risk of AD according to selected risk factors, Norton et al.17 found that 12.7% and 20.3% of AD cases in the world and in Europe in 2010, respectively, were attributed to physical inactivity. Therefore, data from observational studies strongly support PA as a clinically relevant option toward the prevention of AD.
Physical exercise, defined as repetitive and purposeful PA generally used to improve physiological, physical, and functional capacities, is a subtype of PA that also protects against cognitive decline and AD. An American study of 1,740 subjects older than 65 years found that the incidence of dementia was 13.0 per 1,000 person-years for participants who exercised three or more times per week (≥15 min/session of walking, cycling, swimming, aerobics, eurhythmics, aquarobics, strength training, stretching, or other activities) compared with 19.7 per 1,000 person-years for those who exercised fewer than three times per week.22 In a cohort of 347 elderly men (aged 74.6±4.3 years, mean±standard deviation), cognitive decline as measured using the Mini Mental State Examination (MMSE) was higher for individuals who performed PA for less than 1 hour weekly than for those who were significantly more active.23 A longitudinal study carried out in Western Europe (in Finland, Italy, and The Netherlands) over a 10-year period revealed that subjects (n=295) who decreased their daily amount or intensity of PA exhibited a cognitive decline that was greater than that of subjects who maintained their daily amount or intensity of PA.24
Benefits of exercise in Alzheimer's-disease patients
Physical exercise is a powerful instrument for slowing the decline in physical and cognitive function in AD patients.19,25,26,27,28,29 Reductions in depressive symptoms30,31,32 and even mortality33 have been reported in dementia patients involved in PE programs. Improvements in cognitive function were associated with improvements in postural and motor functions in exercise trials.34,35
What is the best physical exercise program to propose to prevent or slow down the course of Alzheimer's disease?
There is currently no consensus regarding the best PE regimen for improving clinical outcomes in AD patients. Aerobic PE is feasible and practical for AD subjects36 and has been repeatedly reported to be associated with better cognitive function.37,38,39,40 For instance, the simple practice of regular walking is associated with a reduced decline in cognitive performance (as assessed on the MMSE) in AD subjects.41,42 Walking also improves the postural and motor functions of AD patients.
Since AD is associated with low muscle mass and strength,43 muscle strength and power training can be crucial for AD patients. Balance training has been shown to improve the postural abilities of subjects who were moderately-to-severely affected by AD, and thus reduce the risk of falling at a later stage.44 Multicomponent exercise training [comprising balance, aerobic exercise (generally walking), and strength training] was shown to be particularly effective for improving postural and motor functioning and reducing the risk of falling in AD subjects.45
While the best PE regimen for AD patients has yet to be defined, there is increasing evidence that multicomponent training (involving aerobic, muscle strength, and power and balance/coordination exercises) provides important health benefits in this population.
Other aspects related to physical exercise
Environmental conditions should also be taken into account since they are likely to influence the effects of PE. Research with AD subjects has shown that walking plus conversation has a better preventive effect than walking alone,46 suggesting that the "socialization effect" of exercise is an important aspect for this population. In another controlled exercise trial, the practice of walking combined with bright light exposure improved sleep among AD patients.47
In animals, the conditions in which PE is practiced also influence the neurobiological adaptations. Mice running freely in a wheel (without a preset speed) exhibited a greater reduction in the concentration of amyloid plaques (or Aβ-plaques) in their brain as well as on memory impairment than mice forced to run in the wheel at a predetermined rotation speed.48 These result and others suggest that the benefits of PE on brain functioning are modulated by other factors (e.g., motivation) than just improved cardiovascular fitness.
Neuroprotective mechanisms induced by physical exercise in subjects affected by Alzheimer's disease
Human studies
In humans, most of the evidence regarding the benefits of PE on brain function has come from imaging technologies. For example, 40 min of PE (ergocycle, treadmill, and stair-climbing) four times weekly for a period of 12 weeks was found to increase the cerebral blood flow in the dentate gyrus of the hippocampus, which may improve neurogenesis.49 Another study showed that 1 year of moderately intense aerobic exercise (3 days/week, 40 min/session, 60-75% of the maximum heart rate reserve from the 7th week) increased (+2%) the hippocampal volume; this exercise training was also associated with an increase in the plasma concentration of brain-derived neurotrophic factor (BDNF) in older healthy subjects.50 Moreover, a significant correlation was found between plasma BDNF levels and the level of PE practiced among people with AD.51 An aerobic program (three weekly 1-hour sessions for a period of 6 months) increased the volumes of both the gray and white matters in certain prefrontal and temporal cortical regions for subjects aged between 60 and 79 years.52 In the same study, the subjects of the same age who participated in stretching and toning (nonaerobic) training did not benefit from the same cortical adaptations. Although these experiments involved healthy subjects, the findings highlighted the positive effect of aerobic PE on brain health.
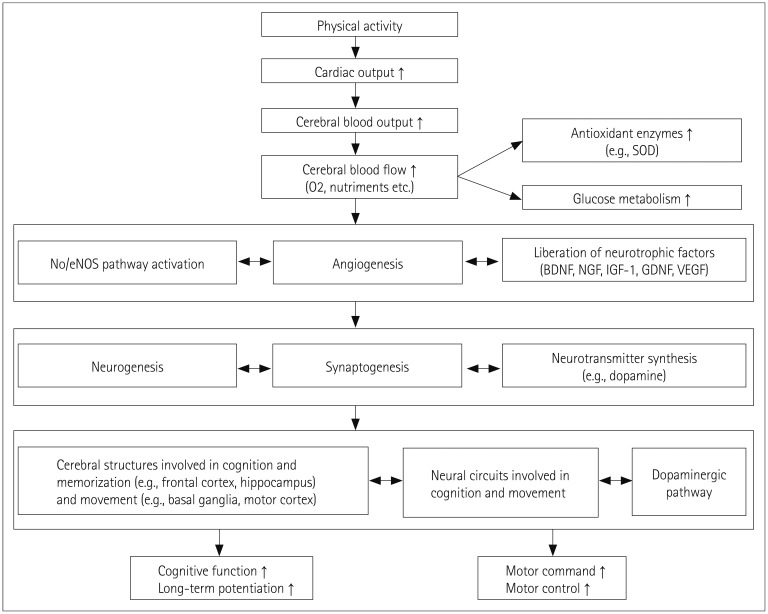
Moreover, Lange-Asschenfeldt and Kojda53 emphasized the interconnection between AD and vascular risk factors and the impact of cerebrovascular and endothelial dysfunction on AD pathophysiology. They described the molecular mechanisms of the beneficial effects of PE on the vasculature, such as activation of the vascular nitric oxide (NO)/endothelial NO synthase (eNOS) pathway (Fig. 1). These authors concluded that PE may counteract AD pathophysiology by building a vascular reserve, as well as by maintaining neuronal plasticity.
Fig. 1. Preventive and slowing down neuroprotective mechanisms induced by regular physical exercise on the cognitive and motor functions. BDNF: brain-derived neurotrophic factors, eNOS: endothelial nitric oxide synthases, GDNF: glial cell line-derived neurotrophic factors, IGF-1: insulin-like growth factors, NGF: nerve growth factors, NO: nitric oxide, SOD: superoxide dismutase, VEGF: vascular endothelial growth factor.
Animal studies
Most of the current knowledge about the potential mechanisms involved in the protective effect of PE has been obtained by studying animal models of AD. The findings thus far suggest that PE reduces the noxious effects of oxidative stress, the production of total cholesterol, and insulin resistance, increases vascularization, and improves energy metabolism (e.g., glucose metabolism).12,39,53,54,55 PE activates neurotrophic functions and angiogenesis, thereby facilitating neurogenesis and synaptogenesis, which improve memory and cognitive functions (Fig. 1).12,26,39,49,53,55
It has been found that moderate-to-vigorous PE in animal models increases the production of antioxidant enzymes (particularly superoxide dismutase), eNOS, BDNF, nerve growth factor, insulin-like growth factor, and vascular endothelial growth factor. PE also reduces the production of free radicals (reactive oxygen species) as well as the concentration of brain Aβ-plaques, in particular in the cerebral regions involved in cognitive function (and notably memory), such as the hippocampus (Fig. 1).12,53,56 PE also improves brain plasticity. For example, after running for 1 hour, 5 days a week, for 16 weeks, Tg2576 mice (a murine AD model) exhibited a more voluminous hippocampus than their control, nonexercising counterparts.48 The hippocampal neurogenesis was associated with synaptogenesis and improvements in learning capacity (spatial memory) in old trained mice compared with sedentary control mice.57
A running program of 16 weeks on a treadmill for transgenic (TgCRND8) mice with AD phenotypes decreased their level of β-amyloid precursor protein (β-APP or Aβ peptide) compared with a control group.54 After 5 months of exercise training, the surface area of the Aβ-plaques of TgCRND8 mice was reduced in the frontal cortex (-38%), the cortex near to the hippocampus (-53%), and the hippocampus (-40%).58 This adaptation occurred mainly during the first month of training and was associated with a decrease in the proteolytic fragments of β-APP. The induced mechanism was independent of mRNA/protein changes in neprilysin and insulin-degrading enzyme, and may involve changes in neuronal metabolism that are known to affect APP processing and to be regulated by PE. In addition, the learning capacities and memory of the TgCRND8 mice improved.58 However, an improvement in cognitive function is not systematically associated with a reduction of the number of Aβ-plaques.59 It was observed that the spatial memory of APP-23 mice placed in a condition of environmental enrichment (a spacious, well-equipped cage) increased at the end of 11 months of training, while the number of cortical and hippocampal Aβ-plaques did not decrease. These cognitive improvements in the mice that had benefited from environmental enrichment arose from increases in BDNF, hippocampal neurotrophin, and activation of hippocampal neurogenesis compared with mice that completed the same training program but without environmental enrichment.59 The combination of the environmental stimulation and PE optimized the effects of the therapeutic program to increase the neural reserve of mouse models affected by AD.
Physical exercise has also been shown to act favorably on neurofibrillary degeneration. After 9 months of training, the concentration of tau protein in the hippocampus was decreased in a mouse model affected by the disease (THY-Tau22).60 In addition, PE may decrease the neuroinflammatory responses activated by the increase in cerebral tau proteins10 and ensure a preventive role against the loss of the expression of choline acetyltransferase.60
PARKINSON'S DISEASE
Preventive effects of physical activity against Parkinson's disease
There is a large amount of epidemiological data suggesting that PA can also prevent the development of PD.9,14 Two studies61,62 found that the risk of developing this disease appeared to be inversely associated with the amount of PA practiced throughout life (0.7, 95% CI=0.5-1.1, p<0.007, and 0.65, 95% CI=0.51-0.83, p<0.0001, respectively).61,62 An important limitation of these observational studies is that subjects predisposed to develop PD may naturally tend to avoid PA61 prior to the onset of the clinical symptoms of the disease. Furthermore, the protective effect of PE against this pathology appears to be particularly large when practiced at young-to-middle adulthood (i.e., around 35-39 years old) and at the end of life.62 Xu et al.62 suggest that people who practice PE during these two periods of their life have a 40% lower risk of PD than people who remained inactive during the same periods. In a study involving 48,574 men and 77,254 women, Chen et al.61 found that the direct relationship between the amount of PA and the risk of developing PD was only significant in men.
For an equal duration of PA, the intensity may also have an effect on the risk of developing the disease. A longitudinal study conducted over a period of 1 year involving 143,325 subjects showed that those who practiced a PA of high intensity such as cycling, aerobics, or tennis at the time of their inclusion had a 40% lower risk than those who did not practice PA or who practiced a low-intensity PA such as walking or dancing.63 Garraux64 reported that the risk of developing PD varies according to the level of work-related physical effort, with the risk being higher in sedentary workers (e.g., teachers, medical doctors, and state employees) than in active workers (e.g., construction workers).
Benefits of physical exercise in Parkinson's-disease patients
Larger amounts of moderate-to-vigorous PE may slow down the evolution of the disease in PD patients.18 It is known that PD patients expend 29% less energy than do healthy subjects, which results in the disease evolving negatively, increased motor deficits, and declines in daily activities.65 PE is a nonpharmacological approach that is usually recommended for PD patients in order to slow down the deleterious effects of the disease.66
An observational Japanese study67 involving 438 PD patients (178 men and 260 women) showed that the ratio of mortality was 1.68 for the group of active subjects (n=151; walking, stretching exercises, and postural exercises) compared with 2.47 for the other participants.
Despite the findings of some studies that the beneficial effects of PE on cognitive performances or psychological domains (i.e., attentional capacities, depressive and anxiety symptoms, and mood state) of the disease are weak or even nonexistent,68,69 it is important to emphasize that PE enables PD subjects to maintain their psychomotor learning abilities. Three weeks of training focusing on sensory stimuli (auditory, visual, and somatosensorial) during the completion of locomotor tasks improved the spatiotemporal characteristics involved in walking for 153 PD subjects;70 the postprogram locomotor improvements observed were preserved 6 weeks after completion of the program. That study indicates that the capacities of psychomotor learning (acquisition, automaticity, and retention) can be maintained for PD subjects. The positive effects of PE on the cognitive and automatic components of motor control in PD subjects result from neuroplastic mechanisms involving the synaptic connections of neuronal networks.71
Regarding motor function, there is solid evidence for the beneficial effects of PE for PD patients. Indeed, strength training has been shown to improve the muscle strength and walking speed of PD patients who are not severely affected by the disease.72,73 A meta-analysis by Herman et al.74 indicates that walking training on a treadmill improves the spatiotemporal parameters of walking, and that this benefit can persist for 2 months. A Cochrane review found that the walking program that produces the biggest improvements involves 30 min of walking, performed five times a week for a period of at least 6 weeks.75 A pilot study showed that a balance training program based on visual feedback, such as those supplied by a videographic game, improved static and dynamic balance, mobility, and functional capacities in PD subjects.76 Tai Chi practiced for 60 min, twice weekly for 24 weeks by patients with mild-to-moderate PD improved postural stability and functional capacities (walking, muscle strength, and performance in the Timed Up-and-Go test) more than the activities of strength training and stretching.77
Multicomponent training improves muscle strength, flexibility, postural balance, walking speed, mobility, functional capacity,78,79 physical performance, and the PAs of daily life,79 especially if the training program lasts more than 10 weeks.80 Aerobic exercise, stretching, strength training, Qigong, and balance training improve motor function, and in particular muscle strength, balance, and walking speed in PD subjects.8,81 These effects on physical functioning may explain, at least in part, why quality of life can be improved after only 6 weeks of training.82
The benefits of PE on fall prevention for PD subjects remain to be determined.83,84 Although PE is of overall benefit for the health and functional capacities of PD subjects, the best exercise regimen remains to be determined.14
Neuroprotective mechanisms induced by physical exercise in subjects affected by Parkinson's disease
The current knowledge about the mechanisms involved in the protective effect of PE against PD relies on data obtained in animal models. It has been shown that PE has a protective effect on the dopaminergic function of PD animals by stimulating the expression of several neurotrophic factors and angiogenesis.11 In response to PE, the concentration of dopamine increases and the receptors of this neurotransmitter enhance their sensitivity.11 More precisely, PE reduces the alteration of the dopaminergic neurons in the substantia nigra and contributes toward reconstituting the function of the basal ganglia involved in the motor command by the adaptive mechanisms of dopamine and glutamate neurotransmission (Fig. 1).85 This action is related to an increased concentration of BDNF.86 In addition, the hyperexcitability often observed in the basal ganglia is decreased.87
Diminished loss of the neurons that produce dopamine is observed in PD mice after 18 months of training, as well as improved movement-balance coordination.88 Mechanistic investigations revealed that the neuronal and behavioral recovery generated by PE is associated with an improvement of the mitochondrial function and increases in the cerebral levels of BDNF and glial-cell-line-derived neurotrophic factors. According to Lau et al.,88 PE not only protects neurons and mitochondria, but also increases the concentration of neurotrophic factors in the substantia nigra (nigrostriatal neurotrophic factors) in PD mice with moderate neurodegeneration.
At another neurological level, aerobic training for PD rats (in sessions lasting 20-60 min) performed 5 days a week for 4 weeks can restore the expression of glial fibrillary acidic protein (GFAP) in the dorsal striatum, indicating that astrocytes may play a role in producing the beneficial effects of PE in PD.89 It was suggested that the reduction in GFAP expression is related to the reduced expansion of astrocytes, probably due to an increase in the synaptic function in the dorsal striatum induced by PE.89 This observation also demonstrates the neuroprotective role of PE.
Furthermore, regular and continuous training of rats (from 5 to 23 months of age) over a period of 18 months, which involved them running on a horizontal treadmill at a speed of 20 m/min for 20 min, twice a day, 5 days a week, also had a neuroprotective effect on the cerebellum,90 a part of the brain that is fundamentally involved in the command and control of movement and balance. Larsen et al.90 reported that sedentary elderly rats had 11% fewer Purkinje cells (cerebellar efferents) and 9% smaller Purkinje cell soma volumes (p=0.02 for both) than exercising elderly rats, with the latter having the same number of Purkinje cells as young rats (5 months of age).
CONCLUSION
The current knowledge supports PA as an important preventive factor against the onset of both AD and PD, and that PE is crucial for the maintenance or slow decline of optimal functional ability levels in AD and PD patients. However, there is currently insufficient information to enable a precise definition of the best exercise regimen for patients with AD or PD. Aerobic exercise is very much favored by therapists because of its role in relation to angiogenesis as well as the liberation of neurotrophic factors (increases in cerebral blood flow and cerebral plasticity). Aerobic exercise is a necessary part of the treatment for AD, but aerobic exercise alone is probably not the best activity. Strength/power training, Tai Chi, balance/coordination, and other types of PE also contribute to reconditioning, maintaining, and improving the cognitive and motor functions of AD and PD subjects. Therefore, it is advisable to combine aerobic PE with other exercises that are beneficial for the neuromuscular system (e.g., strength/power training and stretching), balance function, and the performance of motor coordination. The development of coordination associated with the stimulation of psychomotor capacity seems particularly relevant for PD subjects because of the specificity of their pathology (alteration of the basal ganglia) involving movement command and control processes.
Future studies should quantitatively and qualitatively compare the effects of different types of PE on well-defined outcomes (e.g., disability) in both AD and PD populations. Researchers and health-care workers should pay attention to exercise-program adherence in this elderly, sick, and often polymedicated population, since adherence and compliance are important challenges in clinical populations. When specifying the type, intensity, and overall PA volume that should be practiced in order to prevent the development of AD and PD, future studies will also need to determine the optimal lifetime periods (childhood, adolescence, young adult, middle age, old age, and very old age) during which particularly close attention should be paid. When attempting to determine the nature of optimal PE that should be practiced in order to limit the evolution of these pathologies, future studies will need to assess the impacts of the intensity, duration, and frequency of different exercises.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Bonaconsa M, Colavito V, Pifferi F, Aujard F, Schenker E, Dix S, et al. Cell clocks and neuronal networks: neuron ticking and synchronization in aging and aging-related neurodegenerative disease. Curr Alzheimer Res. 2013;10:597–608. doi: 10.2174/15672050113109990004. [DOI] [PubMed] [Google Scholar]
- 2.Zawia NH, Basha MR. Environmental risk factors and the developmental basis for Alzheimer's disease. Rev Neurosci. 2005;16:325–337. doi: 10.1515/revneuro.2005.16.4.325. [DOI] [PubMed] [Google Scholar]
- 3.Logroscino G. The role of early life environmental risk factors in Parkinson disease: what is the evidence? Environ Health Perspect. 2005;113:1234–1238. doi: 10.1289/ehp.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao HM, Hong JS. Gene-environment interactions: key to unraveling the mystery of Parkinson's disease. Prog Neurobiol. 2011;94:1–19. doi: 10.1016/j.pneurobio.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulson HL, Igo I. Genetics of dementia. Semin Neurol. 2011;31:449–460. doi: 10.1055/s-0031-1299784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibañez V. Les maladies neuro-dégénératives: problmes cliniques. Med Nucl. 2005;29:213–219. [Google Scholar]
- 7.Wenk GL. Neuropathologic changes in Alzheimer's disease. J Clin Psychiatry. 2003;64(Suppl 9):7–10. [PubMed] [Google Scholar]
- 8.Falvo MJ, Schilling BK, Earhart GM. Parkinson's disease and resistive exercise: rationale, review, and recommendations. Mov Disord. 2008;23:1–11. doi: 10.1002/mds.21690. [DOI] [PubMed] [Google Scholar]
- 9.Sutoo D, Akiyama K. Regulation of brain function by exercise. Neurobiol Dis. 2003;13:1–14. doi: 10.1016/s0969-9961(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 10.Leem YH, Lee YI, Son HJ, Lee SH. Chronic exercise ameliorates the neuroinflammation in mice carrying NSE/htau23. Biochem Biophys Res Commun. 2011;406:359–365. doi: 10.1016/j.bbrc.2011.02.046. [DOI] [PubMed] [Google Scholar]
- 11.Zigmond MJ, Cameron JL, Hoffer BJ, Smeyne RJ. Neurorestoration by physical exercise: moving forward. Parkinsonism Relat Disord. 2012;18(Suppl 1):S147–S150. doi: 10.1016/S1353-8020(11)70046-3. [DOI] [PubMed] [Google Scholar]
- 12.Radak Z, Hart N, Sarga L, Koltai E, Atalay M, Ohno H, et al. Exercise plays a preventive role against Alzheimer's disease. J Alzheimers Dis. 2010;20:777–783. doi: 10.3233/JAD-2010-091531. [DOI] [PubMed] [Google Scholar]
- 13.Voelcker-Rehage C, Niemann C. Structural and functional brain changes related to different types of physical activity across the life span. Neurosci Biobehav Rev. 2013;37(9 Pt B):2268–2295. doi: 10.1016/j.neubiorev.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 14.Alonso-Frech F, Sanahuja JJ, Rodriguez AM. Exercise and physical therapy in early management of Parkinson disease. Neurologist. 2011;17(6 Suppl 1):S47–S53. doi: 10.1097/NRL.0b013e31823968ec. [DOI] [PubMed] [Google Scholar]
- 15.Abe K. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;79:1071, author reply 1071. doi: 10.1212/WNL.0b013e31826bd5cf. [DOI] [PubMed] [Google Scholar]
- 16.Karceski S. Preventing Alzheimer disease with exercise? Neurology. 2012;78:e110–e112. doi: 10.1212/WNL.0b013e318255e0c9. [DOI] [PubMed] [Google Scholar]
- 17.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of populationbased data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 18.Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77:288–294. doi: 10.1212/WNL.0b013e318225ab66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitkälä KH, Pöysti MM, Laakkonen ML, Tilvis RS, Savikko N, Kautiainen H, et al. Effects of the Finnish Alzheimer disease exercise trial (FINALEX): a randomized controlled trial. JAMA Intern Med. 2013;173:894–901. doi: 10.1001/jamainternmed.2013.359. [DOI] [PubMed] [Google Scholar]
- 20.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39:3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- 21.Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78:1323–1329. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 23.Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33:772–777. doi: 10.1097/00005768-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 24.van Gelder BM, Tijhuis MA, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Physical activity in relation to cognitive decline in elderly men: the FINE Study. Neurology. 2004;63:2316–2321. doi: 10.1212/01.wnl.0000147474.29994.35. [DOI] [PubMed] [Google Scholar]
- 25.Um HS, Kang EB, Koo JH, Kim HT, Jin-Lee, Kim EJ, et al. Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer's disease. Neurosci Res. 2011;69:161–173. doi: 10.1016/j.neures.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Yu F, Kolanowski AM, Strumpf NE, Eslinger PJ. Improving cognition and function through exercise intervention in Alzheimer's disease. J Nurs Scholarsh. 2006;38:358–365. doi: 10.1111/j.1547-5069.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- 27.Rolland Y, Pillard F, Klapouszczak A, Reynish E, Thomas D, Andrieu S, et al. Exercise program for nursing home residents with Alzheimer's disease: a 1-year randomized, controlled trial. J Am Geriatr Soc. 2007;55:158–165. doi: 10.1111/j.1532-5415.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 28.Buschert V, Bokde AL, Hampel H. Cognitive intervention in Alzheimer disease. Nat Rev Neurol. 2010;6:508–517. doi: 10.1038/nrneurol.2010.113. [DOI] [PubMed] [Google Scholar]
- 29.Forbes D, Thiessen EJ, Blake CM, Forbes SC, Forbes S. Exercise programs for people with dementia. Cochrane Database Syst Rev. 2013;12:CD006489. doi: 10.1002/14651858.CD006489.pub3. [DOI] [PubMed] [Google Scholar]
- 30.Regan C, Katona C, Walker Z, Livingston G. Relationship of exercise and other risk factors to depression of Alzheimer's disease: the LASER-AD study. Int J Geriatr Psychiatry. 2005;20:261–268. doi: 10.1002/gps.1278. [DOI] [PubMed] [Google Scholar]
- 31.Williams CL, Tappen RM. Exercise training for depressed older adults with Alzheimer's disease. Aging Ment Health. 2008;12:72–80. doi: 10.1080/13607860701529932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams CL, Tappen RM. Effect of exercise on mood in nursing home residents with Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2007;22:389–397. doi: 10.1177/1533317507305588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scarmeas N, Luchsinger JA, Brickman AM, Cosentino S, Schupf N, Xin-Tang M, et al. Physical activity and Alzheimer disease course. Am J Geriatr Psychiatry. 2011;19:471–481. doi: 10.1097/JGP.0b013e3181eb00a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernandez SS, Coelho FG, Gobbi S, Stella F. [Effects of physical activity on cognitive functions, balance and risk of falls in elderly patients with Alzheimer's dementia] Rev Bras Fisioter. 2010;14:68–74. [PubMed] [Google Scholar]
- 35.de Andrade LP, Gobbi LT, Coelho FG, Christofoletti G, Costa JL, Stella F. Benefits of multimodal exercise intervention for postural control and frontal cognitive functions in individuals with Alzheimer's disease: a controlled trial. J Am Geriatr Soc. 2013;61:1919–1926. doi: 10.1111/jgs.12531. [DOI] [PubMed] [Google Scholar]
- 36.Yu F, Swartwood RM. Feasibility and perception of the impact from aerobic exercise in older adults with Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2012;27:397–405. doi: 10.1177/1533317512453492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yaffe K. Biomarkers of Alzheimer's disease and exercise: one step closer to prevention. Ann Neurol. 2010;68:275–276. doi: 10.1002/ana.22143. [DOI] [PubMed] [Google Scholar]
- 38.Geda YE, Roberts RO, Knopman DS, Christianson TJ, Pankratz VS, Ivnik RJ, et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 2010;67:80–86. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Archer T. Physical exercise alleviates debilities of normal aging and Alzheimer's disease. Acta Neurol Scand. 2011;123:221–238. doi: 10.1111/j.1600-0404.2010.01412.x. [DOI] [PubMed] [Google Scholar]
- 40.Vidoni ED, Van Sciver A, Johnson DK, He J, Honea R, Haines B, et al. A community-based approach to trials of aerobic exercise in aging and Alzheimer's disease. Contemp Clin Trials. 2012;33:1105–1116. doi: 10.1016/j.cct.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venturelli M, Scarsini R, Schena F. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am J Alzheimers Dis Other Demen. 2011;26:381–388. doi: 10.1177/1533317511418956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winchester J, Dick MB, Gillen D, Reed B, Miller B, Tinklenberg J, et al. Walking stabilizes cognitive functioning in Alzheimer's disease (AD) across one year. Arch Gerontol Geriatr. 2013;56:96–103. doi: 10.1016/j.archger.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hurley BF, Hanson ED, Sheaff AK. Strength training as a countermeasure to aging muscle and chronic disease. Sports Med. 2011;41:289–306. doi: 10.2165/11585920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 44.Hill KD, LoGiudice D, Lautenschlager NT, Said CM, Dodd KJ, Suttanon P. Effectiveness of balance training exercise in people with mild to moderate severity Alzheimer's disease: protocol for a randomised trial. BMC Geriatr. 2009;9:29. doi: 10.1186/1471-2318-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suttanon P, Hill KD, Said CM, Williams SB, Byrne KN, LoGiudice D, et al. Feasibility, safety and preliminary evidence of the effectiveness of a home-based exercise programme for older people with Alzheimer's disease: a pilot randomized controlled trial. Clin Rehabil. 2013;27:427–438. doi: 10.1177/0269215512460877. [DOI] [PubMed] [Google Scholar]
- 46.Tappen RM, Roach KE, Applegate EB, Stowell P. Effect of a combined walking and conversation intervention on functional mobility of nursing home residents with Alzheimer disease. Alzheimer Dis Assoc Disord. 2000;14:196–201. doi: 10.1097/00002093-200010000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCurry SM, Pike KC, Vitiello MV, Logsdon RG, Larson EB, Teri L. Increasing walking and bright light exposure to improve sleep in community-dwelling persons with Alzheimer's disease: results of a randomized, controlled trial. J Am Geriatr Soc. 2011;59:1393–1402. doi: 10.1111/j.1532-5415.2011.03519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuede CM, Zimmerman SD, Dong H, Kling MJ, Bero AW, Holtzman DM, et al. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer's disease. Neurobiol Dis. 2009;35:426–432. doi: 10.1016/j.nbd.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coelho FG, Vital TM, Stein AM, Arantes FJ, Rueda AV, Camarini R, et al. Acute aerobic exercise increases brain-derived neurotrophic factor levels in elderly with Alzheimer's disease. J Alzheimers Dis. 2014;39:401–408. doi: 10.3233/JAD-131073. [DOI] [PubMed] [Google Scholar]
- 52.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 53.Lange-Asschenfeldt C, Kojda G. Alzheimer's disease, cerebrovascular dysfunction and the benefits of exercise: from vessels to neurons. Exp Gerontol. 2008;43:499–504. doi: 10.1016/j.exger.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Um HS, Kang EB, Leem YH, Cho IH, Yang CH, Chae KR, et al. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer's disease in an NSE/APPsw-transgenic model. Int J Mol Med. 2008;22:529–539. [PubMed] [Google Scholar]
- 55.Pérez CA, Cancela Carral JM. Benefits of physical exercise for older adults with Alzheimer's disease. Geriatr Nurs. 2008;29:384–391. doi: 10.1016/j.gerinurse.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 56.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 57.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolf SA, Kronenberg G, Lehmann K, Blankenship A, Overall R, Staufenbiel M, et al. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer's disease. Biol Psychiatry. 2006;60:1314–1323. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Belarbi K, Burnouf S, Fernandez-Gomez FJ, Laurent C, Lestavel S, Figeac M, et al. Beneficial effects of exercise in a transgenic mouse model of Alzheimer's disease-like Tau pathology. Neurobiol Dis. 2011;43:486–494. doi: 10.1016/j.nbd.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 61.Chen H, Zhang SM, Schwarzschild MA, Hernán MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64:664–669. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- 62.Xu Q, Park Y, Huang X, Hollenbeck A, Blair A, Schatzkin A, et al. Physical activities and future risk of Parkinson disease. Neurology. 2010;75:341–348. doi: 10.1212/WNL.0b013e3181ea1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thacker EL, Chen H, Patel AV, McCullough ML, Calle EE, Thun MJ, et al. Recreational physical activity and risk of Parkinson's disease. Mov Disord. 2008;23:69–74. doi: 10.1002/mds.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garraux G. [Preserve brain function...through physical exercice?] Rev Med Liege. 2008;63:293–298. [PubMed] [Google Scholar]
- 65.van Nimwegen M, Speelman AD, Hofman-van Rossum EJ, Overeem S, Deeg DJ, Borm GF, et al. Physical inactivity in Parkinson's disease. J Neurol. 2011;258:2214–2221. doi: 10.1007/s00415-011-6097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ellis T, Cavanaugh JT, Earhart GM, Ford MP, Foreman KB, Fredman L, et al. Factors associated with exercise behavior in people with Parkinson disease. Phys Ther. 2011;91:1838–1848. doi: 10.2522/ptj.20100390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuroda K, Tatara K, Takatorige T, Shinsho F. Effect of physical exercise on mortality in patients with Parkinson's disease. Acta Neurol Scand. 1992;86:55–59. doi: 10.1111/j.1600-0404.1992.tb08054.x. [DOI] [PubMed] [Google Scholar]
- 68.Tanaka K, Quadros AC, Jr, Santos RF, Stella F, Gobbi LT, Gobbi S. Benefits of physical exercise on executive functions in older people with Parkinson's disease. Brain Cogn. 2009;69:435–441. doi: 10.1016/j.bandc.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 69.Cruise KE, Bucks RS, Loftus AM, Newton RU, Pegoraro R, Thomas MG. Exercise and Parkinson's: benefits for cognition and quality of life. Acta Neurol Scand. 2011;123:13–19. doi: 10.1111/j.1600-0404.2010.01338.x. [DOI] [PubMed] [Google Scholar]
- 70.Rochester L, Baker K, Hetherington V, Jones D, Willems AM, Kwakkel G, et al. Evidence for motor learning in Parkinson's disease: acquisition, automaticity and retention of cued gait performance after training with external rhythmical cues. Brain Res. 2010;1319:103–111. doi: 10.1016/j.brainres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease. Lancet Neurol. 2013;12:716–726. doi: 10.1016/S1474-4422(13)70123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hass CJ, Buckley TA, Pitsikoulis C, Barthelemy EJ. Progressive resistance training improves gait initiation in individuals with Parkinson's disease. Gait Posture. 2012;35:669–673. doi: 10.1016/j.gaitpost.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 73.Lima LO, Scianni A, Rodrigues-de-Paula F. Progressive resistance exercise improves strength and physical performance in people with mild to moderate Parkinson's disease: a systematic review. J Physiother. 2013;59:7–13. doi: 10.1016/S1836-9553(13)70141-3. [DOI] [PubMed] [Google Scholar]
- 74.Herman T, Giladi N, Hausdorff JM. Treadmill training for the treatment of gait disturbances in people with Parkinson's disease: a minireview. J Neural Transm. 2009;116:307–318. doi: 10.1007/s00702-008-0139-z. [DOI] [PubMed] [Google Scholar]
- 75.Earhart GM, Williams AJ. Treadmill training for individuals with Parkinson disease. Phys Ther. 2012;92:893–897. doi: 10.2522/ptj.20110471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Esculier JF, Vaudrin J, Bériault P, Gagnon K, Tremblay LE. Home-based balance training programme using Wii Fit with balance board for Parkinson's disease: a pilot study. J Rehabil Med. 2012;44:144–150. doi: 10.2340/16501977-0922. [DOI] [PubMed] [Google Scholar]
- 77.Li F, Harmer P, Fitzgerald K, Eckstrom E, Stock R, Galver J, et al. Tai chi and postural stability in patients with Parkinson's disease. N Engl J Med. 2012;366:511–519. doi: 10.1056/NEJMoa1107911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schenkman M, Hall DA, Barón AE, Schwartz RS, Mettler P, Kohrt WM. Exercise for people in early- or mid-stage Parkinson disease: a 16-month randomized controlled trial. Phys Ther. 2012;92:1395–1410. doi: 10.2522/ptj.20110472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crizzle AM, Newhouse IJ. Is physical exercise beneficial for persons with Parkinson's disease? Clin J Sport Med. 2006;16:422–425. doi: 10.1097/01.jsm.0000244612.55550.7d. [DOI] [PubMed] [Google Scholar]
- 80.States RA, Spierer DK, Salem Y. Long-term group exercise for people with Parkinson's disease: a feasibility study. J Neurol Phys Ther. 2011;35:122–128. doi: 10.1097/NPT.0b013e31822a0026. [DOI] [PubMed] [Google Scholar]
- 81.Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2008;23:631–640. doi: 10.1002/mds.21922. [DOI] [PubMed] [Google Scholar]
- 82.Canning CG, Allen NE, Dean CM, Goh L, Fung VS. Home-based treadmill training for individuals with Parkinson's disease: a randomized controlled pilot trial. Clin Rehabil. 2012;26:817–826. doi: 10.1177/0269215511432652. [DOI] [PubMed] [Google Scholar]
- 83.Allen NE, Sherrington C, Paul SS, Canning CG. Balance and falls in Parkinson's disease: a meta-analysis of the effect of exercise and motor training. Mov Disord. 2011;26:1605–1615. doi: 10.1002/mds.23790. [DOI] [PubMed] [Google Scholar]
- 84.Goodwin VA, Richards SH, Henley W, Ewings P, Taylor AH, Campbell JL. An exercise intervention to prevent falls in people with Parkinson's disease: a pragmatic randomised controlled trial. J Neurol Neurosurg Psychiatry. 2011;82:1232–1238. doi: 10.1136/jnnp-2011-300919. [DOI] [PubMed] [Google Scholar]
- 85.Speelman AD, van de Warrenburg BP, van Nimwegen M, Petzinger GM, Munneke M, Bloem BR. How might physical activity benefit patients with Parkinson disease? Nat Rev Neurol. 2011;7:528–534. doi: 10.1038/nrneurol.2011.107. [DOI] [PubMed] [Google Scholar]
- 86.Wu SY, Wang TF, Yu L, Jen CJ, Chuang JI, Wu FS, et al. Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav Immun. 2011;25:135–146. doi: 10.1016/j.bbi.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 87.Petzinger GM, Fisher BE, Van Leeuwen JE, Vukovic M, Akopian G, Meshul CK, et al. Enhancing neuroplasticity in the basal ganglia: the role of exercise in Parkinson's disease. Mov Disord. 2010;25(Suppl 1):S141–S145. doi: 10.1002/mds.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lau YS, Patki G, Das-Panja K, Le WD, Ahmad SO. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson's disease with moderate neurodegeneration. Eur J Neurosci. 2011;33:1264–1274. doi: 10.1111/j.1460-9568.2011.07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dutra MF, Jaeger M, Ilha J, Kalil-Gaspar PI, Marcuzzo S, Achaval M. Exercise improves motor deficits and alters striatal GFAP expression in a 6-OHDA-induced rat model of Parkinson's disease. Neurol Sci. 2012;33:1137–1144. doi: 10.1007/s10072-011-0925-5. [DOI] [PubMed] [Google Scholar]
- 90.Larsen JO, Skalicky M, Viidik A. Does long-term physical exercise counteract age-related Purkinje cell loss? A stereological study of rat cerebellum. J Comp Neurol. 2000;428:213–222. [PubMed] [Google Scholar]