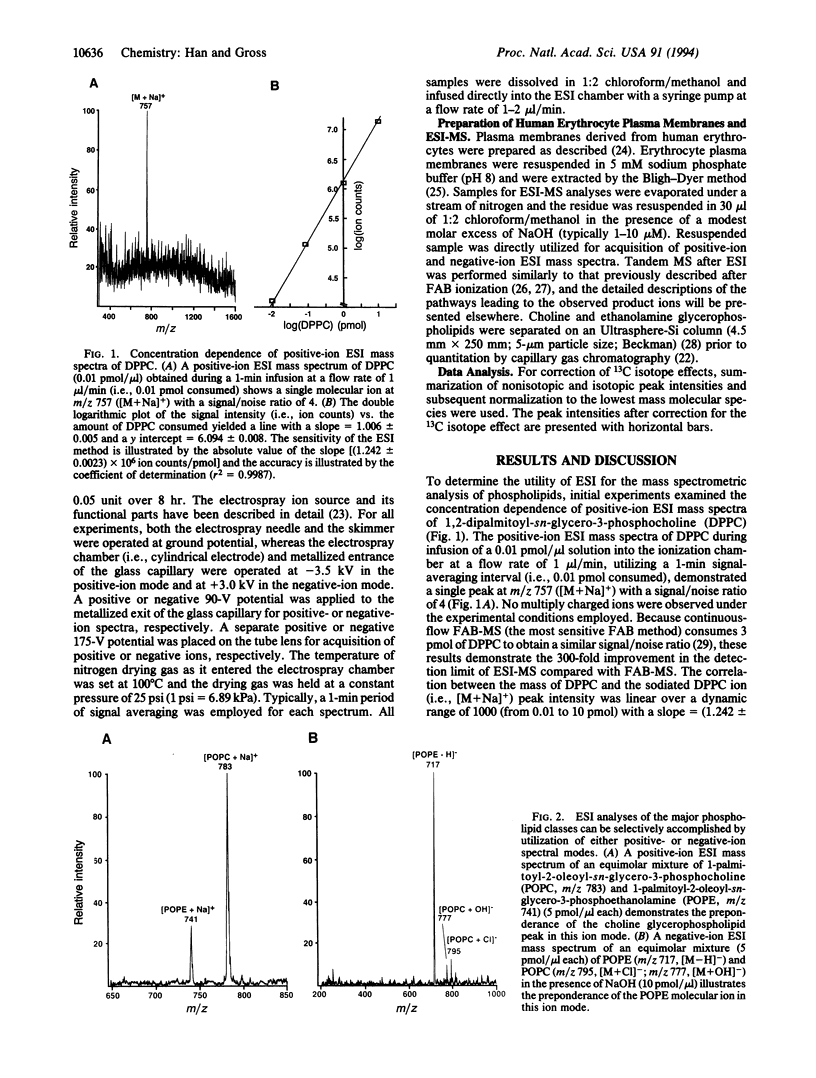
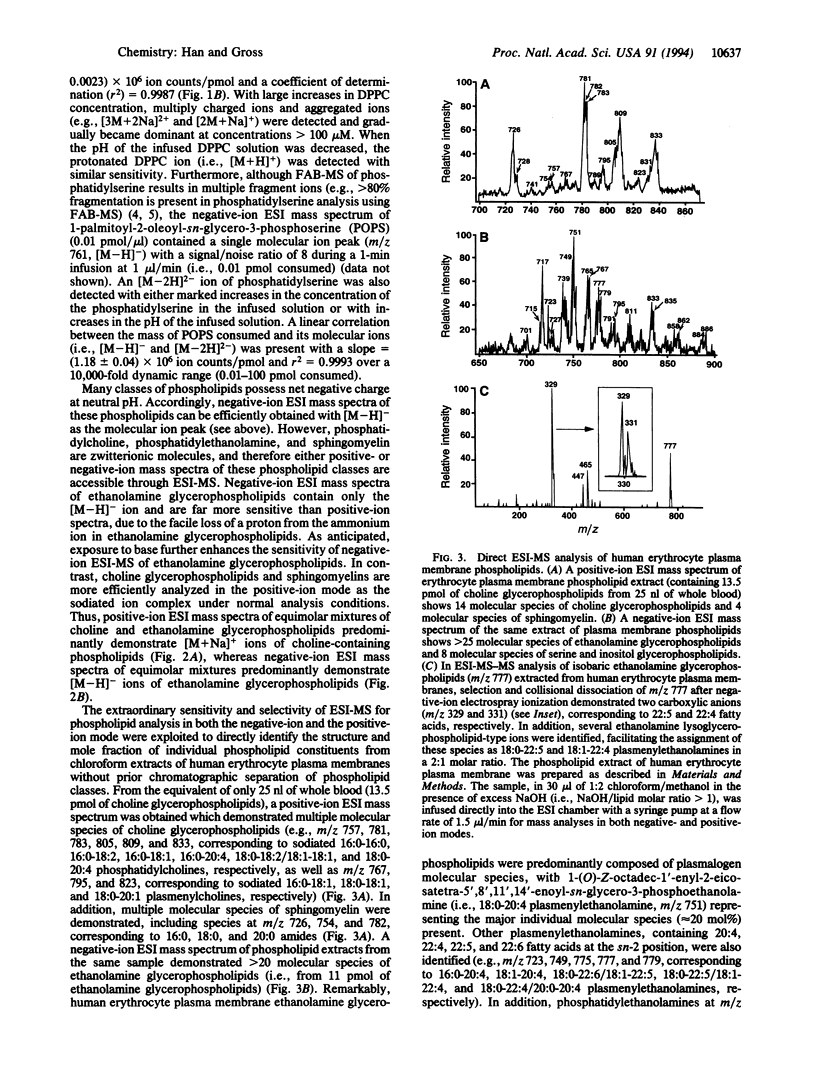
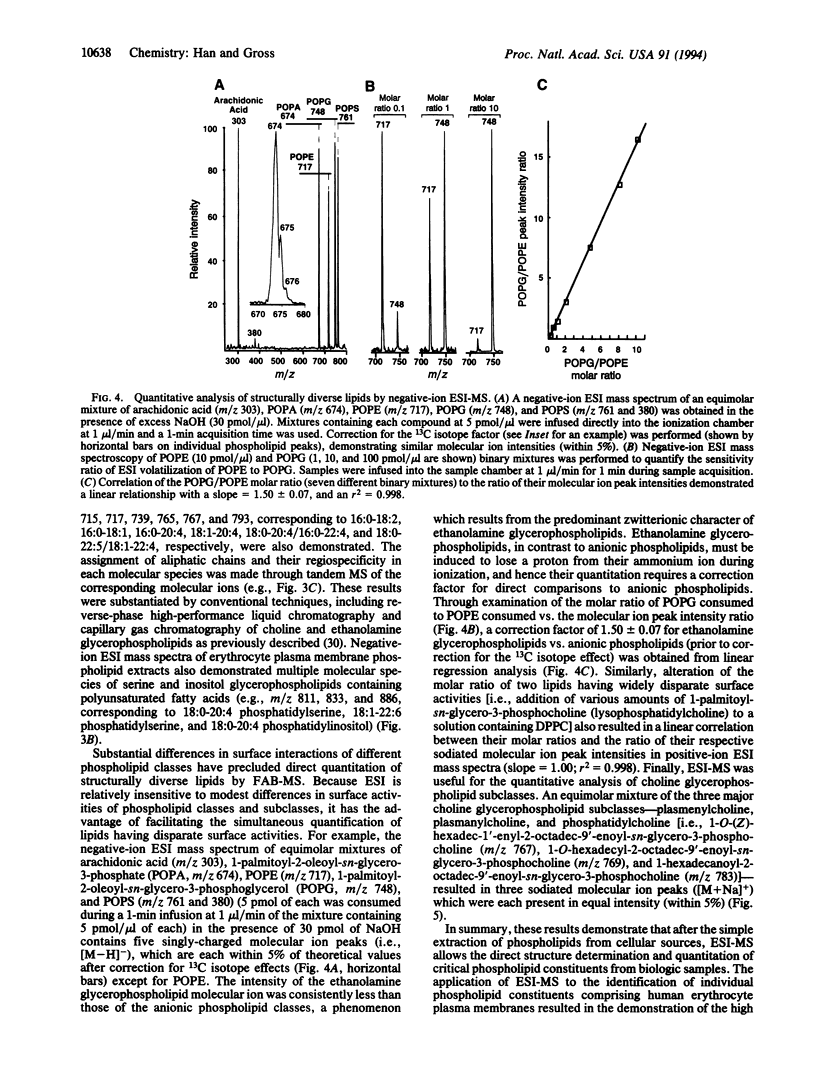
Abstract
Electrospray ionization mass spectrometry (ESI-MS) was utilized for the structural determination and quantitative analysis of individual phospholipid molecular species from subpicomole amounts of human erythrocyte plasma membrane phospholipids. The sensitivity of ESI-MS was 2-3 orders of magnitude greater than that achievable with fast-atom bombardment mass spectrometry (FAB-MS). Phospholipid structure determination and quantitative analysis with ESI-MS can be performed directly from chloroform extracts of biologic samples, obviating the need for prior chromatographic separation of phospholipid classes which has been necessary in FAB-MS phospholipid analyses. Furthermore, ESI-MS is uncomplicated by differential fragmentation of molecular ions and idiosyncratic surface desorption, allowing the quantitation of phospholipids with coefficients of determination (r2) > 0.99 and accuracies > 95%. More than 50 human erythrocyte plasma membrane phospholipid constituents were identified by direct ESI-MS analysis of chloroform extracts of plasma membranes derived from the equivalent of < 1 microliter of whole blood. The major ethanolamine glycerophospholipid subclass in erythrocyte plasma membranes was plasmenylethanolamine that was highly enriched in polyunsaturated fatty acids at the sn-2 position. Collectively, these results demonstrate that ESI-MS of phospholipids is an enabling strategy for the direct structural determination and quantitative analysis of subpicomole amounts of phospholipids from biologic samples.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton D. S., Beddell C. R., Cooper D. J., Green B. N., Oliver R. W., Welham K. J. Some electrospray mass spectrometric evidence for the existence of covalent O-acyl enzyme intermediates. FEBS Lett. 1991 Nov 4;292(1-2):201–204. doi: 10.1016/0014-5793(91)80867-3. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Chen S., Kirschner G., Traldi P. Positive ion fast atom bombardment mass spectrometric analysis of the molecular species of glycerophosphatidylserine. Anal Biochem. 1990 Nov 15;191(1):100–105. doi: 10.1016/0003-2697(90)90394-o. [DOI] [PubMed] [Google Scholar]
- Dennis E. A., Rhee S. G., Billah M. M., Hannun Y. A. Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. 1991 Apr;5(7):2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- Fenn J. B., Mann M., Meng C. K., Wong S. F., Whitehouse C. M. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989 Oct 6;246(4926):64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- Fink K. L., Gross R. W. Modulation of canine myocardial sarcolemmal membrane fluidity by amphiphilic compounds. Circ Res. 1984 Nov;55(5):585–594. doi: 10.1161/01.res.55.5.585. [DOI] [PubMed] [Google Scholar]
- Ford D. A., Gross R. W. Plasmenylethanolamine is the major storage depot for arachidonic acid in rabbit vascular smooth muscle and is rapidly hydrolyzed after angiotensin II stimulation. Proc Natl Acad Sci U S A. 1989 May;86(10):3479–3483. doi: 10.1073/pnas.86.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. W. High plasmalogen and arachidonic acid content of canine myocardial sarcolemma: a fast atom bombardment mass spectroscopic and gas chromatography-mass spectroscopic characterization. Biochemistry. 1984 Jan 3;23(1):158–165. doi: 10.1021/bi00296a026. [DOI] [PubMed] [Google Scholar]
- Gross R. W. Identification of plasmalogen as the major phospholipid constituent of cardiac sarcoplasmic reticulum. Biochemistry. 1985 Mar 26;24(7):1662–1668. doi: 10.1021/bi00328a014. [DOI] [PubMed] [Google Scholar]
- Han X. L., Zupan L. A., Hazen S. L., Gross R. W. Semisynthesis and purification of homogeneous plasmenylcholine molecular species. Anal Biochem. 1992 Jan;200(1):119–124. doi: 10.1016/0003-2697(92)90286-g. [DOI] [PubMed] [Google Scholar]
- Heller D. N., Fenselau C., Cotter R. J., Demirev P., Olthoff J. K., Honovich J., Uy M., Tanaka T., Kishimoto Y. Mass spectral analysis of complex lipids desorbed directly from lyophilized membranes and cells. Biochem Biophys Res Commun. 1987 Jan 15;142(1):194–199. doi: 10.1016/0006-291x(87)90470-0. [DOI] [PubMed] [Google Scholar]
- Jensen N. J., Tomer K. B., Gross M. L. Fast atom bombardment and tandem mass spectrometry of phosphatidylserine and phosphatidylcholine. Lipids. 1986 Sep;21(9):580–588. doi: 10.1007/BF02534056. [DOI] [PubMed] [Google Scholar]
- Kayganich K. A., Murphy R. C. Fast atom bombardment tandem mass spectrometric identification of diacyl, alkylacyl, and alk-1-enylacyl molecular species of glycerophosphoethanolamine in human polymorphonuclear leukocytes. Anal Chem. 1992 Dec 1;64(23):2965–2971. doi: 10.1021/ac00047a015. [DOI] [PubMed] [Google Scholar]
- Knight W. B., Swiderek K. M., Sakuma T., Calaycay J., Shively J. E., Lee T. D., Covey T. R., Shushan B., Green B. G., Chabin R. Electrospray ionization mass spectrometry as a mechanistic tool: mass of human leucocyte elastase and a beta-lactam-derived E-I complex. Biochemistry. 1993 Mar 2;32(8):2031–2035. doi: 10.1021/bi00059a020. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Hayashi A. FAB/mass spectrometry of lipids. Prog Lipid Res. 1991;30(4):301–322. doi: 10.1016/0163-7827(91)90001-l. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Pramanik B. N., Zechman J. M., Das P. R., Bartner P. L. Bacterial phospholipid analysis by fast atom bombardment mass spectrometry. Biomed Environ Mass Spectrom. 1990 Mar;19(3):164–170. doi: 10.1002/bms.1200190312. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Seydel U., Lindner B., Zähringer U., Rietschel E. T., Kusumoto S., Shiba T. Laser desorption mass spectrometry of synthetic lipid A-like compounds. Biomed Mass Spectrom. 1984 Mar;11(3):132–141. doi: 10.1002/bms.1200110308. [DOI] [PubMed] [Google Scholar]
- Smith R. D., Loo J. A., Edmonds C. G., Barinaga C. J., Udseth H. R. New developments in biochemical mass spectrometry: electrospray ionization. Anal Chem. 1990 May 1;62(9):882–899. doi: 10.1021/ac00208a002. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Wahl M. C., Kim H. S., Wood T. D., Guan S., Marshall A. G. Thin gold film-assisted laser desorption/ionization Fourier transform ion cyclotron resonance mass spectrometry of biomolecules. Anal Chem. 1993 Dec 15;65(24):3669–3676. doi: 10.1021/ac00072a021. [DOI] [PubMed] [Google Scholar]
- Weintraub S. T., Pinckard R. N., Hail M. Electrospray ionization for analysis of platelet-activating factor. Rapid Commun Mass Spectrom. 1991 Jul;5(7):309–311. doi: 10.1002/rcm.1290050702. [DOI] [PubMed] [Google Scholar]


