1. INTRODUCTION
Hemoglobin (Hb) is a truly remarkable molecule. Human adult hemoglobin (Hb A) has a tetrameric structure consisting of two α-chains with 141 amino acids each and two β-chains with 146 amino acids each. Figure 1 illustrates features of the molecule that will be discussed. The tertiary structure is the three dimensional structure of the individual protein chains. The quaternary structure is the arrangement of the multiple protein chains into a multi-subunit complex stabilized through non-covalent interactions. Each of the four chains in Hb possesses a heme group, the binding site for ligands, such as oxygen (O2), carbon monoxide (CO), or nitric oxide (NO). It is an essential protein for all vertebrates, designed to facilitate the loading of oxygen molecules in the lungs (or gills) and unloading of oxygen molecules in the tissues efficiently. Hb is one of the first proteins whose structure was determined by X-ray crystallography in the 1960s and has also been used as a paradigm for understanding the structure-function relationship in allosteric proteins. An allosteric protein is one in which binding of a substrate, product, or other effector to a subunit of a multi-subunit protein at a site (allosteric site) other than the functional site alters its conformation and functional properties and can therefore contribute to regulating its physiological properties. For a review of the structure-function relationship of Hb, see ref.1 Most of the published results and conclusions regarding the molecular basis for Hb function, until recently, were based on the information derived from X-ray crystallographic data of Hb, e.g., the classical Monod-Wyman-Changeux (MWC) and Perutz's two-structure stereochemical model for hemoglobin allostery.2-3
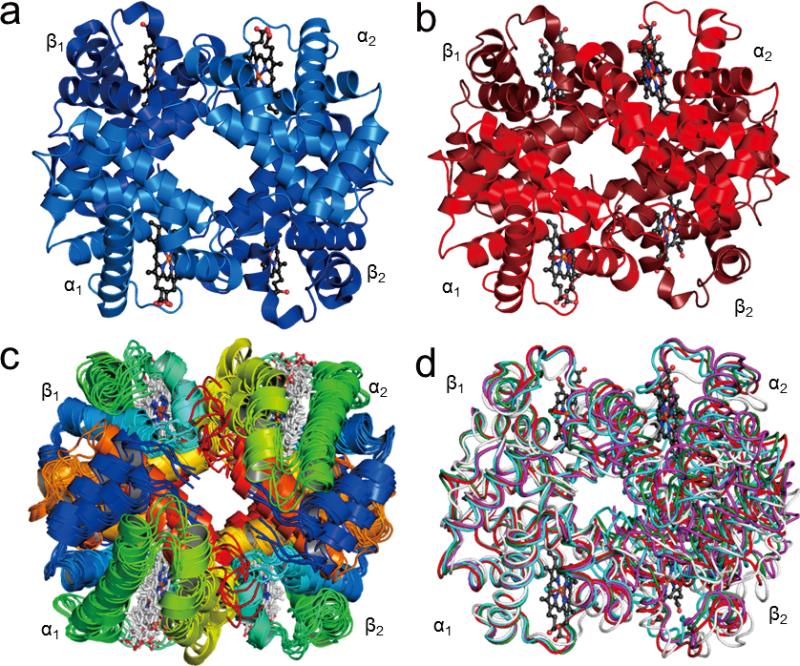
Figure 1.
Structures of Hb A: (a) Crystal structure of deoxy-Hb A (2DN2); (b) Crystal structure of HbCO A (2DN3); (c) The 10 lowest-energy solution structures of HbCO A obtained by NMR spectroscopy (2M6Z); (d) Superimposition of the R (2DN3, colored in red), R2 (1BBB, colored in magenta), RR2 (1MKO, colored in green), and R3 (1YZI, colored in cyan) crystal structures of HbCO A with the average solution structure obtained by NMR (colored in light grey). The structures are aligned according to the α1β1 dimer. Figures were generated with the PyMOL program.61
The classical MWC/Perutz model postulates that all four subunits in Hb have to assume simultaneously either the tense (T)- or relaxed (R)-structure.2-3 Both structures can bind ligands while the affinity towards the ligand changes in transiting from the T- to the R-structure. Noticing the marked differences in the crystal structures of oxy- and deoxy-Hb, Perutz3 put forward his stereochemical mechanism that correlated the T- and R- states of the MWC model to the deoxy- and oxy-structures of Hb. A key feature of the MWC model is that all four subunits must make the switch from T to R or R to T at the same time. In other words, the ligation of one subunit would not affect the ligand affinity of the neighboring subunits within the same quaternary structure. It is a concerted quaternary structural transition model. Perutz's model further postulates that inter- and intra-subunit salt bridges stabilize the Hb molecule in the T-structure. The deoxy- or T-structure has a lower ligand affinity compared to the oxy- or R-structure and the binding of oxygen is cooperative, i.e., binding of the first oxygen molecule increases the affinity of the Hb molecule for additional oxygen molecules.
The induced-fit or sequential model [also known as the Koshland-Nemethy-Filmer (KNF) model] is another classical model for Hb allostery.4-5 It postulates that the binding of a ligand to one subunit can induce the conformational changes in the tertiary structure of its neighboring subunits without their having a bound ligand. Thus, the ligand binding in a multi-subunit protein is a sequential process; there are not just two final states, T and R, but a series of intermediate states. A conformational change in a neighboring subunit can take place in the absence of ligand binding.
Both the MWC and the KNF models can account for the cooperative oxygen binding to Hb, thus the ligand-binding data alone cannot distinguish the KNF model from the MWC/Perutz model. Much work has been done in the last sixty years in order to determine whether the transition from the T to the R state is concerted or sequential and to gain an understanding of the atomic and molecular details of the cooperative oxygenation of Hb A and the mechanism of allostery.
The stereochemical mechanism of Perutz was extended by Szabo and Karplus6 and later refined by Lee and Karplus.7 This statistical-mechanical model derives a partition function that describes the influence of homotropic (oxygen) and heterotropic [e.g., hydrogen ions and 2,3-bisphosphoglycerate (2,3-BPG)] effectors on the Hb structural changes. Two different tertiary structures for each of the two quaternary structures have been included in their formulation. Contrary to Perutz's model, the Szabo-Karplus model takes into account the differences in strength of the salt bridges that stabilize the T-structure and the contributions of the pH-independent steric constraints in reducing the ligand affinity of Hb in the deoxy state.
Yonetani and co-workers proposed a global allostery model.8 This model stipulates that in the absence of heterotropic effectors, the allostery of Hb follows the MWC/Perutz model. Heterotropic effectors, when present, interact with both T and R states of Hb to induce tertiary rather than quaternary structural changes. The changes in oxygen affinity, Bohr effect, and cooperativity of Hb are primarily the consequence of heterotropic effector-induced tertiary structural changes.
The tertiary two-state model of Eaton and co-workers9 can be considered as a variation of the MWC/Perutz model. Within each quaternary structure, the subunits exist in equilibrium of high (r) and low (t) affinity conformers. The R- and T-structures as defined in the MWC/Perutz model favor the r and t formation, respectively. As in the MWC/Perutz model, ligand binding without a quaternary conformation change is non-cooperative. However, the tertiary conformations of individual subunits play the primary role instead of the quaternary conformations.
The molecular code for cooperativity of Ackers and coworkers10-11 points out that there are eight ligation intermediates between the completely unliganded and the fully liganded tetrameric Hb. The tetrameric Hb switches from T- to R-form when at least one subunit of each dimer is liganded. Hence, five ligation intermediates plus the fully liganded tetrameric Hb exist in the R-structure. Within each quaternary state, oxygen binding or releasing “sequentially” modulates the tertiary constraints, which ultimately leads to the quaternary structural switch. Cooperativity is the result of both “concerted” quaternary switching and “sequential” modulation of ligand binding within each quaternary form.
There are many crystal structures determined over the years and several well-characterized T- and R-types of crystal structures of Hb A reported in the literature are summarized in Table 1. This multitude of structures and recent results obtained by other methods clearly show that the classical two-structure MWC/Perutz description for hemoglobin allostery as presented in biochemistry, biophysics, and molecular biology textbooks cannot account for Hb function in details and needs revision. In our last review12 ten years ago, we gave a summary of our experimental results on the molecular basis of the Bohr effect of Hb A and the solution conformation, dynamics, and subunit communication of Hb A as derived from our nuclear magnetic resonance (NMR) studies. Here, we present new results of NMR and wide-angle X-ray scattering (WAXS) studies that are relevant to the structure-function relationship in hemoglobin.
Table 1.
Crystallization conditions and resolutions of various crystal structures.
| PDB code | Resolution [Å] | Space group | R factor | Ligation state/Ligand | Experimental condition | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Crystallization Buffer | pH | T (°C) | ||||||
| 1HHO | 2.10 | P 41212 | 0.223 | R/O2 | 2.25-3.0 M phosphate | 6.8-8.5 | 4 | 45 |
| 1BBBa | 1.70 | P 212121 | 0.184 | R2/CO | 100 mM (CH3)2AsO2Na, 75 mM HCl and 16% PEG 6000 | 5.8 | r.t. | 47 |
| 1MKOab | 2.18 | P 212121 | 0.200 | RR2/CO | 2.25-2.75 M phosphate | 6.7 | r.t. | 53 |
| 1YZIb | 2.07 | P 4122 | 0.208 | R3/CO | 7.1 | |||
| 2DN1b | 1.25 | P 41212 | 0.195 | R/O2 | 2.4 M phosphate, 10% glycerol | 6.7 | 4 | 35 |
| 2DN3b (1IRDa) | 1.25 | P 41212 | 0.183 | R/CO | 20 | |||
| 1LJWb | 2.16 | P 41212 | 0.205 | R/CO | 2.35-2.65 M phosphate | 6.4 | r.t. | 44 |
| 3HXN | 2.00 | P 21 | 0.225 | R/IHP, CO | -- | -- | -- | |
| 4N7N | 2.75 | C 2 | 0.251 | Rmix/met | 10 mM KH2PO4, 18% PEG3350 and 5% glycerol | 6.8 | 20 | 60 |
| 4HHBa | 1.74 | P 21 | 0.135 | T/deoxy | 3.6 M ammonium sulfate/phosphate | 6.5 | r.t. | 42 |
| 2DN2a | 1.25 | P 21 | 0.179 | T/deoxy | 3.6 M ammonium sulfate/phosphate | 6.5 | 20 | 35 |
| 1A3Nab | 1.80 | P 21 | 0.171 | T/deoxy | 3.6 M ammonium sulfate/phosphate | 6.5 | r.t. | 46 |
| 1HGAa | 2.10 | P 21212 | 0.200 | T/deoxy | 100 mM Phosphate, 8% PEG 8000 | 7.0 | r.t. | 56 |
| 1KD2a | 1.87 | P 21212 | 0.198 | T/deoxy | Deionized PEG 3350 | 7.02 | r.t. | 64 |
| 1RQ3a | 1.91 | P 21212 | 0.167 | T/deoxy | 10 mM potassium phosphate, 100 mM potassium chloride 10% PEG 6000 | 7.0 | r.t. | 65 |
| 1XXTa | 1.91 | P 21212 | 0.182 | T/deoxy | 10 mM potassium phosphate, 100 mM potassium chloride 10% PEG 6000 | 7.0 | r.t. | 55 |
| 1BZ0a | 1.50 | P 21 | 0.167 | T/deoxy | 3.6 M ammonium sulfate/phosphate | 6.5 | r.t. | 66 |
| 2D60b | 1.70 | P21 | 0.198 | T/deoxy | 100 mM (CH3)2AsO2Na, 75 mM HCl and 16% PEG 6000 | 5.8 | 20 | 59 |
| 1HGC | 2.10 | P 21212 | 0.200 | T/α-oxy | 100 mM Phosphate, 8% PEG 8000 | 7.0 | r.t. | 56 |
| 1HGB | 2.10 | P 21212 | 0.210 | T/aquamet | 100 mM Phosphate, 8% PEG 8000 and IHP | 7.0 | r.t. | 56 |
| 2D5Zb | 1.45 | P 212121 | 0.187 | T/L35, β-aquomet | 100 mM (CH3)2AsO2Na, 75 mM HCl and 16% PEG 6000, 100 μM L35 | 5.8 | 20 | 59 |
| 1THB | 1.50 | P 21212 | 0.196 | T/IHP, α-oxy | 18-25% PEG1000 | 7.2-7.4 | -- | 58 |
| 1B86 | 2.5 | P 21212 | 0.169 | T/deoxy, DPG | 10 mM sodium phosphate, 18% PEG 1000 and saturated DPG | 7.2-7.4 | 20 | 57 |
| 1YHRa | 2.6 | P 21212 | 0.185 | T/oxy, IHP | 10 mM potassium phosphate, 100 mM potassium chloride, 10% PEG 6000 and 10 mM IHP | 7.0 | r.t. | 55 |
PDB coordinates used in our RDC analysis.
Data collected at cryogenic condition.
During the past 10 years, hemoglobin has remained an active research area for biochemical, biophysical, and computational studies with over 6,500 papers published in the literature. It is interesting to note that a search of PubMed indicates that there were 489 papers with hemoglobin titles published in 2004, 632 papers in 2009, and 813 papers in 2013. This increase in the number of Hb publications indicates that there are new results as well as new thinking in the field of hemoglobin research. This article is not intended to cover all areas of Hb research, but focuses on new findings on the nature of Hb as a dynamic ensemble as related to its properties in solution. For additional readings on Hb and Hb allostery, one could consider a number of relevant articles.13-31
2. STRUCTURE
From a molecular point of view, Hb A can be considered as a dimer of αβ dimers (Figure 1). The α1β1 and α2β2 dimers come into contact and assume a 2-fold symmetry with the axis passing through a water-filled cavity formed by the four subunits. The B, G, and H helices of the unlike subunits make packing contacts that do not change upon oxygen binding. This “BGH frame” was used to compare the rotational motions of the dimers in going from the T- to the R- conformation.32 The C and G helices and FG corner of unlike subunits make “sliding contacts” upon oxygen binding or unloading. Perutz's structural comparison3 between the T- and R-structures of Hb shows that upon oxygenation: (i) the α2β2 dimer rotates approximately 15° relative to the α1β1 dimer; (ii) the heme Fe (II) moves into the porphyrin plane; (iii) six inter-subunit salt bridges between α1 and α2 (α141Arg–α1Val; α141Arg–α126Asp), and between the α- and β-subunits (α40Lys–β146His) are broken; and (iv) two intra β-subunit salt bridges (β146His–β94Asp) are broken. These were the observations that formed his stereochemical mechanism to explain Hb allostery. In addition, the T- to R-structure transition disrupts some interactions (α42Tyr–β99Asp; α97Asn–β99Asp; α91Leu–β40Arg; and α92Arg–β40Arg) and generates new interactions (α38Thr–β97His; α41Thr–β40Arg; and α94Asp–β102Asn) among residues in the α1β2 and α2β1 interfaces.
2.1 Crystal Structures of Hemoglobin Determined by X-ray Crystallography
The low-resolution crystal structures of hemoglobin were initially elucidated by Perutz and his colleagues and reported at 5.5 Å resolution almost 60 years ago.33-34 Since then, more than 200 sets of structural coordinates representing Hb A and its mutants have been deposited with the RCSB (Research Collaboratory Structural Bioinformatics) Protein Data Bank (PDB). The liganded and deoxy forms of Hb A have been reported at 1.25 Å resolution by Park et al.35 while Savino et al.36 solved the structure of the deoxy form of a mutant of Hb A to an even higher resolution (1.07 Å). These newer crystal structures suggest a more complicated picture than the two-structure MWC/Perutz model. Figure 1 shows the crystal structures of Hb A in the deoxy (T-type, panel a) and in the carbonmonoxy (R-type, panel b) forms as well as the solution structures of HbCO A (panel c) determined recently by multi-nuclear NMR spectroscopy. Figure 1d gives a comparison of the average solution structure of HbCO A with R, R2, RR2, and R3 crystal structures.
The methods employed in preparing samples and growing crystals could influence the ultimate structures obtained by X-ray diffraction. Perutz37 crystallized Hb in high salt and pH 6.5. His protocols had been used by others35,38-46 and various representative T and R structures have been obtained and used to compare the structural changes upon ligand binding (Table 1). Alternatively, Hb crystals can be grown in low salt and the presence of polyethylene glycol (PEG). A new form, called R2 was obtained by Silva et al.47 from crystals grown in low salt (100 mM sodium cacodylate, pH 5.8) and 16% PEG. The α1β1 dimer is rotated approximately 11° relative to the α2β2 dimer in the R2 form with respect to R and in a direction different from the T structure.48-49 The R2 structure is not an artifact due to crystallization at low pH. Similar structures had been reported for a Hb A mutant (βD99Y)50-51 crystallized in high salt at pH 6.7 and a Hb A crystallized in low salt and pH 7.4 in the presence of PEG and β-octylglucoside.52 Using the high salt condition, Safo and Abraham53 obtained additional RR2 and R3 quaternary structures at pH 6.7 and 7.1, respectively, for the liganded Hb A. The RR2 model assumes an intermediate conformation between that of the R and R2 structures. The quaternary structural differences between T and R3 are as large as those between T and R2, and the T→R3 and T→R2 transitions are in different directions as defined by a rigid-body screw rotation. Compared to other liganded-Hb structures, the R3 structure has the following features: (i) reduced strain at the α-heme; (ii) reduced steric contact between the ligands and the distal residues in the β-subunits; (iii) reduced iron-iron distances between α1–α2 and β1–β2 subunits; and (iv) both α- and β-clefts are smaller. Hence, R3 has been postulated to represent the conformation actively involved in ligand uptake and or release.54
In addition to the liganded R-structures and unliganded T-structures mentioned above, partially liganded T-structures have also been reported (Table 1).55-59 They have been implicated as intermediates in going from the T- to R-states of Hb A. Recently, Shibayama et al.60 obtained a met-Hb crystal in the space group C2. The isomorphous crystal contains three tetramers, each representing a distinct conformation between the R and R2 structures.
The Cα atoms from various structures can be superimposed pairwise and the PyMOL program61 can then be employed to calculate the root-mean square deviation (RMSD). The calculated RMSD for the R–R2 (1.672 Å) and R–R3 (1.923 Å) pairs are significantly larger than for the R–RR2 (1.120 Å) pair, suggesting that the structure of RR2, not R2 or R3, is closer to that of R. By pairing the R (1IRD) or R2 (1BBB) coordinates with the T (2DN2) structure, we obtained RMSD values of 2.428 Å and 3.481 Å, respectively, for the R–T and R2–T pairs. Therefore, R is closer to T than is R2, as first reported by Silva et al.47 Among the nine T-type deoxy structures used in our residual dipolar coupling (RDC) studies (Table 1), the RMSD values between the pairs are quite small (0.130 to 0.441 Å). However, the 2DN2–1YHR pair62 has an RMSD of 0.990 Å. 1YHR represents a T-type structure with bound IHP and oxygen. These result clearly show that there are different types of T-structure, depending on the experimental conditions.
Dey et al.63 surveyed the crystallographic quaternary structures of 165 human Hb tetramers. The coordinates were superimposed with the SUPERPOSE program. The relative positioning of the dimer pairs in a tetramer, defined by the polar coordinates of the vector connecting the center of masses of the dimer pairs, was plotted. The liganded-Hbs can be divided into two major clusters, R and Y (R2). Structures that represent the RR2 (1MKO) and R3 (1YZI) forms are clearly outside the clusters. The T cluster representing the deoxy-Hbs can be subdivided according to whether the crystals are monoclinic (space group P21) formed in high salt or orthorhombic (space groups P21212 or P212121) formed in low ionic strength and in the presence of PEG. Furthermore, high-oxygen affinity Hb mutants that bind oxygen in the presence of IHP and in the T-structure62 constitute a separate group termed “Thi”. This observation is consistent with the RMSD calculations with the PyMOL program.
By varying the experimental conditions, a series of crystal structures has been obtained for Hb in either the T or the R state. With the summaries presented above, there is enough convincing evidence from these crystallographic studies to suggest that Hb can exist in multiple conformations in each state. Thus, X-ray crystallography gives static snapshot views of proteins, which are, in fact, in motion, fluctuating among many conformations.
2.2 Solution Structures of Hemoglobin and Effects of Inositol Hexaphosphate Investigated by NMR
The development of high-field, multi-nuclear, multi-dimensional NMR, and isotopic labeling techniques have provided powerful tools to investigate the structure and dynamics of proteins in solution. In order to overcome spectral overlap and to facilitate resonance assignments for a protein of the size of hemoglobin (~65,000 Dalton), we have developed a chain-selective labeling technique, in which only one type of Hb chain is isotopically labeled at a time. First, we label the Hb molecule with specific isotopes (2H, 13C, and/or 15N or all three labels) using our Hb expression plasmid,67 pHE2. Second, the purified isotopically labeled Hb A is separated into labeled α- and β-chains. Third, we prepare a chain-selective, isotopically labeled, tetrameric Hb by combining one type of the labeled chain with the other type of unlabeled chain, e.g., combine labeled α-chains with unlabeled β-chains to produce an α-chain selectively labeled Hb molecule, and vice versa for a β-chain selectively labeled Hb. The chain-selective labeling of Hb reduces the spectral complexity by 50%, i.e., only the isotopically labeled chain can be observed by heteronuclear NMR. For details, see ref.68 The solution structures of Hb A in both CO and deoxy forms have been investigated by multi-nuclear NMR methods in two ways, i.e., (i) exploring the quaternary structures by the NMR residual dipolar coupling (RDC) method,69-73 and (ii) determining the solution structure of Hb A, in particular the structure of HbCO A, by using stereo-specifically assigned methyl groups and RDC values.74
2.2.1 Quaternary Structures of HbCO A and Deoxy-Hb A in Solution
The method of weak alignment of proteins and the measurement by NMR of the 15N-1H RDCs of the backbone peptide bonds has opened the possibility to investigate the relative orientation of subunits in multi-subunit proteins.75 The measured RDCs can be compared directly with the calculated ones based on the crystal coordinates of the proteins, an excellent way to compare the quaternary structures of Hb in solution and in crystal.
The RDCs of HbCO A aligned with filamentous bacteriophage Pf1 or in a solution of phospholipid bicelles in water as well as in an isotropic medium were measured and compared with the calculated RDCs based on the coordinates of the four R-type crystal structures,69-70 R (1IRD), R2 (1BBB), RR2 (1MKO), and R3 (1YZI). The top panel of Figure 2 (taken from Figure 1 of ref.73) gives a schematic representation of proteins suspended in isotropic media or in liquid crystals to generate weak alignment in a magnetic field. The bottom panel of Figure 2 (taken from Figure 1 of ref.69) is a superposition of TROSY and HSQC spectra of HbCO A showing the cross-peaks of β65Lys in lipid bicelles (a), Pf1 phage (b), and isotropic media (c). The experimentally determined RDCs can be compared with RDCs predicted by calculations based on the X-ray crystal structures for the α- or β-chains, for an isolated αβ dimer and for the entire tetramer, as presented in Figure 3 (taken from Figure 3 of ref.70). The experimental RDC values for the amino acid residues situated in the rigid α-helices were compared with those calculated separately for the α- and β-chains and for the αβ dimer using the R and R2 crystal structures. We found that there was no significant difference in the goodness of the fit, as these structures differ mainly in the relative orientation of the α2β2 and the α1β1 dimer. It was found, though, that for the entire tetramer, the best match to the experimental data was for a quaternary structure corresponding to a rotation midway between the R and R2 crystal structures, indicating that the quaternary solution structure of HbCO A was neither that of the classical R structure nor that of the R2 structure.69
Figure 2.
(Top): A schematic drawing of proteins and liquid crystals mixture which can generate weak alignment upon application of a magnetic field. Partially aligned molecules (drawing on the left side) yield results in Panels a and b, while proteins in isotropic medium (drawing on the right side) yield results in Panel c. Reprinted with permission from ref. 73. Copyright 2011 Research Signpost. (Bottom): Superimposition of TROSY and HSQC spectra of β-chain specifically (2H,15N)-labeled HbCO A in 90% H2O/10% D2O. Cross-peaks of β65Lys from proteins suspended in: (a) lipid bicelle, (b) Pf1 phage, and (c) isotropic media are shown. Reprinted with permission from ref. 69. Copyright 2003 National Academy of Sciences.
Figure 3.
The observed RDCs plotted against those calculated according to the coordinates of crystal structures R (1IRD), R2 (1BBB), RR2 (1MKO), and R3 (1YZI). The quality factors (Q) of the plots are 14.6, 15.2, 17.9, and 26.4%, respectively.179 The error in each RDC measurement, shown as a horizontal error bar, was calculated from the line width and signal-to-noise ratio of the corresponding cross-peaks.180 Reprinted with permission from ref. 70. Copyright 2006 American Chemical Society.
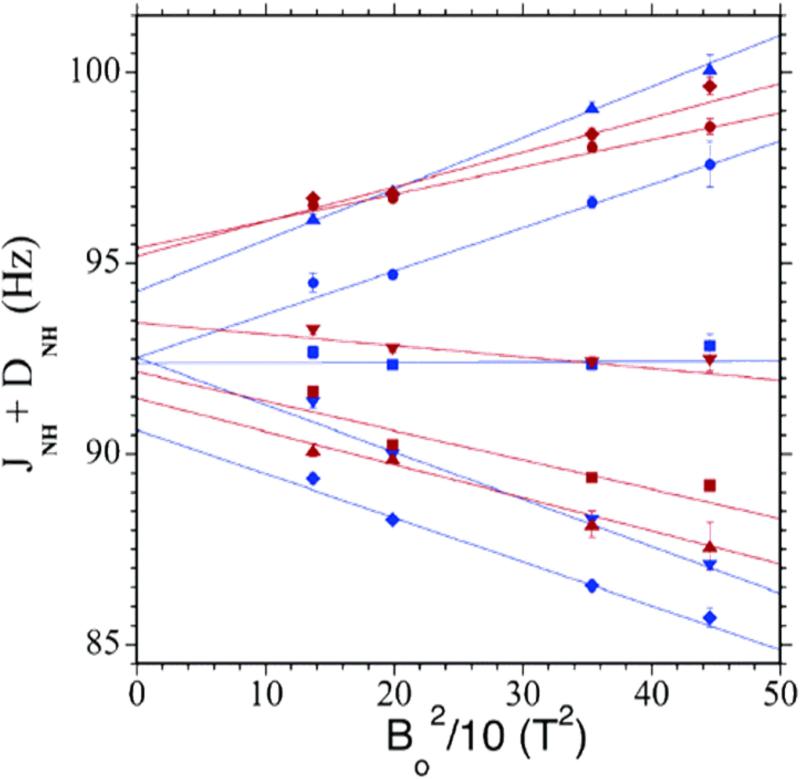
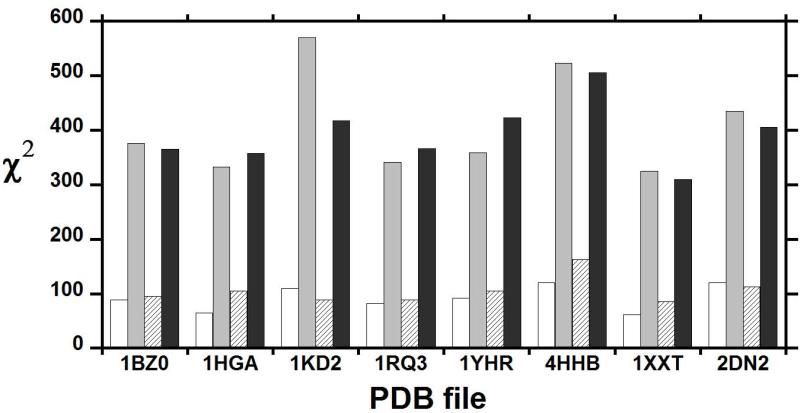
Because deoxy-Hb is a paramagnetic molecule with four unpaired electrons per heme, partial alignment of this molecule can be achieved solely by the effect of the static magnetic field and RDCs can be obtained by measuring the effective J-coupling (JHN + DHN) at several magnetic field strengths (Figure 4).71-72 The calculated RDCs72 were obtained from eight crystal structures of deoxy-Hb A, 4HHB, 1HGA, 1KD2, 1RQ3, 1XXT, 1BZ0, 2DN2, and 1YHR. The scatter in the eight correlation plots between the observed and calculated RDCs is larger than the experimental precision. Among the high-resolution deoxy-Hb A structures, 1XXT (with a resolution of 1.91 Å) provides a better fit than the highest resolution structure (2DN2) at 1.25 Å. The measured RDCs were fitted to the α1β1 dimer and the tetrameric Hb A. Although the best fit is obtained for the dimer, the differences between the dimer and tetramer fits are small, as shown by the very small difference in the reduced χ2 values in Figure 5 (taken from Figure 3 of ref.72). A possible explanation could involve the intra-dimer dynamics detected by the proton solvent exchange at the α1β1 (or α2β2) interface. The RDC analysis suggests that the solution structure of deoxy-Hb A differs from all known crystal structures. It is also apparent that IHP affects at least the dimer structure and possibly also the quaternary structure of deoxy-Hb A as evidenced by the much larger reduced χ2 values in Figure 5.
Figure 4.
Magnetic field dependence of observed (1JNH + 1DNH) couplings of chain-specific (15N,2H)-labeled recombinant deoxy-Hb A. As a demonstration, five amino acid residues each are selected from the α- and β-chains of Hb A: α39Thr (blue ●) and β24Gly (red ●), α45His (blue ◆) and β25Gly (red ◆), α46Phe (blue ▼) and β30Arg (red ▼), α81Ser (blue ■) and β39Gln (red ■), and α96Val (blue ▲) and β42Phe (red ▲). The (1JNH + 1DNH) values in Hz are plotted versus the square of the magnetic field (B0) in T2 (Tesla2). The data points are fitted to the equation: 1JNH =1JNH (iso) + cB02, where c is the slope. The Y-intercept of each plot gives 1JNH (iso), which represents the true isotropic 1JNH value and is used for the determination of RDCs. Reprinted with permission from ref. 72. Copyright 2006 American Chemical Society.
Figure 5.
The fitting quality of experimentally determined RDCs versus those calculated from X-ray crystal structures of deoxy-Hb A. The PDB code of each X-ray crystal structure is shown along the x axis, and the corresponding reduced χ2 values of the fit to either the α1β1 dimer (blank in the absence and grey in the presence of IHP) or the whole tetramer (striped in the absence and black in the presence of IHP) are along the y axis. Adapted with permission from ref. 72. Copyright 2007 American Chemical Society.
The RDC analyses of Hb A in both CO and deoxy forms suggest that the structures of Hb in solution are dynamic ensembles of various structures rather than single static structures.69-72
2.2.2 Solution Structure of HbCO A and Effects of IHP
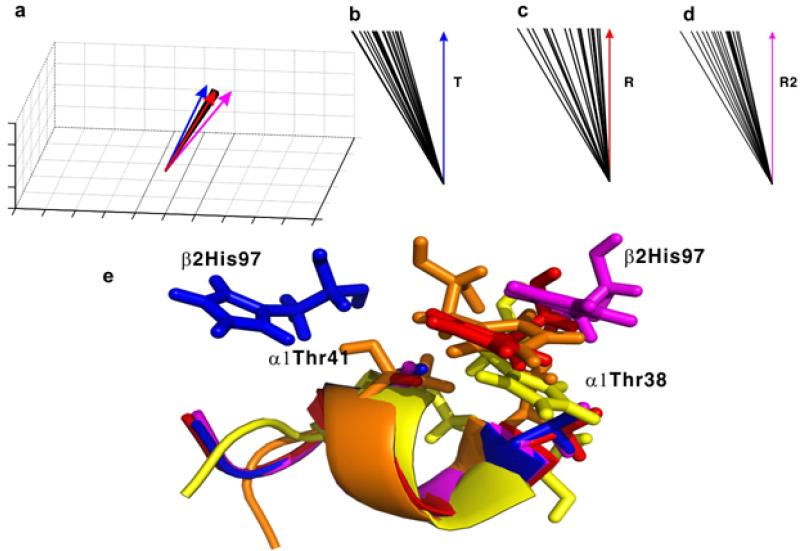
The solution structure of HbCO A determined by NMR has been further refined by using backbone NH RDCs and stereo-specifically assigned methyl groups.69-70,74,76 The NMR experiments were carried out in 0.1 M sodium phosphate at pH 7.0 and at 30 °C. The details of the 20 lowest-energy structures of HbCO A in solution were deposited in PDB (entry 2M6Z).74 For structural statistics for the 20 HbCO A conformers, see Table 1 of ref.74. Figure 1c gives the 10 lowest-energy solution structures of HbCO A.74 Both α- and β-chains assume tertiary structures similar to the X-ray crystal structures. However, the tetrameric quaternary structure determined by NMR exhibits a closer resemblance to the R than to the R2 structure determined by X-ray crystallography as shown in Figure 6c and d (taken from Figure 1 of ref.74). For the switch region of the α1β2 interface, the NMR structures of HbCO A resemble the R and R2 structures and differ significantly from the T structure with respect to the location of β297His (Figure 6e). The complete 3D solution structure of deoxy-Hb A is yet to be determined.
Figure 6.
Comparison of the symmetric axis orientations (a–d) and the switch region in the α1β2 interface (e) of the 20 lowest-energy solution conformations of HbCO A with those of the T, R, and R2 crystal structures. Panel (a) shows the distribution of the C2 axes of different structures in a three-dimensional frame. Angles between the average of the 20 lowest-energy NMR structures (represented with green arrows) and the C2 axes of the T, R, and R2 structures are shown in panels (b), (c) and (d), respectively. The angles shown in panels b–d are drawn off scale and enlarged for better visualization. The C2 axes of the T, the R, the R2, and the 20 solution conformations in panel (a) are shown as blue, red, magenta, and black lines, respectively. The switch regions in the T, R, R2, and one representative solution conformation are colored blue, red, magenta, and yellow, respectively, in panel (e). The backbone atoms of residues 38-44 in the α1 subunit are superimposed to illustrate the relative orientation of β297His. Reprinted with permission from ref. 74. Copyright 2013 American Chemical Society.
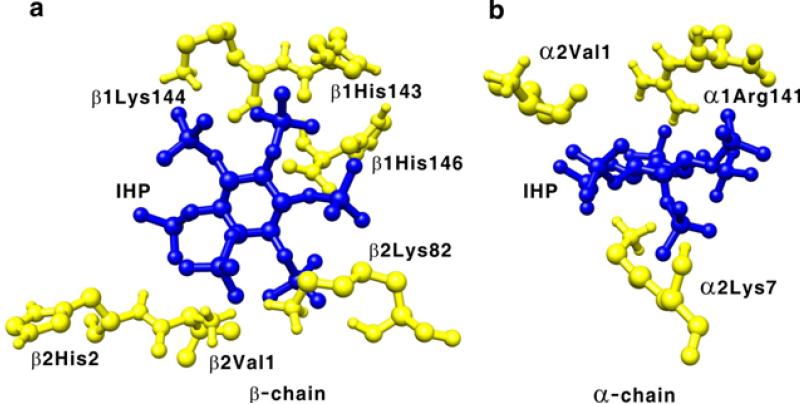
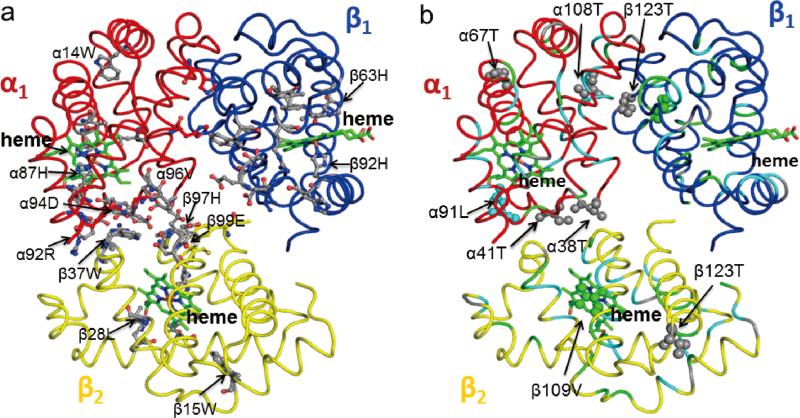
The allosteric effectors, 2,3-BPG and IHP, significantly affect the oxygen binding affinity of Hb A. The binding sites for 2,3-BPG and IHP to deoxy-Hb A were investigated by X-ray crystallography and model building experiments.77-78 However, the exact binding sites for these two allosteric effectors in the Hb molecule remain controversial. Recently, we have investigated by heteronuclear NMR the binding of IHP and its effects on the structure of HbCO A. Using the chemical shift perturbations caused by the presence of IHP, four putative IHP binding regions were identified in HbCO A, namely (i) around the N-termini (α1Val, α3Ser, α7Lys, α9Asn, β1Val, and β3Leu) and the C-termini (α137Thr, β143His, and β146His), (ii) around the EF loop (α78Asn, α79Ala, β82Lys, and β83Gly); (iii) around the switch region of the α1β2 interface composes of the αC helix and the βFG corner (α38Thr, α39Thr, α41Thr, β96Leu, β99Asp, and β101Glu) and around the joint region makes up of the αFG corner and the βC helix (α94Asp, α96Val, α97Asn, α100Leu, and β41Phe); and (iv) around the heme group (α65Ala, α83Leu, α86Leu, α136Leu, β67Val, β70Ala, β88Leu, β98Val, and β141Leu). According to the docking calculations using the solution structure of HbCO A, the central cavity has a single binding site for IHP, while the β-cleft located at the entrance of the central cavity and the α-cleft have two potential binding sites each. For details, see Figure 7 (taken from Figure 4 of ref.74). The chemical shifts for a number of amino acid residues that are not located on the protein surface have also been perturbed. This suggests that IHP binding affects not only local structures, but also disturbs regions away from the binding site. Therefore, it is difficult to determine the exact locations of the binding sites using only the chemical-shift-perturbation data. The perturbation of chemical shifts was found to depend on the IHP concentration and the binding affinity was estimated to be sub-millimolar, but it could not be quantified without knowing the exact number of IHP binding sites. Zuiderweg et al.79 used 31P NMR to approximate the affinity of IHP to HbCO at ~50 μM, compatible with our result.
Figure 7.
Putative IHP binding sites in the β-cleft (a), α-cleft (b), and central cavity (c) of Hb A. The IHP molecule is presented as blue sticks. Reprinted with permission from ref. 74. Copyright 2013 American Chemical Society.
We have found that IHP binding induces significant changes in the chemical shifts of nearly all of the methyl groups around the heme in HbCO A.74 The heme moiety and the methyl side chains located around it cannot be in direct contact with the bound IHP. Therefore, IHP binding induces tertiary structural changes around the heme, and thus causes the observed chemical-shift perturbation for the methyl groups around it. The results support the global allostery model, which stipulates that heterotropic effectors change the tertiary structure of Hb and could alter the oxygen-binding affinity.8,80 IHP binding also changes the chemical shifts of many methyl-containing residues located in the α1β2 interface and several residues in the α1β1 interface. Again, these residues could not be in direct contact with IHP. Thus, these changes in chemical shifts reflect a change in the quaternary structure of HbCO A upon IHP binding. These findings are in agreement with our RDC and backbone relaxation studies.70,81 Hence, upon IHP binding, HbCO A changes its quaternary structure and also the tertiary structures around the heme groups. Both structural changes can affect the affinity of Hb for its ligands, e.g., O2, CO, or NO.
2.3 Wide-angle X-ray Scattering Investigation of Hemoglobin Structures in Solution
Wide-angle X-ray scattering (WAXS) is a sensitive probe of protein structure in solution. This technique has evolved over the past 5 years into a powerful tool to characterize protein conformation and fluctuations in solution as well as to compare the solution and crystal structures of proteins. The breakthrough involved the capability to calculate WAXS data accurately from the atomic coordinate sets with no free parameters.82 WAXS can distinguish between similar structures and provide information on the structural fluctuations of proteins in solution.83-84 This technique was applied to investigate the structural and dynamic properties of Hb in the CO, deoxy, and met forms.84 The Hb samples were prepared in 0.05 M sodium phosphate at pH 7.0 with the Hb concentrations at 50, 20, and 10 mg/mL. WAXS experiments were carried out in a flow cell at 4 °C. Met-Hb A and HbCO A have similar WAXS patterns that are different from that of deoxy-Hb A. The results confirm the well-known relationships among these structures as defined by X-ray crystallography. The discrepancy between the calculated and observed patterns for myoglobin (Mb) (Figure 3c of ref.84) is likely due to small fluctuations in Mb structure in solution as explained previously.85 The much larger discrepancy between the calculated results based on the atomic coordinates for HbCO A (2DN3) and the observed data for Hb cannot be explained by structural fluctuations alone indicating that the quaternary structure of HbCO A in solution is dissimilar from that in the crystal.84 This finding could be explained by a dynamic ensemble of structures of HbCO A in solution consistent with the NMR results described above.
The WAXS pattern from rHbCO (αV96W/βN108K), a low affinity mutant with substitutions at the α1β2 (αV96W) and the α1β1 (βN108K) interfaces, appears to be an intermediate between HbCO A and deoxy-Hb A. These effects appear to be mediated by quaternary structural changes, since NMR studies indicate that this mutant rHb exhibits properties intermediate between the R- and the T-states, and the protein is capable of switching its quaternary structure from the R-state to the T-state even when liganded.86 Another interesting observation is that the WAXS patterns indicate that deoxy-Hb A exhibits substantially larger structural fluctuations than HbCO A, consistent with a recent molecular dynamics study that suggests increased dynamics is associated with lowered oxygen affinity.21 A similar difference in the dynamics between liganded and unliganded HbA was also observed in the intra-dimeric α1β1 and α2β2 interfaces,87 showing increased mobility correlated with decreased affinity as reported by water proton exchange of the NH side-chains of α103His.
Given the extensive crystallographic studies of Hb, the close similarity of all crystal T-state quaternary structures would seem to imply a narrow structural ensemble in solution, whereas the multiple distinct quaternary structures observed for liganded-Hbs might imply sampling of a far broader structural ensemble. However, the relationship between polymorphism in solution and polymorphism of crystallographic structures is not well understood. It could equally well be the case that the energy landscape for unliganded-Hb is smooth and broad, resulting in a single “selected” quaternary structure for many different crystallographic conditions; whereas the energy landscape for liganded-Hb might be rough and relatively narrow, making possible selection of multiple distinct quaternary structures during diverse crystallographic experiments. One might speculate that a broad, smooth energy landscape could provide a kinetic advantage for oxygen binding; whereas an irregular landscape may provide for conformations consistent with different levels of partial ligation.
3. DYNAMICS
Multi-nuclear NMR spectroscopy is also an excellent tool to investigate the dynamic properties of the polypeptide backbone and side-chain residues of Hb A on the timescales of ps to ns and μs to ms. Having completed the assignments of the polypeptide backbones88-89 of deoxy- and CO-forms of Hb A, the indole NH of the Trp residues,68 and the methyl groups74 of HbCO A, we have investigated the dynamic properties of the backbones, Trp side-chains, and methyl groups by NMR in the absence and presence of IHP. In addition, the amide-water proton exchange experiments can provide valuable information in the sub-second timescale for amino acid residues located in the subunit interfaces and the loop regions.
3.1 Backbone Dynamics Analyzed by 15N Relaxation Parameters
The amide N–H bonds of most amino acid residues are rigid in both deoxy- and CO-forms of Hb A on the fast time scale (ps to ns)81,90 as shown by Model-free-based NMR dynamics analysis.91 But, there is considerable flexibility in the loop regions, certain helix-helix connections, the intra-dimer interface (i.e., B, G, and H helices and the GH corner) and for several amino acid residues (e.g., α31Arg, β3Leu, β41Phe, β123Thr, and β146His) that are possibly involved in the allosteric pathway. The residues, α31Arg and β123Thr, neighbors in the intra-dimer (α1β1) interface, appear flexible in deoxy-Hb A, become more rigid upon CO binding. This may imply a role for α31Arg and β123Thr in intra-dimer (α1β1) communication, not predicted from the crystal structure.
Several amino-acid residues in Hb A appear to show slow conformational mobility (μs to ms timescale), which has been characterized by Rex mapping experiments.92 Conformational exchanges occur in several amino acid residues,90 such as β109Val and β132Lys in deoxy-Hb A, and α40Lys in HbCO A.
The mobility of β109Val at the intra-dimer interface (α1β1 or α2β2) of deoxy-Hb A is not consistent with the crystallographic observations that show rigid packing at this site.1,32,42 On the other hand, the mobility of α40Lys in HbCO A shown in the Rex mapping experiments is consistent with the observation from the crystallographic data, which show that the H-bond between α40Lys and β146His in deoxy-Hb A is absent in HbCO A, as a result of breaking a strong interaction in the inter-dimer interfaces (α1β2 or α2β1) due to the allosteric transition.32
Backbone dynamics studies90 indicate that the binding of IHP has distinct effects on the dynamics of the T- and R-states of Hb A. In the absence of IHP, the majority of the polypeptide backbone amino acid residues of HbCO A and deoxy-Hb A are not mobile on the μs-ms timescale with the exception of several amino acid residues, β109Val and β132Lys in deoxy-Hb A and α40Lys in HbCO A. IHP binding appears to rigidify α40Lys in HbCO A, but does not significantly affect the flexibility of β109Val in deoxy-Hb A. Conversely, in the presence of IHP, several amino acid residues, especially those at the inter-dimer (α1β2 or α2β1) interface of HbCO A, exhibit conformational exchange, such as the proximal β92His in the β-subunit heme-pocket as well as other residues located in the flexible joint (βC helix-αFG corner) and the switch region (αC helix-βFG corner) that play an important role in the dimer-dimer rotation of Hb during the oxygenation process. It appears that the IHP-induced quaternary structural fluctuation could be a factor in reducing the ligand affinity of liganded Hb. See also the results discussed in the section on methyl dynamics.
3.2 Dynamics of Tryptophan Residues Analyzed by 15N Relaxation Parameters
There are six Trp residues in the Hb A molecule, three for each αβ dimer. α14Trp and β15Trp are located in inter-helical positions within their respective subunits, while the two β37Trp residues are situated in the inter-subunit (α1β2 and α2β1) interface.1 Due to the symmetry of the molecule, there is only one signal for each subunit type, so that three peaks are observed for the indole NH in the HSQC spectra, one for each Trp type, well resolved form the amide peaks and their chemical shifts change when hemoglobin binds oxygen or CO, the largest change being for β37Trp. The side-chain of β37Trp was found to be involved in a relatively slow conformational exchange on the μs to ms timescale as detected by a Model-free analysis and the transverse relaxation dispersion method93-94 under certain experimental conditions.95
The side-chain of β37Trp is a sensitive reporter for the structural and dynamic changes that occur in the α1β2 and α2β1 subunit interfaces due to the T to R transition. Amino acid substitutions in the α1β2 (or α2β1) subunit interface and the presence of IHP affect the dynamics of β37Trp. For example, β37Trp in HbCO A exhibits conformational exchange only in the presence of IHP, implying a conformational exchange on the ms timescale as evidenced by the relaxation dispersion experiments, while the conformational exchange of β37Trp is observed in a low-affinity mutant, rHb (αV96W), in the CO form even in the absence of IHP and also in a high-affinity mutant, rHb Kempesy (βD99N), in the deoxy-form.95
The other two tryptophan residues, α14Trp and β15Trp, form H-bonds with α67Thr and β72Ser, respectively, to connect the A and E helices in the α- and β-subunits. No conformational exchange on the μs to ms timescale is observed for these residues.95 It should be noted that the dynamical roles of the side-chains of α14Trp and β15Trp were also explored by time-resolved UV resonance Raman spectroscopy96-97 with recombinant site-specific mutants of Hb A. It was found that the absence of these inter-helical H-bonds in the mutants has negligible effects on the oxygen affinity and cooperativity of Hb. However, a full complement of the H-bonds can slowdown the initial quaternary motion in Hb, but accelerate the inter-dimer motions that produce the T-state contacts. These findings provide a new insight into the roles of H-bonds at the intra-dimer α1β1 (or α2β2) interface.
3.3 Dynamics of Methyl Groups Analyzed by 13C Relaxation Parameters
The Saxis2 values of the methyl groups reflect the degree of spatial restriction of the C3 symmetric axis. The average Saxis2 values of HbCO A were found to be 0.70 for the α-chain and 0.65 for the β-chain,74 which are lower than the S2 values for the backbone dynamics. Saxis2 values of the methyl groups vary in a similar range as S2 values,81,90 especially when the methyl group is very close to the backbone, such as that in Ala residue.
The dynamics of the methyl groups is not always the same as that of the backbone. The former is also dependent on how far the methyl group is from the backbone.98 The Saxis2 values of residues Thr, Val, and Leu are less related to the secondary structure. Some residues in the α-helical elements have small (more flexible) Saxis2 values, while others located in the loop regions show relatively large Saxis2 (less flexible) values,74 suggesting that the Saxis2 values of these methyl groups are more likely affected by other factors.
One of the factors that could affect the Saxis2 values is whether the methyl groups are buried or exposed to solvent. Based on the R, R2, and NMR structures, the methyl groups of α39Thr and α108Thr are completely buried inside the protein molecule; their Saxis2 values are 0.95 and 0.81, respectively, and are significantly larger than the average. For partially solvent-exposed methyl groups, the Saxis2 values were found either lower than the average (e.g., 0.58 for α8Thr, 0.51 for β12Thr, and 0.31 for β87Thr), or significantly higher than the average (0.81 for α67Thr, 0.91 for α118Thr, 0.81 for β4Thr, 0.73 for β50Thr, and 0.89 for β123Thr).
The Saxis2 values also relate to the dynamics in the interfaces of Hb A. For example, the Saxis2 values of α38Thr and α41Thr are 0.47 and 0.43, respectively, significantly smaller than the average value (0.70), suggesting that the side-chain methyl groups in the α1β2 interface are more dynamic than those in the α1β1 interface. The larger Saxis2 values of some methyl groups are usually consistent with the existence of H-bonds observed in the X-ray crystal structures, which could be used to identify the local H-bonds. The side-chain oxygen atoms of α118Thr, β4Thr, β50Thr, and β123Thr can form H-bonds with their proximal backbone NH hydrogen atoms of α121Val, β7Glu, β53Ala, and β126Val, respectively. A H-bond between the side-chain OH of α118Thr to the backbone NH of α121Val can be formed because this distance is 3.02 Å, according to the R2 crystal structure (1BBB), for example. Similarly, the corresponding distances for β4Thr, β50Thr and β123Thr's γ-OH to the backbone NH of β7Glu, β53Ala, and β126Val are 2.98, 2.94, and 2.98 Å, respectively.
In the X-ray crystal structures of HbCO A, the H-bond between the OH group of α41Thr and the side-chain NH2 hydrogen atom of β40Arg is predicted in the R structure, not in the R2 structure. If the side-chain hydroxyl group of Thr is involved in H-bonding, the motion of the Cβ–Cγ bond would be greatly restricted and the Saxis2 value should be quite large. In fact, Saxis2 value of α41Thr is small (0.43), suggesting that the side-chain of this residue is flexible. Thus, the local solution conformation at these two residues could be closer to the R2 than the R structure, although the overall solution structure is more similar to the R than the R2 structure in terms of the relative orientation of the two αβ dimers. Another example is the H-bond between the side-chain of α67Thr and the side-chain NεHε of α14Trp. The H-bond can only exist in the R2 structure, not in the R structure.
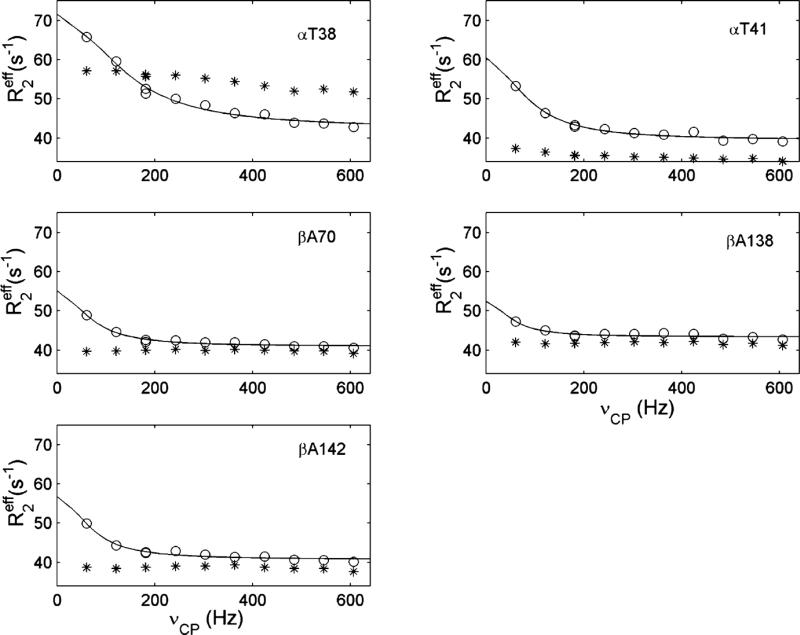
The dynamics of the side-chain methyl groups in the ps to ns and μs to ms timescales can be used to identify the binding sites of IHP in HbCO A and also to demonstrate the allosteric effects of IHP, as summarized in Figures 8 and 9 (taken from Figure 5 and 6 of ref.74). The addition of IHP to HbCO A affects the mobility of several methyl groups, some become more flexible while others become more rigid. The amino acid residues with significant changes in Saxis2 values are distributed mainly in the putative IHP binding sites mentioned earlier.
Figure 8.
Order parameters (Saxis2) describing the degree of spatial restriction of the C3 symmetric axis for methyl groups in the α- and β-chains in the absence (panels a and c, respectively) and presence (panels b and d, respectively) of IHP. The uncertainties in Saxis2 for most methyl groups were ~0.01, and the maximal uncertainty was ~0.02. The Ala, Leu, Met, Thr, and Val methyls are colored magenta, red, green, light blue, and black, respectively. The pro-R and pro-S methyl groups for Val and Leu are represented with empty and filled circles, respectively. Reprinted with permission from ref. 74. Copyright 2013 American Chemical Society.
Figure 9.
Relaxation dispersion profiles of the methyl groups with intrinsic conformational exchange in the absence (asterisks) and presence (circles) of IHP. Solid lines are fitted to the data points according to a two-state exchange model. Each panel represents the amino acid as indicated. Reprinted with permission from ref. 74. Copyright 2013 American Chemical Society.
Slower motions detected by the conformational exchange were only observed for the methyl groups of α38Thr and α41Thr, indicating that the switch region in the α1β2 interface of HbCO A is dynamic on the μs to ms timescale. Meanwhile, the methyl groups of Ala, Met, and other Thr residues in HbCO A do not display relaxation dispersion in the absence of IHP (Figure 9, taken from Figure 6 of ref.74). The conformational exchange described here is consistent with the results from our backbone relaxation study, i.e., the IHP-induced conformational exchange has been detected in three regions, namely, the α1β2 interface, heme pockets, and the presently putative IHP-binding sites.90 Localized conformational exchange in the α1β2 interface was also detected early on for the indole NH group of β37Trp in HbCO A in the presence of IHP.95
The dynamics of the backbone and the side-chain methyl groups show that IHP binds to many sites in HbCO A, which is consistent with early 31P NMR studies99 on binding of IHP to HbCO A with exchange rates greater than 104 s−1. The conformational exchange observed in our experiments could arise from the relative movement of the α1β1 dimer with respect to the α2β2 dimer. These motions could alter the local structures as manifested in changes in the chemical shifts around the α1β2 interface and also around the heme pockets, thus modulating the oxygen affinity of the Hb molecule.
3.4 Amide-Water Exchange Measured by NMR
Amide-water exchange experiments provide a way to measure the dynamics of amino acid residues on the sub-second timescale. Experiments were carried out on chain-specifically 2H- and 15N- labeled HbCO A samples by using the phase-modulated CLEAN chemical exchange with a fast HSQC detection scheme.100 Table 2 (taken from Table 2 of ref.74) gives a summary of the water-amide proton exchange rates of HbCO A with and without the presence of IHP.74 Only about 10% of the amides show relatively large exchange rates. These amides are distributed in the N-terminal region, AB connections (α19Ala-α20His and β18Val-β19Asn), αCD-βCE loop (α44Pro-α52Ser and β41Phe-β52Asp), EF loop (α72His-α81Ser and β78Leu-β87Thr), and GH loop (α114Pro-α117Phe and β118Phe-β122Phe). These results illustrate that the N-termini and all the loops on both α- and β-chains are dynamic on the ms to second timescale. α38Thr is located in the α1β2 interface, but exhibits a large exchange rate (Table 2). Thus, this region of the α1β2 interface is dynamic on the ms to second timescale and is accessible to water.
Table 2.
Water-amide proton exchange rates for HbCO A in the absence and presence of IHP. The measurements were carried out in 0.1 M sodium phosphate at pH 7.0 and 30 °C using a Bruker 800-MHz spectrometer. Reprinted with permission from ref. 74. Copyright 2013 American Chemical Society.
| residue | kex (s–1) without IHP | kex (s–1) with IHP | intrinsic kex (s–1)b |
|---|---|---|---|
| α5Ala | 15.7 ± 0.1 | 34.2 ± 1.0 | 15.0 |
| α20His | 2.6 ± 0.2 | 2.5 ± 0.1 | 43.7 |
| α22Gly | 34.7 ± 0.2 | 35.3 ± 0.6 | 48.6 |
| α38Thr | 38.3 ± 0.7 | 19.3 ± 0.8 | 12.8 |
| α45His | 5.4 ± 0.1 | 4.6 ± 0.4 | 25.1 |
| α50His | 60.3 ± 0.3 | 52.0 ± 0.8 | 87.2 |
| α51Gly | 43.7 ± 0.4 | 39.9 ± 0.6 | 119.0 |
| α52Ser | 2.3 ± 0.1 | 2.4 ± 0.1 | 90.5 |
| α53Ala | 63.1 ± 0.9 | 66.1 ± 0.5 | 52.1 |
| α54Gln | 6.1 ± 0.1 | 6.8 ± 0.2 | 30.0 |
| α74Asp | 3.7 ± 0.1 | 3.7 ± 0.1 | 9.5 |
| α82Ala | 35.6 ± 0.1 | 19.5 ± 0.2 | 52.1 |
| α115Ala | 11.1 ± 0.2 | 11.5 ± 0.3 | 15.0 |
| β3Leu | 4.3 ± 0.3 | 1.5 ± 0.1 | 16.8 |
| β6Glu | 3.7 ± 0.1 | 5.5 ± 0.4 | 4.7 |
| β19Asn | 4.8 ± 0.2 | 5.6 ± 0.1 | 58.4 |
| β41Phe | – a | 40.4 ± 2.0 | 24.9 |
| β44Ser | 28.8 ± 0.5 | 48.7 ± 0.7 | 43.5 |
| β47Asp | 4.2 ± 0.1 | 7.5 ± 0.2 | 17.3 |
| β52Asp | 1.3 ± 0.1 | 4.0 ± 0.2 | 7.6 |
| β78Leu | – a | 1.2 ± 0.1 | 16.8 |
| β79Asp | 6.4 ± 0.2 | 5.7 ± 0.1 | 10.2 |
| β81Leu | 59.6 ± 0.8 | 19.4 ± 0.8 | 14.3 |
| β82Lys | 14.8 ± 0.3 | – a | 14.7 |
| β87Thr | 16.9 ± 0.4 | 17.1 ± 0.3 | 22.2 |
| β120Lys | 5.2 ± 0.2 | 7.2 ± 0.4 | 35.2 |
Exchange rates of <1 s–1, which are undetectable.
Intrinsic exchange rates derived using SPHERE (http://www.fccc.edu/research/labs/roder/sphere/).
The binding of IHP to HbCO A does not change significantly the exchange rates for most amides (Table 2). The result implies that IHP binding does not cause significant secondary structural and dynamic changes in the Hb molecule. The few noticeable exceptions are α5Ala, β41Phe, and β44Ser. The exchange rates for these residues increase by more than 10 s−1. Conversely, the exchange rates for α38Thr, α82Ala, β81Leu, and β82Lys decrease by more than 10 s−1. The reduced exchange rates for β81Leu and β82Lys can be explained by their locations at one of the putative IHP-binding sites and the bound IHP can block water from accessing to the amides of these two residues. α38Thr, β41Phe, and β44Ser residues are located in the switch or joint region of the α1β2 interface. These residues experience significant changes in the exchange rates. The data indicate that IHP binds to HbCO and alters conformation of the α1β2-interface region (i.e., the quaternary structure), and thus could affect the ligand-binding affinity.
4. FUNCTION
The chief physiological function of Hb is to transport oxygen molecules from the lungs to the tissues of air-breathing vertebrates. This process is affected by a number of factors, e.g., the pH (known as the Bohr effect), allosteric effectors (e.g., hydrogen ions, carbon dioxide, 2,3-BPG, IHP, chloride, and phosphate), and the temperature. For a review of the physiological properties of Hb A and mutant Hbs in patients, see Bunn and Forget.101
4.1 Effects of Hydrogen Ions and Allosteric Effectors on Oxygen Affinity
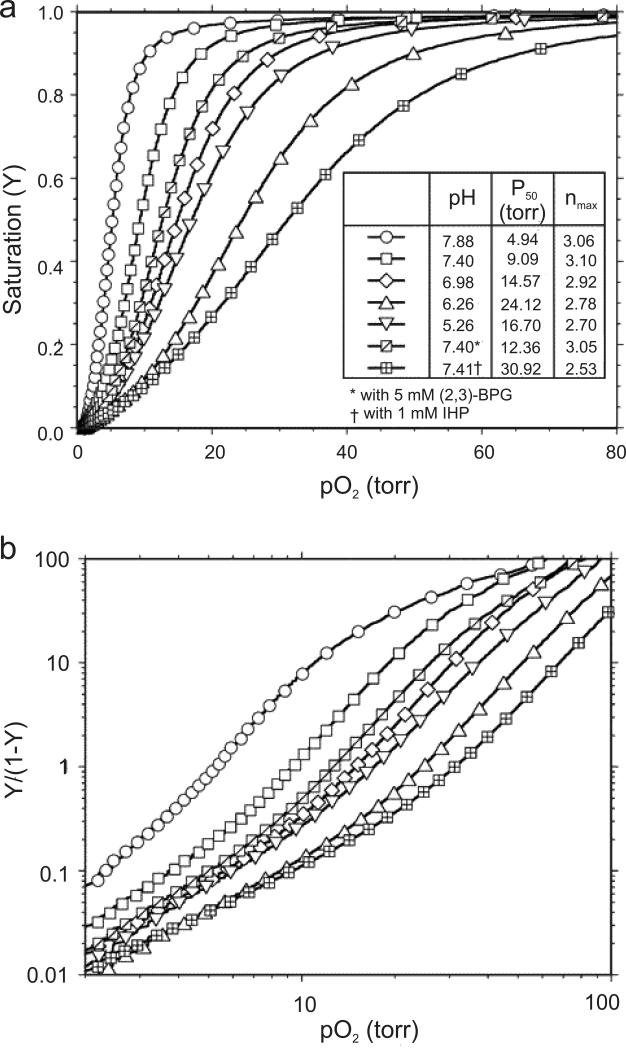
Figure 10 (taken from Figure 4A of ref.12) shows the oxygenation of Hb A in 0.1 M sodium phosphate as a function of pH and in the absence and presence of 5 mM 2,3-BPG or 1 mM IHP at 29 °C. The sigmoidal O2-binding curves and their dependence on pH and allosteric effectors illustrate the fundamental functional properties of hemoglobin that facilitate the loading and unloading of O2 molecules efficiently in the physiological system. The O2 affinity increases in going from pH 6.3 to 7.9. This is known as the alkaline Bohr effect. This effect is important for the ability of Hb to load O2 in the lungs at higher pH and deliver it to tissues, particularly in working muscles, where lactic acid and CO2 are produced and the pH is lower. However, the O2 affinity of Hb A increases again when going from pH 6.3 to 5.7, which is known as the acid Bohr effect. The Bohr effect can be measured by two classical techniques: (i) the difference between the H+ ion binding curves of HbO2 (or HbCO) and deoxy-Hb; and (ii) the change in O2 affinity as a function of pH. According to Wyman's linkage relationship, there is an exact relationship between the change in O2 affinity and the number of H+ ions released as a function of pH.102 The maximum number of H+ ions released per O2 molecule bound is ~0.5 at pH ~7.4 under physiological conditions. The difference in the number of H+ ions released or absorbed is due to a change in the pK values of several amino acid residues in the Hb molecule in going from the deoxy to the liganded state. There are two schools of thought regarding the molecular basis of the Bohr effect.103 One believes that there are only a limited number of amino acid residues involved (e.g., 2 to 3 per αβ dimer) as proposed by Perutz based on his allosteric mechanism for Hb.104 The other believes that any amino acid residue can contribute to the Bohr effect if there is a conformational change of that amino acid residue in going from the deoxy to the liganded state, i.e., if the pK value of that amino acid residue in Hb depends on its environment. In other words, the pK values of a large number of amino acid residues in Hb can change in going from the deoxy to the oxy (or CO) form and these residues contribute to the Bohr effect, positively or negatively. In addition, the amino acid residues that have a differential affinity for anions (e.g., chloride, phosphate, or 2,3-BPG) can influence the Bohr effect of the Hb molecule (known as the anion Bohr effect). Based on their pK values, the following three types of moieties are potential Bohr groups: (i) the imidazoles of His residues; (ii) the α-amino groups from the N-termini; and (iii) other proton-binding sites whose pK values are shifted from their normal values in the physiological pH range due to their unique environments in the Hb molecule. In Hb A, there are 38 His residues and 26 of them are located on the surface of the molecule,1 thus they are likely involved in the Bohr effect.
Figure 10.
(a) Oxygen-binding curves of Hb A measured in 0.1 M phosphate buffer at 29 °C at various pH values in the absence of allosteric effectors, and in the presence of 5 mM 2,3-BPG (pH 7.40) or 1 mM IHP (pH 7.41). (b) Hill plots with values calculated according to the corresponding binding curves shown in panel (a). Reprinted with permission from ref. 12. Copyright 2004 American Chemical Society.
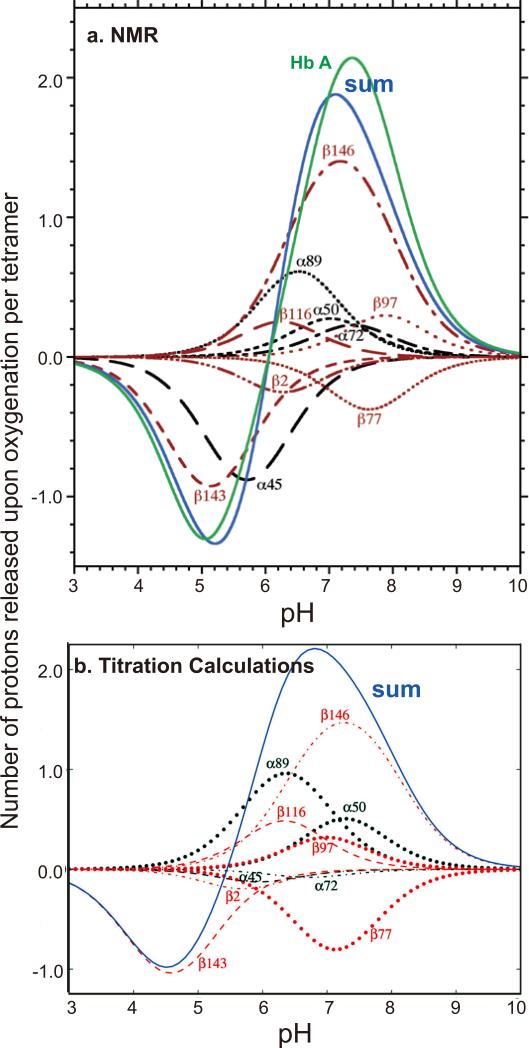
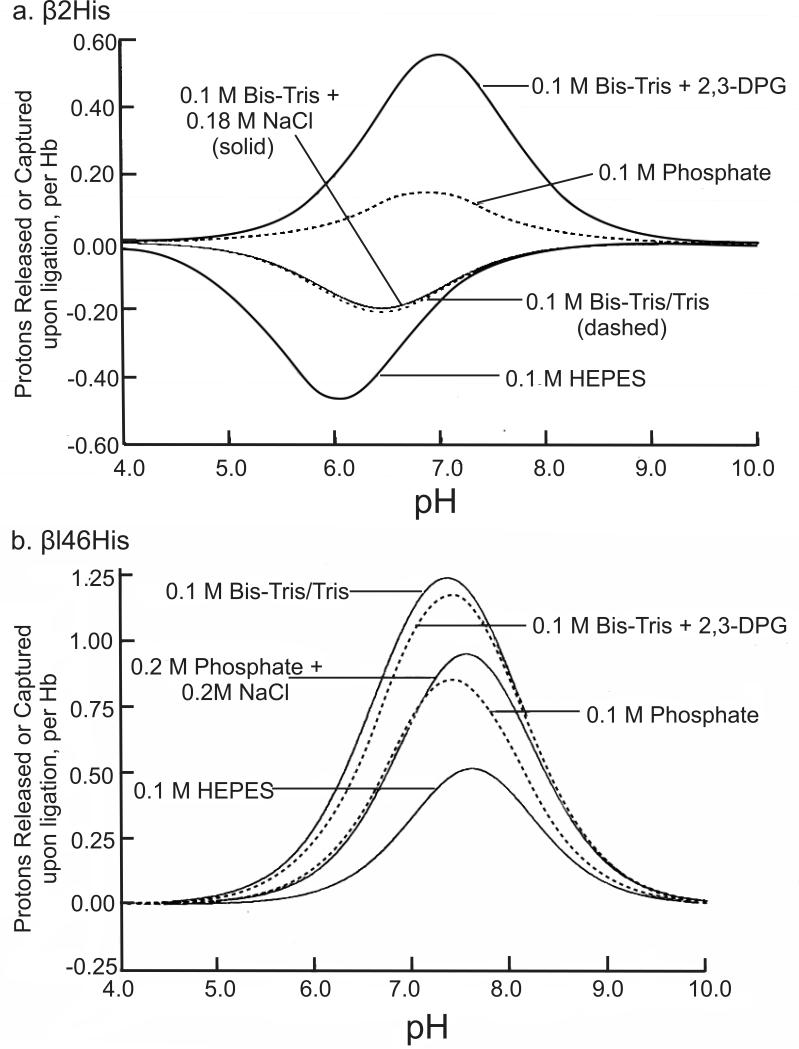
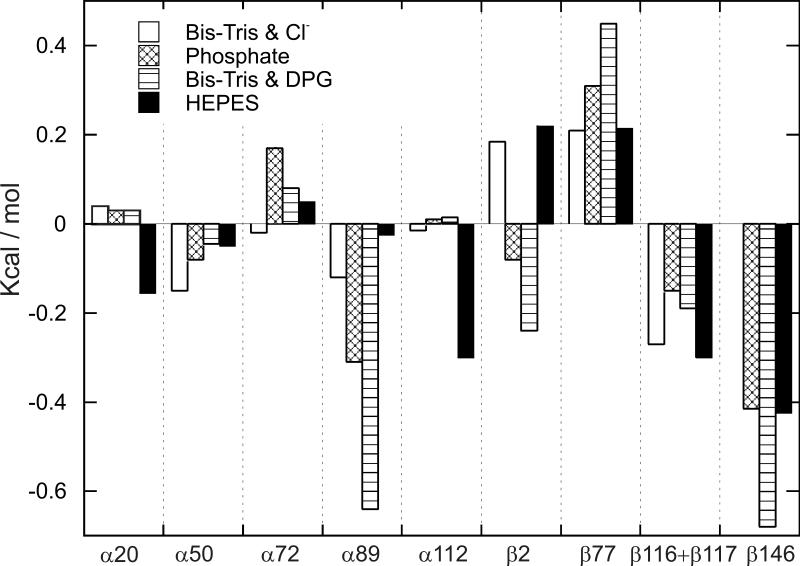
1H-NMR spectroscopy is an excellent tool to investigate the contribution of the 26 surface His residues to the Bohr effect because the resonances of the C2 protons of the His residues can be resolved from other proton resonances and are sensitive to the protonation state of the His residues. Using the site-directed mutagenesis technique to convert specific His residues to Gln or Ser, the resonances of the C2 protons of these 26 His residues were assigned and their respective pK values in the deoxy and CO states were determined.12,105-108 Our experimental results carried out in 0.1 M HEPES plus 0.1 M chloride at 29 °C can be summarized as follows (Table 3, taken from Table 1 of ref.12): (i) With the exception of α20His, α112His, and β117His, the other 23 His residues make very substantial contributions to the observed Bohr effect; (ii) the largest contribution to the alkaline Bohr effect is due to β146His, while α45His and β143His make the largest contribution to the acid Bohr effect; (iii) several His residues (e.g., β2His and β77His) make negative contributions to the alkaline Bohr effect; (iv) the sum of the contributions from the 26 His residues agrees well with the net measured acid Bohr effect, but does not account for the measured alkaline Bohr effect because there must be contributions from other amino acid residues, e.g., the N-terminal residues.109 For details see Table 3 and Figure 11a (taken from Table 1 and Figure 5, respectively of ref.12). Another important finding is that the pK values of several His residues are very sensitive to the presence of various anions and the ionic strength.105,107 Figures 12a and b (taken from Figure 2 and Figure 3, respectively of ref.105) illustrate clearly the effects of buffer and 2,3-BPG on the Bohr effect of Hb A. For example, the pK values for β2His in deoxy-Hb A vary from 5.83 in 0.1 M HEPES plus 0.1 M chloride to 7.13 in 0.1 M Bis-Tris plus 2,3-BPG and the corresponding values are 6.24 and 6.68 for HbCO A. The pK values of β146His in deoxy-Hb A vary from 8.00 in 0.1 M HEPES plus 0.1 M chloride to 8.12 in 0.1 M Bis-Tris plus 2,3-BPG and the corresponding values for β146His in HbCO A are 6.24 and 6.68. Figure 13 (taken from Figure 11 of ref.107) shows the change in free energy of proton dissociation for some of the surface histidyl residues upon Hb oxygenation. It demonstrates clearly that the contribution of surface His residues to the Bohr effect is buffer dependent. These results show that the Bohr effect, a heterotropic effect, depends on the detailed environment and interactions of each His residue with its neighbors in the Hb molecule. Thus, the Bohr effect is an excellent example of a global network of electrostatic interactions, rather than a few specific amino acid residues, playing a dominant role in regulating the physiological function of Hb A.
Table 3.
pK values of histidyl residues in deoxy-Hb A and HbCO A in 0.1 M HEPES plus 0.1 M chloride in D2O at 29 °C.
| Site | pK (CO) | pK (deoxy) |
|---|---|---|
| α20 | 7.08 ± 0.01 | 7.02 ± 0.01 |
| α45 | 6.12 ± 0.02 | 5.25 ± 0.15 |
| α50 | 6.90 ± 0.02 | 7.14 ± 0.01 |
| α72 | 7.27 ± 0.01 | 7.47 ± 0.01 |
| α89 | 6.25 ± 0.03 | 6.80 ± 0.01 |
| α112 | 7.53 ± 0.01 | 7.49 ± 0.01 |
| β2 | 6.39 ± 0.01 | 6.17 ± 0.02 |
| β77 | 7.79 ± 0.01 | 7.46 ± 0.01 |
| β97 | 7.75 ± 0.02 | 8.01 ± 0.01 |
| β116 | 6.13 ± 0.05 | 6.35 ± 0.04 |
| β117 | 6.39 ± 0.02 | 6.43 ± 0.07 |
| β143 | 5.57 ± 0.06 | 4.70 ± 0.05 |
| β146 | 6.42 ± 0.03 | 7.93 ± 0.02 |
Reprinted with permission from ref. 12. Copyright 2004 American Chemical Society.
Figure 11.
(a) The net Bohr effect of Hb A (colored in green) determined from oxygen dissociation studies. The contributions of individual histidyl residues are calculated from pK values determined by NMR spectroscopy. The contributions from 26 surface histidyl residues are represented with the blue line. Reprinted with permission from ref. 12. Copyright 2004 American Chemical Society. (b) Contributions of histidyl residues to the Bohr effect of Hb A. The values were calculated based on crystal structures (titration calculations). The contributions of α20His, α112His, and β117His are negligible and are not shown. The blue line represents the combined contribution from all 26 surface histidyl residues. Reprinted with permission from ref. 110. Copyright 2013 American Chemical Society.
Figure 12.
(a) Protons released or captured by β2His upon Hb A oxygenation. Values were calculated from pH values obtained under various buffer as indicated in the figure. (b) Protons released by β146His residues upon Hb A oxygenation. Values were calculated from pK values obtained under experimental conditions as indicated in the figure. Reprinted with permission from ref. 105. Copyright 1990 Elsevier.
Figure 13.
Change in proton dissociation energy of surface histidines upon Hb A oxygenation under various buffer conditions. Adapted with permission from ref. 107. Copyright 1997 American Chemical Society.
Karplus and coworkers110 have developed a new computational method to investigate the “atomic origin” of the Bohr effect of Hb A. Their method is based on determining the electrostatic interactions between amino acid residues in crystals of Hb A, relative to those residues in solution, by use of the linearized finite difference Poisson-Boltzmann equation and Monte Carlo sampling of the protonation states. Based on the structural information available from the high-resolution structures of Hb A in deoxy and CO (or oxy) forms, they have calculated pK values of the His and other residues (in the absence of 2,3-BPG) and compared them with our NMR results for the His residues. Tables 4 and 5 (taken from Tables 4 and 5 of ref.110) give both their calculated and our experimentally determined values. Structures, 2DN2 (for deoxy) and 2DN3 (for oxy), with the highest resolution, were used in most of their analyses. They reported that the magnitude of the average prediction error is ~ 0.5 pK unit and the maximum error is less than 1 pK unit. Figure 11b (taken from Figure 5b of ref.110) gives a plot of the calculated contributions of His residues to the Bohr effect of Hb A, similar to Figure 12a for results obtained from the NMR experiments. The calculated results for the pKs of the His residues provide an excellent opportunity for a comparison with the experimentally determined pK values for the surface His residues. A major conclusion is that there are many His residues that participate in the Bohr effect, supporting the conclusion derived from our NMR results. However, there are differences between specific pK values determined by NMR in solution and those determined by calculations, depending on which crystal structures were used. As shown in Figures 12 and 13, the pK values of specific His residues and their contributions to the Bohr effect can vary depending on the solution conditions (e.g., buffer, 2.3-BPG, phosphate, chloride, etc.). Since various crystals were grown under various conditions and the crystals were frozen for high-resolution X-ray crystallographic experiments, the conformations of some of the His residues can vary from crystal to crystal, thus giving different pK values in different crystal structures. A possible way to resolve this matter is to carry out the calculations using the NMR solution structure for HbCO A (PDB entry 2M6Z).74
Table 4.
His pKa values in deoxy Hb determined by NMR and calculated from several T-type crystal structures using minimized WHATIF structures, and absolute deviations (numbers in parentheses) of the calculated values from the experimental values.
| Subunit | ResID | NMR | 2DN2 | 4HHB | 1BZ0 | 1RQ3 | 1XXT | 1KD2 |
|---|---|---|---|---|---|---|---|---|
| α | 20 | 7.02 | 6.14 [0.88] | 7.11 [0.09] | 6.93 [0.09] | 6.95 [0.07] | 6.95 [0.07] | 6.78 [0.24] |
| α | 45 | 5.25 | 5.88 [0.63] | 5.90 [0.65] | 5.85 [0.60] | 5.64 [0.39] | 5.56 [0.31] | 6.01 [0.76] |
| α | 50 | 7.14 | 7.50 [0.36] | 7.35 [0.21] | 7.30 [0.16] | 7.15 [0.00] | 7.21 [0.07] | 7.05 [0.09] |
| α | 58 | 4.33 | 4.54 | 4.06 | 3.54 | 3.79 | 4.55 | |
| α | 72 | 7.47 | 6.69 [0.78] | 6.67 [0.80] | 6.72 [0.75] | 6.89 [0.59] | 6.74 [0.73] | 6.68 [0.80] |
| α | 87 | 2.95 | 3.05 | 3.28 | 3.45 | 3.89 | 3.43 | |
| α | 89 | 6.80 | 6.82 [0.02] | 6.78 [0.02] | 6.46 [0.34] | 6.82 [0.02] | 6.77 [0.03] | 6.54 [0.26] |
| α | 103 | 3.92 | 4.19 | 3.58 | 3.60 | 3.55 | 4.37 | |
| α | 112 | 7.49 | 6.64 [0.85] | 6.93 [0.56] | 6.66 [0.83] | 6.60 [0.89] | 6.89 [0.60] | 6.84 [0.65] |
| α | 122 | 0.53 | 0.64 | 0.78 | 0.99 | 0.82 | 1.08 | |
| β | 2 | 6.17 | 5.61 [0.56] | 5.51 [0.66] | 5.27 [0.90] | 4.97 [1.20] | 5.12 [1.05] | 5.70 [0.47] |
| β | 63 | 4.93 | 5.30 | 4.84 | 5.69 | 4.82 | 3.85 | |
| β | 77 | 7.46 | 6.76 [0.70] | 6.70 [0.76] | 6.89 [0.57] | 6.92 [0.54] | 6.83 [0.63] | 7.05 [0.41] |
| β | 92 | 3.61 | 3.70 | 3.56 | 3.22 | 3.14 | 3.73 | |
| β | 97 | 8.01 | 7.13 [0.88] | 7.26 [0.75] | 7.02 [0.99] | 7.12 [0.89] | 7.04 [0.97] | 7.04 [0.97] |
| β | 116 | 6.35 | 6.55 [0.20] | 6.64 [0.29] | 6.36 [0.01] | 5.71 [0.64] | 5.62 [0.73] | 6.74 [0.39] |
| β | 117 | 6.43 | 8.01 [1.58] | 8.11 [1.68] | 7.44 [1.01] | 7.57 [1.14] | 7.77 [1.34] | 6.28 [0.15] |
| β | 143 | 4.70 | 4.08 [0.62] | 3.85 [0.85] | 4.52 [0.19] | 4.82 [0.12] | 4.69 [0.02] | 4.82 [0.12] |
| β | 146 | 7.93 | 8.08 [0.15] | 7.58 [0.35] | 7.86 [0.07] | 7.84 [0.09] | 7.81 [0.12] | 8.12 [0.19] |
| Max error | [1.58] | [1.68] | [1.01] | [1.20] | [1.34] | [0.97] | ||
| Ave. error | [0.63] | [0.59] | [0.50] | [0.51] | [0.51] | [0.42] | ||
Reprinted with permission from ref. 110. Copyright 2013 American Chemical Society.
Table 5.
His pK values of liganded Hb A determined by NMR and calculated from the crystal structure using minimized WHATIF structures, and deviations (numbers in parentheses) of the calculated values from the experimental values.
| Subunit | ResID | NMR | 2DN3 | 1HHO(R) | 1BBB(R2) | 1MKO(RR2) | 1YZI(R3) |
|---|---|---|---|---|---|---|---|
| α | 20 | 7.08 | 7.15 [0.07] | 7.18 [0.10] | 7.05 [0.03] | 7.18 [0.10] | 7.03 [0.05] |
| α | 45 | 6.12 | 5.99 [0.14] | 5.86 [0.26] | 6.14 [0.02] | 6.24 [0.12] | 6.46 [0.34] |
| α | 50 | 6.90 | 7.05 [0.15] | 7.04 [0.14] | 7.40 [0.50] | 7.28 [0.38] | 7.26 [0.36] |
| α | 58 | 3.88 | 3.55 | 5.00 | 4.40 | 5.04 | |
| α | 72 | 7.27 | 6.76 [0.51] | 6.58 [0.69] | 6.68 [0.59] | 6.82 [0.45] | 6.72 [0.55] |
| α | 87 | 3.71 | 3.16 | 3.69 | 3.09 | 3.01 | |
| α | 89 | 6.25 | 5.91 [0.34] | 5.92 [0.33] | 7.12 [0.87] | 5.80 [0.45] | 5.67 [0.59] |
| α | 103 | 3.17 | 2.79 | 3.99 | 2.41 | 3.94 | |
| α | 112 | 7.53 | 7.20 [0.33] | 5.80 [1.73] | 6.24 [1.29] | 6.66 [0.87] | 6.22 [1.32] |
| α | 122 | 0.79 | 1.04 | 0.77 | 0.19 | 1.34 | |
| β | 2 | 6.39 | 5.78 [0.61] | 5.84 [0.55] | 5.93 [0.46] | 5.99 [0.40] | 6.77 [0.38] |
| β | 63 | 5.89 | 5.70 | 6.43 | 5.69 | 5.62 | |
| β | 77 | 7.79 | 7.50 [0.29] | 7.33 [0.46] | 7.14 [0.65] | 7.39 [0.40] | 6.25 [1.54] |
| β | 92 | 4.58 | 4.30 | 3.53 | 4.21 | 5.25 | |
| β | 97 | 7.75 | 6.85 [0.90] | 7.01 [0.74] | 6.77 [0.98] | 6.77 [0.98] | 7.62 [0.13] |
| β | 116 | 6.13 | 6.12 [0.01] | 6.33 [0.20] | 6.73 [0.60] | 6.81 [0.68] | 7.16 [1.03] |
| β | 117 | 6.39 | 6.35 [0.04] | 6.05 [0.34] | 5.87 [0.52] | 6.21 [0.18] | 6.80 [0.41] |
| β | 143 | 5.57 | 5.08 [0.49] | 4.75 [0.82] | 5.43 [0.14] | 4.89 [0.68] | 2.29 [3.28] |
| β | 146 | 6.42 | 6.45 [0.03] | 2.24 [4.18] | 1.30 [5.12] | 5.31 [1.11] | 4.58 [1.84] |
| Max error | [0.90] | [4.18] | [5.12] | [1.11] | [3.28] | ||
| Ave. error | [0.30] | [0.81] | [0.91] | [0.52] | [0.91 | ||
All structures are for HbCO A, except 1HHO, which is for oxy-Hb A. Reprinted with permission from ref. 110. Copyright 2013 American Chemical Society.
Both experimentally measured and calculated results show that β146His makes the largest contribution to the alkaline Bohr effect and its contribution to the Bohr effect is affected by experimental conditions. However, there are differences in the pK values of β146His between these two sets of results (Tables 4 and 5) depending on the crystal structures used in the calculations and the experimental conditions for the NMR measurements. The proton resonance assignment for β146His in HbCO A and its role in the Bohr effect105-106,111-120 were quite challenging and controversial in the 1980s. Figure 14 shows that there are many conformations for β146His in the solution structures of HbCO A and they are distinct from the crystal structures. This could account for the early difficulties in obtaining the correct spectral assignment for this His residue in HbCO A, thus leading to the controversies concerning its role in the alkaline Bohr effect of Hb A. However, the role of β146His in the Bohr effect was established once its correct resonance assignment was made.105-106,116
Figure 14.
The conformations of β146His in the crystal structures of deoxy-Hb A (2DN2, colored in blue), HbCO A in R (2DN3, red), R2 (1BBB, magenta), RR2 (1MKO, green), and R3 (1YZI, cyan) forms, and the five lowest-energy NMR structures (2M6Z, light grey). The structures are superimposed on the backbones of the β1-subunits. The side-chains of β146His are presented as sticks. Figures were generated with the PyMOL program.61
In addition, Karplus and coworkers have also provided the contributions of α1Val to the Bohr effect.110 Their calculated pK values for the N-terminal α1Val are 8.60 and 7.80 for deoxy- and oxy-Hb A, respectively. They found that the number of hydrogen ions released from the two α-chains at pH 8.4 is 0.32, corresponding to 16% of the Bohr effect at that pH.
Based on the NMR and computation studies, we can conclude: (i) β146His plays a very large role in the alkaline Bohr effect of Hb A and its contribution depends on buffer conditions; (ii) α45His and β143His are responsible for the acid Bohr effect; and (iii) in addition to β146His, the other 24 surface His residues plus α1Val also make significant contributions (~30%) to the alkaline Bohr effect of Hb A.
4.2 Effect of Temperature on Oxygen Affinity
Because Hb oxygenation is exothermic, the O2 affinity decreases markedly with increasing temperature and increases with decreasing temperature. For heterothermic mammals, this feature of Hb may pose considerable challenges for O2 delivery to cool extremities and peripheral tissues. Recently,121-123 a smaller temperature effect on oxygen binding has been found for the recombinant woolly mammoth Hb (rHb WM) compared to the Asian elephant Hb (rHb AE). Elephantids are a particularly good model system to investigate the effects of temperature on the structure–function relationship of Hb as they include both warm- and cold-adapted members.
The primary sequence of Asian elephant Hb differs from that of the mammoth at only one position in the α-globin chain (K5N) and at three positions (T12A, A86S, and E101Q) in the β/δ-fusion globin chain. β/δ101Gln is located between β/δ99Asp and β/δ102Asn of the same chain in the inter-subunit α1(β/δ)2 interface. These residues are all critical to the function of Hb. rHb WM and the human mutant Hb Rush,124 both having Gln at the β101 position, are affected similarly by chloride ions. rHb WM exhibits a more significant effect of chloride on the O2 affinity than rHb AE, as does Hb Rush compared to Hb A. Thus, Campbell et al.121 suggested that the β/δ101Gln in rHb WM is responsible for the lower temperature effect by providing additional binding sites for chloride in the central cavity of the Hb molecule. However, X-ray structural analyses125-127 have not shown the specific binding sites for chloride ions in woolly mammoth Hb, Hb A or bovine Hb. Further, the studies of rHb WM and a number of its mutants created at the β/δ101 position, with special reference to Hb A, have shown that chloride can alter the temperature dependence of O2 binding, in addition to its allosteric role in regulating the O2 affinity of hemoglobin.123 A more significant decrease of the temperature effect of rHb WM and its mutants is observed in the presence of both chloride and IHP, two allosteric effectors which both lower the affinity for O2. The amino acid residue β101 is one example that a specific residue could be responsible for the effect of temperature on oxygen affinity. If the replacement of certain amino acid residues in the Hb molecule could cause a stronger response to allosteric effectors, it is possible that those mutant Hbs could exhibit a lower temperature effect. This is an interesting proposition.
4.3 Oxygen Affinity Related to the Distal Hydrogen Bond
On the basis of X-ray crystal structures, electron paramagnetic resonance and resonance Raman spectroscopic measurements,128-129 it was proposed that O2 binding to hemoglobin is stabilized by hydrogen bonds between the oxygen ligand and the side-chain of the two distal histidyl residues, α58His and β63His. Our study by multi-nuclear NMR provided the first direct evidence of such H-bonds in HbO2 A in solution.130 The cross-peaks for the side-chains of α58His and β63His in NMR spectra can be used as markers to demonstrate the strength of the H-bonds, which have been observed for Hb A and five mutant rHbs.131 The changes in these markers are correlated with the effect of pH and/or temperature on the O2-binding affinity, and this relationship is demonstrated by the weakening and even the disappearance of the cross-peaks in the NMR spectra under conditions where the affinity decreases. At higher pH and/or lower temperature, the cross-peaks for both α58His (1Hε2, 15Nε2) and β63His (1Hε2, 15Nε2) are clearly visible in Figure 15 (taken from Figure 2 of ref.131). When pH is decreased and/or at higher temperature, the side-chains of the distal histidines appear to be more mobile, and the exchange with water molecules in the distal heme-pockets is faster. Consequently, the cross peaks lose intensity or even disappear. These H-bond markers in the NMR spectra can also show subtle differences between the distal heme-pockets of the α- and β-chains of Hb A. The H-bond in the heme-pocket of the β-chain is weaker than that in the α-chain and is more sensitive to changes in pH and temperature. IHP has only a minor effect on these H-bond markers compared to the effects of pH and temperature. These H-bonds are found to be sensitive to mutations in the distal heme-pockets but not affected directly by mutations in the quaternary interfaces, i.e., the α1β1 and/or α1β2 subunit interfaces.131
Figure 15.
Horizontal 1D slices along the 1H axis through the cross-peak of the β63His side-chain. Data were extracted from 600 MHz (1H,15N) HSQC spectra of fully 15N-labeled rHbO2 A in H2O under various conditions of pH and temperature. Adapted with permission from ref. 131. Copyright 2010 American Chemical Society.
4.4 Autoxidation
The Fe atom of the porphyrin heme is covalently bound to the proximal F8His (α87His or β92His) and in close proximity to B10Leu (α29Leu or β28Leu), E7His (α58His or β63His) and E11Val (α62Val or β67Val) of the distal pocket.1 The importance of these residues has been studied by various groups.132-143
The heme-iron atom can undergo autoxidation and convert from the ferrous (Fe+2) to the ferric (Fe+3) state.144 The Fe+3 form of the heme-iron cannot bind oxygen and Hb A is inactivated when all four iron atoms of the tetramer are oxidized to form met-Hb resulting in an increased rate of hemin loss and eventual denaturation of the apoglobin.145 This process has been studied extensively using myoglobin (Mb) as a model system.146-150 The reaction initiates by weakening, then breaking the H-bond between the bound oxygen and the Nε atom of the E7His. From this point on, there are two proposed mechanisms for the reaction, summarized in Scheme 1. The Fe+2O2 complex is first protonated. The hydroperoxyl radical (HO2.) then dissociates from the protein and leaves behind an Fe+3 ion for reacting with water to form aquomet-Mb. Alternatively, the oxygen dissociates from the heme and allows the Fe+2 ion of the deoxy-Mb to interact with water. The aquo-Mb and free oxygen then undergo a bimolecular reaction147 to form aquomet-Mb and the superoxide radical (O2.-). In addition, the autoxidation reaction is an acid-catalyzed process147,151-152 and can be promoted by anions such as azide.136,142,150,153 The imidazole ring of the E7His possibly facilitates the movement of a proton from the solvent to the bound oxygen.151 Park et al.35 identified occupied water molecules in the distal heme-pockets of the α-subunits of oxy-Hb A. These water molecules could facilitate the autoxidation process and explain the observation that the α-chain (0.032 hr−1) can oxidize more rapidly than the β-chain (0.0037 hr−1) in the Hb A tetramer at pH 7.2 in an early report.154 A less dramatic difference between the autoxidation rate of the α- (0.078 hr−1) and β-chains (0.011 hr−1) at pH 6.5 was reported by Tsuruga et al.144,152
Scheme 1.
Possible mechanism of Hb A autoxidation. Schematic presentation of reactions in converting the heme-iron atom from the ferrous (Fe+2) to the ferric (Fe+3) state. Scheme adapted from ref. 147. Copyright 1993 American Society for Biochemistry and Molecular Biology.
We have substituted other amino acids for B10Leu and E11Val and gauged the effects on autoxidation or azide-induced oxidation.136,142,153,155 The results are summarized in Table 6. Substituting a bulky aromatic side-chain for B10Leu or E11Val can reduce the capture volume in the distal pocket and possibly exclude water from the α-subunit heme-pocket.133-134 Replacement of E11Val also removes the γ2-methyl group that provides steric hindrance to access the heme Fe.133 Therefore, E11Val mutants, in general, have higher kox and kaz values.142 Among the B10 and E11 mutants,136,153,155 only rHb (αL29F) has kaz lower than Hb A. Jeong et al.136 reported that the kox of rHb (αL29F) was also lower than that of Hb A and gave the reason that the positive edge of the phenyl ring multi-pole might stabilize the bound oxygen.134 However, this lowering in kox has not been detected by Wiltrout et al.153 or Tam et al.142
Table 6.
Autoxidation rate and oxygen binding properties of Hb A and recombinant heme-pocket mutants. Data from refs. 136, 142, 153, and 155.
| koxa | kaza | P50 mm Hg (pH 7.4) | Reference | |
|---|---|---|---|---|
| Hb A | 1 | 1 | 8.0 | 136b |
| rHb(αL29F) | 0.47 | 0.36 | 4.0 | |
| rHb(αL29F,V96W/βN108K) | 1.29 | 0.59 | 22 | |
| Hb(αV96W/βN108K) | 3.8 | 1.6 | 38.1 | |
| rHb(βN108Q) | 2.84 | n.d. | 17.5 | 155c |
| rHb(αL29F/βN108Q) | 1.16 | n.d. | 12.1 | |
| rHb(αL29F,V96W/βN108K) | 2.84 | n.d. | n.d. | |
| rHb(αL29F) | 1 | 0.23 | 4.9 | 153b |
| rHb(αL29W) | 7.7 | 1.2 | 31.5 | |
| rHb(βL28F) | 18.9 | 1.7 | 35.7 | |
| Hb(βL28W) | 21.1 | 4.0 | 64.6 | |
| rHb(αV62L) | 4.6 | 2.3 | 7.5 | 142b |
| rHb(αV62I) | 3.1 | 0.9 | 9.0 | |
| rHb(αV62F) | 5.5 | 3.0 | 7.2 | |
| rHb(βV67L) | 4.4 | 2.5 | 7.5 | |
| rHb(βV67I) | 3.8 | 1.3 | 18.2 | |
| rHb(βV67F) | 6.8 | 3.6 | 2.7 |
kox and kaz results are normalized to that of Hb A.
kox results determined in 0.1 M phosphate and 1 mM EDTA, pH 7.0 at 25 °C.
kox results determined in PlasmaLyte buffer, pH 7.4 and 37 °C.
n.d., not determined.
The factors determining the autoxidation process are mainly: (i) the stability of the H-bond between the bound oxygen and E7His; and (ii) the stereo-placement of E7His, B10Leu and E11Val controlling the entry of reactant (water, oxygen or azide) into the distal pockets. It is of interest to note that the H-bond between the bound oxygen and E7His in the β-subunit is weaker than that in the α-subunit,131 while the β-subunit has also a slower kox. Therefore, the stereo-placement of amino acid residues in the heme-pocket is the major contributing factor in determining the kox of the subunit.
4.5 Effects of Interfacial and Distal Heme-Pocket Mutations on the Structure and Function of Hemoglobin
Kinetic and equilibrium studies of ligand binding as well as structural studies with 1H-NMR have been carried out to investigate recombinant Hb mutants, rHb (αL29F), rHb (αL29W), rHb (αV96W), rHb (βN108K), rHb (αV96W/βN108K), rHb (αL29F/αV96W/βN108K), and rHb (αL29W/αV96W/βN108K).138 The first two rHbs have single amino acid substitutions at the distal heme-pocket of the α-chain. rHb (αV96W) and rHb (βN108K) have a single mutation in the α1β2 and the α1β1 interface, respectively. The double mutant, rHb (αV96W/βN108K), has mutations in both the α1β2 and α1β1 interfaces. The two triple mutants, rHb (αL29F/αV96W/βN108K) and rHb (αL29W/αV96W/βN108K), have mutations at the distal heme-pocket of the α-chain as well as in both the α1β2 and α1β1 interfaces. These recombinant mutants were used to test the hypothesis that the distal heme-pocket mutations produce only local structural and functional changes at the ligand-binding site, while the mutations in the α1β2 and α1β1 interfaces affect the equilibrium between the R- and T-quaternary states. We have found that the placement of Phe or Trp at the α29 position resulted in 40- and 300-fold decreases, respectively, in the rate constant for bimolecular O2 binding to the R-state α-chains measured after partial photolysis the HbO2 tetramers. In contrast, the rate constants for the wild-type β-chains are relatively unaffected by the mutation in their partner α-chains. The βN108K mutation in the α1β1 interface produces a small slow phase for both O2 and CO rebinding after partial photolysis (~25% of the total amplitude). Unlike the distal heme-pocket mutants, this lowered rate constant is not the result of a change in the intrinsic reactivity of the mutated subunit, as is seen for the mutations in the distal heme-pocket, but appears to be the result of shifting the tetramer structure towards the T-quaternary state. Both liganded rHb (βN108K) and rHb (αV96W) can be converted to the T-type structure in fully liganded tetramers86,156 by lowering the temperature and upon the addition of IHP. The ability to switch the quaternary structure in fully liganded forms157 is increased when these two mutations are combined in rHb (αV96W/βN108K). Both NMR spectra157 and WAXS pattern84 suggest that rHb (αV96W/βN108K) assumes a conformation intermediate between that of the T- and the R-forms. Interestingly, the solution structure determined by NMR shows (Figure 14) that some structural elements can span a range of positions in the liganded state (CO) and get close to the deoxy T-structure. The rHb (αL29F) mutant has high while the rHb (αL29W) variant has low O2 affinity. In the triple mutant, rHb (αL29F/αV96W/βN108K), the T to R transition is inhibited by mutating both α1β2 and α1β1 interfaces, thus compensating for the intrinsic increase in O2 affinity caused by the αL29F substitution. Conversely, rHb (αL29W/αV96W/βN108K) exhibits a further drastic drop in O2 affinity. Results from these mutants confirm that the effects of single distal heme-pocket and interface mutations are additive and their individual properties can be used to design novel recombinant Hbs with unique properties. For details, see ref.138.
5. DYNAMIC AND STRUCTURAL BASIS OF HEMOGLOBIN ALLOSTERY
Our recent NMR structural and dynamic studies of Hb A have led to a number of conclusions: (i) Hemoglobin is a flexible and dynamic molecule with a considerable amount of plasticity, exhibiting various types of motions in the backbone and side-chain residues (ranging from ps to ns and μs to ms).74,81,90,95 (ii) RDC measurements for HbCO A and a low oxygen-affinity mutant Hb, rHb (αV96W), in the absence and presence of IHP, have confirmed that the transition pathway69-70 from the unliganded to the liganded state of hemoglobin is likely T → R → R2. (iii) Allosteric effectors, e.g., IHP, alter both the tertiary and quaternary structure of deoxy- and CO-Hb as well as the dynamics of the Hb molecule, thus affecting its ligand affinity.70-72,74 (iv) RDC measurements of Hb A in different aqueous liquid crystalline media and at several magnetic field strengths have shown that in solution, the quaternary conformations for both the CO- and deoxy-forms of Hb A are different from their crystal structures and both exist as dynamic ensembles of various structures.69-72 It is tempting to speculate that the various T- and R-type structures reported by crystallographers (Table 1) may be the so-called “pre-existing protein conformations” and represent some of the conformational ensembles detected by our NMR measurements. Their individual populations can be shifted under different experimental conditions.
Recent experimental and theoretical studies have emphasized the important roles that dynamics and conformational ensembles can play in regulating protein-ligand interactions,158-167 consistent with the conclusions derived from our structural and dynamic results on Hb presented here. The ensemble nature of Hb structure presents a different picture from the classical two-structure allosteric model of MWC/Perutz or the KNF sequential model based on two quaternary structures, namely the unliganded (deoxy) (i.e., T-type) and the liganded (oxy or CO) (i.e., R-type) structures. Thus, in this new picture of Hb allostery, we need to consider the statistical nature of various factors that contribute to the cooperative oxygenation process and the roles of allosteric effectors that fine-tune the functional properties of hemoglobin under physiological conditions. Our NMR results also illustrate that various parts of the Hb molecule are sensitive to changes in experimental conditions, e.g., partial pressure of O2 or CO, pH, nature and concentration of buffers and allosteric effectors, as well as temperature. Through the use of naturally occurring human mutant hemoglobins as well as of a large number of recombinant mutant Hbs produced by using our Hb expression plasmid, we have carried out an extensive array of structural, dynamic, kinetic, and functional studies.67,81,86,90,96,120,131,138,153,156,168-176 Some of the observed structural, dynamic, kinetic and functional changes can be related to the mutations. Some of them can be small, but the mutation at one site could affect the conformation of distant as well as neighboring residues. These types of conformational alterations could play significant roles in the structure-function relationship of Hb. An important consequence of an ensemble nature of allosteric interactions is that it could be related to the allosteric coupling process. It should also be recognized that very rapid fluctuations on ps or ns timescales give an average kinetic or equilibrium property on longer timescales or at equilibrium. For a discussion on this matter, see Petrich et al.177 From a mechanistic point of view, we need to know how the structures and dynamics of various amino acid residues of the partially liganded species play out during the cooperative oxygenation process of Hb A. In order to gain a full insight into the dynamic ensemble nature of the Hb molecule in solution and to correlate this feature with the functional properties of Hb, researchers need to develop novel techniques to obtain a quantitative description of the population distributions of various structures of unliganged (deoxy)- and liganded (oxy and/or CO)-forms as well as various partially liganded species under different experimental conditions. This is a challenging yet essential job for our eventual understanding not only of hemoglobin at the atomic and molecular level but also of other allosteric proteins.
6. CONCLUDING REMARKS
Hemoglobin has been a favorite molecule for researchers in biochemistry, biophysics, chemistry, computational biology, molecular biology, and structural biology. The structural determinations of Hb and Mb played a crucial role in establishing the field of structural biology. The classical two-structure description of Hb allostery pioneered by Perutz3 in 1970, i.e., T ↔ R transition, has also evolved. Figure 16 is an illustration of an evolving view of Hb allostery. Figure 16a shows the superposition of the T- and R-structures of Hb, which allowed Perutz to propose his stereochemical mechanism for the cooperative oxygenation of Hb in 1970. The number of well- characterized T- and R-types of X-ray crystal structures of Hb summarized in Table 1 as well as the results obtained by NMR structural and dynamic studies described in this article clearly illustrate that the picture for Hb allostery is more complex. Figure 16b shows a picture of HbCO A in solution illustrating a collection of 10 best solution structures of HbCO A with one each of the T- and R-type crystal structures superimposed. The solution structure of HbCO A indicates that there are considerable amounts of flexibility in this molecule. There is little doubt that we have learned a great deal about this protein, in terms of dynamics, function, and structure, during the past decade. The NMR structural and dynamic results presented in this review clearly show that there are various types of interactions among many amino acid residues in the deoxy and oxy (or carbonmonoxy) forms and that they contribute to the effects of allosteric effectors on the structural, dynamic, and functional properties of this molecule. The binding of a ligand like O2 or CO as well as allosteric effectors to deoxy-Hb A can initiate various structural and dynamic changes (some might be subtle changes and some can cause large changes in conformations and thus alter the functional properties) in the molecule. Because of the complexity of the structural and dynamic changes in the Hb molecule upon oxygenation, it is a very challenging task to describe them in simple models, especially in a quantitative and statistical manner. Hence, it is not surprising that none of the models proposed for hemoglobin allostery can fully account for the details of cooperative oxygenation of Hb A and the effects of allosteric effectors on the function of Hb A. This is truly an irony in science, i.e., the more we know about a protein the less we are certain about its intimate details!
Figure 16.
An evolving view of hemoglobin allostery from a rigid two-structure model (a) to a dynamic ensemble of the solution structures (b). The T-(unliganded-form, PDB code 4HHB, colored in blue) and the R-(liganded-form, PDB code 2DN3, colored in red) structures of Hb A are superimposed in panel (a). The 10 lowest-energy solution structures of HbCO A obtained by NMR (PDB code 2M6Z, colored in grey) are superimposed with the T- and R-structures of Hb A obtained by X-ray crystallography in panel (b). The structures are aligned according to the α1β1 dimer. Figures were generated with the PyMOL program.61
In order to gain an understanding of the atomic and molecular details of the cooperative oxygenation of Hb A, a new approach is needed making use of new techniques, e.g., NMR in combination with WAXS,84,178 to monitor the structural and dynamic changes of the key amino acid residues of Hb A in the transition from the deoxy (unliganded) form to the oxy or CO (liganded) form as a function of the partial pressure of oxygen or carbon monoxide in the absence and presence of allosteric effectors. As mentioned earlier, WAXS has evolved during the past few years to become a powerful technique for characterization of protein conformations and fluctuations in solution.
The results of multinuclear, multidimensional NMR experiments reported here give an idea of the possibilities available for future research. For example, our RDCs were measured only for the amide NHs, although RDCs can be measured also for other pairs of nuclei. Relaxation properties, dynamics and its interplay with the structure for native as well as mutant Hbs can be investigated under a larger variety of experimental conditions in order to correlate them with the functional studies. Figure 17 is an illustration of some of the key amino acid residues (based on our studies) that can give important information on the allosteric pathways for Hb A during the oxygenation process. There are excellent NMR-based tertiary and quaternary structural markers of the Hb molecule to guide the experiments. The NMR markers are not only correlated with the structural changes upon ligation, but also with the functional properties of Hb A as a function of pH, temperature, and allosteric effectors. In addition, one also has the potential to observe specific structural and dynamic changes of specific amino acid residues under different experimental conditions. From these experiments, one could gain some detailed structural and dynamic insights regarding the signaling pathways from one amino acid to the next one as well as the relationship between tertiary and quaternary structural transitions during the oxygenation process. These are indeed very challenging experiments, but the results obtained will provide much needed information on the molecular basis of hemoglobin allostery. For example, we would like to know the relationship among the tertiary and quaternary structural changes and ligation state as well as the effects of allosteric effectors on the cooperative oxygenation process. These challenging as well as exciting experiments could provide information on the relationship between the tertiary and quaternary changes on oxygenation, i.e., is the quaternary structural change concerted or sequential? This has been a much-debated question in hemoglobin allostery. This approach can also be of value in designing new ways to understand other allosteric proteins in important cellular regulatory pathways.
Figure 17.
(a) Key amino acid residues in the α1β1 and α1β2 interfaces and the heme-pockets of HbCO A. The α2-chain is not shown for clarity. (b) Locations of Val (blue), Leu (green), and Thr (grey) residues. The listed amino acid residues are likely to play important roles in transmitting signals during the cooperative oxygenation of Hb A. Figures were drawn according to coordinates under PDB code 2DN3. Figures were generated with the PyMOL program.61
ACKNOWLEDGEMENT
We thank National Institutes of Health for support of our research on hemoglobin (R01GM084614). We also thank Drs. William A. Eaton, Martin Karplus, Lee Makowski, John S. Olson, E. Ann Pratt, Gordon Rule, and Nico Tjandra for helpful comments and suggestions to improve our manuscript.
ABBREVIATIONS
- Hb
hemoglobin
- Hb A
human normal adult hemoglobin
- HbCO
carbonmonoxy-hemoglobin
- deoxy-Hb
deoxy-hemoglobin
- met-Hb
methemoglobin
- rHb
recombinant hemoglobin
- Mb
myoglobin
- T-structure
tense-structure
- R-structure
relaxed-structure
- HEPES
N-(2-hydroxyethyl)-piperazine-N’-2-ethanesulfonic acid
- PEG
polyethylene glycol
- IHP
inositol hexaphosphate
- 2,3-BPG
2,3-bisphosphoglycerate
- KNF
Koshland, Nemethy, Filmer
- MWC
Monod, Wyman, Changeux
- NMR
nuclear magnetic resonance
- PDB
Protein Data Bank
- R1
longitudinal relaxation rate
- R2
transverse relaxation rate
- S2
generalized order parameter
- Saxis2
order parameter for the methyl rotation axis
- Rex
conformational exchange contribution to transverse relaxation rate
- RCSB
Research Collaboratory Structural Bioinformatics
- RDC
residual dipolar coupling
- HSQC
heteronuclear single-quantum coherence
- TROSY
transverse relaxation-optimized spectroscopy
- RMSD
root-mean-square deviation
- WAXS
wide-angle X-ray scattering
- JHN
scalar coupling between H and N of the amide group
- DHN
dipolar coupling
- kox
autoxidation rate constant
- kaz
azide-induced autoxidation rate constant
Biography
 Yue Yuan was Senior Research Biologist in the Department of Biological Sciences at Carnegie Mellon University, Pittsburgh, PA. She received her PhD in Pharmaceutical Chemistry in 1995 from Beijing Institute of Pharmacology and Toxicology under the guidance of Prof. Kefang Jiao. She started working on protein NMR in 1995 at Beijing University with Prof. Xiaocheng Gu. As a Postdoctoral Fellow, she joined Prof. Chien Ho's research group at Carnegie Mellon University in 1999, and then the group of Prof. B. Mario Pinto at Simon Fraser University in Canada in 2003. She returned to Carnegie Mellon University with Prof. Chien Ho in 2008 as a Research Biologist. She is currently working as a Senior Scientist at University of Notre Dame, IN. Her research interests are applications of NMR to study structure-function relationships of proteins.
Yue Yuan was Senior Research Biologist in the Department of Biological Sciences at Carnegie Mellon University, Pittsburgh, PA. She received her PhD in Pharmaceutical Chemistry in 1995 from Beijing Institute of Pharmacology and Toxicology under the guidance of Prof. Kefang Jiao. She started working on protein NMR in 1995 at Beijing University with Prof. Xiaocheng Gu. As a Postdoctoral Fellow, she joined Prof. Chien Ho's research group at Carnegie Mellon University in 1999, and then the group of Prof. B. Mario Pinto at Simon Fraser University in Canada in 2003. She returned to Carnegie Mellon University with Prof. Chien Ho in 2008 as a Research Biologist. She is currently working as a Senior Scientist at University of Notre Dame, IN. Her research interests are applications of NMR to study structure-function relationships of proteins.
 Ming F. Tam did his undergraduate work at University of California, Davis, and received his B.S. degree in chemistry in 1974. He then studied under Prof. Walter E. Hill at University of Montana and received his Ph.D. degree in 1981. He did postdoctoral research with Prof. Masayasu Nomura at the Enzyme Institute of University of Wisconsin at Madison from 1982-1984. From 1984-1986, he was a Research Scientist at Pharmacia Inc., designing instruments for liquid chromatography and DNA synthesis. From 1986-2011, he was an Associate Research Fellow, then Research Fellow at the Institute of Molecular Biology, Academia Sinica in Taiwan. He joined the Department of Biological Sciences at Carnegie Mellon University as Senior Research Biologist in 2011. He is a protein chemist who has worked with glutathione S-transferases, arginine methyltransferases, and hemoglobin. His current interest is in elucidating the allosteric mechanism of hemoglobin oxygen binding.
Ming F. Tam did his undergraduate work at University of California, Davis, and received his B.S. degree in chemistry in 1974. He then studied under Prof. Walter E. Hill at University of Montana and received his Ph.D. degree in 1981. He did postdoctoral research with Prof. Masayasu Nomura at the Enzyme Institute of University of Wisconsin at Madison from 1982-1984. From 1984-1986, he was a Research Scientist at Pharmacia Inc., designing instruments for liquid chromatography and DNA synthesis. From 1986-2011, he was an Associate Research Fellow, then Research Fellow at the Institute of Molecular Biology, Academia Sinica in Taiwan. He joined the Department of Biological Sciences at Carnegie Mellon University as Senior Research Biologist in 2011. He is a protein chemist who has worked with glutathione S-transferases, arginine methyltransferases, and hemoglobin. His current interest is in elucidating the allosteric mechanism of hemoglobin oxygen binding.
 Virgil Simplaceanu is NMR Spectroscopist in the Department of Biological Sciences at Carnegie Mellon University in Pittsburgh, PA. He graduated from the Polytechnical Institute in Bucharest, Romania in 1969 as an electronics engineer in the specialty of technical physics, then worked as a Research Associate at the Institute for Atomic Physics in Magurele, Bucharest, Romania in the NMR laboratory of Prof. Ioan Ursu. Since 1981 he works with Prof. Chien Ho at Carnegie Mellon University and his interests are NMR instrumentation and applications of NMR to biology and chemistry.
Virgil Simplaceanu is NMR Spectroscopist in the Department of Biological Sciences at Carnegie Mellon University in Pittsburgh, PA. He graduated from the Polytechnical Institute in Bucharest, Romania in 1969 as an electronics engineer in the specialty of technical physics, then worked as a Research Associate at the Institute for Atomic Physics in Magurele, Bucharest, Romania in the NMR laboratory of Prof. Ioan Ursu. Since 1981 he works with Prof. Chien Ho at Carnegie Mellon University and his interests are NMR instrumentation and applications of NMR to biology and chemistry.
 Chien Ho did his undergraduate training at Williams College and received his B.A. degree in Chemistry in 1957. He then went to Yale University for his graduate work in physical chemistry with Julian M. Sturtevant and received his Ph.D. degree in 1961. From 1960-61, he was a Research Chemist for Linde Company, Union Carbide Corporation working on the liquid nitrogen temperature preservation of human blood. From 1961-64, he did postdoctoral research with George Scatchard and Jancinto Steinhardt in Department of Chemistry and with David F. Waugh in the Department of Biology, Massachusetts Institute of Technology. From 1964-79, he was an assistant professor, associate professor, and professor of molecular biology at the University of Pittsburgh. In 1979, he joined Carnegie Mellon University as the head and professor of the Department of Biological Sciences. Since 1985, he is Alumni Professor of Biological Sciences at Carnegie Mellon University. His research goal is to understand the relationship between structure and function in biological systems by correlating information obtained from biochemical, biophysical, and molecular biological techniques. His current research is focused in two areas. The first centers on a study of human normal and abnormal hemoglobins in order to correlate the structure, dynamics, and function of hemoglobin in solution. He received a MERIT Award in 1986 from the National Heart, Lung, and Blood Institute for his research on hemoglobin. The second centers on the application of magnetic resonance imaging (MRI) and spectroscopy (MRS) to biomedical systems. He is currently developing non-invasive techniques to monitor the migration/accumulation of immune cells in vivo by MRI as a means to detect early signs of graft rejection following solid organ transplantation.
Chien Ho did his undergraduate training at Williams College and received his B.A. degree in Chemistry in 1957. He then went to Yale University for his graduate work in physical chemistry with Julian M. Sturtevant and received his Ph.D. degree in 1961. From 1960-61, he was a Research Chemist for Linde Company, Union Carbide Corporation working on the liquid nitrogen temperature preservation of human blood. From 1961-64, he did postdoctoral research with George Scatchard and Jancinto Steinhardt in Department of Chemistry and with David F. Waugh in the Department of Biology, Massachusetts Institute of Technology. From 1964-79, he was an assistant professor, associate professor, and professor of molecular biology at the University of Pittsburgh. In 1979, he joined Carnegie Mellon University as the head and professor of the Department of Biological Sciences. Since 1985, he is Alumni Professor of Biological Sciences at Carnegie Mellon University. His research goal is to understand the relationship between structure and function in biological systems by correlating information obtained from biochemical, biophysical, and molecular biological techniques. His current research is focused in two areas. The first centers on a study of human normal and abnormal hemoglobins in order to correlate the structure, dynamics, and function of hemoglobin in solution. He received a MERIT Award in 1986 from the National Heart, Lung, and Blood Institute for his research on hemoglobin. The second centers on the application of magnetic resonance imaging (MRI) and spectroscopy (MRS) to biomedical systems. He is currently developing non-invasive techniques to monitor the migration/accumulation of immune cells in vivo by MRI as a means to detect early signs of graft rejection following solid organ transplantation.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Dickerson RE, Geis I. Hemoglobin: Structure, Function, Evolution, and Pathology. Benjamin/Cummings; Menlo Park, CA: 1983. [Google Scholar]
- 2.Monod J, Wyman J, Changeux JP. J. Mol. Biol. 1965;12:88. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 3.Perutz MF. Nature. 1970;228:726. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- 4.Koshland DE., Jr. J. Cell. Comp. Physiol. 1959;54:245. doi: 10.1002/jcp.1030540420. [DOI] [PubMed] [Google Scholar]
- 5.Koshland DE, Jr., Nemethy G, Filmer D. Biochemistry. 1966;5:365. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- 6.Szabo A, Karplus M. J. Mol. Biol. 1972;72:163. doi: 10.1016/0022-2836(72)90077-0. [DOI] [PubMed] [Google Scholar]
- 7.Lee AW, Karplus M. Proc. Natl. Acad. Sci. U. S. A. 1983;80:7055. doi: 10.1073/pnas.80.23.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yonetani T, Park S, Tsuneshige A, Imai K, Kanaori K. J. Biol. Chem. 2002;277:34508. doi: 10.1074/jbc.M203135200. [DOI] [PubMed] [Google Scholar]
- 9.Henry ER, Bettati S, Hofrichter J, Eaton WA. Biophys. Chem. 2002;98:149. doi: 10.1016/s0301-4622(02)00091-1. [DOI] [PubMed] [Google Scholar]
- 10.Ackers GK, Doyle ML, Myers D, Daugherty MA. Science. 1992;255:54. doi: 10.1126/science.1553532. [DOI] [PubMed] [Google Scholar]
- 11.Ackers GK. Adv. Protein Chem. 1998;51:185. doi: 10.1016/s0065-3233(08)60653-1. [DOI] [PubMed] [Google Scholar]
- 12.Lukin JA, Ho C. Chem. Rev. 2004;104:1219. doi: 10.1021/cr940325w. [DOI] [PubMed] [Google Scholar]
- 13.Hopfield JJ. J. Mol. Biol. 1973;77:207. doi: 10.1016/0022-2836(73)90332-x. [DOI] [PubMed] [Google Scholar]
- 14.Shulman RG, Hopfield JJ, Ogawa SQ. Rev. Biophys. 1975;8:325. doi: 10.1017/s0033583500001840. [DOI] [PubMed] [Google Scholar]
- 15.Perutz MF, Wilkinson AJ, Paoli M, Dodson GG. Annu. Rev.Biophys. Biomol. Struct. 1998;27:1. doi: 10.1146/annurev.biophys.27.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Eaton WA, Henry ER, Hofrichter J, Mozzarelli A. Nature Struct. Biol. 1999;6:351. doi: 10.1038/7586. [DOI] [PubMed] [Google Scholar]
- 17.Shibayama N. J. Mol. Biol. 1999;285:1383. doi: 10.1006/jmbi.1998.2407. [DOI] [PubMed] [Google Scholar]
- 18.Ackers GK, Holt JM, Huang Y, Grinkova Y, Klinger AL, Denisov I. Proteins. 2000;(Suppl 4):23. doi: 10.1002/1097-0134(2000)41:4+<23::aid-prot30>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 19.Shulman RG. IUBMB Life. 2001;51:351. doi: 10.1080/152165401753366104. [DOI] [PubMed] [Google Scholar]
- 20.Yun KM, Morimoto H, Shibayama N. J. Biol. Chem. 2002;277:1878. doi: 10.1074/jbc.M108494200. [DOI] [PubMed] [Google Scholar]
- 21.Yonetani T, Laberge M. Biochim. Biophys. Acta. 2008;1784:1146. doi: 10.1016/j.bbapap.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safo M, Ahmed M, Ghatge M, Boyiri T. Biochim. Biophys. Acta. 2011;1814:797. doi: 10.1016/j.bbapap.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Bellelli A, Brunori M. Biochim. Biophys. Acta. 2011;1807:1262. doi: 10.1016/j.bbabio.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Changeux JP, Edelstein S. F1000 Biol. Rep. 2011;3:19. doi: 10.3410/B3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Changeux J-P. Annu. Rev. Biophys. 2012;41:103. doi: 10.1146/annurev-biophys-050511-102222. [DOI] [PubMed] [Google Scholar]
- 26.Jones EM, Balakrishnan G, Spiro TG. J. Am. Chem. Soc. 2012;134:3461. doi: 10.1021/ja210126j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yonetani T, Kanaori K. Biochim. Biophys. Acta. 2013;1834:1873. doi: 10.1016/j.bbapap.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Tekpinar M, Zheng W. Proteins. 2013;81:240. doi: 10.1002/prot.24180. [DOI] [PubMed] [Google Scholar]
- 29.Brunori M. FEBS J. 2014;281:633. doi: 10.1111/febs.12586. [DOI] [PubMed] [Google Scholar]
- 30.Jones EM, Monza E, Balakrishnan G, Blouin GC, Mak PJ, Zhu Q, Kincaid JR, Guallar V, Spiro TG. J. Am. Chem. Soc. 2014;136:10325. doi: 10.1021/ja503328a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viappiani C, Abbruzzetti S, Ronda L, Bettati S, Henry ER, Mozzarelli A, Eaton WA. Proc. Natl. Acad. Sci. U. S. A. 2014;111:12758. doi: 10.1073/pnas.1413566111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldwin J, Chothia C. J. Mol. Biol. 1979;129:175. doi: 10.1016/0022-2836(79)90277-8. [DOI] [PubMed] [Google Scholar]
- 33.Perutz MF. Brookhaven Symp. Biol. 1960;13:165. [PubMed] [Google Scholar]
- 34.Muirhead H, Perutz MF. Nature. 1963;199:633. doi: 10.1038/199633a0. [DOI] [PubMed] [Google Scholar]
- 35.Park S-Y, Yokoyama T, Shibayama N, Shiro Y, Tame JRH. J. Mol. Biol. 2006;360:690. doi: 10.1016/j.jmb.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 36.Savino C, Miele AE, Draghi F, Johnson KA, Sciara G, Brunori M, Vallone B. Biopolymers. 2009;91:1097. doi: 10.1002/bip.21201. [DOI] [PubMed] [Google Scholar]
- 37.Perutz MF. J. Cryst. Growth. 1968;2:54. [Google Scholar]
- 38.Abassi Z, Kotob S, Pieruzzi F, Abouassali M, Keiser HR, Fratantoni JC, Alayash AI. J. Lab. Clin. Med. 1997;129:603. doi: 10.1016/s0022-2143(97)90194-3. [DOI] [PubMed] [Google Scholar]
- 39.Vasquez GB, Ji XH, Fronticelli C, Gilliland GL. Acta Crystallogr. D Biol. Crystallogr. 1998;54:355. doi: 10.1107/s0907444997012250. [DOI] [PubMed] [Google Scholar]
- 40.Derewenda Z, Dodson G, Emsley P, Harris D, Nagai K, Perutz M, Reynaud JP. J. Mol. Biol. 1990;211:515. doi: 10.1016/0022-2836(90)90262-k. [DOI] [PubMed] [Google Scholar]
- 41.Fermi G. J. Mol. Biol. 1975;97:237. doi: 10.1016/s0022-2836(75)80037-4. [DOI] [PubMed] [Google Scholar]
- 42.Fermi G, Perutz MF, Shaanan B, Fourme R. J. Mol. Biol. 1984;175:159. doi: 10.1016/0022-2836(84)90472-8. [DOI] [PubMed] [Google Scholar]
- 43.Pechik I, Ji X, Fidelis K, Karavitis M, Moult J, Brinigar WS, Fronticelli C, Gilliland GL. Biochemistry. 1996;35:1935. doi: 10.1021/bi9519967. [DOI] [PubMed] [Google Scholar]
- 44.Safo MK, Burnett JC, Musayev FN, Nokuri S, Abraham DJ. Acta Crystallogr. D Biol. Crystallogr. 2002;58:2031. doi: 10.1107/s0907444902015809. [DOI] [PubMed] [Google Scholar]
- 45.Shaanan B. J. Mol. Biol. 1983;171:31. doi: 10.1016/s0022-2836(83)80313-1. [DOI] [PubMed] [Google Scholar]
- 46.Tame JRH, Vallone B. Acta Crystallogr. D Biol. Crystallogr. 2000;56:805. doi: 10.1107/s0907444900006387. [DOI] [PubMed] [Google Scholar]
- 47.Silva MM, Rogers PH, Arnone A. J. Biol. Chem. 1992;267:17248. [PubMed] [Google Scholar]
- 48.Mueser TC, Rogers PH, Arnone A. Biochemistry. 2000;39:15353. doi: 10.1021/bi0012944. [DOI] [PubMed] [Google Scholar]
- 49.Srinivasan R, Rose GD. Proc. Natl. Acad. Sci. U.S.A. 1994;91:11113. doi: 10.1073/pnas.91.23.11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janin J, Wodak SJ. Proteins. 1993;15:1. doi: 10.1002/prot.340150102. [DOI] [PubMed] [Google Scholar]
- 51.Smith FR, Lattman EE, Carter CW. Proteins. 1991;10:81. doi: 10.1002/prot.340100202. [DOI] [PubMed] [Google Scholar]
- 52.Smith FR, Simmons KC. Proteins. 1994;18:295. doi: 10.1002/prot.340180310. [DOI] [PubMed] [Google Scholar]
- 53.Safo MK, Abraham DJ. Biochemistry. 2005;44:8347. doi: 10.1021/bi050412q. [DOI] [PubMed] [Google Scholar]
- 54.Jenkins JD, Musayev FN, Danso-Danquah R, Abraham DJ, Safo MK. Acta Crystallogr. D Biol. Crystallogr. 2009;65:41. doi: 10.1107/S0907444908037256. [DOI] [PubMed] [Google Scholar]
- 55.Kavanaugh JS, Rogers PH, Arnone A. Biochemistry. 2005;44:6101. doi: 10.1021/bi047813a. [DOI] [PubMed] [Google Scholar]
- 56.Liddington R, Derewenda Z, Dodson E, Hubbard R, Dodson G. J. Mol. Biol. 1992;228:551. doi: 10.1016/0022-2836(92)90842-8. [DOI] [PubMed] [Google Scholar]
- 57.Richard V, Dodson GG, Mauguen Y. J. Mol. Biol. 1993;233:270. doi: 10.1006/jmbi.1993.1505. [DOI] [PubMed] [Google Scholar]
- 58.Dodson GG, Richard VR, Tolley SP, Waller DA, Weber RE. J. Mol. Biol. 1990;211:691. doi: 10.1016/0022-2836(90)90069-X. [DOI] [PubMed] [Google Scholar]
- 59.Yokoyama T, Neya S, Tsuneshige A, Yonetani T, Park SY, Tame JRH. J. Mol. Biol. 2006;356:790. doi: 10.1016/j.jmb.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 60.Shibayama N, Sugiyama K, Tame JR, Park SY. J. Am. Chem. Soc. 2014;136:5097. doi: 10.1021/ja500380e. [DOI] [PubMed] [Google Scholar]
- 61.DeLano WL. The PyMOL Molecular Graphics System, Version 1.5.0.4. Schrodinger, LLC.; Palo Alto, CA, USA.: 2002. [Google Scholar]
- 62.Kavanaugh JS, Weydert JA, Rogers PH, Arnone A. Biochemistry. 1998;37:4358. doi: 10.1021/bi9708702. [DOI] [PubMed] [Google Scholar]
- 63.Dey S, Chakrabarti P, Janin J. Proteins. 2011;79:2861. doi: 10.1002/prot.23112. [DOI] [PubMed] [Google Scholar]
- 64.Seixas FAV, de Azevedo WF, Colombo MF. Acta Crystallogr. D Biol. Crystallogr. 1999;55:1914. doi: 10.1107/s0907444999009750. [DOI] [PubMed] [Google Scholar]
- 65.Chan NL, Kavanaugh JS, Rogers PH, Arnone A. Biochemistry. 2004;43:118. doi: 10.1021/bi030172j. [DOI] [PubMed] [Google Scholar]
- 66.Kavanaugh JS, Moopenn WF, Arnone A. Biochemistry. 1993;32:2509. doi: 10.1021/bi00061a007. [DOI] [PubMed] [Google Scholar]
- 67.Shen TJ, Ho NT, Simplaceanu V, Zou M, Green BN, Tam MF, Ho C. Proc. Natl. Acad. Sci. U. S. A. 1993;90:8108. doi: 10.1073/pnas.90.17.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simplaceanu V, Lukin JA, Fang TY, Zou M, Ho NT, Ho C. Biophys. J. 2000;79:1146. doi: 10.1016/S0006-3495(00)76368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lukin JA, Kontaxis G, Simplaceanu V, Yuan Y, Bax A, Ho C. Proc. Natl. Acad. Sci.U.S.A. 2003;100:517. doi: 10.1073/pnas.232715799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gong QG, Simplaceanu V, Lukin JA, Giovannelli JL, Ho NT, Ho C. Biochemistry. 2006;45:5140. doi: 10.1021/bi052424h. [DOI] [PubMed] [Google Scholar]
- 71.Sahu SC, Simplaceanu V, Gong QG, Ho NT, Glushka JG, Prestegard JH, Ho C. J. Am. Chem. Soc. 2006;128:6290. doi: 10.1021/ja060023z. [DOI] [PubMed] [Google Scholar]
- 72.Sahu SC, Simplaceanu V, Gong Q, Ho NT, Tian F, Prestegard JH, Ho C. Biochemistry. 2007;46:9973. doi: 10.1021/bi700935z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ho C, Yuan Y, Simplaceanu V. In: Hemoglobin: Recent Developments and Topics. Nagai M, editor. Research Signpost; Kerala, India: 2011. [Google Scholar]
- 74.Fan JS, Zheng Y, Choy WY, Simplaceanu V, Ho NT, Ho C, Yang D. Biochemistry. 2013;52:5809. doi: 10.1021/bi4005683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tjandra N, Bax A. Science. 1997;278:1697. doi: 10.1126/science.278.5340.1111. [DOI] [PubMed] [Google Scholar]
- 76.Xu Y, Zheng Y, Fan J-S, Yang D. Nat. Methods. 2006;3:931. doi: 10.1038/nmeth938. [DOI] [PubMed] [Google Scholar]
- 77.Arnone A. Nature. 1972;237:146. doi: 10.1038/237146a0. [DOI] [PubMed] [Google Scholar]
- 78.Arnone A, Perutz MF. Nature. 1974;249:34. doi: 10.1038/249034a0. [DOI] [PubMed] [Google Scholar]
- 79.Zuiderweg ER, Hamers LF, de Bruin SH, Hilbers CW. Eur. J. Biochem. 1981;118:85. doi: 10.1111/j.1432-1033.1981.tb05489.x. [DOI] [PubMed] [Google Scholar]
- 80.Nagatomo S, Nagai M, Mizutani Y, Yonetani T, Kitagawa T. Biophys. J. 2005;89:1203. doi: 10.1529/biophysj.104.049775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song XJ, Yuan Y, Simplaceanu V, Sahu SC, Ho NT, Ho C. Biochemistry. 2007;46:6795. doi: 10.1021/bi602654u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park S, Bardhan JP, Roux B, Makowski L. J. Chem. Phys. 2009;130:134114. doi: 10.1063/1.3099611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Makowski L, Rodi DJ, Mandava S, Minh DD, Gore DB, Fischetti RF. J. Mol. Biol. 2008;375:529. doi: 10.1016/j.jmb.2007.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Makowski L, Bardhan J, Gore D, Lal J, Mandava S, Park S, Rodi DJ, Ho NT, Ho C, Fischetti RF. J. Mol. Biol. 2011;408:909. doi: 10.1016/j.jmb.2011.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Makowski L, Gore D, Mandava S, Minh D, Park S, Rodi DJ, Fischetti RF. Biopolymers. 2011;95:531. doi: 10.1002/bip.21631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsai CH, Shen TJ, Ho NT, Ho C. Biochemistry. 1999;38:8751. doi: 10.1021/bi990286o. [DOI] [PubMed] [Google Scholar]
- 87.Mihailescu MR, Russu IM. Proc. Natl. Acad. Sci.U.S.A. 2001;98:3773. doi: 10.1073/pnas.071493598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lukin JA, Kontaxis G, Simplaceanu V, Yuan Y, Bax A, Ho C. J. Biomol. NMR. 2004;28:203. doi: 10.1023/B:JNMR.0000013816.64039.6f. [DOI] [PubMed] [Google Scholar]
- 89.Sahu SC, Simplaceanu V, Ho NT, Giovannelli JL, Ho C. J. Biomol. NMR. 2006;36:1. doi: 10.1007/s10858-005-2455-z. [DOI] [PubMed] [Google Scholar]
- 90.Song XJ, Simplaceanu V, Ho NT, Ho C. Biochemistry. 2008;47:4907. doi: 10.1021/bi7023699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mandel AM, Akke M, Palmer AG. J. Mol. Biol. 1995;246:144. doi: 10.1006/jmbi.1994.0073. [DOI] [PubMed] [Google Scholar]
- 92.Wang CY, Rance M, Palmer AG. J. Am. Chem. Soc. 2003;125:8968. doi: 10.1021/ja035139z. [DOI] [PubMed] [Google Scholar]
- 93.Loria JP, Rance M, Palmer AG. J. Am. Chem. Soc. 1999;121:2331. [Google Scholar]
- 94.Millet O, Loria JP, Kroenke CD, Pons M, Palmer AG. J. Am. Chem. Soc. 2000;122:2867. [Google Scholar]
- 95.Yuan Y, Simplaceanu V, Lukin JA, Ho C. J. Mol. Biol. 2002;321:863. doi: 10.1016/s0022-2836(02)00704-0. [DOI] [PubMed] [Google Scholar]
- 96.Balakrishnan G, Tsai CH, Wu Q, Case MA, Pevsner A, McLendon GL, Ho C, Spiro TG. J. Mol. Biol. 2004;340:857. doi: 10.1016/j.jmb.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 97.Balakrishnan G, Case MA, Pevsner A, Zhao XJ, Tengroth C, McLendon GL, Spiro TG. J. Mol. Biol. 2004;340:843. doi: 10.1016/j.jmb.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 98.Yang DW, Mittermaier A, Mok YK, Kay LE. J. Mol. Biol. 1998;276:939. doi: 10.1006/jmbi.1997.1588. [DOI] [PubMed] [Google Scholar]
- 99.Zuiderweg ER, Hamers LF, Rollema HS, de Bruin SH, Hilbers CW. Eur. J. Biochem. 1981;118:95. doi: 10.1111/j.1432-1033.1981.tb05490.x. [DOI] [PubMed] [Google Scholar]
- 100.Hwang TL, van Zijl PCM, Mori S. J. Biomol. NMR. 1998;11:221. doi: 10.1023/a:1008276004875. [DOI] [PubMed] [Google Scholar]
- 101.Bunn HF, Forget BG. Hemoglobin: molecular, genetic and clinical aspects. W.B. Saunders; Philadelphia, PA: 1986. [Google Scholar]
- 102.Wyman J. Adv. Protein Chem. 1964;19:223. doi: 10.1016/s0065-3233(08)60190-4. [DOI] [PubMed] [Google Scholar]
- 103.Ho C, Yuan Y. Encyclopedia of Life Sciences. John Wiley & Sons, Ltd.; Chichester: 2010. [Google Scholar]
- 104.Perutz MF. Nature. 1970;228:734. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- 105.Busch MR, Ho C. Biophys. Chem. 1990;37:313. doi: 10.1016/0301-4622(90)88031-m. [DOI] [PubMed] [Google Scholar]
- 106.Busch MR, Mace JE, Ho NT, Ho C. Biochemistry. 1991;30:1865. doi: 10.1021/bi00221a020. [DOI] [PubMed] [Google Scholar]
- 107.Sun DZP, Zou M, Ho NT, Ho C. Biochemistry. 1997;36:6663. doi: 10.1021/bi963121d. [DOI] [PubMed] [Google Scholar]
- 108.Fang TY, Zou M, Simplaceanu V, Ho NT, Ho C. Biochemistry. 1999;38:13423. doi: 10.1021/bi9911379. [DOI] [PubMed] [Google Scholar]
- 109.Kilmartin JV, Rossi-Bernardi L. Nature. 1969;222:1243. doi: 10.1038/2221243a0. [DOI] [PubMed] [Google Scholar]
- 110.Zheng G, Schaefer M, Karplus M. Biochemistry. 2013;52:8539. doi: 10.1021/bi401126z. [DOI] [PubMed] [Google Scholar]
- 111.Russu IM, Ho NT, Ho C. Biochemistry. 1980;19:1043. doi: 10.1021/bi00546a033. [DOI] [PubMed] [Google Scholar]
- 112.Perutz MF, Kilmartin JV, Nishikura K, Fogg JH, Butler PJG, Rollema HS. J. Mol. Biol. 1980;138:649. doi: 10.1016/s0022-2836(80)80022-2. [DOI] [PubMed] [Google Scholar]
- 113.Kilmartin JV, Fogg JH, Perutz MF. Biochemistry. 1980;19:3189. doi: 10.1021/bi00555a013. [DOI] [PubMed] [Google Scholar]
- 114.Perutz MF, Gronenborn AM, Clore GM, Shib DT, Craescu CT. J. Mol. Biol. 1985;186:471. doi: 10.1016/0022-2836(85)90119-6. [DOI] [PubMed] [Google Scholar]
- 115.Perutz MF, Gronenborn AM, Clore GM, Fogg JH, Shih DTB. J. Mol. Biol. 1985;183:491. doi: 10.1016/0022-2836(85)90016-6. [DOI] [PubMed] [Google Scholar]
- 116.Russu IM, Ho C. Biochemistry. 1986;25:1706. doi: 10.1021/bi00355a040. [DOI] [PubMed] [Google Scholar]
- 117.Ho C, Russu IM. Biochemistry. 1987;26:6299. doi: 10.1021/bi00394a001. [DOI] [PubMed] [Google Scholar]
- 118.Shih DTB, Perutz MF. J. Mol. Biol. 1987;195:419. doi: 10.1016/0022-2836(87)90660-7. [DOI] [PubMed] [Google Scholar]
- 119.Shih DTB, Perutz MF, Gronenborn AM, Clore GM. J. Mol. Biol. 1987;195:453. doi: 10.1016/0022-2836(87)90666-8. [DOI] [PubMed] [Google Scholar]
- 120.Russu IM, Wu SS, Ho NT, Kellogg GW, Ho C. Biochemistry. 1989;28:5298. doi: 10.1021/bi00438a057. [DOI] [PubMed] [Google Scholar]
- 121.Campbell KL, Roberts JEE, Watson LN, Stetefeld J, Sloan AM, Signore AV, Howatt JW, Tame JRH, Rohland N, Shen TJ, Austin JJ, Hofreiter M, Ho C, Weber RE, Cooper A. Nat. Genet. 2010;42:536. doi: 10.1038/ng.574. [DOI] [PubMed] [Google Scholar]
- 122.Yuan Y, Shen TJ, Gupta P, Ho NT, Simplaceanu V, Tam TC, Hofreiter M, Cooper A, Campbell KL, Ho C. Biochemistry. 2011;50:7350. doi: 10.1021/bi200777j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yuan Y, Byrd C, Shen T-J, Simplaceanu V, Tam TCS, Ho C. Biochemistry. 2013;52:8888. doi: 10.1021/bi401087d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shih DT, Jones RT, Imai K, Tyuma I. J. Biol. Chem. 1985;260:5919. [PubMed] [Google Scholar]
- 125.Noguchi H, Campbell KL, Ho C, Unzai S, Park SY, Tame JR. Acta Crystallogr. D Biol. Crystallogr. 2012;68:1441. doi: 10.1107/S0907444912029459. [DOI] [PubMed] [Google Scholar]
- 126.Kavanaugh JS, Rogers PH, Arnone A. Biochemistry. 1992;31:8640. doi: 10.1021/bi00151a034. [DOI] [PubMed] [Google Scholar]
- 127.Perutz MF, Fermi G, Poyart C, Pagnier J, Kister J. J. Mol. Biol. 1993;233:536. doi: 10.1006/jmbi.1993.1530. [DOI] [PubMed] [Google Scholar]
- 128.Yonetani T, Yamamoto H, Iizuka T. J. Biol. Chem. 1974;249:2168. [PubMed] [Google Scholar]
- 129.Kitagawa T, Ondrias MR, Rousseau DL, Ikedasaito M, Yonetani T. Nature. 1982;298:869. doi: 10.1038/298869a0. [DOI] [PubMed] [Google Scholar]
- 130.Lukin JA, Simplaceanu V, Zou M, Ho NT, Ho C. Proc. Natl. Acad. Sci.U.S.A. 2000;97:10354. doi: 10.1073/pnas.190254697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yuan Y, Simplaceanu V, Ho NT, Ho C. Biochemistry. 2010;49:10606. doi: 10.1021/bi100927p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Birukou I, Schweers RL, Olson JS. J. Biol. Chem. 2010;285:8840. doi: 10.1074/jbc.M109.053934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Birukou I, Maillett DH, Birukova A, Olson JS. Biochemistry. 2011;50:7361. doi: 10.1021/bi200923k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Jr., Olson JS. Biochemistry. 1996;35:6976. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 135.Fronticelli C, Brinigar WS, Olson JS, Bucci E, Gryczynski Z, O'Donnell JK, Kowalczyk J. Biochemistry. 1993;32:1235. doi: 10.1021/bi00056a006. [DOI] [PubMed] [Google Scholar]
- 136.Jeong ST, Ho NT, Hendrich MP, Ho C. Biochemistry. 1999;38:13433. doi: 10.1021/bi991271t. [DOI] [PubMed] [Google Scholar]
- 137.Karavitis M, Fronticelli C, Brinigar WS, Vasquez GB, Militello V, Leone M, Cupane A. J. Biol. Chem. 1998;273:23740. doi: 10.1074/jbc.273.37.23740. [DOI] [PubMed] [Google Scholar]
- 138.Maillett DH, Simplaceanu V, Shen TJ, Ho NT, Olson JS, Ho C. Biochemistry. 2008;47:10551. doi: 10.1021/bi800816v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mathews AJ, Olson JS, Renaud JP, Tame J, Nagai K. J. Biol. Chem. 1991;266:21631. [PubMed] [Google Scholar]
- 140.Mathews AJ, Rohlfs RJ, Olson JS, Tame J, Renaud JP, Nagai K. J. Biol. Chem. 1989;264:16573. [PubMed] [Google Scholar]
- 141.Nagai K, Luisi B, Shih D, Miyazaki G, Imai K, Poyart C, De Young A, Kwiatkowsky L, Noble RW, Lin SH, Yu NT. Nature. 1987;329:858. doi: 10.1038/329858a0. [DOI] [PubMed] [Google Scholar]
- 142.Tam MF, Rice NW, Maillett DH, Simplaceanu V, Ho NT, Tam TC, Shen TJ, Ho C. J. Biol. Chem. 2013;288:25512. doi: 10.1074/jbc.M113.474841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tucker PW, Phillips SEV, Perutz MF, Houtchens R, Caughey WS. Proc. Natl. Acad. Sci.U.S.A. 1978;75:1076. doi: 10.1073/pnas.75.3.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tsuruga M, Shikama K. Biochim. Biophys. Acta. 1997;1337:96. doi: 10.1016/s0167-4838(96)00156-2. [DOI] [PubMed] [Google Scholar]
- 145.Macdonald VW. Methods Enzymol. Vol. 231. Academic Press Inc.; San Diego: 1994. p. 480. [DOI] [PubMed] [Google Scholar]
- 146.Brancaccio A, Cutruzzola F, Allocatelli C, Brunori M, Smerdon S, Wilkinson A, Dou Y, Keenan D, Ikeda-Saito M, Brantley R., Jr J. Biol. Chem. 1994;269:13843. [PubMed] [Google Scholar]
- 147.Brantley RE, Jr., Smerdon SJ, Wilkinson AJ, Singleton EW, Olson JS. J. Biol. Chem. 1993;268:6995. [PubMed] [Google Scholar]
- 148.Carver TE, Brantley RE, Jr., Singleton EW, Arduini RM, Quillin ML, Phillips GN, Jr., Olson JS. J. Biol. Chem. 1992;267:14443. [PubMed] [Google Scholar]
- 149.Quillin ML, Li T, Olson JS, Phillips GN, Jr., Dou Y, Ikeda-Saito M, Regan R, Carlson M, Gibson QH, Li H, et al. J. Mol. Biol. 1995;245:416. doi: 10.1006/jmbi.1994.0034. [DOI] [PubMed] [Google Scholar]
- 150.Wallace WJ, Houtchens RA, Maxwell JC, Caughey WS. J. Biol. Chem. 1982;257:4966. [PubMed] [Google Scholar]
- 151.Suzuki T, Watanabe YH, Nagasawa M, Matsuoka A, Shikama K. Eur. J. Bioch. 2000;267:6166. doi: 10.1046/j.1432-1327.2000.01685.x. [DOI] [PubMed] [Google Scholar]
- 152.Tsuruga M, Matsuoka A, Hachimori A, Sugawara Y, Shikama K. J. Biol. Chem. 1998;273:8607. doi: 10.1074/jbc.273.15.8607. [DOI] [PubMed] [Google Scholar]
- 153.Wiltrout ME, Giovannelli JL, Simplaceanu V, Lukin JA, Ho NT, Ho C. Biochemistry. 2005;44:7207. doi: 10.1021/bi048289a. [DOI] [PubMed] [Google Scholar]
- 154.Mansouri A, Winterhalter KH. Biochemistry. 1973;12:4946. doi: 10.1021/bi00748a020. [DOI] [PubMed] [Google Scholar]
- 155.Tsai CH, Fang TY, Ho NT, Ho C. Biochemistry. 2000;39:13719. doi: 10.1021/bi001116a. [DOI] [PubMed] [Google Scholar]
- 156.Kim HW, Shen TJ, Sun DP, Ho NT, Madrid M, Ho C. J. Mol. Biol. 1995;248:867. doi: 10.1006/jmbi.1995.0267. [DOI] [PubMed] [Google Scholar]
- 157.Tsai CH, Ho C. Biophys. Chem. 2002;98:15. doi: 10.1016/s0301-4622(02)00081-9. [DOI] [PubMed] [Google Scholar]
- 158.Kern D, Zuiderweg ERP. Curr. Opin. Struct. Biol. 2003;13:748. doi: 10.1016/j.sbi.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 159.Tang C, Schwieters CD, Clore GM. Nature. 2007;449:1078. doi: 10.1038/nature06232. [DOI] [PubMed] [Google Scholar]
- 160.Eaton WA, Henry ER, Hofrichter J, Bettati S, Viappiani C, Mozzarelli A. IUBMB Life. 2007;59:586. doi: 10.1080/15216540701272380. [DOI] [PubMed] [Google Scholar]
- 161.Velyvis A, Yang YR, Schachman HK, Kay LE. Proc. Natl. Acad. Sci.U.S.A. 2007;104:8815. doi: 10.1073/pnas.0703347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cui Q, Karplus M. Protein Sci. 2008;17:1295. doi: 10.1110/ps.03259908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lakomek NA, Walter KFA, Fares C, Lange OF, de Groot BL, Grubmuller H, Bruschweiler R, Munk A, Becker S, Meiler J, Griesinger C. J. Biomol. NMR. 2008;41:139. doi: 10.1007/s10858-008-9244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Boehr DD, Nussinov R, Wright PE. Nat. Chem. Biol. 2009;5:789. doi: 10.1038/nchembio.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gua Y, Shrivastava IH, Amara SG, Bahar I. Proc. Natl. Acad. Sci.U.S.A. 2009;106:2589. doi: 10.1073/pnas.0812299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Velyvis A, Schachman HK, Kay LE. J. Mol. Biol. 2009;387:540. doi: 10.1016/j.jmb.2009.01.066. [DOI] [PubMed] [Google Scholar]
- 167.Motlagh HN, Wrabl JO, Li J, Hilser VJ. Nature. 2014;508:331. doi: 10.1038/nature13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fung LWM, Minton AP, Lindstrom TR, Pisciotta AV, Ho C. Biochemistry. 1977;16:1452. doi: 10.1021/bi00626a033. [DOI] [PubMed] [Google Scholar]
- 169.Takahashi S, Lin A, Ho C. Biochemistry. 1980;19:5196. doi: 10.1021/bi00564a007. [DOI] [PubMed] [Google Scholar]
- 170.Miura S, Ho C. Biochemistry. 1982;21:6280. doi: 10.1021/bi00267a037. [DOI] [PubMed] [Google Scholar]
- 171.Russu IM, Ho NT, Ho C. Biochim. Biophys. Acta. 1987;914:40. doi: 10.1016/0167-4838(87)90159-2. [DOI] [PubMed] [Google Scholar]
- 172.Kim HW, Shen TJ, Sun DP, Ho NT, Madrid M, Tam MF, Zou M, Cottam PF, Ho C. Proc. Natl. Acad. Sci.U.S.A. 1994;91:11547. doi: 10.1073/pnas.91.24.11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Barrick D, Ho NT, Simplaceanu V, Dahlquist FW, Ho C. Nat. Struct. Biol. 1997;4:78. doi: 10.1038/nsb0197-78. [DOI] [PubMed] [Google Scholar]
- 174.Barrick D, Ho NT, Simplaceanu V, Ho C. Biochemistry. 2001;40:3780. doi: 10.1021/bi002165q. [DOI] [PubMed] [Google Scholar]
- 175.Chang CK, Simplaceanu V, Ho C. Biochemistry. 2002;41:5644. doi: 10.1021/bi011919d. [DOI] [PubMed] [Google Scholar]
- 176.Kneipp J, Balakrishnan G, Chen RP, Shen TJ, Sahu SC, Ho NT, Giovannelli JL, Simplaceanu V, Ho C, Spiro TG. J. Mol. Biol. 2006;356:335. doi: 10.1016/j.jmb.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 177.Petrich JW, Lambry JC, Kuczera K, Karplus M, Poyart C, Martin JL. Biochemistry. 1991;30:3975. doi: 10.1021/bi00230a025. [DOI] [PubMed] [Google Scholar]
- 178.Cammarata M, Levantino M, Wulff M, Cupane A. J. Mol. Biol. 2010;400:951. doi: 10.1016/j.jmb.2010.05.057. [DOI] [PubMed] [Google Scholar]
- 179.Cornilescu G, Marquardt JL, Ottiger M, Bax A. J. Am. Chem. Soc. 1998;120:6836. [Google Scholar]
- 180.Bax A, Kontaxis G, Tjandra N. In Methods Enzymol. Vol. 339. Academic Press Inc; San Diego: 2001. p. 127. [DOI] [PubMed] [Google Scholar]