Abstract
Background
Long-term inhaled corticosteroids (ICS) may reduce growth velocity and final height of children with asthma. We aimed to evaluate the association between ICS use of >12 months and growth.
Methods
We initially searched MEDLINE and EMBASE in July 2013, followed by a PubMed search updated to December 2014. We selected RCTs and controlled observational studies of ICS use in patients with asthma. We conducted random effects meta-analysis of mean differences in growth velocity (cm/year) or final height (cm) between groups. Heterogeneity was assessed using the I2 statistic.
Results
We found 23 relevant studies (twenty RCTs and three observational studies) after screening 1882 hits. Meta-analysis of 16 RCTs showed that ICS use significantly reduced growth velocity at one year follow-up (mean difference -0.48 cm/year (95% CI -0.66 to -0.29)). There was evidence of a dose-response effect in three RCTs. Final adult height showed a mean reduction of -1.20 cm (95% CI -1.90 cm to -0.50 cm) with budesonide versus placebo in a high quality RCT. Meta-analysis of two lower quality observational studies revealed uncertainty in the association between ICS use and final adult height, pooled mean difference -0.85 cm (95% CI -3.35 to 1.65).
Conclusion
Use of ICS for >12 months in children with asthma has a limited impact on annual growth velocity. In ICS users, there is a slight reduction of about a centimeter in final adult height, which when interpreted in the context of average adult height in England (175 cm for men and 161 cm for women), represents a 0.7% reduction compared to non-ICS users.
Introduction
Inhaled corticosteroids (ICS) are amongst the most important treatment options in persistent asthma because of their efficacy in suppressing the inflammatory response. The clinical benefits of long-term therapy with ICS are consistently emphasized in national and international guidelines (United Kingdom and United States). [1, 2] However, there have been widespread, long-standing concerns regarding adverse events (such as fractures, and reduction of growth in children) with corticosteroids. [3, 4] The James Lind Alliance (in partnership with the British Thoracic Society and Asthma UK) reported on a recent prioritization exercise involving patients and health care professionals where adverse effects of corticosteroids was judged to be a top research priority. [5]
One of the major areas of concern and uncertainty is the potential for reduction in growth velocity and final height of children who are long-term users of inhaled corticosteroids (ICS). In 2006, Pedersen conducted a systematic review on randomized controlled trials (RCTs) of > 12 months ICS use in children, and concluded that there was possibly a small decrease in statural growth. [6] In an expert symposium, Skoner et al. noted a similar small decrease in growth, and an increase in cataracts, but argued that the earlier studies were methodologically weak, or based on drugs no longer in common use. [7]
Recent recommendations on comprehensive evaluations of adverse effects have suggested that a wide range of study designs (beyond just RCTs) may need to considered, depending on the features of the adverse outcome of interest. [8] Rare adverse events, or those that occur only after prolonged therapy, can be evaluated with non-randomized studies, perhaps even requiring a meta-analysis of various study designs. [8] Hence we aimed to analyse the effects of long-term (>12 months) ICS use in children with asthma, concentrating on growth velocity and final adult height in randomized and non-randomized studies.
Methods
Study Selection Criteria
As we were interested in long-term adverse effects that may be of relatively small magnitude, we selected studies with> 20 users of each ICS formulation, and follow-up of at least 52 weeks duration.
Our inclusion criteria for RCTs were (1) parallel-group RCT; (2) children with asthma of any severity; (3) ICS as the intervention vs a control treatment, where the comparison groups consisted of ICS vs other treatment, or ICS in combination with LABA vs a LABA alone; and (4) stated aim to evaluate growth velocity and/or final adult height.
We also evaluated controlled observational studies (case control, prospective cohort or retrospective cohort) reporting on growth velocity and/or height with any ICS exposure compared to those without ICS exposure.
We excluded studies that recruited children where the diagnosis of asthma had not been established. We excluded crossover trials and studies that considered only oral corticosteroid use without reporting the effects of inhaled corticosteroids.
Search Strategy
We initially searched MEDLINE and EMBASE in June 2013 using a broad strategy for a wide range of adverse effects, and we updated this with a more focused PubMed search in December 2014 (see S1 Appendix for search terms and restrictions). We also checked the bibliographies of included studies and existing systematic reviews for relevant articles.
Study Selection
Two reviewers (MT and PB) independently, and in duplicate considered all titles and abstracts. At this point, we excluded reports that were clearly not RCTs or observational studies of ICS in asthma. We then retrieved full text versions of potentially relevant articles and carried out further screening to determine if the study objectives included the evaluation of growth. A third researcher (YKL) finalized the decision during discussion with the two reviewers.
Study Characteristics and Data Extraction
We extracted data onto pre-formatted tables with details on study design, participants, definition of asthma, drug therapy (dose, device and frequency), and duration of follow-up. Two reviewers independently extracted data (MT and PB) on final adult height and growth velocity (cm per year). A third reviewer (YKL) corrected any discrepancies after rechecking the source papers.
Risk of Bias Assessment
Two reviewers (MT and PB) independently assessed the reporting of blinding, allocation concealment, withdrawals and the loss to follow-up in RCTs. In accordance with recommendations on assessing adverse effects, we extracted information on participant selection, ascertainment of exposure and outcomes, and methods of addressing confounding in observational studies. [9] A third reviewer (YKL) adjudicated and made the final decision on discrepant items after rechecking the source papers.
We aimed to use a funnel plot to assess publication bias if there were >10 studies in the meta-analysis, with no significant heterogeneity seen. [10]
Statistical Analysis
We pooled trial data using Review Manager (RevMan) version 5.3.2 (Nordic Cochrane Center, Copenhagen, Denmark). We used the inverse variance method to pool mean differences in growth velocity (cm per year) or final adult height (cm) between ICS users and non-users. We calculated the I2 statistic to for statistical heterogeneity with I2> 50% indicating a substantial level of heterogeneity. [11]
If a study had multiple arms involving different ICS doses, we attempted to analyse data on the licensed dose for children where available, otherwise we combined all the intervention arms together as recommended by the Cochrane Handbook. [12] For studies with more than one group of non-ICS users, we analysed data from the placebo arm (wherever possible) in preference to data from active comparators such as nedocromil or montelukast. If the study reported growth velocity values at different points over a number of years, we analysed the data based on the first year, but also recorded the overall change over the complete follow-up, as well as at different annual intervals. For studies that did not explicitly report growth velocity, we extracted data on the mean change in height from baseline to the 52 week follow-up. In accordance with the recommendations of the Cochrane Handbook, we imputed any standard deviations from 95% confidence intervals or p-values. [13]
Subgroup Analysis
We aimed to conduct subgroup analysis where growth data were available for head to head comparisons of different treatment regimens (dose / duration), or for children of different ages within the same study.
We do not have a pre-registered protocol.
Results
We screened 1882 potentially relevant articles, and finally included 23 studies in our systematic review (comprising twenty RCTs, [14–33] and three observational studies [34–36]). The process of study selection is shown in S1 Fig.
S1 and S2 Tables show the characteristics of the included RCTs, and the observational studies respectively. S3 and S4 Tables report on study validity and outcomes.
The study with the longest treatment duration was four years (CAMP—Childhood Asthma Management Programme), [24] while the remaining trials had ICS therapy for between 52–156 weeks. The ICS formulations reported in the trials included beclometasone, budesonide, ciclesonide, flunisolide, fluticasone, and mometasone. There was variation in the choice of control intervention, with placebo, nedocromil, montelukast, sodium cromoglicate being used in some trials. We identified three trials where budesonide and fluticasone were compared head-to-head, without any non-ICS control arm. [14, 18, 19]
The observational studies looked at narrow range of ICS users, primarily focusing on budesonide (two studies), [34, 35] whilst one study did not specify ICS formulation.[36]
Study Validity
Randomized Controlled Trials (n = 20)
Nine of the RCTs reported an appropriate method of sequence generation, eleven provided details on how concealment of allocation was achieved whilst 13 reported the use of double blinding. (S2 Table). Ascertainment of height was usually done through stadiometry. S2 Table also shows that discontinuations and substantial losses to follow-up (particularly where height was not available at final timepoint) are major limitation affecting almost all the trials. The vast majority of trials had involvement of pharmaceutical industry sponsors.
Observational studies (n = 3)
Overall, we considered the studies to be at moderate to high risk of bias due to potentially confounded comparisons arising from the relatively limited matching and lack of multivariate adjustment.
Outcomes: Growth Velocity
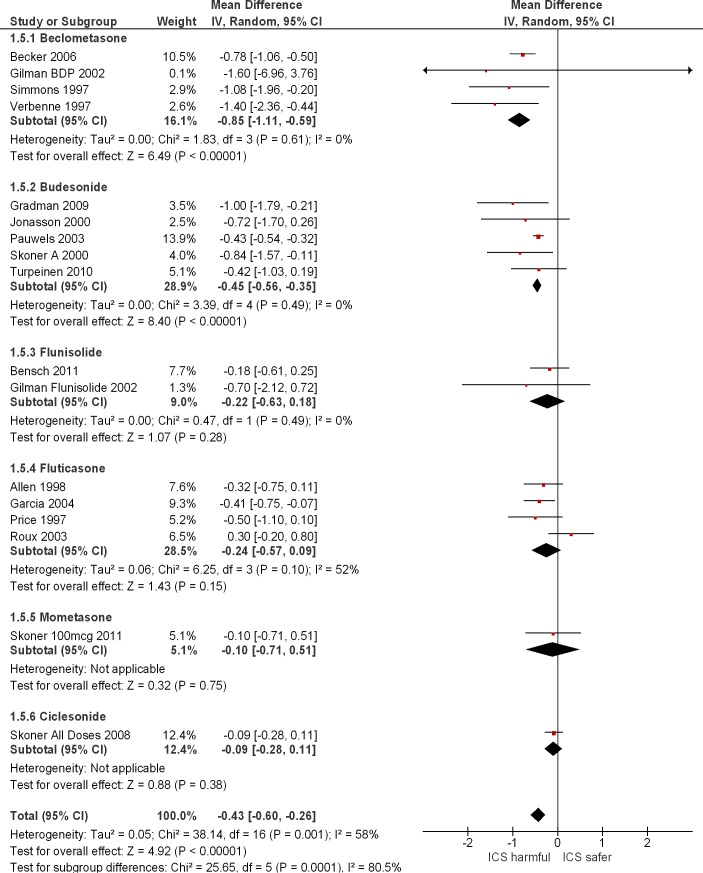
Sixteen RCTs and one observational study reported on comparative difference in growth velocity (cm/year) in children. (Fig 1) Overall, ICS use was associated with significant reductions in growth velocity as compared to controls in RCTs (pooled Mean difference -0.48 cm/year1; 95% CI -0.66–0.29 cm/year; I2 = 48%). We demonstrated that agents such as beclometasone, budesonide, and fluticasone were all individually associated with significant reductions in growth velocity compared to non-users. However, there was only sparse data available for other formulations such as ciclesonide (one trial), [29] flunisolide (two trials), [17, 21] or mometasone (one trial), [30] and the broad confidence intervals reflect considerable uncertainty about treatment effects.
Fig 1. Growth Velocity in RCTS at 12 months follow-up.
The magnitude of reduction in growth velocity in the meta-analysis of RCTs was similar to that seen with an observational study with 2.5 years’ follow-up in the Netherlands (Mean difference -0.44 cm/year; 95% CI -1.25 to 0.37 cm/year). [35] However, this study lacked power to detect a difference as there were only 66 children available for analysis.
Outcomes: Final Adult Height
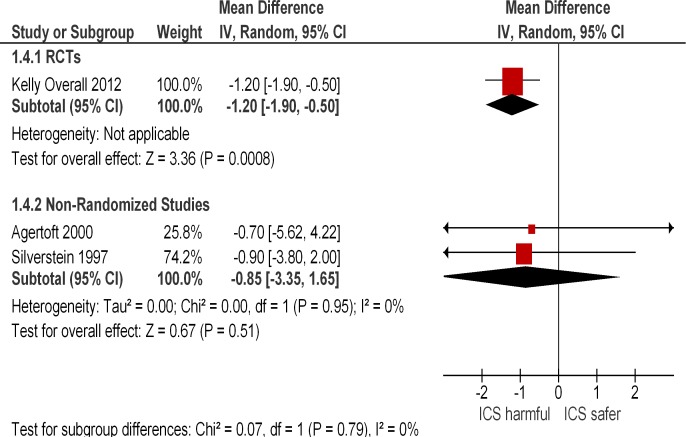
We identified one large long-term RCT of budesonide that captured final adult height in >90% of the originally enrolled children. [24] This trial found that four years of budesonide use was associated with a mean difference in final height of -1.2 cm (95% CI -1.9 − -0.5 cm) as compared to those on placebo.
We identified two observational studies that reported reduction of between 0.7–0.9 cm in final adult height. [34, 36] However, the sample sizes were small (leading to imprecise estimates with broad confidence intervals) and a meta-analysis of the two observational studies showed a mean difference of -0.85cm (95% CI -3.35–1.65, I2 = 0%). (Fig 2)
Fig 2. Final Adult Height, ICS users vs. non-users.
Subgroup Analysis Dose Response
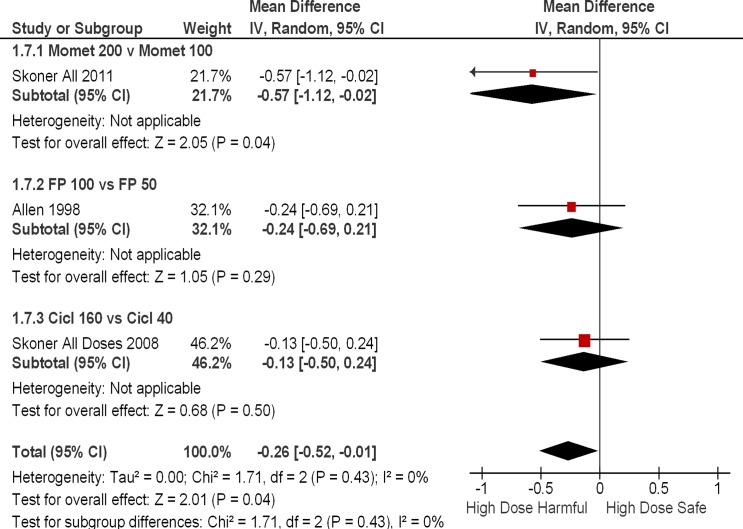
We identified 3 RCTS where we were able to conduct head to head comparisons of growth velocity between a higher dose and a lower dose of the same compound. [15, 29, 30] The trials were not powered to identify statistically significant differences in growth velocity with ascending ICS doses or different ICS formulations. However, we noted a consistent finding towards reduced growth velocities in the higher-dose arms relative to the lower-dose arms of the ICS, with a pooled estimate confirming greater harm (p = 0.04)with higher doses overall. (Fig 3).
Fig 3. Mean Differences in Growth Velocity with Direct Comparison of Higher Dose vs. Lower Dose.
Subgroup Analysis Effect of Different ICS Compounds
Visual inspection of the Forest plot (Fig 1) suggests that magnitude of growth reduction may differ amongst the ICS compounds. When we conducted subgroup testing for differences between fluticasone and other ICS, we found that budesonide was not significantly worse (p = 0.15), whereas beclometasone had a significantly greater adverse impact (p = 0.007).
This was corroborated in a direct head-to-head comparison where there was a significant reduction in growth velocity of 0.91cm/year (95% CI 0.63–1.20) with beclometasone 400 mcg daily compared to fluticasone 400 mcg daily. [18] In contrast, we identified divergent findings in direct randomized trials comparing budesonide against fluticasone. Acun’s trial of budesonide 400 mcg vs. fluticasone 250 mcg showed better growth velocity with budesonide (+0.37 cm/year), [14] whereas Ferguson’s study of budesonide 400 mcg vs fluticasone 200 mcg reported significant adverse impact on growth velocity with budesonide of -0.9 cm/year (SE 0.19) compared to fluticasone. [19]
Subgroup Analysis Change in Growth Velocity by Duration of Follow-Up
We identified findings in three RCTs where ICS appeared to have the greatest adverse impact on growth velocity in the first 12 months of the trial, whereas the reduction in growth velocity was less problematic at 24 or 36 months follow-up. [25, 27, 37] For instance, the large CAMP trial found a 1.7 cm/year reduction in growth velocity in the first 12 months for ICS versus control, but the difference in growth velocity was only about 0.1 cm at the 36 month follow-up. [37]
In contrast, a smaller trial did not demonstrate any variation in effect of ICS on growth velocity between the 12 and 24 months follow-up. [23]
Subgroup Analysis Age of Participants
Three trials reported the impact of ICS on growth for children of different age groups. [23–25] Both Jonasson et al. and Pauwels et al. found that reduction in growth velocity with ICS was more marked in those age ≤11 years as compared to older children. [23, 25] The large CAMP trial found that when compared to controls, ICS reduced final adult height by -1.9 cm (95% CI -3.2 to -0.6 cm) in children entering the trial at age 5–8 years, whereas the reduction in final adult height was -0.5 cm (95% CI -1.7 to -0.6 cm) in those entering at age 9–13 years. [24]
Reporting Biases
As there was substantial heterogeneity in the main analysis (Fig 1), we did not proceed to construct a funnel plot. The potential direction of reporting biases in the included studies remains unclear because we cannot judge conclusively if authors were more inclined to report significant adverse effects, or conversely, to play down any indication of harm. However, as mostof the studies involved commercial sponsors, we should be aware of the possibility of benefits being emphasized, whilst harms are presented in less detail.
Discussion
Our meta-analysis of 23 studies confirms that long-term use >12 months of ICS is associated with a slight reduction in growth velocity and final adult height in children. The reliability of these results is supported by the concordance between the findings of the randomized and non-randomized studies in direction and magnitude of effect. Moreover, we also identified dose-responsiveness from direct randomized comparisons where lower doses of ICS were less harmful than higher doses. Subgroup data indicates that adverse effects on growth may be more prominent in younger children as opposed to older children. Equally, in trials where children were followed-up for 24–36 months, we found that the reduction in growth velocity appeared to be most prominent in the first year of therapy, and the magnitude of the adverse effect seemed less problematic with time. This would tie in with the relatively limited diminution in final adult height, whereas we would have expected a cumulative decrement of several centimeters if children had continued to fall behind by 0.5 cm for every cumulative year of ICS therapy.
The effect size should be interpreted in the context of the average adult height of the population. For instance, in England, the average adult height is 175.3 cm for men, and 161.6 cm for women. [38] In an adult male, the reduction of around 1.2 cm would represent only a 0.68% absolute diminution (from 175.3 cm to 174.1 cm). This might be considered as an almost imperceptible or clinically insignificant change, particularly when weighted up against of the proven benefits of ICS therapy. We should be conscious too that the reported 1.2 cm diminution probably represents a worst case scenario where participants in the RCT were using Budesonide 400 mcg daily continuously for a three year period, whereas real-world have their doses titrated up and down according to disease severity. [39] In addition, adherence to therapy, and therefore adverse effects of medication, is likely to be greater in a RCT than a real-world setting.
There are a number of potential explanations for these findings. With longer durations of follow-up, the initial adverse consequences on growth velocity may become less problematic if the child has better-controlled asthma (thus enabling greater physical activity) and fewer exacerbations (associated with reduced need for acute short-courses of oral corticosteroids). Moreover, Pedersen has pointed out that children have different growth phases according to age. [6] Hence, susceptibility to the adverse effects of ICS seems to be less of a problem in those above the age of 10 years or more, which is a consistent finding in a number of trials.
Our findings are similar to those of recent Cochrane systematic reviews that have focused principally on data from RCTs. [40, 41] In a meta-analysis of 15 RCTs, Zhang et al. reported that ICS use was associated with significantly reduced growth velocity, mean difference of -0.48 cm/year as compared to controls. [41] In contrast to our review, Zhang et al. had selection criteria that included RCTs of <12 months, but excluded observational studies. Pruteanu et al. conducted a meta-analysis which demonstrated that higher ICS doses were associated with greater reductions in growth velocity (a finding that is similar to ours), but they were unable to identify significant differences amongst the ICS compounds. [40]
There are a number of limitations to our systematic review. Owing to the wide variety of possible combinations of drug compounds, inhaler devices, and daily doses, we felt that it would be scientifically inappropriate to draw conclusions through confounded indirect comparisons on dose or drug compound. Instead, we have limited our subgroup analyses to trials that performed direct comparisons of dose or different ICS, and we do not have consistent evidence that any specific compound is safer. The studies in our review date back 10–20 years and the findings may be less applicable to current-day children in the face of better nutrition and innovations in asthma management.
There are also a number of important limitations relating to validity of the primary studies. Owing to the inherent difficulties in measuring long-term adverse events, there is potential for considerable risk of bias (particularly from attrition) within this dataset. Moreover, the vast majority of the trials were sponsored by the pharmaceutical industry, and the study methodology (e.g. when choosing interventional dose and device) may have been designed towards obtaining favourable results for the sponsored product. Equally, we should be wary of potential reporting biases where positive findings are emphasized whereas negative ones are downplayed. This diversity amongst trial sponsors may have contributed towards the divergent findings and increased heterogeneity in results.
Lack of information and inaccurate interpretation of the benefits and harms of ICS may hinder medication adherence in patients with asthma. [42] Although the evidence may be imperfect, our systematic review helps to address some of the concerns and uncertainty surrounding the exact magnitude of growth reduction with ICS use. There is some indication of potential differences between ICS formulations, with beclometasone possibly having a greater adverse impact on growth. Prescribers and patients should aim for the lowest effective dose, particularly if initiating ICS therapy for patients in the younger age groups who may be more susceptible to adverse effects.
Supporting Information
(DOCX)
(PDF)
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
This manuscript presents a systematic review commissioned by Asthma UK (AUK-PG-2012-181),and we are grateful to the Asthma UK Research team for their guidance. The views expressed in this paper are those of the authors.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This manuscript presents a systematic review commissioned by Asthma UK (AUK-PG-2012-181), www.asthma.org.uk, and the authors are grateful to the Asthma UK Research team for their guidance. The views expressed in this paper are those of the authors, and the funders had no role in the data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. British-Thoracic-Society/Scottish-Intercollegiate-Guidelines-Network. British Guideline on the Management of Asthma. Thorax. 2008;63 Suppl 4:iv1–121. 10.1136/thx.2008.097741 [DOI] [PubMed] [Google Scholar]
- 2. National-Asthma-Education-and-Prevention-Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. [DOI] [PubMed] [Google Scholar]
- 3. Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: A systematic review and meta-analysis. Arch Intern Med. 1999;159(9):941–55. [DOI] [PubMed] [Google Scholar]
- 4. Wong CA, Walsh LJ, Smith CJ, Wisniewski AF, Lewis SA, Hubbard R, et al. Inhaled corticosteroid use and bone-mineral density in patients with asthma. Lancet. 2000;355(9213):1399–403. [DOI] [PubMed] [Google Scholar]
- 5.James-Lind-Alliance. Research activity following Asthma Treatment Uncertainty Priority Setting Exercise 2010 [5 January 2015]. Available from: http://www.lindalliance.org/pdfs/Asthma/Post_JLA_priorities_Asthma_January_2010.pdf.
- 6. Pedersen S. Clinical safety of inhaled corticosteroids for asthma in children: an update of long-term trials. Drug Saf. 2006;29(7):599–612. [DOI] [PubMed] [Google Scholar]
- 7. Skoner JD, Schaffner TJ, Schad CA, Kwon AY, Skoner DP. Addressing steroid phobia: improving the risk-benefit ratio with new agents. Allergy Asthma Proc. 2008;29(4):358–64. 10.2500/aap.2008.29.3132 [DOI] [PubMed] [Google Scholar]
- 8. Loke YK, Golder SP, Vandenbroucke JP. Comprehensive evaluations of the adverse effects of drugs: importance of appropriate study selection and data sources. Ther Adv Drug Saf. 2011;2(2):59–68. 10.1177/2042098611401129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loke YK, Price D, Herxheimer A. Chapter 14: Adverse effects In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons; 2008. [Google Scholar]
- 10. Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ. 2007;176(8):1091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins JPT, Deeks JJ, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons; 2008. [Google Scholar]
- 13. Higgins JPT, Deeks JJ, Altman DG. Chapter 16. Special topics in statistics In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons; 2008. [Google Scholar]
- 14. Acun C, Tomac N, Ermis B, Onk G. Effects of inhaled corticosteroids on growth in asthmatic children: A comparison of fluticasone propionate with budesonide. Allergy and Asthma Proceedings. 2005;26 (3):204–6. [PubMed] [Google Scholar]
- 15. Allen DB, Bronsky EA, LaForce CF, Nathan RA, Tinkelman DG, Vandewalker ML, et al. Growth in asthmatic children treated with fluticasone propionate. Fluticasone Propionate Asthma Study Group. Journal of Pediatrics. 1998;132(3 Pt 1):472–7. [DOI] [PubMed] [Google Scholar]
- 16. Becker AB, Kuznetsova O, Vermeulen J, Soto-Quiros ME, Young B, Reiss TF, et al. Linear growth in prepubertal asthmatic children treated with montelukast, beclomethasone, or placebo: A 56-week randomized double-blind study. Annals of Allergy, Asthma and Immunology. 2006;96 (6):800–7. [DOI] [PubMed] [Google Scholar]
- 17. Bensch GW, Greos LS, Gawchik S, Kpamegan E, Newman KB. Linear growth and bone maturation are unaffected by 1 year of therapy with inhaled flunisolide hydrofluoroalkane in prepubescent children with mild persistent asthma: A randomized, double-blind, placebo-controlled trial. Annals of Allergy, Asthma and Immunology. 2011;107 (4):323–9. 10.1016/j.anai.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 18. De Benedictis FM, Teper A, Green RJ, Boner AL, Williams L, Medley H. Effects of 2 inhaled corticosteroids on growth: Results of a randomized controlled trial. Archives of Pediatrics and Adolescent Medicine. 2001;155 (11):1248–54. [DOI] [PubMed] [Google Scholar]
- 19. Ferguson AC, Van Bever HP, Teper AM, Lasytsya O, Goldfrad CH, Whitehead PJ. A comparison of the relative growth velocities with budesonide and fluticasone propionate in children with asthma. Respiratory Medicine. 2007;101 (1):118–29. [DOI] [PubMed] [Google Scholar]
- 20. Garcia Garcia ML, Wahn U, Gilles L, Swern A, Tozzi CA, Polos P. Montelukast, compared with fluticasone, for control of asthma among 6- to 14-year-old patients with mild asthma: the MOSAIC study. Pediatrics. 2005;116(2):360–9. [DOI] [PubMed] [Google Scholar]
- 21. Gillman SA, Anolik R, Schenkel E, Newman K. One-year trial on safety and normal linear growth with flunisolide HFA in children with asthma. Clinical Pediatrics. 2002;41 (5):333–40. [DOI] [PubMed] [Google Scholar]
- 22. Gradman J, Wolthers OD. A randomized trial of lower leg and height growth in children with asthma treated with inhaled budesonide from a new dry powder inhaler. Pediatric allergy and immunology: official publication of the European Society of Pediatric Allergy and Immunology. 2010;21 (1 Pt 2):e206–12. [DOI] [PubMed] [Google Scholar]
- 23. Jonasson G, Carlsen KH, Jonasson C, Mowinckel P. Low-dose inhaled budesonide once or twice daily for 27 months in children with mild asthma. Allergy: European Journal of Allergy and Clinical Immunology. 2000;55 (8):740–8. [DOI] [PubMed] [Google Scholar]
- 24. Kelly HW, Sternberg AL, Lescher R, Fuhlbrigge AL, Williams P, Zeiger RS, et al. Effect of inhaled glucocorticoids in childhood on adult height. New England Journal of Medicine. 2012;367 (10):904–12. 10.1056/NEJMoa1203229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. Early intervention with budesonide in mild persistent asthma: A randomised, double-blind trial. Lancet. 2003;361 (9363):1071–6. [DOI] [PubMed] [Google Scholar]
- 26. Price JF, Russell G, Hindmarsh PC, Weller P, Heaf DP, Williams J. Growth during one year of treatment with fluticasone propionate or sodium cromoglycate in children with asthma. Pediatric Pulmonology. 1997;24 (3):178–86. [DOI] [PubMed] [Google Scholar]
- 27. Roux C, Kolta S, Desfougeres JL, Minini P, Bidat E. Long-term safety of fluticasone propionate and nedocromil sodium on bone in children with asthma. Pediatrics. 2003;111 (6 Pt 1):e706–13. [DOI] [PubMed] [Google Scholar]
- 28. Simons FE. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. Canadian Beclomethasone Dipropionate-Salmeterol Xinafoate Study Group. New England Journal of Medicine. 1997;337(23):1659–65. [DOI] [PubMed] [Google Scholar]
- 29. Skoner DP, Maspero J, Banerji D, Ahrens R, Aldrey O, Atkinson D, et al. Assessment of the long-term safety of inhaled ciclesonide on growth in children with asthma. Pediatrics. 2008;121 (1):e1–e14. [DOI] [PubMed] [Google Scholar]
- 30. Skoner DP, Meltzer EO, Milgrom H, Stryszak P, Teper A, Staudinger H. Effects of inhaled mometasone furoate on growth velocity and adrenal function: A placebo-controlled trial in children 49 years old with mild persistent asthma. Journal of Asthma. 2011;48 (8):848–59. 10.3109/02770903.2011.604883 [DOI] [PubMed] [Google Scholar]
- 31. Skoner DP, Szefler SJ, Welch M, Walton-Bowen K, Cruz-Rivera M, Smith JA. Longitudinal growth in infants and young children treated with budesonide inhalation suspension for persistent asthma. Journal of Allergy and Clinical Immunology. 2000;105 (2 I):259–68. [DOI] [PubMed] [Google Scholar]
- 32. Turpeinen M, Pelkonen AS, Nikander K, Sorva R, Selroos O, Juntunen-Backman K, et al. Bone mineral density in children treated with daily or periodical inhaled budesonide: The Helsinki early intervention childhood asthma study. Pediatric Research. 2010;68 (2):169–73. 10.1203/00006450-201011001-00329 [DOI] [PubMed] [Google Scholar]
- 33. Verberne AA, Frost C, Roorda RJ, van der Laag H, Kerrebijn KF. One year treatment with salmeterol compared with beclomethasone in children with asthma. The Dutch Paediatric Asthma Study Group. American Journal of Respiratory & Critical Care Medicine. 1997;156(3 Pt 1):688–95. [DOI] [PubMed] [Google Scholar]
- 34. Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. New England Journal of Medicine. 2000;343 (15):1064–9. [DOI] [PubMed] [Google Scholar]
- 35. Merkus PJ, van Essen-Zandvliet EE, Duiverman EJ, van Houwelingen HC, Kerrebijn KF, Quanjer PH. Long-term effect of inhaled corticosteroids on growth rate in adolescents with asthma. Pediatrics. 1993;91(6):1121–6. [PubMed] [Google Scholar]
- 36. Silverstein MD, Yunginger JW, Reed CE, Petterson T, Zimmerman D, Li JTC, et al. Attained adult height after childhood asthma: Effect of glucocorticoid therapy. Journal of Allergy and Clinical Immunology. 1997;99 (4):466–74. [DOI] [PubMed] [Google Scholar]
- 37. The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. The New England journal of medicine. 2000;343(15):1054–63. [DOI] [PubMed] [Google Scholar]
- 38.Office-of-National-Statistics-UK. ‘Average’ Briton highlighted on UN World Statistics Day 2010 [6 January 2015]. Available from: http://www.ons.gov.uk/ons/about-ons/get-involved/events/events/un-world-statictics-day/-average--briton-highlighted-on-un-world-statistics-day.pdf.
- 39. Bush A. Inhaled corticosteroid and children's growth. Archives of disease in childhood. 2014;99(3):191–2. 10.1136/archdischild-2012-303105 [DOI] [PubMed] [Google Scholar]
- 40. Pruteanu AI, Chauhan BF, Zhang L, Prietsch SO, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: dose-response effects on growth. The Cochrane database of systematic reviews. 2014;7:Cd009878 10.1002/14651858.CD009878.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang L, Prietsch SO, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: effects on growth. The Cochrane database of systematic reviews. 2014;7:Cd009471 10.1002/14651858.CD009471.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rossi GA, Cerasoli F, Cazzola M. Safety of inhaled corticosteroids: Room for improvement. Pulmonary Pharmacology & Therapeutics. 2007;20(1):23–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(PDF)
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.