Abstract
Rotator cuff (RC) disease is an extremely common condition associated with shoulder pain, reduced functional capacities and impaired quality of life. It primarily involves alterations in tendon health and mechanical properties that can ultimately lead to tendon failure. RC tendon tears induce progressive muscular changes that negatively impact surgical reparability of the RC tendons and clinical outcomes. At the same time, a significant base of clinical data suggests a relatively weak relationship between RC integrity and clinical presentation, emphasizing the multifactorial aspects of RC disease. This review aims to summarize the potential contribution of peripheral, spinal and supraspinal neural factors that may: (i) exacerbate structural and functional muscle changes induced by tendon tear, (ii) compromise the reversal of these changes during surgery and rehabilitation, (iii) contribute to pain generation and persistence of pain, iv) impair shoulder function through reduced proprioception, kinematics and muscle recruitment, and iv) help to explain interindividual differences and response to treatment. Given the current clinical and scientific interest in peripheral nerve injury in the context of RC disease and surgery, we carefully reviewed this body of literature with a particular emphasis for suprascapular neuropathy that has generated a large number of studies in the past decade. Within this process, we highlight the gaps in current knowledge and suggest research avenues for scientists and clinicians.
Keywords: Shoulder, rotator cuff tear, pain, muscle, nerve, spinal cord, brain
Introduction
The human shoulder complex exhibits a unique anatomical design to allow a wide range of motion at various speed and force levels. The shoulder joint complex has an unstable bony configuration secured by connective tissues and dynamic stabilizers (rotator cuff muscles) controlled by a sophisticated neuromuscular system156; 160. As a consequence, shoulder structures, particularly rotator cuff (RC) tendons, are prone to various injuries and degenerative disorders19; 120. RC tendon tears are common in the general population103; 122 and can lead to shoulder pain, impaired functional capacities, and reduced quality of life87; 163.
RC tendon tears are not necessarily associated with pain or patient-reported loss of shoulder function90; 163; 164, however, asymptomatic patients may develop symptoms in a relatively short period of time106. Symptomatic patients usually undergo surgery when nonoperative and pharmacological options have been exhausted111; 129. Surgical management decisions are mainly driven by patients’ pain, disability, and functional requirements rather than the severity of local-tissue damage15. In the short-term, nonoperative treatment may be effective in a fraction of patients35; 50; 75 but tissue damage and symptoms may progress over time90; 106; 163, further limiting surgery and rehabilitation78; 91; 95; 97. RC tendon repair is not universally successful, ~25% of repairs fail to reestablish the integrity of the rotator cuff97 (up to 70% in massively retracted tears36) and patient-reported improvements are limited78; 97. Pre-operative factors such as age, chronicity, and severity of muscle-tendon unit impairments have been repeatedly associated with higher retear-rates and poorer clinical outcomes78; 97. Paradoxically, two recent meta-analyses97; 129 suggested that patients with intact repairs might not have significant differences in symptom improvement compared to patients with recurrent tears. Another major concern is that muscle impairments do not seem to reverse, even when repair is intact and function improved at follow-up26.
During the past decades, RC disease has been extensively investigated within the framework of tendon pathophysiology, tendon-to-bone healing, and muscular changes following tendon tear30;71. A smaller set of studies have investigated how peripheral, spinal, and central neural factors are likely to contribute to muscle-tendon unit changes, impaired shoulder function, and responses to treatment. Expanding our knowledge, or at least considering the potential involvement of both peripheral and central nervous system is critical to improve our understanding of RC disease and our ability to appropriately intervene along the continuum of RC injury processes. Therefore, this review aims to scrutinize and highlight the gaps in current knowledge regarding the nervous system that may be altered in patients with RC disease from the peripheral receptors to the brain and from the brain to the neuromuscular junction. We summarized how these factors may (i) exacerbate structural and functional muscle changes induced by tendon tear, (ii) compromise the reversal of these changes during surgery and rehabilitation, (iii) contribute to pain generation and persistence iv) impair shoulder function by impairing shoulder proprioception, kinematics and muscle recruitment, iv) contribute to explain interindividual differences in symptoms and response to treatment. Given the current and lively interest for peripheral nerves injuries in the context of RC disease and surgery, we carefully reviewed this body of literature with a particular emphasis for suprascapular nerve injury that has generated a large number of studies in the past decade. Within this process, we highlighted the gaps in current knowledge and suggested research avenues for scientists and clinicians.
Proprioceptors and Related Spinal Reflexes
Shoulder movements and positional changes induce a deformation of tissues surrounding joints, including skin, muscles, tendons, fascia, joint capsules, and ligaments24; 27; 47; 121; 143; 155. All these tissues are innervated by mechanically sensitive receptors termed proprioceptors that relay information to the central nervous system regarding movement, position, and forces exerted on shoulder structures (e.g. muscle spindles, Golgi tendon organs, Ruffini endings Pacinian and Meissner corpuscles). The distribution and the function of proprioceptors in shoulder joints and soft tissue have been investigated in both animal and human studies 40; 51; 138; 140; 143; 146; 155).
Glenohumeral joint and ligaments receptors probably play a minor role in shoulder proprioception121 as illustrated by the small proprioceptive deficit observed after shoulder arthroplasty21. However they may act as limit detectors triggering protective and synergistic reflex muscle activity during movement27; 46; 64; 140; 148; 157. In RC muscles and tendons, a large concentration of muscles spindles and Golgi tendon organs have been demonstrated in rabbits and rats3; 22; 104; 165 but no human data exist. Current theory suggests that muscle spindles are the most important proprioceptors, especially during movement121. They also play a critical role in regulating muscle contraction via spinal reflexes, that are essential for joint stability and accurate motor control100. Golgi tendon organs are equally important proprioceptors, signaling information about force and mass and are also involved in the regulation of muscle contraction121.
The effect of tendon disruption on muscle spindles and Golgi tendon organs has been studied in a limited number of animal experiments concerning hind limb muscles only. Following tenotomy, muscle shortening and changes in the surrounding extrafusal tissue modify the morphology of muscle spindles that become slack and distorted168. In the chronically tenotomized muscle, atrophy of intrafusal fibers, degeneration of supplying axons and fibrotic thickening of the capsule have been reported67; 94 Functionally, acute tenotomy decreases muscle spindle discharge56; 159; 168 but interestingly, responsiveness of muscle spindles from the chronically tenotomized muscle has been shown to increase56; 57; 168. Shortening of intrafusal fibers, increased preliminary stretch caused by kinking of intrafusal fibers, change in passive mechanical properties or increased sensitivity of spindles have been subsequently proposed as potential explanations for this phenomenon. These increases in muscle/tendon afferent outflow have also been suggested to result from nonproprioceptive discharge57; 77. Increase in the amplitude of the monosynaptic reflex has also been repeatedly observed in the chronically tenotomized muscle10; 61; 74; 159, suggesting adaptive changes in motoneurons excitability consistent with the decrease in muscle mechanical loading98. In the Golgi tendon organs, tenotomy also induces morphological changes, but the physiological consequences remain to be investigated67. To the best of our knowledge, only one study related to proprioceptors function in RC tendon tear have been conducted and reported that experimentally-induced inflammation within rabbit RC sensitize and increase the firing of mechanical receptors165.
Based on the findings of the aforementioned studies, it is reasonable to speculate that RC tendon tear is associated with structural and functional alterations of proprioceptors. Either reduced or inconsistent proprioceptive information from the injured muscle-tendon unit and altered muscle reflex activity may impair shoulder proprioception and contribute to impaired kinematics and muscle recruitment (see also section “Impact of RC Disease on Shoulder Muscle Activity”). Finally, the effects of tendon repair on the structure and the function of proprioceptors remain entirely unknown. Further experimentations are therefore required to assess the relative contribution of these mechanisms to anatomical and clinical impairments associated with RC disease.
Central Processing of Proprioceptive information
Proprioceptive information from the shoulder and more broadly from the upper limb are conveyed via the spinothalamic tracts and relayed to the somatosensory cortex where it is referred to a central body map allowing the conscious awareness of arm position and movement in space. Unconscious proprioceptive tracts (i.e. spinocerebellar tracts, projecting in the ipsilateral cerebellum) and the cervical propriospinal system are also involved the coordination movements involving multiple joints of the arm121; 124.
Measurement of errors in the perceived position, movement detection latency, or ability to reproduce a determined force level can be used to globally assess shoulder proprioception6; 85; 107; 125; 131. A large fraction of studies involving shoulder proprioception assessment have been conducted in patients with shoulder instability6; 107; 125. In the overhead athlete with isolated infraspinatus atrophy caused by SSN compression, impaired sense of movement associated with different brain activation pattern has been reported suggesting an important contribution of RC muscle to shoulder proprioception131. Decreased sense of movement88; 4; 130 and a tendency to overestimate the target during force reproduction tests89 have been reported in patients with RC tendinopathy but no data exist in patients with RC tendon tears. In conditions such as knee disorders, functional brain MRI demonstrated reduced activation of sensorimotor cortical areas and increased activation in proprioception-related brain regions, however no data exist in patients with RC disease69. In healthy subjects, transcranial magnetic stimulation (TMS) combined with peripheral nerve stimulation has been used to assess the modulation of the propriospinal system124 of the upper limb which is a important determinant for synergies between forearm, hand, and shoulder muscles. This system remains to be investigated in patients with RC disease.
Proprioception has been insufficiently assessed in patients with RC disease despite its recognized importance in other musculoskeletal conditions121. Therefore, further studies are required to assess proprioception in patients with RC disease and patients who have undergone RC reconstruction.
Nociceptors, Peripheral and Central Pain Processing
Nociceptors are high threshold receptors that detect signals from damaged tissue or tissue on the verge of damage. They can be found in the shoulder, skin, muscles, joints, soft-tissue, and bone32; 40; 41; 51; 143; 148; 149. RC disease is associated with local-tissue damage and inflammation within the RC and surrounding structures, which release a variety of substances that sensitize nociceptors by decreasing their activation threshold (peripheral sensitization) resulting in hyperalgesia at the site of injury23; 34; 41. Prolonged release of neuropeptides by nociceptive afferent fibers at the dorsal horn may sensitize nocineurons and cause long-term changes in pain processing at the spinal level and higher centers that result in pain hypersensitivity within, but also outside the original zone of injury162. As previously observed in other musculoskeletal conditions53, sensory abnormalities have been observed on the injured but also on the non-injured side of patients with RC disease, illustrating the involvement of central mechanisms39; 48; 55. Interestingly, patients with a RC tendon tear and signs of central sensitization have been shown to have worse clinical outcomes after surgery48. Pain may have profound effects on motor behavior mediated at various level of the nervous system and impact on numerous motor parameters such as reflex amplitude, muscle activity, kinematics, movement planning and brain activation5 (see section “Shoulder Muscle Activity and Kinematics”).
Pain remains poorly characterized in patients with RC disease, but the use of existing pain assessment tools and the development of biological markers have the potential for enhancement in our understanding of pain in RC disease24. Interindividual differences in the magnitude of these changes and their persistence after local-tissue damage has healed may explain differences in clinical presentation and response to therapies24.
Motor Nerves and Neuromuscular Junction
The motor innervation of the RC muscles is achieved by nerves emerging from the posterior and the superior trunks of the brachial plexus, all originating from the C5–C6 cervical roots and C4 nerve root in some individuals2; 80; 136; 166. The architecture and the high mobility of the shoulder complex predispose nerves to various dynamic or static compressive and/or traction injuries147. Cervical radiculopathy, brachial plexopathy and peripheral nerve trunk injuries are potential comorbidities of RC tendon tear52; 135. Motoneuron damage immediately reduces muscle activation and induces progressive muscle changes proportional to the severity of nerve injury145. Over time, the muscle tissue can virtually disappear while connective tissue and fat accumulate84 as recently illustrated in the human supraspinatus14; 79; 101. A particular interest has been placed in the suprascapular nerve (SSN) since it innervates the most affected muscles in RC disease (i.e. supraspinatus and infraspinatus) and because it is particularly prone to entrapment105; 135. SSN injury can cause shoulder weakness and pain that overlap with the signs of RC disease105.
SSN injury associated with RC tendon tear, Anatomical Studies
SSN injury is possible given the surgical manipulation of previously retracted muscle(s) during RC repair procedures133. In vivo studies have shown that lateral advancement during supraspinatus repair initiates a stretch of the SSN44; 161. The main trunk of the SSN may be prone to damage but also its smaller branches may be injured44. Following a similar principle, medial retraction of the supraspinatus and/or infraspinatus muscles caused by tendon tear has been suggested to place excessive traction on the SSN and to promote compressive injuries at the suprascapular and/or spinoglenoid notch. In cadavers, supraspinatus tenotomy changes the course of the SSN1; 93. Various anatomical variations have also been suggested to promote suprascapular entrapment (e.g. deep and narrow shaped suprascapular notch60; 108; 118; 123; 150, shape/ossification of the superior transverse scapular ligament (STSL)117; 119; 150, arrangements of blood vessels119; 167, configuration of the fascia securing the suprascapular nerve to the supraspinatus fossa28, close relationship of the subscapularis muscle7). However, the incidence of these anatomical predispositions in patients with a RC tendon tear and concomitant neuropathy has never been studied. In addition, the potential occurrence of dynamic stretch/compressive strain of the SSN promoted by biomechanical and kinematic impairments in patients with RC disease should not be neglected20; 116.
These anatomical studies must be acknowledged as the original incentive for investigating SSN function in RC tendon tears105. However they have not addressed the question of whether these changes are physiologically relevant and whether smaller nerve branches are also likely to be insulted clinically.
Prevalence of SSN injury in patients with RC tendon tear
In patients, diagnosis of SSN injury is confirmed by electrodiagnosis that combined needle electromyography (EMG) and nerve conduction studies (NCS). Various clinical reports, retrospective studies, and prospective studies regarding the prevalence and the impact of peripheral nerve injuries before and/or after surgery have been published (see Table I for supporting material).
Table I.
Prevalence of suprascapular neuropathy in patients with rotator cuff tears before and/or after surgical repair.
| Tear etiology | Tear severity | Time of Electrodiagnosis | Prevalence of Suprascapular neuropathy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| Studies | n | Trauma | Chronic | Mild/Partial | Massive/Full | Pre surgery | Post surgery | Pre-Post surgery | Time from surgery or trauma (months) | Before surgery | After surgery |
| Ha’eri et al. 198174 | 5 | / | / | / | / | 0 | 5 | 0 | / | / | 0% (0/5) |
|
| |||||||||||
| Kaplan et al. 1984101 | 6 | 6 | 0 | 5 | 1 | 5 | 1 | 1 | / | 100% (5/5) | 100% (1/1) |
|
| |||||||||||
| Zanotti et al. 1997231 | 10 | 0 | 10 | 0 | 10 | 1 | 10 | 1 | (24–36) | / | 10% (1/10) |
|
| |||||||||||
| Goutallier et al. 199664 | 24 | / | / | / | / | 19 | 24 | 19 | 8.5 | / | ~21% (4/19) |
|
| |||||||||||
| Hoellrich et al. 200588 | 9 | 0 | 9 | / | / | 9 | 17 (6–28) | / | 0% (0/9) | ||
|
| |||||||||||
| Vad et al. 2003211 | 25 | 8 | 17 | 17 | 8 | 25 | 0 | 0 | / | 8% overall (2/25) | / |
|
| |||||||||||
| Mallon et al. 2006131 | 8 | 0 | 8 | 0 | 9 | 8 | 4 | 4 | 6 | 100% (8/8) | 0 % (0/6) |
|
| |||||||||||
| Costouros et al. 200726 | 26 | 19 | 7 | 0 | 26 | 26 | 6 | 6 | 8 (3–12)(trauma) 6 (surgery) |
27% (7/26, all trauma) | 0% (0/6) |
|
| |||||||||||
| Boykin et al. 201119 | 44 | / | / | 6 | 38 | 44 | 0 | 0 | / | 0% (partial tear) 60% (massive tear) |
/ |
|
| |||||||||||
| Shi et al. 2013189 | 60 | / | / | SS (14) IS (15) |
SS (46) IS (30) |
60 | 0 | 0 | / | 29% overall (26/87) 50 % (partial SS tear) 54% (full SS tear/minor retraction) 17% (full SS tear/>5cm retraction) 20% (partial IS tear) 71% (full IS tear/minor retraction) 23 % (full SS tear/>5cm retraction) |
/ |
|
| |||||||||||
| Collin et al. 201424 | 49 | 24 | 25 | 0 | 49 | 49 | 0 | 0 | / | 2% | |
SS, Supraspinatus; IS, Infraspinatus; /, undocumented or unavailable information.
Following tendon repair, a low risk of iatrogenic nerve injury has been reported25; 49; 59; 169 but comparisons of pre- and post-surgery EMG/NCS data have not been systematically performed18; 42; 92; 169. Goutallier et al.42 achieved such comparisons in the largest sample of patients and findings confirmed the low incidence of SSN dysfunction after RC repair previously reported. In these studies, the long time delay between surgery and electrodiagnosis may have allowed nerve recovery. Some case reports also suggested that supraspinatus repair may restore the normal course of the SSN, therefore reducing nerve strain and allowing its recovery but larger studies are required to prove this concept18; 92. The large undocumented occurrence of traumatic events that could have caused direct nerve injury often limits data interpretation (see Table I).
Studies suggesting a greater prevalence of SSN motor neuropathy in patients with RC tendon tears involve important recruitment bias. In the studies of Boykin et al.12 and Shi et al.134, patients were sent for electrophysiological examination for persistent pain and/or severe muscle changes; Similarly, Costouros et al.18 and Mallon et al.92 selected patients with severe muscle atrophy and fatty infiltration. While some studies are consistent with a higher risk of SSN injury in severely versus slightly retracted tears13; 92, the study of Shi et al.134 involving a larger spectrum of RC tears severity does not support this hypothesis. These data thus call into question the concept of SSN injury as a direct consequence of muscle retraction. Prospective and carefully conducted studies indicate a rare occurrence of isolated motor SSN injury in patients with RC tendon tears, even in massive and/or traumatic RC tendon tear16; 153. Within the largest patient series in this topic area16, peripheral neuropathy was found in 12% of patients and only one patient exhibited positive signs of SSN injury.
Heterogeneous and incompletely documented EMG/NCS methods are also major limitations when comparing results between these studies13; 92; 134; 153. Some categorize EMG findings based upon the isolated or combined occurrence of positive EMG signs134 while others use graded scoring based on semi-quantitative assessments of EMG abnormalities16; 18; 134. Regarding NCS, some compare latencies to previously published values13; 16 and/or to the contralateral side16; 18; 153 while others compared latencies of patients with positive and negative EMG findings13. Severe retraction, ultrastructural muscle changes and/or non-uniform denervation may also complicate EMG/NCS in RC muscles8. US imaging113 and multisite EMG assessments may help overcome some of these limitations. Standardization of procedure and quantification methods170 must be pursued to enhance the sensitivity of EMG. Recent progress in nerve imaging techniques81; 115; 144 may also allow enhancement of our ability to study peripheral nerve injuries in vivo.
Relative Contribution of Denervation to Muscle Changes associated with RC Tendon Tears: Clinical Data and Animal Models
In humans, imaging techniques cannot discriminate muscle impairments related to tendon tear or denervation when they happen simultaneously8. EMG/NCS is limited and an objective test such as nerve biopsy cannot be reasonably performed in patients. Consequently various animal models of RC disease and/or nerve injury have been developed to understand cellular and molecular mechanism underlying muscle changes30.
In rabbits and rodents, tenotomy associated with full nerve transection has been shown to produce severe atrophy and fatty infiltration and these data are frequently used to support the role of SSN injury in human RC muscle changes65; 66; 72; 82; 126. However if nerve injury occurs in humans, denervation is more likely to be incomplete with higher capacity for recovery. In rabbits, fatty infiltration has been repeatedly observed following isolated supraspinatus tenotomy126; 128; 152 even in absence of retraction151, and independent from denervation38, further clouding the cause-effect relationship between nerve injury and fatty infiltration. There are many transcriptional pathways that control various aspects of the adipogenic, fibrogenic and myogenic programs68. However, distinct pathways may be triggered by RC tenotomy or denervation as recently reported in rodents65; 82. Although small animal models have a limited ability to replicate human RC disease, previously developed transgenic mice associated with tendon and/or nerve injury have great potential to further understand RC disease pathophysiology72; 83. Increased availability in human tissue may also allow further investigations of muscle impairments and comparison of data obtained in animal models.
Direct Consequences of RC Tendon Tears on Nerves and Neuromuscular Junction
Studies that investigated the consequences of tendon tear on motor nerve and neuromuscular junction provide equivocal results61. These effects have been investigated in animal models of RC tendon tears in rabbit only. Signs of degenerative histological changes in the subscapular nerve after tenotomy of the subscapularis muscle have been reported126 but characteristics of these nerve abnormalities remain unclear. Gayton et al.38 reported that motor endplates were not significantly affected after tenotomy in rabbits; confirmation is required given the small sample size of this work (n=4). A critical point that has not been addressed is whether neuromuscular junctions are altered in patients with isolated RC tendon tears.
Sensory Nerves
Sensory nerve injuries have received less interest than the motor neuropathies discussed above. However the RC and surrounding structures receive sensory innervation from numerous sensory nerve branches29; 158 that are equally susceptible to injury. Injury within a peripheral nerve trunk induces a local inflammatory response that causes changes in afferent fibers and in the central nervous system and may lead to neurogenic pain (see section “Nociceptors and Pain Mediating Systems” and Ref.31 for more details). Damage to afferent fibers may also contribute to the impairment of the transduction of proprioceptive information. SSN block has demonstrated effectiveness in the management of post-operative pain63 and pulsed radiofrequency modulation has been reported to provide promising long-lasting pain relief in experimental models154 and in patients with shoulder pain62. These data highlight the important contribution of shoulder nerves in the transmission of nociceptive information in patients with RC disease, making them important targets for shoulder pain management63; 154.
Shoulder Muscle Activity and Kinematics
Alterations in shoulder muscle activity and kinematics of the glenohumeral and scapulothoracic joints have been widely reported in patients with RC disease86; 96; 127. One potential contributing factor may be that patients with symptomatic tears display different motor control patterns during movement compared to asymptomatic patients127.
Kelly et al.70 observed that symptomatic patients retain supraspinatus and infraspinatus activity despite tendon tears but are unable to activate intact deep muscles (i.e. subscapularis) as efficient co-contractors and that they may preferentially rely on periscapular muscles during elevation. These results have been partially reproduced by Cordasco et al.17 and suggest that symptomatic patients fail to develop alternative muscle activation strategies to compensate for weakened RC muscles and the resulting altered shoulder biomechanics. Importantly, they suggest that RC muscles may continue to be activated despite tendon damage. Shinozaki et al.137 recently used positron emission tomography with fluorodeoxyglucose (FDG)76; 112 to assess shoulder muscle activity differences between asymptomatic and symptomatic patients. They observed increased trapezius activity and lower deltoid activity in the symptomatic group but no differences in RC muscles activity compared to asymptomatic patients. This technique appears promising but further developments are required, particularly regarding quantification.
An important issue is whether different muscle activity patterns observed in symptomatic patients are the cause or the result of pain, or both. Experimentally-induced pain has been shown to increase activity in the antagonist muscle during abduction (i.e. latissimus), probably in an attempt to limit the compression of painful subacromial structures. Similar adaptations have been observed in patients with massive RC tendon tears17; 142. Masking pain may reduce these protective mechanisms and further promote local-tissue damage. Stackhouse et al.141 reported that pain reduced shoulder strength in external rotation in association with a decrease in voluntary activation using the twitch interpolation technique102. Sole et al.139 also pointed out that motor adaptation to acute pain may be individual- and task-specific58. Given the acute nature of experimentally induced pain5, precautions should be taken when trying to generalize these results in patients with chronic RC disease.
In patients with RC disease, pain reduction has been shown to improve glenohumeral motion and to reduce scapular contribution during arm elevation132. Dramatic increases of peak torque and power have also been reported9. Surprisingly, when assessed with isometric contractions, pain reduction has been shown to have no relevant effect on shoulder strength33; 114 suggesting that pain-related motor impairments may be particularly visible during movement.
These experiments observed muscle activity pattern changes under pathophysiologic and simulated conditions, however, the relative contribution of muscle-tendon unit impairments, biomechanical abnormalities, pain, impaired proprioception, and deterioration of motor control in shoulder dyskinesia and weakness remain unclear. Poor coping strategies in muscle activation patterns in response to biomechanical changes and pain may contribute to worsen local-tissue damage and pain. Interestingly, motor adaptations may also differ between individuals, in particular between symptomatic and asymptomatic patients.
Motor Cortical Changes
As in various other conditions, RC disease may induce structural and functional changes in the motor cortex that could partly explain changes in motor control and affect muscle activation. Little is known about the cortical organization of motoneurons related to proximal muscles of the arm, and even less regarding RC muscles99. Functional MRI has been previously used but is not discriminant for motor cortical mapping of individual RC muscles73. The output of the primary motor cortex (M1) can be objectively measured by motor evoked potentials (MEPs) elicited by TMS, providing direct insight on the cortical representation and the function of the corticospinal tracts45. Mapping of the infraspinatus muscle has been recently described in healthy subjects110 and the same group observed positive correlation between pain chronicity and reduced M1 excitability in patients with RC disease109 supporting an indirect inhibitory effect of pain on corticospinal excitability in line with current concepts5. However, the effects of limb disuse and other spinal/supraspinal neural factors cannot be excluded. Similarly bilateral alterations of corticospinal excitability in the deltoid and the first interosseous muscles have been reported in patients with RC tendon tears11. However it should be notified that spinal motoneuron excitability must be properly assessed to verify that the change in MEPs size is not mediated at the spinal level37. C3–4 propriospinal neurons may also influence the excitability of premotoneuronal sites and therefore the amplitude of MEPs43; 124. Peripheral nerve stimulation associated with TMS has been recently used in healthy subjects to assess the modulation of afferent signals on M1 output54 thus opening the possibility for its application in patients with RC disease. Further TMS studies are required to confirm the effects of RC disease on the motor cortex and to understand how these alterations may impair muscle activation, motor control, and shoulder function.
Conclusion and Perspectives
In this review, we identified a large number of neural structures and mechanisms that may contribute to pain and shoulder dysfunction in patients with RC disease. These structures and mechanisms are summarized in Figure 1. However, numerous questions remain unanswered (see Table II). Current data suggest that inflammation and muscle-tendon unit impairment disrupt proprioceptive function and reflex muscle activity. Alterations of proprioceptive afferents may impair proprioception and motor control, therefore contributing to poor muscle activation and impaired shoulder kinematics. However motor control and proprioception impairments in patients with RC diseases have been insufficiently assessed and require further investigations. Current advances in the understanding of pain pathophysiology encourage the enhancement of pain assessment and sensory abnormalities that remain poorly characterized in the clinical setting in patients with RC disease. Recent experiments suggest that the occurrence of motor nerve injury appears to be less frequent than first assumed, yet peripheral nerve dysfunction remains a non-negligible aggravating factor. Thus, this problem must be considered (perhaps with improved diagnostic tools) in clinical practice and further explored through both anatomical and physiological studies. Some data also highlight that tendon disruption, disuse, and inflammation may have a direct impact on neuromuscular junction and motoneurons but further studies are needed for confirmation. Increased availability of human tissue obtained during surgeries and animals models of RC disease will also improve our understanding of RC disease physiopathology and will help to define markers able to improve the detection of muscle denervation process. Damage inflicted to sensory nerves should not be neglected because it may contribute to the generation of pain and disrupt the afferent transduction of proprioceptive information. Evidence that RC disease induces significant motor adaptations and the important role of pain in these changes has been clearly demonstrated. However, the contribution of proprioception deficits, motor cortical changes, and modified brain activity in patients with RC disease remains to be explored. The problem of motor nervous system dysfunction is particularly relevant as the field begins to explore the mechanisms of reduced muscle force generation after reconstruction. If these problems are induced or aggravated by poor muscle activation, the nervous system impairments may need to be addressed first, and perhaps, in a way that is consistent with neurorehabilitation instead of standard musculoskeletal physical therapy. In the clinical setting, all these factors may contribute to explain why clinical presentations and responses to treatments can vary considerably between individuals despite similar peripheral tissue damage. Therefore, our final proposal is that different profiles involving different degrees of biomechanical, motor control, proprioceptive, and nociceptive impairments exist amongst patients with RC disease. The development of standardized tests achievable in the clinical setting to assess each of these aspects is necessary to provide comprehensive assessment and refine the management of these patients.
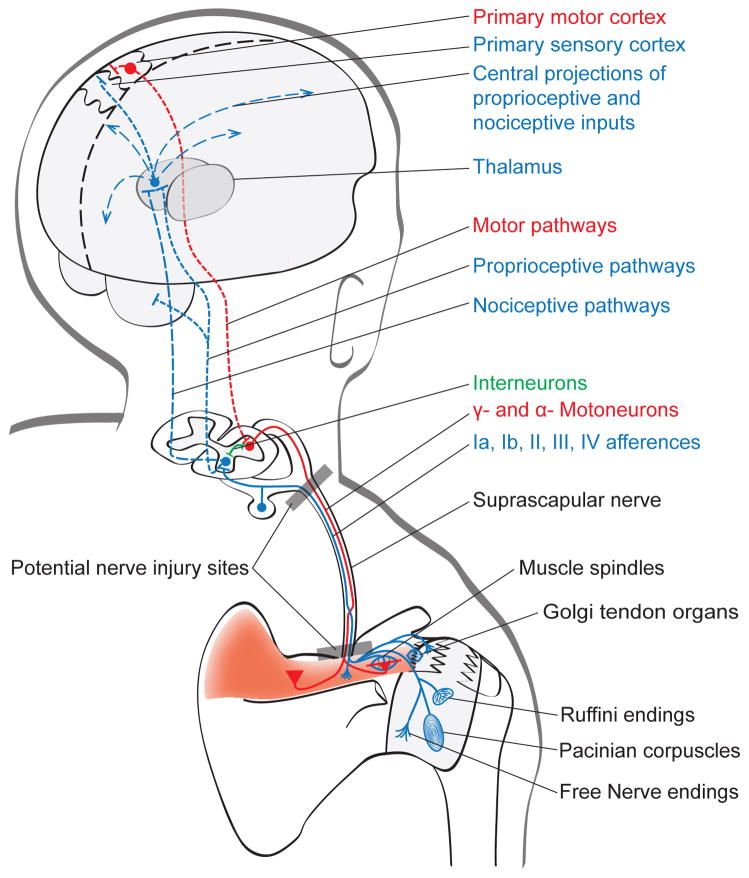
Figure 1. Potential sites for sensory and motor impairments associated with supraspinatus tendon tear.
Tendon tear, soft tissue and/or joints damage, and local inflammatory environment sensitize peripheral nociceptors (mechanical or chemical high-threshold peripheral nociceptors (e.g. Free endings) that cause pain and increase the sensitivity of central pain centers (peripheral and central sensitization, respectively). They may also induce impairments in proprioceptive outputs (Muscle spindles; Golgi tendon organ, Ruffini endings; Pacinian corpuscles) and in the central processing of proprioceptive information (proprioceptive pathways; primary sensory cortex. Motoneurons innervating both extrafusal and intrafusal muscle fibers (α- and γ– motoneurons, respectively) may equally undergo remodeling and impairments. Neuromuscular junction may also be altered as a result of reduced neural activity, muscle impairments, and central alterations within the motor nervous system. At the nerve level, stretch and/or compression caused by muscle retraction, mass compression, and manipulation of the previously retracted muscle or direct nerve manipulation during surgery can result in injury of both sensory and motor axons. The suprascapular nerve may be damaged at any point of its path but the suprascapular notch and the cervical roots are identified as the most common sites for injury. Nerve(s) damage can further increase pain, limit the afferent transduction of proprioceptive information, and aggravate muscle changes.
Table II.
Suggested deleterious nervous consequences of rotator cuff (RC) disease in studies cited in the current review.
| Structures/mechanisms | Consequences | Human RC studies | Animal RC studies | Human or Animal non-RC studies |
|---|---|---|---|---|
| Proprioceptors, Afferences and Related Spinal Reflexes | Structural/Functional impairments of proprioceptors | 226 | 7; 32; 85; 86; 98; 99; 112; 128; 135; 146; 153; 217; 226; 229. | |
| ↑ Motoneuron excitability | 16; 91; 108; 217 | |||
|
| ||||
| Central Processing of Proprioceptive Afferences | ↓ Sense of position | 180; 182 | ||
| ↓ Sense of movement | 126 | |||
| ↓ Sense of force | 8; 127 | |||
| Modified brain activity | 102 | |||
|
| ||||
| Nociceptors, Peripheral and Central Pain Processing | Peripheral sensitization | 51; 62; 63; 207 | 204 | |
| Central sensitization | 56; 73; 83 | |||
|
| ||||
| Motor Nerves | ± Injury: | |||
| Iatrogenic | 74 101 231 231 160] 66; 222 | |||
| Direct consequence of RC disease | 101; 131; 211 19; 24; 131 4; 14; 134 | 96; 97; 106; 118; 119; 176; 178; 209; 210 | ||
|
| ||||
| Neuromuscular junction | ↓= Acetyl choline receptors ↓ Cholinergic/non-cholinergic muscle stimulation |
55; 132; 176 | 92 | |
|
| ||||
| Sensory Nerves | ± Injury | 75; 158 | 85; 135; 217; 229 | |
|
| ||||
| Shoulder Muscle Activity and Kinematics | Modifications of muscle recruitment and kinematics | 103; 123; 137; 177 25; 185; 192; 194; 197; 198 144 | ||
| ↓ Voluntary activation ↑= Strength with pain reduction |
15; 47; 162 | |||
|
| ||||
| Cortical changes | ↓ Corticospinal excitability | 17; 155 | ||
Numbers refer to references; ↓, decreased; ↑, increased; =, unchanged; review articles excluded.
Acknowledgments
Funding: The source of funding for this study was NIH R01 HD073180.
Footnotes
Disclaimer: none;
Ethical approval: No ethical approval was required.
Level of evidence: Narrative Review
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albritton MJ, Graham RD, Richards RS, 2nd, Basamania CJ. An anatomic study of the effects on the suprascapular nerve due to retraction of the supraspinatus muscle after a rotator cuff tear. J Shoulder Elbow Surg. 2003;12:497–500. doi: 10.1016/s1058-2746(03)00182-4. http://dx.doi.org/10.1016/S1058-2746(03)00182-4. [DOI] [PubMed] [Google Scholar]
- 2.Aszmann OC, Dellon AL, Birely BT, McFarland EG. Innervation of the human shoulder joint and its implications for surgery. Clin Orthop Relat Res. 1996:202–207. doi: 10.1097/00003086-199609000-00027. [DOI] [PubMed] [Google Scholar]
- 3.Backenkohler U, Halata Z, Strasmann TJ. The sensory innervation of the shoulder joint of the mouse. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft. 1996;178:173–181. doi: 10.1016/S0940-9602(96)80040-9. [DOI] [PubMed] [Google Scholar]
- 4.Bandholm T, Rasmussen L, Aagaard P, Jensen BR, Diederichsen L. Force steadiness, muscle activity, and maximal muscle strength in subjects with subacromial impingement syndrome. Muscle Nerve. 2006;34:631–639. doi: 10.1002/mus.20636. http://dx.doi.org/10.1002/mus.20636. [DOI] [PubMed] [Google Scholar]
- 5.Bank PJ, Peper CE, Marinus J, Beek PJ, van Hilten JJ. Motor consequences of experimentally induced limb pain: a systematic review. Eur J Pain. 2013;17:145–157. doi: 10.1002/j.1532-2149.2012.00186.x. http://dx.doi.org/10.1002/j.1532-2149.2012.00186.x. [DOI] [PubMed] [Google Scholar]
- 6.Barden JM, Balyk R, Raso VJ, Moreau M, Bagnall K. Dynamic upper limb proprioception in multidirectional shoulder instability. Clin Orthop Relat Res. 2004:181–189. doi: 10.1097/00003086-200403000-00025. http://dx.doi.org/10.1097/00003086-200403000-00025. [DOI] [PubMed]
- 7.Bayramoglu A, Demiryurek D, Tuccar E, Erbil M, Aldur MM, Tetik O, et al. Variations in anatomy at the suprascapular notch possibly causing suprascapular nerve entrapment: an anatomical study. Knee Surg Sports Traumatol Arthrosc. 2003;11:393–398. doi: 10.1007/s00167-003-0378-3. http://dx.doi.org/10.1007/s00167-003-0378-3. [DOI] [PubMed] [Google Scholar]
- 8.Beeler S, Ek ET, Gerber C. A comparative analysis of fatty infiltration and muscle atrophy in patients with chronic rotator cuff tears and suprascapular neuropathy. J Shoulder Elbow Surg. 2013;22:1537–1546. doi: 10.1016/j.jse.2013.01.028. http://dx.doi.org/10.1016/j.jse.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Yishay A, Zuckerman JD, Gallagher M, Cuomo F. Pain inhibition of shoulder strength in patients with impingement syndrome. Orthopedics. 1994;17:685–688. doi: 10.3928/0147-7447-19940801-06. [DOI] [PubMed] [Google Scholar]
- 10.Beranek R, Hnik P. Long-term effects of tenotomy on spinal monosynaptic response in the cat. Science. 1959;130:981–982. doi: 10.1126/science.130.3381.981-a. [DOI] [PubMed] [Google Scholar]
- 11.Berth A, Pap G, Neuman W, Awiszus F. Central neuromuscular dysfunction of the deltoid muscle in patients with chronic rotator cuff tears. J Orthop Traumatol. 2009;10:135–141. doi: 10.1007/s10195-009-0061-7. http://dx.doi.org/10.1007/s10195-009-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boykin RE, Friedman DJ, Higgins LD, Warner JJ. Suprascapular neuropathy. J Bone Joint Surg Am. 2010;92:2348–2364. doi: 10.2106/JBJS.I.01743. http://dx.doi.org/10.2106/JBJS.I.01743. [DOI] [PubMed] [Google Scholar]
- 13.Boykin RE, Friedman DJ, Zimmer ZR, Oaklander AL, Higgins LD, Warner JJ. Suprascapular neuropathy in a shoulder referral practice. J Shoulder Elbow Surg. 2011;20:983–988. doi: 10.1016/j.jse.2010.10.039. http://dx.doi.org/10.1016/j.jse.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 14.Carlson BM, Borissov G, Dedkov EI, Dow DE, Kostrominova TY. The Biology and Restorative Capacity of Long-Term Denervated Skeletal Muscle. Basic Appl Myol. 2002;12:247–254. [Google Scholar]
- 15.Chaudhury S, Gwilym SE, Moser J, Carr AJ. Surgical options for patients with shoulder pain. Nat Rev Rheumatol. 2010;6:217–226. doi: 10.1038/nrrheum.2010.25. http://dx.doi.org/10.1038/nrrheum.2010.25. [DOI] [PubMed] [Google Scholar]
- 16.Collin P, Treseder T, Ladermann A, Benkalfate T, Mourtada R, Courage O, et al. Neuropathy of the suprascapular nerve and massive rotator cuff tears: a prospective electromyographic study. J Shoulder Elbow Surg. 2014;23:28–34. doi: 10.1016/j.jse.2013.07.039. http://dx.doi.org/10.1016/j.jse.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 17.Cordasco FA, Chen NC, Backus SI, Kelly BT, Williams RJ, 3rd, Otis JC. Subacromial injection improves deltoid firing in subjects with large rotator cuff tears. HSS J. 2010;6:30–36. doi: 10.1007/s11420-009-9127-6. http://dx.doi.org/10.1007/s11420-009-9127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costouros JG, Porramatikul M, Lie DT, Warner JJ. Reversal of suprascapular neuropathy following arthroscopic repair of massive supraspinatus and infraspinatus rotator cuff tears. Arthroscopy. 2007;23:1152–1161. doi: 10.1016/j.arthro.2007.06.014. http://dx.doi.org/10.1016/j.arthro.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Craik JD, Mallina R, Ramasamy V, Little NJ. Human evolution and tears of the rotator cuff. Int Orthop. 2014;38:547–552. doi: 10.1007/s00264-013-2204-y. http://dx.doi.org/10.1007/s00264-013-2204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cummins CA, Messer TM, Nuber GW. Suprascapular nerve entrapment. J Bone Joint Surg Am. 2000;82:415–424. doi: 10.2106/00004623-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Cuomo F, Birdzell MG, Zuckerman JD. The effect of degenerative arthritis and prosthetic arthroplasty on shoulder proprioception. J Shoulder Elbow Surg. 2005;14:345–348. doi: 10.1016/j.jse.2004.07.009. http://dx.doi.org/10.1016/j.jse.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 22.de Castro Pochini A, Ejnisman B, de Seixas Alves MT, Uyeda LF, Nouailhetas VL, Han SW, et al. Overuse of training increases mechanoreceptors in supraspinatus tendon of rats SHR. J Orthop Res. 2011;29:1771–1774. doi: 10.1002/jor.21320. http://dx.doi.org/10.1002/jor.21320. [DOI] [PubMed] [Google Scholar]
- 23.Dean BJ, Franklin SL, Carr AJ. The peripheral neuronal phenotype is important in the pathogenesis of painful human tendinopathy: a systematic review. Clin Orthop Relat Res. 2013;471:3036–3046. doi: 10.1007/s11999-013-3010-y. http://dx.doi.org/10.1007/s11999-013-3010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dean BJ, Gwilym SE, Carr AJ. Why does my shoulder hurt? A review of the neuroanatomical and biochemical basis of shoulder pain. Br J Sports Med. 2013;47:1095–1104. doi: 10.1136/bjsports-2012-091492. http://dx.doi.org/10.1136/bjsports-2012-091492. [DOI] [PubMed] [Google Scholar]
- 25.Debeyre J, Patie D, Elmelik E. Repair of Ruptures of the Rotator Cuff of the Shoulder. Br J Sports Med. 1965;47:36–42. [PubMed] [Google Scholar]
- 26.Deniz G, Kose O, Tugay A, Guler F, Turan A. Fatty degeneration and atrophy of the rotator cuff muscles after arthroscopic repair: does it improve, halt or deteriorate? Arch Orthop Trauma Surg. 2014;134:985–990. doi: 10.1007/s00402-014-2009-5. http://dx.doi.org/10.1007/s00402-014-2009-5. [DOI] [PubMed] [Google Scholar]
- 27.Diederichsen L, Krogsgaard M, Voigt M, Dyhre-Poulsen P. Shoulder reflexes. J Electromyogr Kinesiol. 2002;12:183–191. doi: 10.1016/s1050-6411(02)00019-6. http://dx.doi.org/10.1016/S1050-6411(02)00019-6. [DOI] [PubMed] [Google Scholar]
- 28.Duparc F, Coquerel D, Ozeel J, Noyon M, Gerometta A, Michot C. Anatomical basis of the suprascapular nerve entrapment, and clinical relevance of the supraspinatus fascia. Arch Orthop Trauma Surg. 2010;32:277–284. doi: 10.1007/s00276-010-0631-7. http://dx.doi.org/10.1007/s00276-010-0631-7. [DOI] [PubMed] [Google Scholar]
- 29.Ebraheim NA, Whitehead JL, Alla SR, Moral MZ, Castillo S, McCollough AL, et al. The suprascapular nerve and its articular branch to the acromioclavicular joint: an anatomic study. J Shoulder Elbow Surg. 2011;20:e13–17. doi: 10.1016/j.jse.2010.09.004. http://dx.doi.org/10.1016/j.jse.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Edelstein L, Thomas SJ, Soslowsky LJ. Rotator cuff tears: what have we learned from animal models? J Musculoskelet Neuronal Interact. 2011;11:150–162. [PubMed] [Google Scholar]
- 31.Ellis A, Bennett DL. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth. 2013;111:26–37. doi: 10.1093/bja/aet128. http://dx.doi.org/10.1093/bja/aet128. [DOI] [PubMed] [Google Scholar]
- 32.Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2011;27:581–592. doi: 10.1016/j.arthro.2010.10.014. http://dx.doi.org/10.1016/j.arthro.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Farshad M, Jundt-Ecker M, Sutter R, Schubert M, Gerber C. Does subacromial injection of a local anesthetic influence strength in healthy shoulders?: a double-blinded, placebo-controlled study. J Bone Joint Surg Am. 2012;94:1751–1755. doi: 10.2106/JBJS.K.00855. http://dx.doi.org/10.2106/JBJS.K.00855. [DOI] [PubMed] [Google Scholar]
- 34.Franklin SL, Dean BJ, Wheway K, Watkins B, Javaid MK, Carr AJ. Up-regulation of Glutamate in Painful Human Supraspinatus Tendon Tears. Am J Sports Med. 2014;42:1955–1962. doi: 10.1177/0363546514532754. http://dx.doi.org/10.1177/0363546514532754. [DOI] [PubMed] [Google Scholar]
- 35.Fucentese SF, von Roll AL, Pfirrmann CW, Gerber C, Jost B. Evolution of nonoperatively treated symptomatic isolated full-thickness supraspinatus tears. J Bone Joint Surg Am. 2012;94:801–808. doi: 10.2106/JBJS.I.01286. http://dx.doi.org/10.2106/JBJS.I.01286. [DOI] [PubMed] [Google Scholar]
- 36.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86–A:219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 38.Gayton JC, Rubino LJ, Rich MM, Stouffer MH, Wang Q, Boivin GP. Rabbit supraspinatus motor endplates are unaffected by a rotator cuff tear. J Orthop Res. 2013;31:99–104. doi: 10.1002/jor.22192. http://dx.doi.org/10.1002/jor.22192. [DOI] [PubMed] [Google Scholar]
- 39.Ge HY, Fernandez-de-Las-Penas C, Madeleine P, Arendt-Nielsen L. Topographical mapping and mechanical pain sensitivity of myofascial trigger points in the infraspinatus muscle. Eur J Pain. 2008;12:859–865. doi: 10.1016/j.ejpain.2007.12.005. http://dx.doi.org/10.1016/j.ejpain.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Gohlke F, Janssen E, Leidel J, Heppelmann B, Eulert J. Histopathological findings in the proprioception of the shoulder joint. Der Orthopade. 1998;27:510–517. doi: 10.1007/s001320050263. [DOI] [PubMed] [Google Scholar]
- 41.Gotoh M, Hamada K, Yamakawa H, Inoue A, Fukuda H. Increased substance P in subacromial bursa and shoulder pain in rotator cuff diseases. J Orthop Res. 1998;16:618–621. doi: 10.1002/jor.1100160515. [DOI] [PubMed] [Google Scholar]
- 42.Goutallier D, Postel JM, Boudon R, Lavau L, Bernageau J. A study of the neurologic risk in tendino-muscular advancement of supra-spinatus and infra-spinatus in the repair of large rotator cuff rupture. Rev Chir Orthop Reparatrice Appar Mot. 1996;82:299–305. [PubMed] [Google Scholar]
- 43.Gracies JM, Meunier S, Pierrot-Deseilligny E, Simonetta M. Pattern of propriospinal-like excitation to different species of human upper limb motoneurones. J Physiol. 1991;434:151–167. doi: 10.1113/jphysiol.1991.sp018463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greiner A, Golser K, Wambacher M, Kralinger F, Sperner G. The course of the suprascapular nerve in the supraspinatus fossa and its vulnerability in muscle advancement. J Shoulder Elbow Surg. 2003;12:256–259. doi: 10.1016/s1058-2746(02)00034-4. http://dx.doi.org/10.1016/S1058-2746(02)00034-4. [DOI] [PubMed] [Google Scholar]
- 45.Gruet M, Temesi J, Rupp T, Levy P, Millet GY, Verges S. Stimulation of the motor cortex and corticospinal tract to assess human muscle fatigue. Neuroscience. 2013;231:384–399. doi: 10.1016/j.neuroscience.2012.10.058. http://dx.doi.org/10.1016/j.neuroscience.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 46.Guanche C, Knatt T, Solomonow M, Lu Y, Baratta R. The synergistic action of the capsule and the shoulder muscles. Am J Sports Med. 1995;23:301–306. doi: 10.1177/036354659502300308. [DOI] [PubMed] [Google Scholar]
- 47.Guanche CA, Noble J, Solomonow M, Wink CS. Periarticular neural elements in the shoulder joint. Orthopedics. 1999;22:615–617. doi: 10.3928/0147-7447-19990601-12. [DOI] [PubMed] [Google Scholar]
- 48.Gwilym SE, Oag HC, Tracey I, Carr AJ. Evidence that central sensitisation is present in patients with shoulder impingement syndrome and influences the outcome after surgery. J Bone Joint Surg Am. 2011;93:498–502. doi: 10.1302/0301-620X.93B4.25054. http://dx.doi.org/10.1302/0301-620X.93B4.25054. [DOI] [PubMed] [Google Scholar]
- 49.Ha’eri GB, Wiley AM. Advancement of the supraspinatus muscle in the repair of ruptures of the rotator cuff. J Bone Joint Surg Am. 1981;63:232–238. [PubMed] [Google Scholar]
- 50.Harris JD, Pedroza A, Jones GL, Group MS. Predictors of pain and function in patients with symptomatic, atraumatic full-thickness rotator cuff tears: a time-zero analysis of a prospective patient cohort enrolled in a structured physical therapy program. Am J Sports Med. 2012;40:359–366. doi: 10.1177/0363546511426003. http://dx.doi.org/10.1177/0363546511426003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hashimoto T, Hamada T, Sasaguri Y, Suzuki K. Immunohistochemical approach for the investigation of nerve distribution in the shoulder joint capsule. Clin Orthop Relat Res. 1994:273–282. [PubMed] [Google Scholar]
- 52.Hattrup SJ, Cofield RH. Rotator cuff tears with cervical radiculopathy. J Shoulder Elbow Surg. 2010;19:937–943. doi: 10.1016/j.jse.2010.05.007. http://dx.doi.org/10.1016/j.jse.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 53.Heales LJ, Lim EC, Hodges PW, Vicenzino B. Sensory and motor deficits exist on the non-injured side of patients with unilateral tendon pain and disability--implications for central nervous system involvement: a systematic review with meta-analysis. Br J Sports Med. 2014;48:1400–1406. doi: 10.1136/bjsports-2013-092535. http://dx.doi.org/10.1136/bjsports-2013-092535. [DOI] [PubMed] [Google Scholar]
- 54.Hendy KA, Visser A, Hordacre B, Bradnam LV. Afferent inhibition of infraspinatus primary motor cortex by stimulation of the suprascapular nerve. Brain stimulation. 2014;7:338–339. doi: 10.1016/j.brs.2013.12.015. http://dx.doi.org/10.1016/j.brs.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 55.Hidalgo-Lozano A, Fernandez-de-las-Penas C, Alonso-Blanco C, Ge HY, Arendt-Nielsen L, Arroyo-Morales M. Muscle trigger points and pressure pain hyperalgesia in the shoulder muscles in patients with unilateral shoulder impingement: a blinded, controlled study. Exp Brain Res. 2010;202:915–925. doi: 10.1007/s00221-010-2196-4. http://dx.doi.org/10.1007/s00221-010-2196-4. [DOI] [PubMed] [Google Scholar]
- 56.Hnik P, Beranek R, Vyklicky L, Zelena J. Sensory outflow from chronically tenotomized muscles. Physiol Bohemoslov. 1963;12:23–29. [PubMed] [Google Scholar]
- 57.Hnik P, Lessler MJ. Alterations in spindle activity during long-term tenotomy in the rat gastrocnemius muscle. Exp Neurol. 1973;40:232–242. doi: 10.1016/0014-4886(73)90138-6. [DOI] [PubMed] [Google Scholar]
- 58.Hodges PW, Tucker K. Moving differently in pain: a new theory to explain the adaptation to pain. Pain. 2011;152:S90–98. doi: 10.1016/j.pain.2010.10.020. http://dx.doi.org/10.1016/j.pain.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 59.Hoellrich RG, Gasser SI, Morrison DS, Kurzweil PR. Electromyographic evaluation after primary repair of massive rotator cuff tears. J Shoulder Elbow Surg. 2005;14:269–272. doi: 10.1016/j.jse.2004.09.013. http://dx.doi.org/10.1016/j.jse.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 60.Iqbal K, Iqbal R. Classification of suprascapular notch according to anatomical measurements in human scapulae. J Coll Physicians Surg Pak. 2011;21:169–170. [PubMed] [Google Scholar]
- 61.Jamali AA, Afshar P, Abrams RA, Lieber RL. Skeletal muscle response to tenotomy. Muscle Nerve. 2000;23:851–862. doi: 10.1002/(sici)1097-4598(200006)23:6<851::aid-mus3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 62.Jang JS, Choi HJ, Kang SH, Yang JS, Lee JJ, Hwang SM. Effect of pulsed radiofrequency neuromodulation on clinical improvements in the patients of chronic intractable shoulder pain. J Korean Neurosurg Soc. 2013;54:507–510. doi: 10.3340/jkns.2013.54.6.507. http://dx.doi.org/10.3340/jkns.2013.54.6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jerosch J, Saad M, Greig M, Filler T. Suprascapular nerve block as a method of preemptive pain control in shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2008;16:602–607. doi: 10.1007/s00167-008-0520-3. http://dx.doi.org/10.1007/s00167-008-0520-3. [DOI] [PubMed] [Google Scholar]
- 64.Jerosch J, Thorwesten L. Proprioceptive abilities of patients with post-traumatic instability of the glenohumeral joint. Z Orthop Ihre Grenzgeb. 1998;136:230–237. doi: 10.1055/s-2008-1054228. [DOI] [PubMed] [Google Scholar]
- 65.Joshi SK, Kim HT, Feeley BT, Liu X. Differential ubiquitin-proteasome and autophagy signaling following rotator cuff tears and suprascapular nerve injury. J Orthop Res. 2014;32:138–144. doi: 10.1002/jor.22482. http://dx.doi.org/10.1002/jor.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joshi SK, Liu X, Samagh SP, Lovett DH, Bodine SC, Kim HT, et al. mTOR regulates fatty infiltration through SREBP-1 and PPARgamma after a combined massive rotator cuff tear and suprascapular nerve injury in rats. J Orthop Res. 2013;31:724–730. doi: 10.1002/jor.22254. http://dx.doi.org/10.1002/jor.22254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jozsa L, Kvist M, Kannus P, Jarvinen M. The effect of tenotomy and immobilization on muscle spindles and tendon organs of the rat calf muscles. A histochemical and morphometrical study. Acta Neuropathol. 1988;76:465–470. doi: 10.1007/BF00686385. [DOI] [PubMed] [Google Scholar]
- 68.Kang JR, Gupta R. Mechanisms of fatty degeneration in massive rotator cuff tears. J Shoulder Elbow Surg. 2012;21:175–180. doi: 10.1016/j.jse.2011.11.017. http://dx.doi.org/10.1016/j.jse.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 69.Kapreli E, Athanasopoulos S, Gliatis J, Papathanasiou M, Peeters R, Strimpakos N, et al. Anterior cruciate ligament deficiency causes brain plasticity: a functional MRI study. Am J Sports Med. 2009;37:2419–2426. doi: 10.1177/0363546509343201. http://dx.doi.org/10.1177/0363546509343201. [DOI] [PubMed] [Google Scholar]
- 70.Kelly BT, Williams RJ, Cordasco FA, Backus SI, Otis JC, Weiland DE, et al. Differential patterns of muscle activation in patients with symptomatic and asymptomatic rotator cuff tears. J Shoulder Elbow Surg. 2005;14:165–171. doi: 10.1016/j.jse.2004.06.010. http://dx.doi.org/10.1016/j.jse.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 71.Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. Recent advances in shoulder research. Arthritis Res Ther. 2012;14:214. doi: 10.1186/ar3846. http://dx.doi.org/10.1186/ar3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim HM, Galatz LM, Lim C, Havlioglu N, Thomopoulos S. The effect of tear size and nerve injury on rotator cuff muscle fatty degeneration in a rodent animal model. J Shoulder Elbow Surg. 2012;21:847–858. doi: 10.1016/j.jse.2011.05.004. http://dx.doi.org/10.1016/j.jse.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kocak M, Ulmer JL, Sahin Ugurel M, Gaggl W, Prost RW. Motor homunculus: passive mapping in healthy volunteers by using functional MR imaging--initial results. Radiology. 2009;251:485–492. doi: 10.1148/radiol.2512080231. http://dx.doi.org/10.1148/radiol.2512080231. [DOI] [PubMed] [Google Scholar]
- 74.Kozak W, Westerman RA. Plastic changes of spinal monosynaptic responses from tenotomized muscles in cats. Nature. 1961;189:753–755. doi: 10.1038/189753b0. [DOI] [PubMed] [Google Scholar]
- 75.Kuhn JE, Dunn WR, Sanders R, An Q, Baumgarten KM, Bishop JY, et al. Effectiveness of physical therapy in treating atraumatic full-thickness rotator cuff tears: a multicenter prospective cohort study. J Shoulder Elbow Surg. 2013;22:1371–1379. doi: 10.1016/j.jse.2013.01.026. http://dx.doi.org/10.1016/j.jse.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kurokawa D, Sano H, Nagamoto H, Omi R, Shinozaki N, Watanuki S, et al. Muscle activity pattern of the shoulder external rotators differs in adduction and abduction: an analysis using positron emission tomography. J Shoulder Elbow Surg. 2014;23:658–664. doi: 10.1016/j.jse.2013.12.021. http://dx.doi.org/10.1016/j.jse.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 77.Laurin J, Gondin J, Dousset E, Decherchi P. Effect of tenotomy on metabosensitive afferent fibers from tibialis anterior muscle. Exp Brain Res. 2008;186:87–92. doi: 10.1007/s00221-007-1210-y. http://dx.doi.org/10.1007/s00221-007-1210-y. [DOI] [PubMed] [Google Scholar]
- 78.Le BT, Wu XL, Lam PH, Murrell GA. Factors predicting rotator cuff retears: an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med. 2014;42:1134–1142. doi: 10.1177/0363546514525336. http://dx.doi.org/10.1177/0363546514525336. [DOI] [PubMed] [Google Scholar]
- 79.Leclere LE, Shi LL, Lin A, Yannopoulos P, Higgins LD, Warner JJ. Complete Fatty infiltration of intact rotator cuffs caused by suprascapular neuropathy. Arthroscopy. 2014;30:639–644. doi: 10.1016/j.arthro.2014.01.010. http://dx.doi.org/10.1016/j.arthro.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 80.Lee HY, Chung IH, Sir WS, Kang HS, Lee HS, Ko JS, et al. Variations of the ventral rami of the brachial plexus. J Korean Med Sci. 1992;7:19–24. doi: 10.3346/jkms.1992.7.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lehmann HC, Zhang J, Mori S, Sheikh KA. Diffusion tensor imaging to assess axonal regeneration in peripheral nerves. Exp Neurol. 2010;223:238–244. doi: 10.1016/j.expneurol.2009.10.012. http://dx.doi.org/10.1016/j.expneurol.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu X, Joshi SK, Samagh SP, Dang YX, Laron D, Lovett DH, et al. Evaluation of Akt/mTOR activity in muscle atrophy after rotator cuff tears in a rat model. J Orthop Res. 2012;30:1440–1446. doi: 10.1002/jor.22096. http://dx.doi.org/10.1002/jor.22096. [DOI] [PubMed] [Google Scholar]
- 83.Liu X, Laron D, Natsuhara K, Manzano G, Kim HT, Feeley BT. A mouse model of massive rotator cuff tears. J Bone Joint Surg Am. 2012;94:e41. doi: 10.2106/JBJS.K.00620. http://dx.doi.org/10.2106/JBJS.K.00620. [DOI] [PubMed] [Google Scholar]
- 84.Lu DX, Huang SK, Carlson BM. Electron microscopic study of long-term denervated rat skeletal muscle. Anat Rec. 1997;248:355–365. doi: 10.1002/(SICI)1097-0185(199707)248:3<355::AID-AR8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 85.Lubiatowski P, Ogrodowicz P, Wojtaszek M, Kaniewski R, Stefaniak J, Dudzinski W, et al. Measurement of active shoulder proprioception: dedicated system and device. Eur J Orthop Surg Traumatol. 2013;23:177–183. doi: 10.1007/s00590-012-0950-y. http://dx.doi.org/10.1007/s00590-012-0950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ludewig PM, Reynolds JF. The association of scapular kinematics and glenohumeral joint pathologies. J Orthop Sports Phys Ther. 2009;39:90–104. doi: 10.2519/jospt.2009.2808. http://dx.doi.org/10.2519/jospt.2009.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.MacDermid JC, Ramos J, Drosdowech D, Faber K, Patterson S. The impact of rotator cuff pathology on isometric and isokinetic strength, function, and quality of life. J Shoulder Elbow Surg. 2004;13:593–598. doi: 10.1016/j.jse.2004.03.009. http://dx.doi.org/10.1016/j.jse.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 88.Machner A, Merk H, Becker R, Rohkohl K, Wissel H, Pap G. Kinesthetic sense of the shoulder in patients with impingement syndrome. Acta Orthop Scand. 2003;74:85–88. doi: 10.1080/00016470310013716. http://dx.doi.org/10.1080/00016470310013716. [DOI] [PubMed] [Google Scholar]
- 89.Maenhout AG, Palmans T, De Muynck M, De Wilde LF, Cools AM. The impact of rotator cuff tendinopathy on proprioception, measuring force sensation. J Shoulder Elbow Surg. 2012;21:1080–1086. doi: 10.1016/j.jse.2011.07.006. http://dx.doi.org/10.1016/j.jse.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 90.Mall NA, Kim HM, Keener JD, Steger-May K, Teefey SA, Middleton WD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92:2623–2633. doi: 10.2106/JBJS.I.00506. http://dx.doi.org/10.2106/JBJS.I.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mall NA, Tanaka MJ, Choi LS, Paletta GA., Jr Factors affecting rotator cuff healing. J Bone Joint Surg Am. 2014;96:778–788. doi: 10.2106/JBJS.M.00583. http://dx.doi.org/10.2106/JBJS.M.00583. [DOI] [PubMed] [Google Scholar]
- 92.Mallon WJ, Wilson RJ, Basamania CJ. The association of suprascapular neuropathy with massive rotator cuff tears: a preliminary report. J Shoulder Elbow Surg. 2006;15:395–398. doi: 10.1016/j.jse.2005.10.019. http://dx.doi.org/10.1016/j.jse.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 93.Massimini DF, Singh A, Wells JH, Li G, Warner JJ. Suprascapular nerve anatomy during shoulder motion: a cadaveric proof of concept study with implications for neurogenic shoulder pain. J Shoulder Elbow Surg. 2013;22:463–470. doi: 10.1016/j.jse.2012.04.018. http://dx.doi.org/10.1016/j.jse.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 94.Matsumoto DE, Baker JH. Degeneration and alteration of axons and intrafusal muscle fibers in spindles following tenotomy. Exp Neurol. 1987;97:482–498. doi: 10.1016/0014-4886(87)90108-7. [DOI] [PubMed] [Google Scholar]
- 95.Matthews TJ, Hand GC, Rees JL, Athanasou NA, Carr AJ. Pathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increases. J Bone Joint Surg Br. 2006;88:489–495. doi: 10.1302/0301-620X.88B4.16845. http://dx.doi.org/10.1302/0301-620X.88B4.16845. [DOI] [PubMed] [Google Scholar]
- 96.McClure PW, Michener LA, Karduna AR. Shoulder function and 3-dimensional scapular kinematics in people with and without shoulder impingement syndrome. Phys Ther. 2006;86:1075–1090. [PubMed] [Google Scholar]
- 97.McElvany MD, McGoldrick E, Gee AO, Neradilek MB, Matsen FA., 3rd Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43:491–500. doi: 10.1177/0363546514529644. http://dx.doi.org/10.1177/0363546514529644. [DOI] [PubMed] [Google Scholar]
- 98.McNeil CJ, Butler JE, Taylor JL, Gandevia SC. Testing the excitability of human motoneurons. Front Hum Neurosci. 2013;7:152. doi: 10.3389/fnhum.2013.00152. http://dx.doi.org/10.3389/fnhum.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Melgari JM, Pasqualetti P, Pauri F, Rossini PM. Muscles in “concert”: study of primary motor cortex upper limb functional topography. PLoS One. 2008;3:e3069. doi: 10.1371/journal.pone.0003069. http://dx.doi.org/10.1371/journal.pone.0003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mense S. Functional Anatomy of Muscle: Muscle, Nociceptors and Afferent Fibers. In: Mense S, Gerwin RD, editors. Muscle Pain: Understanding the Mechanisms. Springer; Berlin Heidelberg: 2010. pp. 17–48. http://dx.doi.org/10.1007/978-3-540-85021-2_2. [Google Scholar]
- 101.Midrio M. The denervated muscle: facts and hypotheses. A historical review. Eur J Appl Physiol. 2006;98:1–21. doi: 10.1007/s00421-006-0256-z. http://dx.doi.org/10.1007/s00421-006-0256-z. [DOI] [PubMed] [Google Scholar]
- 102.Millet GY, Bachasson D, Temesi J, Wuyam B, Feasson L, Verges S, et al. Potential interests and limits of magnetic and electrical stimulation techniques to assess neuromuscular fatigue. Neuromuscul Disord. 2012;22 (Suppl 3):S181–186. doi: 10.1016/j.nmd.2012.10.007. http://dx.doi.org/10.1016/j.nmd.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 103.Minagawa H, Yamamoto N, Abe H, Fukuda M, Seki N, Kikuchi K, et al. Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: From mass-screening in one village. J Orthop. 2013;10:8–12. doi: 10.1016/j.jor.2013.01.008. http://dx.doi.org/10.1016/j.jor.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Minaki Y, Yamashita T, Takebayashi T, Ishii S. Mechanosensitive afferent units in the shoulder and adjacent tissues. Clin Orthop Relat Res. 1999:349–356. doi: 10.1097/00003086-199912000-00037. [DOI] [PubMed] [Google Scholar]
- 105.Moen TC, Babatunde OM, Hsu SH, Ahmad CS, Levine WN. Suprascapular neuropathy: what does the literature show? J Shoulder Elbow Surg. 2012;21:835–846. doi: 10.1016/j.jse.2011.11.033. http://dx.doi.org/10.1016/j.jse.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 106.Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95:1249–1255. doi: 10.2106/JBJS.L.00185. http://dx.doi.org/10.2106/JBJS.L.00185. [DOI] [PubMed] [Google Scholar]
- 107.Myers JB, Wassinger CA, Lephart SM. Sensorimotor contribution to shoulder stability: effect of injury and rehabilitation. Man Ther. 2006;11:197–201. doi: 10.1016/j.math.2006.04.002. http://dx.doi.org/10.1016/j.math.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 108.Natsis K, Totlis T, Tsikaras P, Appell HJ, Skandalakis P, Koebke J. Proposal for classification of the suprascapular notch: a study on 423 dried scapulas. Clin Anat. 2007;20:135–139. doi: 10.1002/ca.20318. http://dx.doi.org/10.1002/ca.20318. [DOI] [PubMed] [Google Scholar]
- 109.Ngomo S, Mercier C, Bouyer LJ, Savoie A, Roy JS. Alterations in central motor representation increase over time in individuals with rotator cuff tendinopathy. Clin Neurophysiol. 2015;126:365–371. doi: 10.1016/j.clinph.2014.05.035. http://dx.doi.org/10.1016/j.clinph.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 110.Ngomo S, Mercier C, Roy JS. Cortical mapping of the infraspinatus muscle in healthy individuals. BMC Neurosci. 2013;14:52. doi: 10.1186/1471-2202-14-52. http://dx.doi.org/10.1186/1471-2202-14-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oh LS, Wolf BR, Hall MP, Levy BA, Marx RG. Indications for rotator cuff repair: a systematic review. Clin Orthop Relat Res. 2007;455:52–63. doi: 10.1097/BLO.0b013e31802fc175. http://dx.doi.org/10.1097/BLO.0b013e31802fc175. [DOI] [PubMed] [Google Scholar]
- 112.Omi R, Sano H, Ohnuma M, Kishimoto KN, Watanuki S, Tashiro M, et al. Function of the shoulder muscles during arm elevation: an assessment using positron emission tomography. J Anat. 2010;216:643–649. doi: 10.1111/j.1469-7580.2010.01212.x. http://dx.doi.org/10.1111/j.1469-7580.2010.01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Padua L, Martinoli C. From square to cube: ultrasound as a natural complement of neurophysiology. Clin Neurophysiol. 2008;119:1217–1218. doi: 10.1016/j.clinph.2008.02.005. http://dx.doi.org/10.1016/j.clinph.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 114.Park JY, Lee WS, Lee ST. The strength of the rotator cuff before and after subacromial injection of lidocaine. J Shoulder Elbow Surg. 2008;17:8S–11S. doi: 10.1016/j.jse.2007.06.010. http://dx.doi.org/10.1016/j.jse.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 115.Pham M, Baumer T, Bendszus M. Peripheral nerves and plexus: imaging by MR-neurography and high-resolution ultrasound. Curr Opin Neurol. 2014;27:370–379. doi: 10.1097/WCO.0000000000000111. http://dx.doi.org/10.1097/WCO.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 116.Plancher KD, Luke TA, Peterson RK, Yacoubian SV. Posterior shoulder pain: a dynamic study of the spinoglenoid ligament and treatment with arthroscopic release of the scapular tunnel. Arthroscopy. 2007;23:991–998. doi: 10.1016/j.arthro.2007.03.098. http://dx.doi.org/10.1016/j.arthro.2007.03.098. [DOI] [PubMed] [Google Scholar]
- 117.Polguj M, Jedrzejewski K, Podgorski M, Majos A, Topol M. A proposal for classification of the superior transverse scapular ligament: variable morphology and its potential influence on suprascapular nerve entrapment. J Shoulder Elbow Surg. 2013;22:1265–1273. doi: 10.1016/j.jse.2012.11.017. http://dx.doi.org/10.1016/j.jse.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 118.Polguj M, Jedrzejewski K, Podgorski M, Topol M. Morphometric study of the suprascapular notch: proposal of classification. Arch Orthop Trauma Surg. 2011;33:781–787. doi: 10.1007/s00276-011-0821-y. http://dx.doi.org/10.1007/s00276-011-0821-y. [DOI] [PubMed] [Google Scholar]
- 119.Polguj M, Rozniecki J, Sibinski M, Grzegorzewski A, Majos A, Topol M. The variable morphology of suprascapular nerve and vessels at suprascapular notch: a proposal for classification and its potential clinical implications. Knee Surg Sports Traumatol Arthrosc. 2014 doi: 10.1007/s00167-014-2937-1. Epub ahead of print http://dx.doi.org/10.1007/s00167-014-2937-1. [DOI] [PMC free article] [PubMed]
- 120.Prescher A. Anatomical basics, variations, and degenerative changes of the shoulder joint and shoulder girdle. Eur J Radiol. 2000;35:88–102. doi: 10.1016/s0720-048x(00)00225-4. [DOI] [PubMed] [Google Scholar]
- 121.Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev. 2012;92:1651–1697. doi: 10.1152/physrev.00048.2011. http://dx.doi.org/10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- 122.Reilly P, Macleod I, Macfarlane R, Windley J, Emery RJ. Dead men and radiologists don’t lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann R Coll Surg Engl. 2006;88:116–121. doi: 10.1308/003588406X94968. http://dx.doi.org/10.1308/003588406X94968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rengachary SS, Neff JP, Singer PA, Brackett CE. Suprascapular entrapment neuropathy: a clinical, anatomical, and comparative study. Part 1: clinical study. Neurosurgery. 1979;5:441–446. doi: 10.1227/00006123-197910000-00006. [DOI] [PubMed] [Google Scholar]
- 124.Roberts LV, Stinear CM, Lewis GN, Byblow WD. Task-dependent modulation of propriospinal inputs to human shoulder. J Neurophysiol. 2008;100:2109–2114. doi: 10.1152/jn.90786.2008. http://dx.doi.org/10.1152/jn.90786.2008. [DOI] [PubMed] [Google Scholar]
- 125.Rokito AS, Birdzell MG, Cuomo F, Di Paola MJ, Zuckerman JD. Recovery of shoulder strength and proprioception after open surgery for recurrent anterior instability: a comparison of two surgical techniques. J Shoulder Elbow Surg. 2010;19:564–569. doi: 10.1016/j.jse.2009.09.010. http://dx.doi.org/10.1016/j.jse.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 126.Rowshan K, Hadley S, Pham K, Caiozzo V, Lee TQ, Gupta R. Development of fatty atrophy after neurologic and rotator cuff injuries in an animal model of rotator cuff pathology. J Bone Joint Surg Am. 2010;92:2270–2278. doi: 10.2106/JBJS.I.00812. http://dx.doi.org/10.2106/JBJS.I.00812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roy JS, Moffet H, McFadyen BJ. Upper limb motor strategies in persons with and without shoulder impingement syndrome across different speeds of movement. Clin Biomech (Bristol, Avon) 2008;23:1227–1236. doi: 10.1016/j.clinbiomech.2008.07.009. http://dx.doi.org/10.1016/j.clinbiomech.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 128.Rubino LJ, Stills HF, Jr, Sprott DC, Crosby LA. Fatty infiltration of the torn rotator cuff worsens over time in a rabbit model. Arthroscopy. 2007;23:717–722. doi: 10.1016/j.arthro.2007.01.023. http://dx.doi.org/10.1016/j.arthro.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 129.Russell RD, Knight JR, Mulligan E, Khazzam MS. Structural integrity after rotator cuff repair does not correlate with patient function and pain: a meta-analysis. J Bone Joint Surg Am. 2014;96:265–271. doi: 10.2106/JBJS.M.00265. http://dx.doi.org/10.2106/JBJS.M.00265. [DOI] [PubMed] [Google Scholar]
- 130.Safran MR, Borsa PA, Lephart SM, Fu FH, Warner JJ. Shoulder proprioception in baseball pitchers. J Shoulder Elbow Surg. 2001;10:438–444. doi: 10.1067/mse.2001.118004. [DOI] [PubMed] [Google Scholar]
- 131.Salles JI, Cossich VR, Amaral MV, Monteiro MT, Cagy M, Motta G, et al. Electrophysiological correlates of the threshold to detection of passive motion: an investigation in professional volleyball athletes with and without atrophy of the infraspinatus muscle. Biomed Res Int. 2013;2013:634891. doi: 10.1155/2013/634891. http://dx.doi.org/10.1155/2013/634891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Scibek JS, Mell AG, Downie BK, Carpenter JE, Hughes RE. Shoulder kinematics in patients with full-thickness rotator cuff tears after a subacromial injection. J Shoulder Elbow Surg. 2008;17:172–181. doi: 10.1016/j.jse.2007.05.010. http://dx.doi.org/10.1016/j.jse.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 133.Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21:717–726. doi: 10.5435/JAAOS-21-12-717. http://dx.doi.org/10.5435/JAAOS-21-12-717. [DOI] [PubMed] [Google Scholar]
- 134.Shi LL, Boykin RE, Lin A, Warner JJ. Association of suprascapular neuropathy with rotator cuff tendon tears and fatty degeneration. J Shoulder Elbow Surg. 2014;23:339–346. doi: 10.1016/j.jse.2013.06.011. http://dx.doi.org/10.1016/j.jse.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 135.Shi LL, Freehill MT, Yannopoulos P, Warner JJ. Suprascapular nerve: is it important in cuff pathology? Adv Orthop. 2012;(2012):516985. doi: 10.1155/2012/516985. http://dx.doi.org/10.1155/2012/516985. [DOI] [PMC free article] [PubMed]
- 136.Shin C, Lee SE, Yu KH, Chae HK, Lee KS. Spinal root origins and innervations of the suprascapular nerve. Arch Orthop Trauma Surg. 2010;32:235–238. doi: 10.1007/s00276-009-0597-5. http://dx.doi.org/10.1007/s00276-009-0597-5. [DOI] [PubMed] [Google Scholar]
- 137.Shinozaki N, Sano H, Omi R, Kishimoto KN, Yamamoto N, Tashiro M, et al. Differences in muscle activities during shoulder elevation in patients with symptomatic and asymptomatic rotator cuff tears: analysis by positron emission tomography. J Shoulder Elbow Surg. 2014;23:e61–67. doi: 10.1016/j.jse.2013.06.009. http://dx.doi.org/10.1016/j.jse.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 138.Snow BJ, Narvy SJ, Omid R, Atkinson RD, Vangsness CT., Jr Anatomy and histology of the transverse humeral ligament. Orthopedics. 2013;36:e1295–1298. doi: 10.3928/01477447-20130920-23. http://dx.doi.org/10.3928/01477447-20130920-23. [DOI] [PubMed] [Google Scholar]
- 139.Sole G, Osborne H, Wassinger C. Electromyographic response of shoulder muscles to acute experimental subacromial pain. Man Ther. 2014;19:343–348. doi: 10.1016/j.math.2014.03.001. http://dx.doi.org/10.1016/j.math.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 140.Solomonow M, Guanche C, Wink C, Knatt T, Baratta RV, Lu Y. Mechanoreceptors and reflex arc in the feline shoulder. J Shoulder Elbow Surg. 1996;5:139–146. doi: 10.1016/s1058-2746(96)80009-7. [DOI] [PubMed] [Google Scholar]
- 141.Stackhouse SK, Eisennagel A, Eisennagel J, Lenker H, Sweitzer BA, McClure PW. Experimental pain inhibits infraspinatus activation during isometric external rotation. J Shoulder Elbow Surg. 2013;22:478–484. doi: 10.1016/j.jse.2012.05.037. http://dx.doi.org/10.1016/j.jse.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 142.Steenbrink F, de Groot JH, Veeger HE, Meskers CG, van de Sande MA, Rozing PM. Pathological muscle activation patterns in patients with massive rotator cuff tears, with and without subacromial anaesthetics. Man Ther. 2006;11:231–237. doi: 10.1016/j.math.2006.07.004. http://dx.doi.org/10.1016/j.math.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 143.Steinbeck J, Bruntrup J, Greshake O, Potzl W, Filler T, Liljenqvist U. Neurohistological examination of the inferior glenohumeral ligament of the shoulder. J Orthop Res. 2003;21:250–255. doi: 10.1016/S0736-0266(02)00155-9. http://dx.doi.org/10.1016/S0736-0266(02)00155-9. [DOI] [PubMed] [Google Scholar]
- 144.Subhawong TK, Wang KC, Thawait SK, Williams EH, Hashemi SS, Machado AJ, et al. High resolution imaging of tunnels by magnetic resonance neurography. Skeletal Radiol. 2012;41:15–31. doi: 10.1007/s00256-011-1143-1. http://dx.doi.org/10.1007/s00256-011-1143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sulaiman W, Gordon T. Neurbiology of peripheral nerve injury, regeneration, and functional recovery: from bench top research to bedside application. Ochsner J. 2013;13:100–108. NO doi. [PMC free article] [PubMed] [Google Scholar]
- 146.Tamai M, Okajima S, Fushiki S, Hirasawa Y. Quantitative analysis of neural distribution in human coracoacromial ligaments. Clin Orthop Relat Res. 2000:125–134. doi: 10.1097/00003086-200004000-00015. [DOI] [PubMed] [Google Scholar]
- 147.Tapadia M, Mozaffar T, Gupta R. Compressive neuropathies of the upper extremity: update on pathophysiology, classification, and electrodiagnostic findings. J Hand Surg Am. 2010;35:668–677. doi: 10.1016/j.jhsa.2010.01.007. http://dx.doi.org/10.1016/j.jhsa.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tarumoto R, Murakami M, Imai S, Maeda T, Hukuda S. A morphometric analysis of protein gene product 9. 5-, substance P-, and calcitonin gene-related peptide immunoreactive innervation in the shoulder joint of the Japanese macaque. J Shoulder Elbow Surg. 1998;7:522–528. doi: 10.1016/s1058-2746(98)90206-3. [DOI] [PubMed] [Google Scholar]
- 149.Tosounidis T, Hadjileontis C, Georgiadis M, Kafanas A, Kontakis G. The tendon of the long head of the biceps in complex proximal humerus fractures: a histological perspective. Injury. 2010;41:273–278. doi: 10.1016/j.injury.2009.09.015. http://dx.doi.org/10.1016/j.injury.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 150.Tubbs RS, Nechtman C, D’Antoni AV, Shoja MM, Mortazavi MM, Loukas M, et al. Ossification of the suprascapular ligament: A risk factor for suprascapular nerve compression? Int J Shoulder Surg. 2013;7:19–22. doi: 10.4103/0973-6042.109882. http://dx.doi.org/10.4103/0973-6042.109882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Uhthoff HK, Coletta E, Trudel G. Intramuscular fat accumulation and muscle atrophy in the absence of muscle retraction. Bone Join Res. 2014;3:117–122. doi: 10.1302/2046-3758.34.2000275. http://dx.doi.org/10.1302/2046-3758.34.2000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Uhthoff HK, Matsumoto F, Trudel G, Himori K. Early reattachment does not reverse atrophy and fat accumulation of the supraspinatus--an experimental study in rabbits. J Orthop Res. 2003;21:386–392. doi: 10.1016/S0736-0266(02)00208-5. http://dx.doi.org/10.1016/S0736-0266(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 153.Vad VB, Southern D, Warren RF, Altchek DW, Dines D. Prevalence of peripheral neurologic injuries in rotator cuff tears with atrophy. J Shoulder Elbow Surg. 2003;12:333–336. doi: 10.1016/s1058-2746(03)00040-5. http://dx.doi.org/10.1016/S1058-2746(03)00040-5. [DOI] [PubMed] [Google Scholar]
- 154.Vallejo R, Tilley DM, Williams J, Labak S, Aliaga L, Benyamin RM. Pulsed radiofrequency modulates pain regulatory gene expression along the nociceptive pathway. Pain Physician. 2013;16:E601–613. NO doi. [PubMed] [Google Scholar]
- 155.Vangsness CT, Jr, Ennis M, Taylor JG, Atkinson R. Neural anatomy of the glenohumeral ligaments, labrum, and subacromial bursa. Arthroscopy. 1995;11:180–184. doi: 10.1016/0749-8063(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 156.Veeger HE, van der Helm FC. Shoulder function: the perfect compromise between mobility and stability. J Biomech. 2007;40:2119–2129. doi: 10.1016/j.jbiomech.2006.10.016. http://dx.doi.org/10.1016/j.jbiomech.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 157.Voigt M, Jakobsen J, Sinkjaer T. Non-noxious stimulation of the glenohumeral joint capsule elicits strong inhibition of active shoulder muscles in conscious human subjects. Neurosci Lett. 1998;254:105–108. doi: 10.1016/s0304-3940(98)00665-x. [DOI] [PubMed] [Google Scholar]
- 158.Vorster W, Lange CP, Briet RJ, Labuschagne BC, du Toit DF, Muller CJ, et al. The sensory branch distribution of the suprascapular nerve: an anatomic study. J Shoulder Elbow Surg. 2008;17:500–502. doi: 10.1016/j.jse.2007.10.008. http://dx.doi.org/10.1016/j.jse.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 159.Vrbova G. Changes in the motor reflexes produced by tenotomy. J Physiol. 1963;166:241–250. doi: 10.1113/jphysiol.1963.sp007103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ward SR, Hentzen ER, Smallwood LH, Eastlack RK, Burns KA, Fithian DC, et al. Rotator cuff muscle architecture: implications for glenohumeral stability. Clin Orthop Relat Res. 2006;448:157–163. doi: 10.1097/01.blo.0000194680.94882.d3. http://dx.doi.org/10.1097/01.blo.0000194680.94882.d3. [DOI] [PubMed] [Google Scholar]
- 161.Warner JP, Krushell RJ, Masquelet A, Gerber C. Anatomy and relationships of the suprascapular nerve: anatomical constraints to mobilization of the supraspinatus and infraspinatus muscles in the management of massive rotator-cuff tears. J Bone Joint Surg Am. 1992;74:36–45. [PubMed] [Google Scholar]
- 162.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. http://dx.doi.org/10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 2006;88:1699–1704. doi: 10.2106/JBJS.E.00835. http://dx.doi.org/10.2106/JBJS.E.00835. [DOI] [PubMed] [Google Scholar]
- 164.Yamamoto A, Takagishi K, Kobayashi T, Shitara H, Osawa T. Factors involved in the presence of symptoms associated with rotator cuff tears: a comparison of asymptomatic and symptomatic rotator cuff tears in the general population. J Shoulder Elbow Surg. 2011;20:1133–1137. doi: 10.1016/j.jse.2011.01.011. http://dx.doi.org/10.1016/j.jse.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 165.Yamashita T, Minaki Y, Takebayashi T, Sakamoto N, Ishii S. Neural response of mechanoreceptors to acute inflammation in the rotator cuff of the shoulder joint in rabbits. Acta Orthop Scand. 1999;70:137–140. doi: 10.3109/17453679909011251. [DOI] [PubMed] [Google Scholar]
- 166.Yan J, Horiguchi M. The communicating branch of the 4th cervical nerve to the brachial plexus: the double constitution, anterior and posterior, of its fibers. Arch Orthop Trauma Surg. 2000;22:175–179. doi: 10.1007/s00276-000-0175-3. [DOI] [PubMed] [Google Scholar]
- 167.Yang HJ, Gil YC, Jin JD, Ahn SV, Lee HY. Topographical anatomy of the suprascapular nerve and vessels at the suprascapular notch. Clinical Anat. 2012;25:359–365. doi: 10.1002/ca.21248. http://dx.doi.org/10.1002/ca.21248. [DOI] [PubMed] [Google Scholar]
- 168.Yellin H, Eldred E. Spindle activity of the tenotomized gastrocnemius muscle in the cat. Exp Neurol. 1970;29:513–533. doi: 10.1016/0014-4886(70)90077-4. [DOI] [PubMed] [Google Scholar]
- 169.Zanotti RM, Carpenter JE, Blasier RB, Greenfield ML, Adler RS, Bromberg MB. The low incidence of suprascapular nerve injury after primary repair of massive rotator cuff tears. J Shoulder Elbow Surg. 1997;6:258–264. doi: 10.1016/s1058-2746(97)90014-8. [DOI] [PubMed] [Google Scholar]