Abstract
Contrasting results have been reported regarding the associations between plasma total homocysteine (tHcy) and B vitamin levels and age-related macular degeneration (AMD) risk. Thus, we aimed to systematically evaluate these associations. Relevant case control studies in English were identified via a thorough search of the PubMed, Medline, and Embase databases from inception to June 2014. The results were pooled using Review Manager 5.2.1. Eleven studies (including 1072 cases and 1202 controls) were eligible for analysis of tHcy levels; additionally, 3 studies (including 152 cases and 98 controls) were eligible for analysis of folic acid and vitamin B12 levels. The cumulative results demonstrated that the plasma tHcy level among the AMD cases was 2.67 μmol/L (95% confidence interval [CI], 1.60-3.74) higher than that among the controls. In contrast, the vitamin B12 level among the AMD cases was 64.16 pg/mL (95% CI, 19.32-109.00) lower than that among the controls. Subgroup analyses showed that the folic acid level was 1.66 ng/mL (95% CI, 0.10-3.21) lower for the wet type. Together, the results demonstrated that AMD is associated with elevated tHcy levels and decreased vitamin B12 levels. Plasma tHcy may act as a modulator of the risk for AMD based on the current evidence.
Age-related macular degeneration (AMD) is the primary cause of permanent vision loss among individuals greater than 50 years of age in industrialised countries and affects 25-35 million people worldwide1,2,3. Generally, AMD is stratified into two stages, namely, early and late age-related maculopathy (ARM), according to the International Classification and Grading System designed by the International ARM Epidemiological Study Group4. Advanced (late) AMD can be further classified into non-neovascular (dry, atrophic) and neovascular (wet, exudative) types. Few therapeutic or preventative strategies are currently available for the dry type, which constitutes approximately 80% of all late AMD cases5. Anti-vascular endothelial growth factor (VEGF) therapy has demonstrated great benefit for the wet type, although issues, such as the need for repeated injections and non-responses, continue to occur6. Accordingly, new therapies are anticipated.
The exact pathogenesis of AMD remains poorly understood. Several risk factors have been suggested, including advanced age, Caucasian ethnicity, smoking, blue light irradiation, oxidative stress, and genetic factors. Recent epidemiological evidence has implicated a direct association between plasma total homocysteine (tHcy) levels and AMD risk. Plasma tHcy levels may be influenced by many factors. Nutritional factors, including serum vitamin B6, folic acid, and vitamin B12, are common and important regulators of plasma tHcy levels that can be modulated by diet, suggesting a simple homocysteine-lowering therapy. Hyperhomocysteinemia has been demonstrated to be an independent risk factor for cardiovascular disease (CVD) and atherosclerosis7. Interestingly, a link between AMD, atherosclerosis, and CVD has been observed8,9. Moreover, AMD patients exhibit an elevated cardiovascular risk profile and increased prospective CVD risk. These findings imply a common causal pathway among AMD, atherosclerosis and CVD and hyperhomocysteinemia may act as a common etiological role in these diseases, specifically in the induction of endothelial injury and atherosclerosis, both of which are involved in these diseases.
Many studies have been conducted to elucidate the association between AMD and homocysteine. However, the results were inconsistent10,11,12,13,14,15,16,17,18,19,20. Thus, we aimed to combine the current evidence to elucidate the relationship between serum tHcy, folic acid, and vitamin B12 levels and the risk of AMD.
Results
Study characteristics
A flow chart of the screening progress is shown in Fig. 1. Ultimately, 11 studies were included, among which 11 studies (including 1072 cases and 1202 controls) were eligible for analysis of tHcy levels, 3 studies (including 152 cases and 98 controls) were eligible for analysis of folic acid levels, and 3 (including 152 cases and 98 controls) were eligible for analysis of vitamin B9 levels. The number of studies that examined the association of vitamin B6 levels or a polymorphism of methylenetetrahydrofolate reductase (MTHFR), methionine synthase (MS), or cystathionine β-synthase (CBS) with AMD was less than two. Therefore, we did not perform a pooled analysis for these factors. Details regarding the included studies are presented in Table 1.
Figure 1.
Flow chart of the study search and selection strategies.
Table 1. Study design and baseline characteristics of the included studies.
| Study | Year and location | Study design | Study content | AMD type | Number of patients | Age, years mean (SD) | PCT of males (%) | AMD grading method | AMD classification and grading system |
|---|---|---|---|---|---|---|---|---|---|
| Axer-Siegel et al.10 | 2004, Israel | Clinic-based, prospective, cross-sectional | tHcy | group 1. wAMD | group 1. n = 59 | 78 ± 8.4 | 42.37 | Ophthalmologist examination | NA |
| group 2. dAMD | group 2. n = 58 | 76.3 ± 8.4 | 41.38 | ||||||
| group 3. control | group 3. n = 56 | 76.3 ± 8.4 | 48.21 | ||||||
| Nowak et al.11 | 2005, Poland | Clinic-based, case-control | tHcy | group 1. wAMD | group 1. n = 30 | 66.2 ± 3.6 | NA | Ophthalmologist examination | NA |
| B9, B12 | group 2. control | group 2. n = 20 | 65.8 ± 5.2 | NA | |||||
| Coral et al.12 | 2006, India | Clinic-based, case-control | tHcy | group 1. wAMD | group 1. n = 16 | 66 (51–82) | 68.75 | Photographic grading | AREDS classification system |
| GSH, tSH | group 2. control | group 2. n = 20 | 62 (55–75) | 40 | |||||
| Kamburoglu et al.13 | 2006, Turkey | Clinic-based, prospective, cross-sectional | tHcy | group 1. wAMD | group 1. n = 30 | 69.7 ± 7.2 | 50 | Ophthalmologist examination | NA |
| B9, B12 | group 2. dAMD | group 2. n = 30 | 69.9 ± 6.8 | 43.33 | |||||
| group 3. control | group 3. n = 30 | 69.9 ± 7.0 | 36.67 | ||||||
| Seddon et al.14 | 2006, USA | Population-based, cross-sectional, case-control | tHcy | group 1. Late AMD | group 1. n = 222 | 71 ± 5.1 | 45 | Photographic grading | AREDS classification system |
| group 2. control | group 2. n = 184 | 67 ± 4.2 | 36 | ||||||
| Wang et al.15 | 2008, Australia | Population-based, case-control | tHcy | group 1. Early and late AMD | group 1. n = 278 | 75.6 ± 8.5 | NA | Photographic grading | Wisconsin age-related maculopathy grading system |
| group 2. control | group 2. n = 557 | 74.9 ± 7.9 | NA | ||||||
| Ates et al.16 | 2009, Turkey | Clinic-based, cross-sectional | tHcy | group 1. wAMD | group 1. n = 40 | 63.3 ± 5 | 45 | Ophthalmologist examination | NA |
| group 2. control | group 2. n = 40 | 61 ± 4 | NA | ||||||
| Javadzadeh et al.17 | 2010, Iran | Clinic-based, case-control | tHcy | group 1. wAMD | group 1. n = 45 | 71 ± 7 | 40 | Ophthalmologist examination | NA |
| group 2. control | group 2. n = 45 | 69 ± 5 | 40 | ||||||
| Ghosh et al.18 | 2013, India | Clinic-based, case-control | tHcy | group 1. wAMD | group 1. n = 12 | 67.4 ± 6.5 | 41.67 | Ophthalmologist examination | NA |
| group 2. dAMD | group 2. n = 20 | 45 | |||||||
| group 3. control | group 3. n = 32 | 66.5 ± 5.9 | 43.75 | ||||||
| Obeid et al.19 | 2013, Germany | Clinic-based, case-control | tHcy | group 1. wAMD | group 1. n = 31 | 78 (67–86) | 51.61 | Ophthalmologist examination | NA |
| B9, B12 | group 2. dAMD | group 2. n = 38 | 77 (68–86) | 26.32 | |||||
| group 3. control | group 3. n = 48 | 74 (60–81) | 48.94 | ||||||
| Mulero et al.20 | 2014, Spain | Clinic-based, cross-sectional, case-control | tHcy | group 1. wAMD | group 1. n = 163 | 71 ± 7.3 | 49 | Ophthalmologist examination | NA |
| group 2. control | group 2. n = 170 | 71 ± 6.7 | 52 | Ophthalmologist examination | NA |
AMD: age-related macular degeneration; PCT: percentage; tHcy: total homocysteine; NA: not available; AREDS: Age-Related Eye Disease Study; GSH: glutathione; tSH: thiol content.
Pooled analysis
Total homocysteine
The combined difference in the serum homocysteine levels of the eligible studies and the corresponding odds ratio (OR) and 95% confidence interval (CI) are shown in Fig. 2. The dots indicate the estimated mean differences (MDs), and the length of the lines indicates the associated 95% CI. The values to the right of the longitudinal line at 0 represent higher tHcy levels in the AMD patients, whereas the values to the left of the longitudinal line represent higher tHcy levels in the control subjects.
Figure 2.
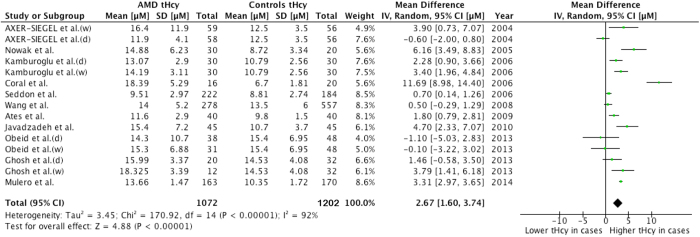
Forest plots for the association of the plasma tHcy levels with AMD.
Overall, the pooled results showed that the serum tHcy level among the AMD patients was 2.67 μmol/L higher than that among the controls; this difference was significant (96% CI, 1.60-3.74) with extreme heterogeneity (I2=92%, P<0.00001). Sensitivity analyses indicated that this result was not excessively influenced by any particular study.
Folic acid
Figure 3 presents the forest plot of the serum folic acid levels in the AMD cases and the controls. This figure can be interpreted in the same manner as Fig. 2 except that the results are expressed in ng/mL. The values to the left of the longitudinal line at 0 represent lower serum folic acid levels in the AMD patients, whereas the values to the right of the longitudinal line indicate lower serum folic acid levels in the controls.
Figure 3.
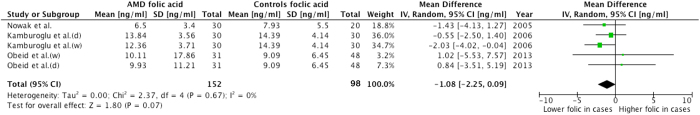
Forest plots for the association of the serum folic acid levels with AMD.
The combined results revealed no difference in the serum folic acid levels between the AMD patients and the controls. The mean difference was −1.08 ng/mL (95% CI, −2.25-0.09), and no between-study heterogeneity was observed (I2=0%, P=0.67).
Vitamin B12
The differences in the pooled plasma vitamin B12 levels between the AMD cases and the controls (Fig. 4) can also be interpreted as described above, with the exception that the results are expressed in pg/mL. The values to the left of the vertical line at 0 represent lower serum vitamin B12 levels in the AMD patients, whereas the values to the right of the longitudinal line indicate lower serum vitamin B12 levels in the controls.
Figure 4.
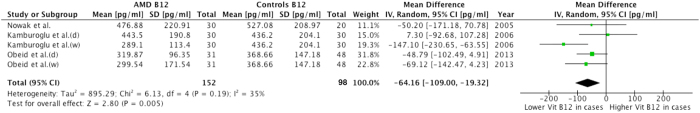
Forest plots for the association of the serum vitamin B12 levels with AMD.
The pooled results showed that the mean serum vitamin B12 level among the AMD patients was 64.16 pg/mL (95% CI, 19.32-109.00) lower than that among the controls, and moderate heterogeneity was observed (I2 = 35%, P = 0.19).
Subgroup analyses
Factors influencing the plasma tHcy levels or the AMD status may bias the pooled results. Therefore, we conducted subgroup analyses according to the AMD stage (late AMD or any AMD), clinical subtype of late AMD (wet AMD or dry AMD), AMD diagnostic method (ophthalmic or photographic), and age and gender differences between the cases and controls.
Only one study (Wang et al. 2008) combined both early and late AMD (any AMD) and revealed a small and non-significant association with the tHcy level (0.50 μmol/L, 95%CI, −0.29-1.29). Studies including only late AMD cases revealed a high and significant association with the estimated tHcy level (2.87 μmol/L, 95%CI, 1.74-4.00). Studies of the folic acid and vitamin B12 levels included only late AMD cases. Therefore, subgroup analysis was not performed in this respect.
The wet and dry AMD types differ significantly with respect to the natural disease course. Regarding tHcy levels, subgroup analyses showed a higher level of homocysteine among the wet AMD patients than among all AMD patients (pooled mean difference 4.14 μmol/L, 95% CI, 2.78-5.51 vs. 0.75 μmol/L, 95% CI, −0.95-2.45). However, the dry type was associated with an insignificant elevation of the plasma tHcy level, in contrast to the result for all AMD patients. Regarding folic acid, the analysis of only the exudative AMD patients revealed significantly lower plasma folic acid levels in these patients but not in all patients (1.66 ng/mL, 95% CI, 0.10-3.21 vs. 1.08 ng/mL, 95% CI, −0.09-2.25). Regarding vitamin B12 levels, subgroup analyses revealed an insignificant difference in levels between the dry AMD patients and the controls, in contrast to the results for all AMD patients. In summary, only exudative AMD was associated with significantly higher tHcy levels and lower folic acid and vitamin B12 levels.
Three studies (Coral et al. 2006; Seddon et al. 2006; Wang et al. 2006) based on photographic grading yielded a slightly higher association between the tHcy level and any AMD type (3.74, 95% CI, 0.69-6.80). The studies based on ophthalmic examination revealed a lower association between tHcy levels and late AMD (2.47 μmol/L, 95% CI, 1.49-3.44). All studies on folic acid and vitamin B12 levels utilised ophthalmic examination. Thus, no subgroup analysis was performed on these factors.
The level of plasma homocysteine was approximately 10% higher in healthy males than in healthy females. Two studies revealed a gender difference between the cases and controls. One of these two studies (Coral et al. 2006), which included a higher percentage of males among the cases, demonstrated higher tHcy levels in the AMD patients, whereas the other study (Obeid et al.), which included a lower percentage of males among the cases, demonstrated a non-significant difference in the tHcy levels between the two groups (11.69 μmol/L, 95% CI, 8.98-14.40, −0.49 μmol/L, 95% CI, −2.93-1.96, respectively).
The incidence of AMD increases with age. In all the eligible studies, the cases and controls were either age-matched or were not significantly different with respect to gender. Furthermore, the mean age of the participants in all of the included studies was greater than 60 years. Therefore, subgroup analysis was not performed for age.
The results of the analysis of publication bias, which was evaluated using the fail-safe number (Nfs), are shown in Table 2. Additional caution should be taken regarding the subtype analyses of the association of folic acid with wet/dry AMD and of vitamin B12 with dry AMD, as the corresponding Nfs values were smaller than the number of included studies, suggesting marked publication bias.
Table 2. Fail-safe number.
| Comparison | Nfs |
|---|---|
| tHcy with any AMD | 1292.03 |
| tHcy with late AMD | 1239.02 |
| tHcy with wet AMD | 900.91 |
| tHcy with dry AMD | 9.47 |
| Folic acid with late AMD | 3.81 |
| Folic acid with wet AMD | 1.17 |
| Folic acid with dry AMD | -1.68 |
| Vitamin B12 with late AMD | 20.97 |
| Vitamin B12 with wet AMD | 10.88 |
| Vitamin B12 with dry AMD | −0.63 |
Nfs: fail-safe number; tHcy: total homocysteine; AMD: age-related macular degeneration.
Discussion
We primarily examined the association of the tHcy level with the risk of AMD in this meta-analysis. Briefly, our study suggested that the plasma tHcy level was elevated but the vitamin B12 level was reduced in AMD patients compared with healthy controls. This association was found to be enhanced for the wet AMD type but insignificant for the dry AMD type.
The overall meta-analyses of the eligible studies confirmed a significantly elevated plasma level of tHcy in the AMD patients. This elevation was significant for the late AMD patients but not for the study including both early and late AMD patients. This trend suggested that the plasma tHcy level may increase with disease progression. Thus, plasma tHcy level may serve as a biomarker of AMD with which to monitor disease status. Moreover, the abnormal metabolism of tHcy may play an etiological role in the development of AMD, particularly for the exudative type. These findings suggest a future direction of research.
The mechanisms underlying hyperhomocysteinemia in AMD remain unclear, but several reasons are implied based on increasing evidence. First, oxidative stress may play a major role. The retina is particularly susceptible to reactive oxygen species (ROS) because of 1) direct exposure to light, 2) high consumption of oxygen, and 3) high concentrations of polyunsaturated fatty acids in the photoreceptors21. Homocysteine is an active oxidising agent that can exacerbate oxidative stress-induced injury22. Second, increased serum homocysteine levels can cause direct epithelial damage and retinal pigment epithelium (RPE) junction disruption23, both of which can lead to neovascularisation. Third, elevated homocysteine levels can promote inflammatory processes that ultimately induce atherosclerosis24. The mechanisms noted above all contribute to the underlying pathogenesis of AMD and atherosclerosis.
Sensitivity analyses of the tHcy levels were performed by excluding one study at a time to demonstrate the effect of each study on the overall pooled results. The results remained within the CI, which indicated that the results were stable. The Nfs, reflecting publication bias, was quite large, indicating that the publication bias was minor.
The classifications of AMD were not uniform in the included studies. One study used the Wisconsin grading system25, two studies used the AREDS classification26, and the other studies simply used the terms “Dry AMD” and “Wet AMD” without clarification (Table 1). However, the definitions of neovascular AMD are similar between each classification system. Thus, the use of different classification systems would not affect these results. Alternatively, some patients in the “intermittent AMD group” based on the AREDS classification who exhibited geographic atrophy may have been included in the “late AMD group” according to other classification systems. This discrepancy may weaken the associations between elevated homocysteine levels and AMD risk. Moreover, the classification of the control groups may also contain discrepancies. As defined in the AREDS classification system, patients displaying drusen with a maximum size < 63 μm and a total diameter < 125 μm are included in the “No AMD group”; however, some of these patients would be included in the “early stage AMG group” according to other classification systems. This discrepancy would also weaken these associations. In this regard, among the currently available systems, the AREDS classification system is highly recommended in future studies because of its good repeatability and accuracy.
The current pooled data showed a non-significant difference in the folic acid levels between the cases and controls, although subgroup analyses showed a significant difference between the AMD wet group and controls. Regarding vitamin B12, the AMD patients exhibited significantly lower levels. This association was stronger in the wet AMD subgroup but was not detected in the dry AMD subgroup. The difference in the results between the wet and dry AMD cases and the controls may be attributed to their apparently distinct disease characteristics.
The causal role of folic acid and vitamin B12 in AMD cannot be well established based on our combined data due to the small number of studies included. Thus, conclusions based on these analyses require further supportive results. Despite the small number of previously published articles, our findings were in accordance with two high-quality studies27,28. The Blue Mountains Eye Study, which reported the 10-year incidence of AMD, revealed that an elevated serum level of total homocysteine increased the probability of developing AMD by approximately 30% but that an increased serum concentration of vitamin B12 decreased the probability of developing AMD by approximately 30%29. The other high-quality randomised placebo-controlled trial with 7.3 years of follow-up, the Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS), reported that the incidence of AMD doubled among individuals with folate or vitamin B12 deficiency at baseline and that daily supplementation with vitamins B6/B9/B12 reduced the probability of developing AMD by 35-40%30. This beneficial effect emerged in the second year of treatment.
The concept that vitamin B supplementation prevents the development of AMD is of great interest; however, this treatment is far from being recommended for clinical use at this stage31. The role of other nutritional supplements has been studied extensively, and the results have been promising. The original Age-Related Eye Disease Study (AREDS) showed that daily supplementation with vitamin C, vitamin E, β-carotene, and zinc reduced the risk of progression by 25% in 5 years32. The AREDS-2 treatment paradigm substituted carotene for lutein and zeaxanthin and also showed benefits33. Whether B vitamins may serve as auxiliary dietary supplements requires further investigation. In addition, we should seriously consider the findings from studies of cardiovascular disease. Although observational studies showed elevated levels of homocysteine and decreased levels of B vitamins, homocysteine-lowering interventions failed to reduce the risk of CVD in randomised placebo-controlled trials34. In conclusion, the limited studies on B vitamins may cause bias in the pooled results regarding folic acid and vitamin B12; thus, these results should be interpreted cautiously. It is also too early to recommend any treatment for clinical use to prevent AMD development.
There are some limitations of our study that should be considered during its interpretation. First, the small number of included studies on B vitamins may lead to publication bias and selective reporting. Second, AMD is a multi-factorial disease that is both genetically and environmentally influenced. An ideal study design would adjust for covariates. Our studies were not adjusted due to the limitations of the original study design. Important covariates, including age, gender, ethnicity, smoking status, atherosclerotic cardiovascular disease, and glucose levels, were adjusted in a portion of the included studies. Third, the distinct classification systems of AMD subtypes may weaken the difference in the homocysteine and B vitamin levels between late AMD or dry AMD patients and controls, although the “wet AMD type” was nearly consistently defined. The AREDS classification system is recommended for future studies. In short, further prospective multi-centre RCTs using a clarified united classification of AMD, larger samples, and various ethnicity that adjust for confounding risk factors may overcome the aforementioned limitations of previous data collection and analysis methods.
Despite the shortcomings mentioned above, the current study revealed preliminarily useful clinical results by providing evidence supporting a potential intervention for both types of AMD. Supplementation of vitamin B or folic acid for AMD prevention/control is biologically plausible based on the currently available evidence. Additional randomised clinical trials in different races are anticipated to aid in determining the efficiency and safety of homocysteine-lowering therapy.
Methods
Our meta-analysis strictly complies with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement35.
Literature search
Two investigators (P.R. Huang and J.H. Jiang) were involved in the literature search of PubMed, Medline, and Embase from inception to June 2014. The search terms used were “age-related macular degeneration”, “homocysteine”, “pyridocine”, “folic acid”, “cobalamin”, “vitamin B6”, “vitamin B9”, “vitamin B12”, “methylenetetrahydrofolate reductase (MTHFR)”, “methionine synthase (MS)”, and “cystathionine β-synthase (CBS)” in various combinations. Related citations in PubMed, along with the references of each retrieved study, were also examined. The searches were restricted to studies published in the English language and performed in humans. Common agreement between two researchers was a prerequisite for the final inclusion of a qualified article. If two or more studies were based on the same cohort, the more definitive study was included.
Inclusion and exclusion criteria
Articles were only included under the following conditions: (1) case control study consisting of a laboratory assessment of (2) plasma total homocysteine, (3) vitamin B (including vitamin B6, B9, and B12), or (4) a polymorphism of MTHFR, MS, or CBS. Studies were excluded if they were non-controlled, in the non-English literature, or reported as abstracts from academic conferences.
Study selection
From the initial 456 relevant articles identified in the databases, 22 full texts were assessed for eligibility. Two reports shared the same study sample, so only one study was included. Nine studies did not report relevant data to enable calculation of the effect size. Ultimately, eleven studies were included.
Data extraction
Two authors (X.D. Sun and P.R. Huang) independently selected the qualified studies according to the inclusion and exclusion criteria listed above. The following data were collected: (1) the homocysteine levels; (2) the vitamin B levels; (3) MTHFR, MS, and CBS polymorphisms; and (4) characteristics of the included studies, such as the first author, the year and geographical location of the study, mean age, gender ratio, type of AMD, AMD grading method, and AMD classification and grading system. Other relevant data that were missing from the reports were acquired from the respective authors.
Statistical analysis
The data were collected and analysed using RevMan software (version 5.2.1, The Nordic Cochrane Centre, Copenhagen, Denmark). The MD and 95% CI were calculated separately for tHcy, folic acid, and cobalamin. The difference between the AMD patients and the controls was displayed using a forest plot. The I2 statistic (ranging from 0 to 100%) was applied to quantify between-study heterogeneity not attributed to chance (I2 = 0-25%, no heterogeneity; I2 = 25-50%, moderate heterogeneity; I2 = 50-75%, large heterogeneity; and I2 = 75-100%, extreme heterogeneity). A random-effects model was employed in this study.
Publication bias was assessed using the fail-safe number (Nfs), and the statistical threshold was 0.05. A calculated Nfs smaller than the number of included studies in a given comparison was considered to indicate significant publication bias. We calculated the significance of Nfs using the formula Nfs0.05 = (∑Z/1.64)2−k, where k represents the number of included studies36,37,38,39.
Additional Information
How to cite this article: Huang, P. et al. Homocysteine and risk of age-related macular degeneration: a systematic review and meta-analysis. Sci. Rep. 5, 10585; doi: 10.1038/srep10585 (2015).
Acknowledgments
This study was supported by the National Basic Research Program of China “973 Program” (2011CB707506), the National Natural Science Foundation of China (81271030), a Shanghai Key Basic Research Grant (11JC141601), a Shanghai Scholar Leadership Grant (12XD1404100, XBR2013081), and a Shanghai Creative Key Medical Research Grant (1341195400).
Footnotes
Author Contributions P.H. and X.S. conceived and designed the experiments; P.H., F.W. and X.S. performed the experiments; J.J. analysed the data; Z.N. and R.W. contributed reagents/materials/analysis tools; and P.H., F.W. and B.S. wrote the manuscript. All authors reviewed the manuscript.
References
- Jager R. D., Mieler W. F. & Miller J. W. Age-related macular degeneration. N Engl J Med 358, 2606–17 (2008). [DOI] [PubMed] [Google Scholar]
- Nussenblatt R. B. et al. Immune Responses in Age Related Macular Degeneration and a possible Long Term Therapeutic Strategy for Prevention. Am J Ophthalmol 158, 5–11 e2 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L. S., Mitchell P., Seddon J. M., Holz F. G. & Wong T. Y. Age-related macular degeneration. Lancet 379, 1728–38 (2012). [DOI] [PubMed] [Google Scholar]
- Bird A. C. et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol 39, 367–74 (1995). [DOI] [PubMed] [Google Scholar]
- Chew E. Y. et al. Long-term effects of vitamins C and E, beta-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology 120, 1604–11 e4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld P. J. et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355, 1419–31 (2006). [DOI] [PubMed] [Google Scholar]
- Wu J., Uchino M., Sastry S. M. & Schaumberg D. A. Age-related macular degeneration and the incidence of cardiovascular disease: a systematic review and meta-analysis. PLoS One 9, e89600 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerling J. R. et al. Age-related macular degeneration is associated with atherosclerosis. The Rotterdam Study. Am J Epidemiol 142, 404–9 (1995). [DOI] [PubMed] [Google Scholar]
- Snow K. K. & Seddon J. M. Do age-related macular degeneration and cardiovascular disease share common antecedents? Ophthalmic Epidemiol 6, 125–43 (1999). [DOI] [PubMed] [Google Scholar]
- Axer-Siegel R. et al. Association of neovascular age-related macular degeneration and hyperhomocysteinemia. Am J Ophthalmol 137, 84–9 (2004). [DOI] [PubMed] [Google Scholar]
- Nowak M. et al. Homocysteine, vitamin B12, and folic acid in age-related macular degeneration. Eur J Ophthalmol 15, 764–7 (2005). [DOI] [PubMed] [Google Scholar]
- Coral K. et al. Plasma homocysteine and total thiol content in patients with exudative age-related macular degeneration. Eye (Lond) 20, 203–7 (2006). [DOI] [PubMed] [Google Scholar]
- Kamburoglu G., Gumus K., Kadayifcilar S. & Eldem B. Plasma homocysteine, vitamin B12 and folate levels in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 244, 565–9 (2006). [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Gensler G., Klein M. L. & Milton R. C. Evaluation of plasma homocysteine and risk of age-related macular degeneration. Am J Ophthalmol 141, 201–3 (2006). [DOI] [PubMed] [Google Scholar]
- Wang J. J. et al. The LOC387715 polymorphism, inflammatory markers, smoking, and age-related macular degeneration. A population-based case-control study. Ophthalmology 115, 693–9 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ates O. et al. Decreased serum paraoxonase 1 activity and increased serum homocysteine and malondialdehyde levels in age-related macular degeneration. Tohoku J Exp Med 217, 17–22 (2009). [DOI] [PubMed] [Google Scholar]
- Javadzadeh A. et al. Plasma oxidized LDL and thiol-containing molecules in patients with exudative age-related macular degeneration. Mol Vis 16, 2578–84 (2010). [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Saha M. & Das D. A study on plasma homocysteine level in age-related macular degeneration. Nepal J Ophthalmol 5, 195–200 (2013). [DOI] [PubMed] [Google Scholar]
- Obeid R. et al. Aqueous humor glycation marker and plasma homocysteine in macular degeneration. Clin Chem Lab Med 51, 657–63 (2013). [DOI] [PubMed] [Google Scholar]
- Mulero J., Manresa N., Zafrilla P. & Losada M. Markers of cardiovascular risk in elderly patients with age-related macular degeneration. Clin Hemorheol Microcirc 58, 447–453 (2014). [DOI] [PubMed] [Google Scholar]
- Beatty S., Koh H., Phil M., Henson D. & Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 45, 115–34 (2000). [DOI] [PubMed] [Google Scholar]
- Welch G. N. & Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med 338, 1042–50 (1998). [DOI] [PubMed] [Google Scholar]
- Nappo F. et al. Impairment of endothelial functions by acute hyperhomocysteinemia and reversal by antioxidant vitamins. JAMA 281, 2113–8 (1999). [DOI] [PubMed] [Google Scholar]
- Silverman M. D. et al. Homocysteine upregulates vascular cell adhesion molecule-1 expression in cultured human aortic endothelial cells and enhances monocyte adhesion. Arterioscler Thromb Vasc Biol 22, 587–92 (2002). [DOI] [PubMed] [Google Scholar]
- Klein R. et al. The Wisconsin age-related maculopathy grading system. Ophthalmology 98, 1128–34 (1991). [DOI] [PubMed] [Google Scholar]
- The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study Report Number 6. Am J Ophthalmol 132, 668–81 (2001). [DOI] [PubMed] [Google Scholar]
- Heuberger R. A. et al. Relation of blood homocysteine and its nutritional determinants to age-related maculopathy in the third National Health and Nutrition Examination Survey. Am J Clin Nutr 76, 897–902 (2002). [DOI] [PubMed] [Google Scholar]
- Albert C. M. et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA 299, 2027–36 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath B., Flood V. M., Rochtchina E., Wang J. J. & Mitchell P. Homocysteine, folate, vitamin B-12, and 10-y incidence of age-related macular degeneration. Am J Clin Nutr 98, 129–35 (2013). [DOI] [PubMed] [Google Scholar]
- Christen W. G., Glynn R. J., Chew E. Y., Albert C. M. & Manson J. E. Folic acid, pyridoxine, and cyanocobalamin combination treatment and age-related macular degeneration in women: the Women’s Antioxidant and Folic Acid Cardiovascular Study. Arch Intern Med 169, 335–41 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. Should we be taking B vitamins to prevent age-related macular degeneration? Not yet, but worth doing more research. Am J Clin Nutr 98, 4–5 (2013). [DOI] [PubMed] [Google Scholar]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 119, 1417–36 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew E. Y. et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol 132, 142–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti-Carvajal A. J., Sola I., Lathyris D., Karakitsiou D. E. & Simancas-Racines D. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev 1, CD006612 (2013). [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. & Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151, 264–9, W64 (2009). [DOI] [PubMed] [Google Scholar]
- Niu W., Qi Y., Qian Y., Gao P. & Zhu D. The relationship between apolipoprotein E epsilon2/epsilon3/epsilon4 polymorphisms and hypertension: a meta-analysis of six studies comprising 1812 cases and 1762 controls. Hypertens Res 32, 1060–6 (2009). [DOI] [PubMed] [Google Scholar]
- Niu W. & Qi Y. Association of alpha-adducin and G-protein beta3 genetic polymorphisms with hypertension: a meta-analysis of Chinese populations. PLoS One 6, e17052 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue P., Niu W. Q., Jiang Z. Y., Zheng M. H. & Fei J. A meta-analysis of apolipoprotein E gene epsilon2/epsilon3/epsilon4 polymorphism for gallbladder stone disease. PLoS One 7, e45849 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P., Niu W., Ni Z., Wang R. & Sun X. A meta-analysis of anti-vascular endothelial growth factor remedy for macular edema secondary to central retinal vein occlusion. PLoS One 8, e82454 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]