Abstract
A robust and sensitive ultra-low flow liquid chromatography (UFLC) method that can reproducibly, at reasonable cost, detect low concentrations of piperine from human plasma is necessary. Piperine in plasma was separated and quantified by a gradient method using ultraviolet detection at a maximal absorbance wavelength of 340 nm. An aliquot was injected onto a reversed-phase column Waters SymmetryShield, 2.1 × 100 mm, 3.5 μm, C18 column, attached to a Waters absorbosphere, 4.6 × 30 mm, C18 guard column and eluted with a mobile phase containing a mixture of acetonitrile/water/ acetic acid (25:74.9:0.1, v/v/v) on line A and acetonitrile/acetic acid (99.9:0.1, v/v) on line B. The flow rate was 0.3 mL/min. The gradient method consisted of an opening condition of 20% pump B, with a linear increase to 37% pump B over 8 min, then a linear increase to 100% pump B at 11 min, 2 min at 100% pump B, and then a return to the opening condition (20% pump B) via a linear gradient over 2 min, followed by 5 min re-equilibration at opening conditions. The total run time was 20 min for each sample. All samples were processed protected from ambient light to avoid isomerization of piperine. The plasma assay was linear with R = 0.9995, with a lower limit of detection [signal-to-noise (S/N) > 5:1] of 100 pg of piperine loaded into the analytical system with acceptable accuracy and precision. Extraction recoveries of piperine from human plasma were 88% for quality control high (QCH), 93% for quality control medium (QCM), and 90% for quality control low (QCL), and the matrix effect was <12%. Piperine was quantifiable from a 50 mg oral dose given to human volunteers. A UFLC method for the rapid assay of human plasma with sensitivity to detect as low as 5 ng/mL piperine was developed. The method sensitivity equals that of liquid chromatography/tandem mass spectrometry (LC/MSMS) methods with much less cost.
Keywords: Liquid chromatography assay, piperine, natural compounds, bioavailability
Introduction
Natural compounds, such as alkaloids and polyphenols, from spices and fruits have been shown to have medicinal properties, such as anti-inflammatory and anticarcinogenic activity in many pre-clinical studies (1–7). Despite highly active, multi-mechanism anticarcinogenesis efficacy of polyphenolic compounds, such as curcumin, resveratrol, and epigallocatechin gallate, in vitro and in vivo, early phase human clinical trials have been limited by poor bioavailability (8,9). Piperine (1-piperoyl piperidine) is a major alkaloid of black pepper and hot peppers (10). Piperine may be a useful, bioavailable, cancer-risk-reductive intervention (“chemopreventive agent”). A 50 mg/kg body weight piperine dose reversed the benzo(a)pyrene-induced model of lung carcinogenesis in mice (11). Piperine has single-agent anti-neoplastic activity in melanoma and lung cancer cell lines (12, 13) and inhibits proliferation of colon cancer cell lines (14). Piperine has also been shown to have significant anti-inflammatory and anti-arthritic effects in vitro and in rat arthritis models (15)
In addition to its anti-inflammatory and anticarcinogenic activities, piperine enhances the absorption of drugs and many dietary compounds through multiple mechanisms. For example, piperine increases intestinal brush border membrane (BBM) fluidity and microvilli length (16). It inhibits hepatic CYP3A4 drug metabolism and blocks the P-glycoprotein (ABCB1 or MDR1) drug efflux pump in rodents and humans (17). Piperine downregulates intestinal and hepatic glucuronidation and sulfation. Through these mechanisms, piperine enhances bioavailability of dietary polyphenols, such as curcumin (17). Piperine, 5–50 mg/day in human clinical trials, enhances blood concentrations of many drugs, such as vasicine, pyrazinamide, rifampicin, isoniazid, propranolol, theophylline, and phenytoin, in humans (18–20) and dietary substances, such as β-carotene, water-soluble vitamin B6, vitamin C, selenium, epigallocatechin gallate, and curcumin (10, 20–22). Piperine has mild toxicities, including grade 1 nausea and diarrhea or dyspepsia at high doses (50 mg/day), and is classified as generally regarded as safe (GRAS) (18). Piperine is currently marketed as a nutraceutical to enhance the bioavailability of other micronutrients, such as vitamins and coenzyme Q10, as well as some anti-seizure medications, such as phenytoin (23, 24).
A robust and sensitive analytical method that can reproducibly, at reasonable cost, detect low concentrations of piperine from human plasma is necessary for future studies of piperine. Assays described to date include high-performance liquid chromatography (HPLC), magnetic resonance spectroscopy, and mass spectroscopy methods (25–27). There are no published liquid chromatography/tandem mass spectrometry (LC/MSMS) methods for the assay of piperine from human plasma. Although such a method would be highly sensitive, it is too expensive for clinical use to repetitively assay hundreds of biosamples. A HPLC–ultraviolet (UV) assay in rat plasma was shown to have good sensitivity (28). However, the ubiquitousness of piperine in the human diet and the presence of lipids in the plasma of humans makes the assay of piperine from human plasma more complex than rodent plasma. The aim in this study is to develop and validate a cost-effective ultra-low flow liquid chromatography (UFLC) assay capable of detecting low nanograms per milliliter concentrations of piperine specifically from human plasma.
Materials and Methods
Chemicals and Reagents
Piperine and β-17-estradiol acetate were obtained from Sigma (St. Louis, MO). HPLC-grade acetonitrile, methanol, water, and ethyl acetate were purchased from Honeywell Burdick (Jackson, MI) and acetic acid was purchased from EMD Chemicals, Inc. (Darmstadt, Germany).
Instrumentation and Equipment
The UFLC system consisted of a Shimadzu LC-2010 model with an autosampler, a UV–vis detector operated using EZStart chromatography software.
Mobile-Phase Reagent Preparation
In a 1 L glass container, the following reagents were measured: 750 mL of double-distilled water and 249 mL of acetronitrile with 1 mL of acetic acid for line A and 999 mL of acetonitrile with 1 mL of acetic acid for line B. The mixed reagents were degassed using sonication.
Extracting Reagent Preparation
A total of 190 mL of ethyl acetate and 10 mL of methanol were mixed together. This reagent can be stored at room temperature (RT) and is stable for 4 weeks.
Preparation of Calibration Standards in Human Plasma
Piperine (5 mg) was dissolved in methanol and made to a volume of 25 mL in a volumetric flask to achieve a final concentration of 200 μg/mL as the working stock solution. This stock solution was prepared fresh as required. On the assay day, the working solution of 200 μg/mL was diluted serially 10-fold to obtain lower working solutions, with lowest concentration of 10 ng/mL. Calibration was performed by spiking pooled plasma obtained from volunteer healthy adult humans to produce the following piperine concentrations: lowest at 10 ng/mL to highest at 10 μg/mL (20 pg to 200 ng of piperine injected into the analytical system). For quality control samples, human plasma was spiked with piperine 10 ng/mL as low, 250 ng/mL as medium, and 9500 ng/mL as high concentrations. For the lower limit of quantitation (LLOQ), 5 ng/mL piperine was spiked into plasma. A subset of the prepared piperine standards was re-assayed.
Human plasma samples were collected using heparinized vacutainer tubes from healthy volunteers. The samples were stored at −80 °C. A total of nine independent plasma samples were prepared. Each sample was spiked with varying amounts of piperine from the previously prepared stock solution.
Internal Standard Preparation
A total of 6.25 mg of β-17-estradiol acetate was weighed and dissolved in 1 mL of methanol. Methanol was added to the solution and brought to volume in a 25 mL volumetric flask to obtain a final concentration of 250 μg/mL. This reagent when stored at −80 °C and is stable for 3 months in a volumetric flask.
Sample Preparation
For quality control, similar to the method of Heath et al. (29), a known concentration of piperine standard was pipeted into 200 μL of pooled, normal human plasma using a Hamilton syringe. Unknown and quality control plasma samples were each pipeted, using a micropipet (Rainin, Emeryville, CA), into 2 mL microcentrifuge tubes (USA Scientific, Ocala, FL). All sample processing was performed in a minimal light environment. To each tube, 80 μL of double-distilled water was added. The tubes were capped and mixed for 30 s at high speed by vortex (Fisher Scientific). A total of 20 μL of internal standard (250 μg/mL β-17-estradiol acetate) was added to each tube, along with 20 μL of piperine stock varying from the lowest concentration of 0.005 μg/mL to highest concentration of 10 μg/mL. A total of 200 μL of plasma was added to each tube. The tubes were capped and mixed by vortexing for 30 s. Then, 500 μL of the extraction reagent (95% ethyl acetate/5% methanol) was added to each tube. The tubes were capped, vortexed at high speed for 30 s, and then centrifuged at 1000 rpm for 5 min in an Eppendorf microcentrifuge (Brinkmann Instruments, Westbury, NY). After centrifugation, the supernatant organic layer was extracted 3 times and approximately 420 μL was carefully removed into a clean microcentrifuge tube and dried under a stream of room air using a low heat setting. The dried extract was resuspended in 100 μL of methanol, capped, and vortexed at medium speed for 30 s. Contents of each tube were then transferred to an injection sample vial (Chromacol 1.1 mL vials) (about 80 μL), and 10 μL was injected into the UFLC column for assay.
UFLC Analytical Method
Piperine in plasma was separated and quantified by a gradient method using UV detection at a maximal absorbance wavelength of 340 nm. An aliquot (10 μL) was injected onto a reversed-phase column Waters SymmetryShield, 2.1 × 100 mm, 3.5 μm, C18 column, attached to a Waters absorbosphere, 4.6 × 30 mm, C18 guard column (Waters, Milford, MA) and eluted with a mobile phase containing a mixture of acetonitrile/water/acetic acid (25:74.9:0.1, v/v/v) on line A and acetonitrile/acetic acid (99.9:0.1, v/v) on line B. The flow rate was 0.3 mL/min. The gradient method consisted of an opening condition of 20% pump B, with a linear increase to 37% pump B over 8 min, then a linear increase to 100% pump B at 11 min, 2 min at 100% pump B, and then a return to the opening condition (20% pump B) via a linear gradient over 2 min, followed by 5 min re-equilibration at opening conditions. The total run time was 20 min for each sample. The quantitation of the piperine peak area ratio (piperine to internal standard) is based on standard curves in plasma. A linear curve was generated from analysis of six different standard concentrations.
Validation Procedures
Calibration Curve and LLOQ of Piperine in Methanol
Three separate calibration curves with eight points of known concentrations of piperine, including 10, 50, 100, 500, 1000, 5000, 10 000, and 50 000 ng/mL (100 pg to 500 ng loaded onto the column) in methanol and methanol blanks, were performed within one 24 h period for intraday variability. For interday variability, three separate calibration curves with duplicates were performed on 3 consecutive days. HPLC-grade methanol was used to prepare standards and as the blank. LLOQ in methanol was determined with a signal-to-noise (S/N) ratio > 5:1.
Calibration Curve of Piperine in Human Plasma
The plasma calibration curve concentrations were 5, 10, 50, 100, 500, 5000, and 10 000 ng/mL (100 pg to 200 ng piperine loaded onto the column). These plasma samples were injected into the analytical system at decreasing concentrations to achieve a S/N ratio of >5:1. The analyte/internal standard ratio was calculated for each plasma sample by dividing the area of the piperine peak by the area of the internal standard, β-estradiol acetate peak. Standard curves of piperine in plasma were constructed by linear regression analysis of the ratio of the piperine peak area/internal standard area to the piperine concentration.
Accuracy and Precision
The accuracy of the assay was determined by analyzing five separate plasma samples spiked with piperine at the LLOQ of 5 ng/mL, quality control low (QCL) limit of 10 ng/mL, quality control medium (QCM) limit of 250 ng/mL, and quality control high (QCH) limit of 9500 ng/mL concentrations in replicates in five analytical runs together with an independently prepared, triplicate calibration curve. Accuracy was calculated at each test concentration as (mean measured concentration/ nominal concentration) × 100%. The assay precision was determined by assaying five replicates of low, medium, and high concentrations (as detailed above) prepared from one sample. Assay precision was calculated by analysis of variation (ANOVA). Back-calculated concentrations of calibration and QC samples were entered with the run number as a factor. The intra- and interassay precisions were calculated from the resulting mean squares of the within runs and mean squares of the between runs, respectively.
Selectivity and Specificity
To determine whether endogenous matrix constituents interfered with the assay, six individual batches of control, piperine-free plasma were processed and analyzed according to the described procedures. Responses of piperine at the LLOQ concentration were compared to the blank samples.
Extraction Recovery and Matrix Effect
We calculated the extraction recovery of piperine from human plasma by comparing the absolute response of an extract of control plasma to which piperine was added after extraction to the absolute response of an extract of plasma to which the same amount of piperine was added before extraction for low, medium, and high concentrations of piperine. Samples were run in two sets with replicates. The matrix effect on piperine recovery by plasma matrix components was defined as the effect on the signal when comparing the absolute response of an extract of control plasma to which piperine was added after the extraction to the absolute response of reconstitution solvent to which the same amount of piperine was added.
Mass Spectrometry
The metabolites from plasma and neat standard piperine were subjected to LC/MSMS studies on Thermo Finnigan Surveyor HPLC fitted with a diode array detector (set for 200–600 nm) and a LTQ linear ion-trap mass spectrometer. The Waters C18 reverse-phase column was used for the UFLC assay. The electrospray ionization (ESI) was used in positive mode to produce mass spectra in the scan range of 150–2000. The capillary temperature was 275 °C; the tube lens voltage was 73; the sheath gas flow rate was 45; and the aux gas was 10. The MSMS conditions were optimized by infusion experiments with piperine standard.
Demonstration of Applicability to Biological Samples
To demonstrate the applicability of the method, the concentration of piperine in the plasma of two healthy human volunteers who consumed a single dose of 50 mg of piperine (donated by Sabinsa Co., Piscataway, NJ) orally was assayed, with the University of Michigan Institutional Review Board (IRB) approval. Blood samples were collected into heparinized tubes at 0.5, 1, 1.5, 2, 3, 5, and 24 h post-dose. The plasma supernatant was removed after the sample was centrifuged at 1258g (2500 rpm) at 4 °C with an Eppendorf 5810R centrifuge.
Results and Discussion
Chromatography
Piperine was diluted in methanol and injected into the starting mobile-phase conditions [80% acetonitrile/water/acetic acid (75:24.9:0.1, v/v/v) on line A and 20% acetonitrile/acetic acid (99.5:0.5, v/v) on line B]. Under the chromatographic conditions described above, the retention time of piperine was in the range of 7.0–7.2 min (Figure 1). The internal standard β-17-estradiol acetate, used in all quantification experiments, had a retention time of 14–14.4 min (Figure 1).
Figure 1.
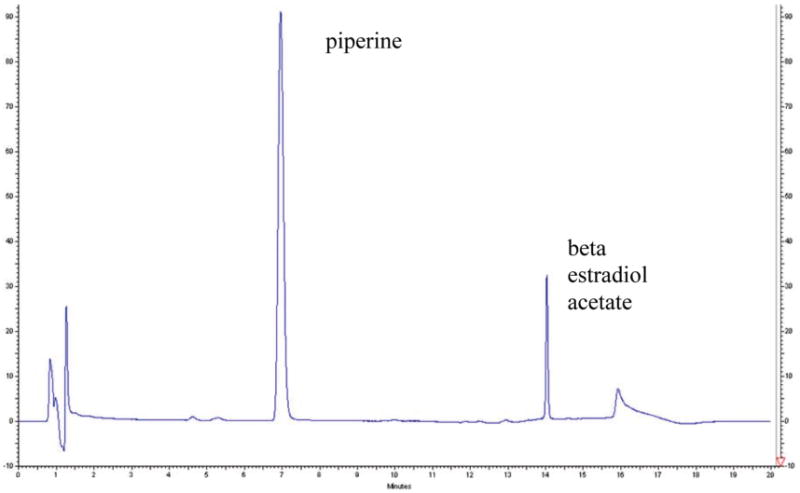
Chromatogram showing piperine and β-estradiol acetate internal standard peaks in plasma.
Light and pH Effects
Piperine (Figure 1 in the Supporting Information) is an alkaloid from black and long peppers, with maximal absorbance at 340 nm. The photo sensitivity of piperine was demonstrated by a series of experiments, with sample processing in full ambient light and red light yielding split peaks, while processing in minimal ambient light resulted in a single peak (Figure 2 in the Supporting Information). As little as 30 min of exposure to ambient light caused isomerization to chavicine, isochavicine, and isopiperine, as previously reported (25). Under red light conditions, we observed isomer formation at 6 h of red-light exposure. Protection from ambient daylight or fluorescent lighting during sample processing results in no isomerization and a single piperine peak.
Selectivity and Specificity
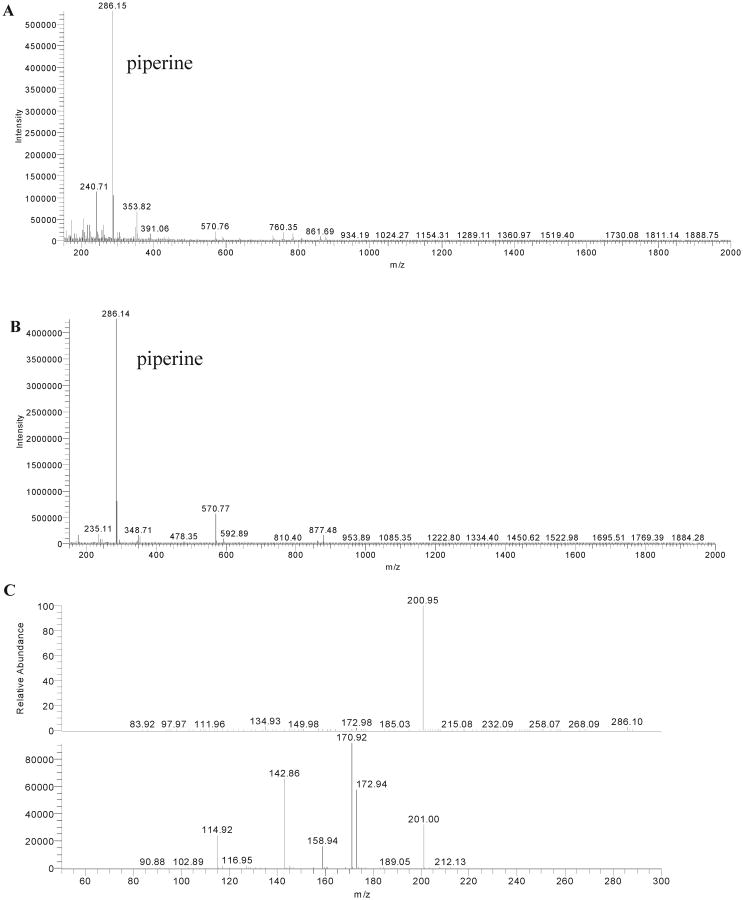
To determine whether endogenous matrix constituents interfered with the assay, blank human plasma samples from six different subjects were assayed and found to have a small peak at 7 min of retention time with the spectrum of piperine at 340 nm in every sample. To confirm whether this peak was indeed piperine, LC/MSMS analysis was performed. The extracted ion chromatogram (XIC) from the total ion chromatogram (TIC) was obtained (data not shown). The complete fragmentation pattern of the unknown endogenous peak at 7 min of retention time from plasma was obtained by LC/ MS (Figure 2B), LC/MSMS (Figure 2C), and further with MS3 studies and compared to that of the piperine standard in methanol (Figure 2A) (full fragmentation pattern of the piperine standard in methanol is not shown but was found to be an exact duplicate of that in plasma). The molecular mass of the metabolite from plasma was found to be equal to the molecular mass of the metabolite from piperine of 285 Da. The piperine standard in methanol (molecular mass of 285 Da) gave a [M + H]+ peak of m/z 286 Da, with a fragment ion of m/z 201 Da formed by the loss of the piperidine moiety with a molecular mass of 85 Da (mass spectrum not shown). The molecular ion peak profile of the metabolite from plasma (Figure 2C) was superimposable with piperine in methanol of 286 Da[M + H]+, with a base peak at m/z 201 Da formed by the loss of a fragment of 85 Da, along with a superimposable UV profile at 341 nm and the same RT on LC, confirming that the metabolite from plasma was piperine.
Figure 2.
LC/MS for (A) piperine in methanol and (B) piperine in blank human plasma extraction and (C) LC/MSMS for fragmentation of piperine in human plasma.
Therefore, for subsequent plasma calibration standards, accuracy, and precision experiments and for detection in biological samples, blank plasma extractions were assayed and the area under the curve for the resultant endogenous piperine peak was subtracted from the post-spiking extraction peak to obtain the corrected area under the curve corresponding to the known concentration of piperine that was spiked into each sample.
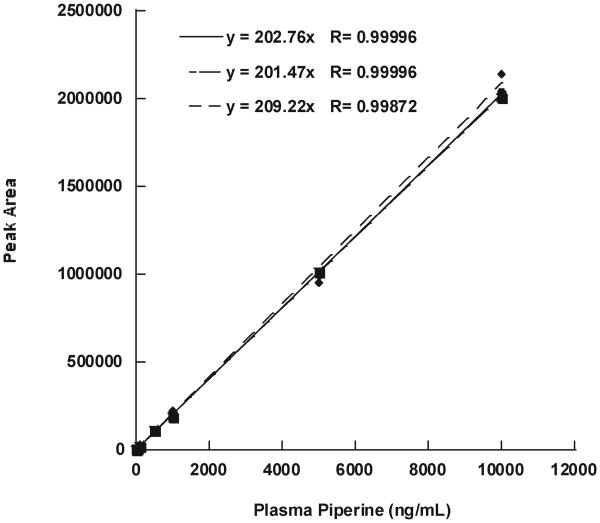
Calibration Curve and LLOQ
For calibration in plasma, the assay was linear, with regression coefficients >0.99955 for each of the three standard curves when forced to a y intercept of 0 (Figure 3). The lower limit of detection of piperine in human plasma was 1 ng/mL, with a S/N ratio of >5:1, similar to the HPLC method developed for rat plasma (28). However, the United States Food and Drug Administration defines LLOQ as the point within 3 times the lower limit of detection, which has less than 20% coefficient of variability in precision and is linear with the rest of the standard curve. The endogenous piperine peak caused variability of precision at concentrations lower than 10 ng/ mL; therefore, 10 ng/mL was defined as LLOQ in plasma, and 5 ng/mL was defined as the lower limit of detection, despite the S/N ratio being > 10:1 at this concentration. The sensitivity and linearity in humans is similar to that demonstrated in rats by Bajad et al. using HPLC–UV detection, despite the more complex lipid-rich human plasma matrix and endogenous peak (28). There are no published LC/MSMS methods for assaying piperine in human plasma for a direct comparison.
Figure 3.
Three intraday piperine calibration curves in plasma.
Accuracy and Precision
Data in Table 1 show precision and accuracy of piperine extracted from plasma at QCL, QCM, and QCH concentrations.
Table 1. Assay Performance and Recoveries of Piperine from Human Plasma and Matrix Effect with Coefficient of Variation (CV) for the Quantitation of QCL, QCM, and QCH in Human Plasma.
| plasma concentration (ng/mL) | accuracy (%) | intra-assay precision (%) | recovery (%) | CV (%) | matrix effect (%) | CV (%) |
|---|---|---|---|---|---|---|
| 10 (QCL) | 131.82 | 7.27 | 90.43 | 18.17 | 9.57 | 171.75 |
| 250 (QCM) | 89.40 | 0.40 | 93.48 | 3.08 | 6.52 | 44.21 |
| 9500 (QCH) | 98.91 | 0.40 | 88.49 | 11.13 | 11.51 | 85.61 |
Extraction
Extraction recoveries of piperine from human plasma were 88% for high concentration, 93% for medium concentration, and 90% for low concentration, and the matrix effect was less than 12% (Table 1). CV of matrix effect was large due to endogenous piperine in the plasma.
Stability
Three calibration curves were performed within one 24 h period, as well as calibration curves on 3 separate days from one set of stocks, to test stability of piperine in methanol solution at RT. Storage, refrigeration at 4 °C, and freezing at −30 °C of piperine stock in methanol for weeks did not change the slope of our calibration curves significantly if samples were protected from light. Piperine is stable in methanol over weeks and at different temperature storage conditions. It is, however, not stable and generates isomers with light exposure (30), as shown in Figure 2A in the Supporting Information. Setting the temperature of the column using the column oven at (25 °C) versus running at RT with minor variance with light/dark cycles also did not change our calibration curve slopes.
Demonstration of Applicability to Biological Samples
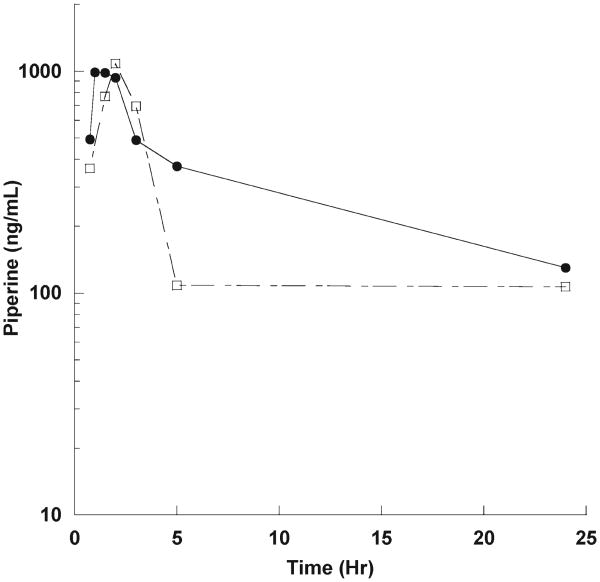
To demonstrate applicability of this UFLC assay to human biosamples, we assayed the plasma of two healthy male volunteers who took a single 50 mg oral dose of piperine, under an IRB approved protocol. Following oral piperine dosing, blood was taken at 45 min to 24 h time points. The time concentrations curves are shown in Figure 4. The peak concentration was 949 ng/mL at 1 h for subject 1 and 782 ng/mL at 3 h for subject 2 after a single 50 mg oral dose.
Figure 4.
Time concentration curve of oral piperine (50 mg) in two healthy male volunteers.
These data strongly suggest but do not conclusively establish that piperine is bioavailable with oral dosing. A formal pharmacokinetic study in 10–15 subjects per dose level must be conducted to define pharmacokinetics. This UFLC assay can be applied to human clinical trials studying the potential effects of piperine in drug bioavailability enhancement (18, 31), cancer prevention (11, 32), or cancer treatment, in concert with chemotherapy (33).
Supplementary Material
Footnotes
Supporting InformationAvailable: Piperine molecular structure (Figure 1) and piperine chromatogram in (A) ambient light, (B) red light, and (C) minimal light processing (Figure 2). This material is available free of charge via the Internet at http://pubs.acs.org.
Literature Cited
- 1.Beltz LA, Bayer DK, Moss AL, Simet IM. Mechanisms of cancer prevention by green and black tea polyphenols. Anticancer Agents Med Chem. 2006;6:389–406. doi: 10.2174/187152006778226468. [DOI] [PubMed] [Google Scholar]
- 2.Biesalski HK. Polyphenols and inflammation: Basic interactions. Curr Opin Clin Nutr Metab Care. 2007;10:724–728. doi: 10.1097/MCO.0b013e3282f0cef2. [DOI] [PubMed] [Google Scholar]
- 3.Bonfili L, Cecarini V, Amici M, Cuccioloni M, Angeletti M, Keller JN, Eleuteri AM. Natural polyphenols as proteasome modulators and their role as anti-cancer compounds. FEBS J. 2008;275:5512–5526. doi: 10.1111/j.1742-4658.2008.06696.x. [DOI] [PubMed] [Google Scholar]
- 4.D'Archivio M, Filesi C, Di Benedetto R, Gargiulo R, Giovannini C, Masella R. Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanita. 2007;43:348–361. [PubMed] [Google Scholar]
- 5.Kampa M, Nifli AP, Notas G, Castanas E. Polyphenols and cancer cell growth. Rev Physiol Biochem Pharmacol. 2007;159:79–113. doi: 10.1007/112_2006_0702. [DOI] [PubMed] [Google Scholar]
- 6.Magrone T, Candore G, Caruso C, Jirillo E, Covelli V. Polyphenols from red wine modulate immune responsiveness: Biological and clinical significance. Curr Pharm Des. 2008;14:2733–2748. doi: 10.2174/138161208786264098. [DOI] [PubMed] [Google Scholar]
- 7.Kumar N, Shibata D, Helm J, Coppola D, Malafa M. Green tea polyphenols in the prevention of colon cancer. Front Biosci. 2007;12:2309–2315. doi: 10.2741/2233. [DOI] [PubMed] [Google Scholar]
- 8.Zick SM, Djuric Z, Ruffin MT, Litzinger AJ, Normolle DP, Alrawi S, Feng MR, Brenner DE. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol, Biomarkers Prev. 2008;17:1930–1936. doi: 10.1158/1055-9965.EPI-07-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, Brenner DE. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol, Biomarkers Prev. 2008;17:1411–1417. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khajuria A, Thusu N, Zutshi U, Bedi KL. Piperine modulation of carcinogen induced oxidative stress in intestinal mucosa. Mol Cell Biochem. 1998;189:113–118. doi: 10.1023/a:1006877614411. [DOI] [PubMed] [Google Scholar]
- 11.Selvendiran K, Banu SM, Sakthisekaran D. Protective effect of piperine on benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Clin Chim Acta. 2004;350:73–78. doi: 10.1016/j.cccn.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Pradeep CR, Kuttan G. Piperine is a potent inhibitor of nuclear factor-κB (NF-κB), c-Fos, CREB, ATF-2 and proinflammatory cytokine gene expression in B16F-10 melanoma cells. Int Immunopharmacol. 2004;4:1795–1803. doi: 10.1016/j.intimp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Pradeep CR, Kuttan G. Effect of piperine on the inhibition of lung metastasis induced B16F-10 melanoma cells in mice. Clin Exp Metastasis. 2002;19:703–708. doi: 10.1023/a:1021398601388. [DOI] [PubMed] [Google Scholar]
- 14.Duessel S, Heuertz RM, Ezekiel UR. Growth inhibition of human colon cancer cells by plant compounds. Clin Lab Sci. 2008;21:151–157. [PubMed] [Google Scholar]
- 15.Bang JS, Oh da H, Choi HM, Sur BJ, Lim SJ, Kim JY, Yang HI, Yoo MC, Hahm DH, Kim KS. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1β-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res Ther. 2009;11:R49. doi: 10.1186/ar2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khajuria A, Thusu N, Zutshi U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: Influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine. 2002;9:224–231. doi: 10.1078/0944-7113-00114. [DOI] [PubMed] [Google Scholar]
- 17.Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J Pharmacol Exp Ther. 2002;302:645–650. doi: 10.1124/jpet.102.034728. [DOI] [PubMed] [Google Scholar]
- 18.Bano G, Raina RK, Zutshi U, Bedi KL, Johri RK, Sharma SC. Effect of piperine on bioavailability and pharmacokinetics of propranolol and theophylline in healthy volunteers. Eur J Clin Pharmacol. 1991;41:615–617. doi: 10.1007/BF00314996. [DOI] [PubMed] [Google Scholar]
- 19.Hiwale AR, Dhuley JN, Naik SR. Effect of co-administration of piperine on pharmacokinetics of β-lactam antibiotics in rats. Indian J Exp Biol. 2002;40:277–281. [PubMed] [Google Scholar]
- 20.Lambert JD, Hong J, Kim DH, Mishin VM, Yang CS. Piperine enhances the bioavailability of the tea polyphenol (–)-epigallocatechin-3-gallate in mice. J Nutr. 2004;134:1948–1952. doi: 10.1093/jn/134.8.1948. [DOI] [PubMed] [Google Scholar]
- 21.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 22.Zutshi RK, Singh R, Zutshi U, Johri RK, Atal CK. Influence of piperine on rifampicin blood levels in patients of pulmonary tuberculosis. J Assoc Physicians India. 1985;33:223–224. [PubMed] [Google Scholar]
- 23.Badmaev V, Majeed M, Prakash L. Piperine derived from black pepper increases the plasma levels of coenzyme Q10 following oral supplementation. J Nutr Biochem. 2000;11:109–113. doi: 10.1016/s0955-2863(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 24.Bano G, Amla V, Raina RK, Zutshi U, Chopra CL. The effect of piperine on pharmacokinetics of phenytoin in healthy volunteers. Planta Med. 1987;53:568–569. doi: 10.1055/s-2006-962814. [DOI] [PubMed] [Google Scholar]
- 25.Ternes W, Krause EL. Characterization and determination of piperine and piperine isomers in eggs. Anal Bioanal Chem. 2002;374:155–160. doi: 10.1007/s00216-002-1416-6. [DOI] [PubMed] [Google Scholar]
- 26.Bajad S, Johri RK, Singh K, Singh J, Bedi KL. Simple high-performance liquid chromatography method for the simultaneous determination of ketoconazole and piperine in rat plasma and hepatocyte culture. J Chromatogr, A. 2002;949:43–47. doi: 10.1016/s0021-9673(01)01260-2. [DOI] [PubMed] [Google Scholar]
- 27.Bajad S, Coumar M, Khajuria R, Suri OP, Bedi KL. Characterization of a new rat urinary metabolite of piperine by LC/NMR/MS studies. Eur J Pharm Sci. 2003;19:413–421. doi: 10.1016/s0928-0987(03)00143-x. [DOI] [PubMed] [Google Scholar]
- 28.Bajad S, Singla AK, Bedi KL. Liquid chromatographic method for determination of piperine in rat plasma: Application to pharmacokinetics. J Chromatogr, B: Anal Technol Biomed Life Sci. 2002;776:245–249. doi: 10.1016/s1570-0232(02)00352-5. [DOI] [PubMed] [Google Scholar]
- 29.Heath DD, Pruitt MA, Brenner DE, Rock CL. Curcumin in plasma and urine: Quantitation by high-performance liquid chromatography. J Chromatogr, B: Anal Technol Biomed Life Sci. 2003;783:287–295. doi: 10.1016/s1570-0232(02)00714-6. [DOI] [PubMed] [Google Scholar]
- 30.Kozukue N, Park MS, Choi SH, Lee SU, Ohnishi-Kameyama M, Levin CE, Friedman M. Kinetics of light-induced cis–trans isomerization of four piperines and their levels in ground black peppers as determined by HPLC and LC/MS. J Agric Food Chem. 2007;55:7131–7139. doi: 10.1021/jf070831p. [DOI] [PubMed] [Google Scholar]
- 31.Atal CK, Dubey RK, Singh J. Biochemical basis of enhanced drug bioavailability by piperine: Evidence that piperine is a potent inhibitor of drug metabolism. J Pharmacol Exp Ther. 1985;232:258–262. [PubMed] [Google Scholar]
- 32.Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, Liu S, Dontu G, Wicha MS. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0612-x. doi:0.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bezerra DP, de Castro FO, Alves AP, Pessoa C, de Moraes MO, Silveira ER, Lima MA, Elmiro FJ, de Alencar NM, Mesquita RO, Lima MW, Costa-Lotufo LV. In vitro and in vivo antitumor effect of 5-FU combined with piplartine and piperine. J Appl Toxicol. 2008;28:156–163. doi: 10.1002/jat.1261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.