Abstract
Background
Pollen is the most common aeroallergen to cause conjunctivitis. In this study, we established a short ragweed (SRW)-induced mouse model of allergic conjunctivitis (AC) and aimed to explore the potential role of miR-146a and its downstream molecules in the development of ocular allergic inflammation.
Material/Methods
The mouse model of challenge pollen was used for in vivo study. The culture model of primary human limbal epithelium (HLE) exposed to lipopolysaccharide (LPS) was performed for in vitro research. The numbers of eosinophils and total inflammatory cells were examined using Giemsa staining. The expression of mRNA and miR-146a was determined by quantitative RT-PCR, and protein production was evaluated by Western blotting.
Results
In vivo of mice, pollen challenge induced conjunctiva inflammatory response indicated by increased number of eosinophils and total inflammatory cells. Interestingly, pollen significantly attenuated miR-146a expression while it enhanced expression of thymic stromal lymphopoietin (TSLP) and its downstream molecules, including TSLP receptor (TSLPR)/ OX40 ligand (OX40L)/CD11C. In vitro of HCE, downregulation effect of miR-146a expression induced by LPS was reversed by Bay treatment, an inhibitor for nuclear factor kappa B (NF-κB), and LPS-induced cell inflammation is mediated by miR-146a-TSLP/TSLPR/OX40L/CD11C signaling pathway. This was further demonstrated by overexpression of miR-146a in mouse abrogated pollen-triggered conjunctiva inflammatory reaction as well as pollen-induced activity of TSLP/TSLPR/OX40L/CD11C signaling.
Conclusions
Down-regulation of miR-146a expression induces allergic conjunctivitis in mice by increasing TSLP level.
Keywords: Conjunctivitis, Allergic; MicroRNAs; Pollen
Background
Allergic conjunctivitis (AC) is one of the most common ocular surface diseases. The disease encompasses a variety of pathological conditions, and can be subdivided into seasonal and perennial allergic conjunctivitis based on immunopathological mechanisms [1]. AC is an abnormal immune-hypersensitivity response to allergens, including animal dander and house dust mites, as well as pollen, which commonly triggers seasonal conjunctivitis [2]. Pollen is a ubiquitous substance and is regarded as the most difficult allergen to control [3]. There is no effective therapy due to lack of evidence of the underlying molecular mechanism by which pollen induces allergic inflammation of conjunctiva.
Thymic stromal lymphopoietin (TSLP), an epithelium-derived cytokine, is regarded as a novel pro-allergic molecule and can strongly activate dendritic cells through interaction with the TSLP receptor (TSLPR) to induce an inflammatory Th2-type response that is essential for initiating allergic inflammation [4]. The mechanism underlying TSLP-induced inflammation has been pointed out that TSLP-treated dendritic cells express OX40 ligand (OX40L), which interacts with OX40 to prime CD4+ T cells to produce the pro-allergic cytokines IL-4, IL-13, and IL-5 [4]. TSLP was found to be highly expressed in inflamed conjunctiva and its combining with its downstream signaling pathway TSLPR/OX40L/CD11C has proved to be involved in short ragweed (SRW) pollen-induced allergic inflammation [5,6]. Due to its important role in the initiation and maintenance of the allergic immune response, TSLP may become an important biomarker and therapeutic target for intervention in allergic inflammatory responses. Thus, a comprehensive elaboration on discovering the underlying mechanism of TSLP-mediated AC is urgently needed.
MicroRNAs are small non-coding RNAs that bind to multiple target mRNAs to control gene expression post-transcriptionally by inhibiting translation. In mammalian cells, microRNAs play important roles in a diverse array of cellular processes (e.g., cell proliferation and differentiation) [7,8]. Importantly, alterations in their levels may compromise cellular function, predisposing to disease [9]. Although there is no direct evidence focusing on miRNAs in AC, miRNAs have been reported to regulate allergic inflammation-related TSLP-signaling pathway in rhinitis and asthma [10]. For example, miR-21 is a negative regulator of Toll-like receptor 4 (TLR4) signaling and of the activation of nuclear factor kappa B (NF-κB) [11]. Up-regulated expression of 1 candidate miRNA, miR-126, was dependent on both TLR4 and MyD88, linking expression with activation of this innate immune pathway and the induction of allergic disease [12]. miR-21 was correlated with HSV-induced BD-like inflammation in mice as well as in Behçet’s Disease (BD) patients [13]. miR-146a, initially linked with the inflammatory response by virtue of their potent up-regulation in multiple immune cell lineages by Toll-like receptor ligands, inflammatory cytokines, and specific antigens, is involved in many inflammatory response in several diseases, including keratinocytes [14]. However, the pathogenic role of the miR-146a in the development of AC remains unknown.
To uncover the novel molecular signaling pathways involved in pollen-induced allergic inflammation, the present study was conducted using a well-characterized mouse model of allergic conjunctivitis induced by SRW pollen in BALB/c, as well as a lipopolysaccharide (LPS)-stimulated inflammatory model of cultured human corneal limbal epithelial cells.
Material and Methods
Regents
Ambrosia artemisiifolia short ragweed (SRW) pollen was purchased from Greer Lab (Lenoir, NC). Lipopolysaccharides (LPS), isolated from Escherichia coli, were purchased from Sigma (Sigma-Aldrich Co., USA). Bay 11-7082, an inhibitor for NF-κB via inhibiting cytokine-induced IκB-α phosphorylation, was purchased from Sigma (Sigma-Aldrich Co., MO, USA). Inject Alum Adjuvant was purchased from Pierce Biotechnology (Rockford, IL, USA)
Animals and Model of experimental allergic conjunctivitis (EAC)-induced by SRW pollen
The animal experimental protocols were approved by the Institutional Animal and Care Committee of Zhejiang University. BALB/c mice, aged 4–6 weeks, were obtained from Nanjing Jun Biological Engineering Co., LTD (Nanjing, China) and maintained in specific pathogen-free conditions in micro-isolator cages according to the guidelines provided in Zhejiang University Laboratory Animal Center.
The mouse model of allergic conjunctivitis was induced by SRW pollen. Briefly, mice were previously immunized with SRW pollen combined with Inject Alum Adjuvant (50 μg in 5 mg) by footpad injection. Ten days after immunization, allergic conjunctivitis was induced by SRW pollen drops mixed with phosphate-buffered saline (PBS, pH 7.2; 1.5 mg in 10 μl) once a day (n=5). PBS drops treatment was used as control (n=5). Animals’ action was examined on minute 20 after SRW pollen treatment. Twenty-four hours after SRW challenge, the corneal epithelium and conjunctiva were harvested for gene expression assays.
Culture of human corneal limbal epithelium
Human corneal limbal epithelium (HLE) was cultured from explants of human donor corneoscleral rims, provided by the First Affiliated Hospital of Zhejiang University. Each corneoscleral rim was trimmed and digested by Dispase for 15 minutes. The digestive mixture was dissected and applied to 6-well plastic dishes with a drop of FBS overnight. The cells were cultured in supplemented hormonal epithelial medium (SHEM) containing equal amounts of DMEM and Ham’s F12 medium, supplemented with 5% FBS, 0.5% dimethyl sulfoxide, 2 ng/ml EGF, 5 mg/ml insulin, 5 mg/ml transferrin, 5 ng/ml selenium, 0.5 mg/ml hydrocortisone, 30 ng/ml cholera toxin A, 50 mg/ml gentamicin, and 1.25 mg/ml amphotericin B at 37°C and 5% CO2. Cultures were maintained for 10 to 14 days until confluence up to 90% and then switched to the serum-free medium described above before the addition of treatments.
To demonstrate the effect of NF-κB LPS-induced inflammation of the corneal epithelium, primary cultures of HLE were treated with 5 μmol/L Bay or in combination with 0.5 μg/ml bacterial LPS. These treatments were maintained for 24 hours. After treatment, the culture supernatant was collected from each well, centrifuged, and stored in −80°C until assayed by qRT-PCR.
Giemsa staining
To detect inflammatory cell infiltration, eyes, including eyelids and conjunctiva, were exenterated and fixed in 4% paraformaldehyde for 24 hours. Then, they were cut into cross-sections 3-mm thick and stained with toluidine blue and hematoxylin-eosin for detection of total inflammatory cells and eosinophils, respectively. Infiltrating cells in the lamina propria mucosae of the palpebral and bulbar conjunctivas were presented by 2 masked observers (Palpebral zone and bulbar conjunctiva). The slides were evaluated by microscope at magnification ×400. Data are presented as mean ±SD per mm2.
Quantitative RT-PCR
For miR-146a and TSLP/TSLPR/OX40L/CD11C mRNA expression analysis, total miRNAs were isolated from mouse tissue specimens and human cells using TRIzol Reagent (Invitrogen, Carlsbad, USA) according to the manufacturer’s protocol. The purity of all RNA samples was quantified by NanoDrop® ND-1000 Spectrophotometer and stored at −80°C. The first strand cDNA was synthesized by RT from 1 μg of total RNA using superscript III reverse transcriptase (Takara, Tokyo, Japan). Real-time PCR was performed with specific primers and probes using TaqMan® gene expression assays and TaqMan® master mix (Applied Biosystems, Foster City, CA, USA). The results were analyzed by the comparative threshold cycle (Ct) method. miR-146a expression level is normalized by U6 and TSLP/TSLPR/OX40L/CD11C expression is normalized by GAPDH, respectively.
Western blot
Western blotting was performed to evaluate the expression of TSLP protein in HLE cells and conjunctiva tissue. Briefly, tissues or transfected cells were washed twice with cold phosphate-buffered saline (PBS) and were lysed with iced RIPA buffer containing 1% PMSF (KeyGen, Nanjin, China). After total protein detection using a BCA kit (Beyotime, Shanghai, China), protein lysates were separated on 8% SDS polyacrylamide gel, transferred to PVDF membranes, and blocked in 0.1% Tween 20 and 5% skim milk protein in Tris Buffer Saline at room temperature for 2 hours. Target proteins were probed with rabbit anti-TSLP antibody (1:500; Santa Cruz, USA), and rabbit anti-β-Actin antibody (1:2000; acted as an internal control, Zhongshan, Inc., Beijing, China) overnight at 4°C. The membranes were washed twice and visualized with horseradish peroxidase (HRP)-conjugated secondary antibodies for 2 hours. Tagged proteins were detected by enhanced chemiluminescence (KeyGen, Nanjing, China).
Knockdown and overexpression experiments
In human corneal limbal epithelial cells, for miR-146a knockdown, cells were transfected with miRCURY LNATM microRNA Power Inhibitors (Exiqon) for miR-146a, with nonsense strand as negative control (NC). For miR-146a overexpression, the cells were transfected with miRIDIAN Mimics for miR-146a (Dharmacon) with nonsense strand as Pre-negative control (Pre-NC). All transfections were carried out using lipofectamine 2000 according to the manufacturers’ protocol and cells were harvested after 48 hours for subsequent experiments. In BALB/c mice, for miR-146a overexpression, the mice were intravenously injected with miR-146a mimic (10 mg/kg). Forty-eight hours after injection, the mice were prepared for subsequent experimentation.
Dual luciferase reporter assays
Bioinformatics analysis of miR-146a target sites was performed using TargetScan Human Release 6.2 (http://www.targetscan.org/). Oligonucleotide pair was designed to contain miR-146a target sequences in 3′-UTR of TLSP. The pair was connected into the pmirGLO Dual-Luciferase miRNA Target Expression Vector which maintained the miRNA target region in the correct 5′ to 3′ orientation. HLE cells plated on 24-well plates were co-transfected with 50 nmol of -miR-146a mimics, inhibitor, or their NC and with 200 ng reporter plasmid using Lipofectamine 2000 Transfection Reagent according to the manufacturer’s instructions. Cells were lysed and the relative luciferase activities were quantified at hour 48 after transfection using the Dual Glo Luciferase Assay System (Promega, WI, USA). All transfections were carried out in triplicate.
Statistical analysis
All data were analyzed with SPSS software (version 16.0; SPSS Inc., Chicago, Illinois, United States). Student’s t-test was used to compare differences between the 2 groups. The data are presented as mean ±s.d. P values <0.05 were considered statistically significant.
Results
Inflammatory cells and signaling molecules in an allergic conjunctivitis mouse model induced by SRW
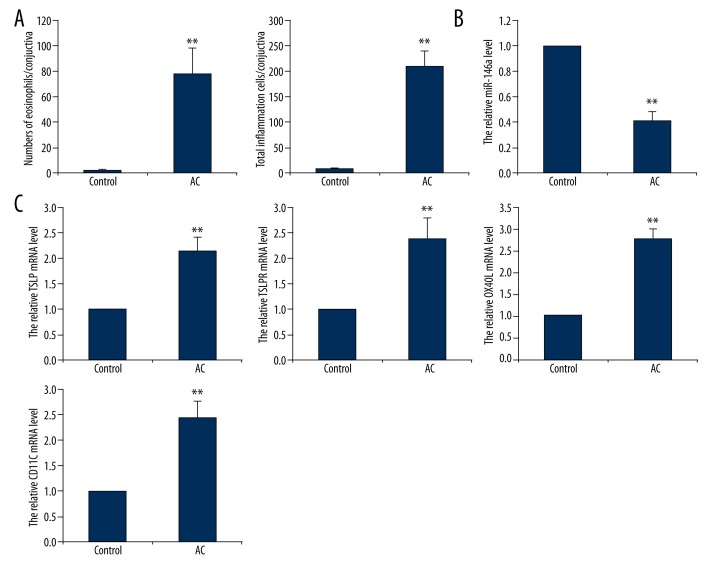
To explore the role of miR-146a and its possible downstream signaling pathway in pollen-induced inflammation of the conjunctiva, a model of allergic conjunctivitis (AC) was performed in BALB/c mice challenged by SRW pollen, with PBS-treated mice as control. Pollen challenge brought about human conjunctivitis behavior in mice, including eyelid edema, conjunctival congestion and edema, sweeping and frequent scratching eyelid. These pathologic signs lasted for 8~12 hours. One hour after the model was established, conjunctiva of mice were removed for further analysis. We counted the numbers of eosinophils cell and total inflammatory cells using Giemsa staining assay. As shown in Figure 1A, numbers of eosinophils and total inflammatory cells were substantially increased in AC mice compared to control mice. Evaluated by RT-qPCR, miR-146a expression was significantly downregulated (Figure 1B) in AC mice. On the contrary, pollen treatment enhanced expression of TSLP mRNA as well as increased mRNA levels of TSLPR, OX40L, and CD11C (Figure 1C).
Figure 1.
Signaling molecules expression in SRW-stimulated conjunctiva of mice. BALB/c mice were sensitized with SRW with Inject Alum Adjuvant for 10 days with PBS as control. After immune, individual mice were challenged with SRW through ocular instillation. Animals were sacrificed 24 hours after the challenge for following detection. (A) Quantitative numbers of eosinophils and total inflammatory cells in conjunctiva tissue using Giemsa staining. qRT-PCR was used to detect (B) level of miR-146a expression, and (C) mRNA levels of TSLP, TSLPR, OX40L and CD11C expression. AC, allergic conjunctivitis. Data are presented as mean ±s.d. ** P<0.01 compared with control with no SRW treatment.
NF-κB mediated inflammation-induced miR-146a and TSLP expression in LPS-stimulated human corneal limbal epithelial cells
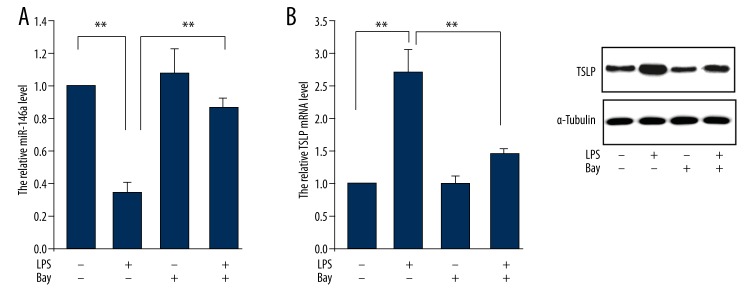
To further investigate the role of miR-146a in pollen-triggered conjunctivitis, we established an in vitro inflammation model of corneal cells by cells incubated with LPS (0.5 ug/ml). The cell was pretreated with or without Bay, an inhibitor for NF-κB, to examine the effect of NF-κB on the underlying molecule of conjunctiva inflammation. The result showed that LPS treatment induced expressional inhibition of miR-146a, which is consistent with that in pollen-stimulated allergic conjunctivitis. This inhibitory action was abrogated by Bay treatment (Figure 2A). Next, we evaluated TSLP expression in both mRNA and protein levels. The data showed that LPS increased TSLP expression while this promotion effect was inhibited by Bay treatment (Figure 2B).
Figure 2.
Effect of NF-κB on signaling molecule expression in LPS-treated human corneal limbal epithelial cells. Human corneal limbal epithelial cells were treated with LPS (0.5 ug/ml) and/or Bay (5 μmol/L) for 24 hours for following examination. (A) miR-146 expression and (B) mRNA and protein levels of TSLP were determined. Data are presented as mean ±s.d. ** P<0.01 compared with corresponding control.
MiR-146a negatively regulates TSLP expression in human corneal limbal epithelial cells
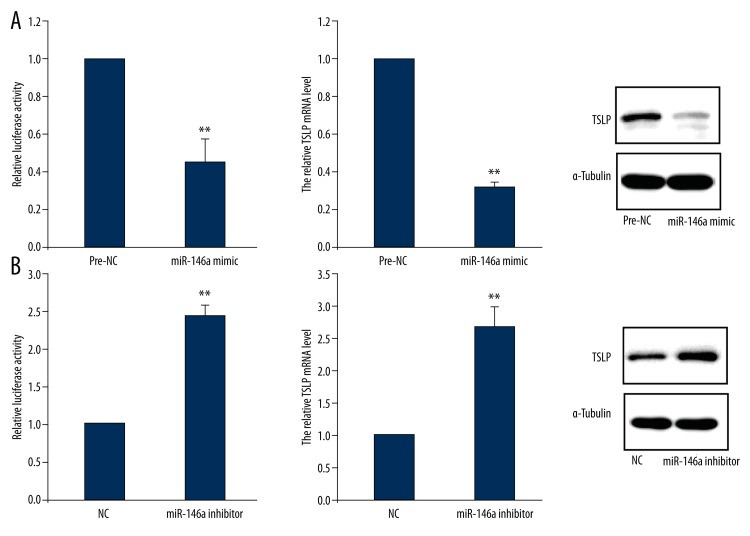
To determine the possible regulatory role of miR-146a on TSLP expression, we treated cells with miR-146a mimic or miR-146a inhibitor as well as their negative control accompanied by luciferase-labelled TSLP gene. TSLP expression was determined 48 hours after transfection. The data from the luciferase report plasmid analysis showed that overexpression of miR-146a attenuated TSLP mRNA 3′UTR activity. Accordingly, it also inhibited TSLP expression in both levels of miRNA and protein (Figure 3A). On the contrary, knock-down of miR-146a induced increase of activity of TSLP mRNA 3′UTR, as well as enhanced miR-146a expression of mRNA and protein (Figure 3B).
Figure 3.
miR-146a regulates TSLP expression in human corneal limbal epithelial cells. (A) Cells were treated with miR-146a mimic and then lysed for analysis of TSLP 3′UTR activity and TSLP expression. (B) Cells were treated with miR-146a inhibitor and then lysed for analysis of TSLP 3′UTR activity and TSLP expression. NC, negative control. Data are presented as mean ±s.d. ** P<0.01 compared with pre-NC or NC.
Overexpression of miR-146a abrogated LPS-induced TSLP expression in human corneal limbal epithelial cells
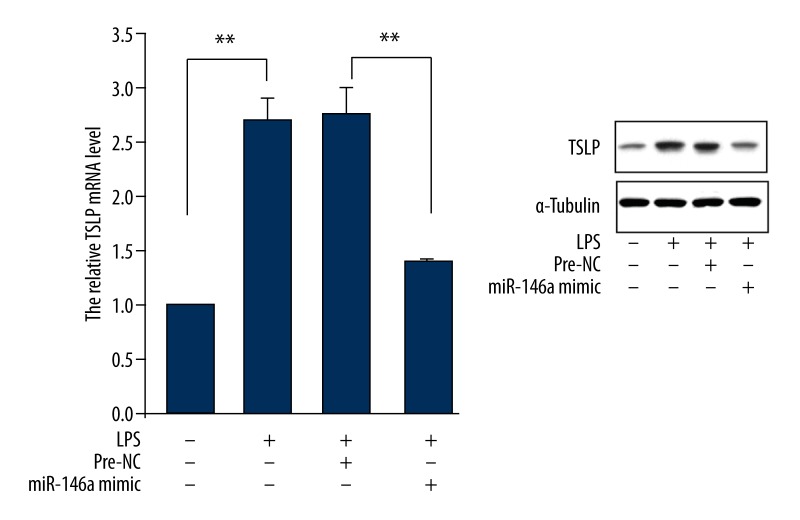
To verify regulated TSLP by miR-146a in LPS-stimulated corneal limbal epithelial cells, we treated cell with miR-146a mimic and observed TSLP expression. The result showed that LPS-induced increase expression of TSLP mRNA and protein was inhibited by overexpression of miR-146a (Figure 4).
Figure 4.
Effect of miR-146a overexpression on TSLP expression in LPS-treated human corneal limbal epithelial cells. Cells were pretreated with miR-146a mimic for 48 hours and stimulated by LPS (0.5 ug/ml), and 24 hours later, mRNA and protein levels of TSLP expression were determined. Data are presented with mean ±s.d. ** P<0.01 compared with corresponding control.
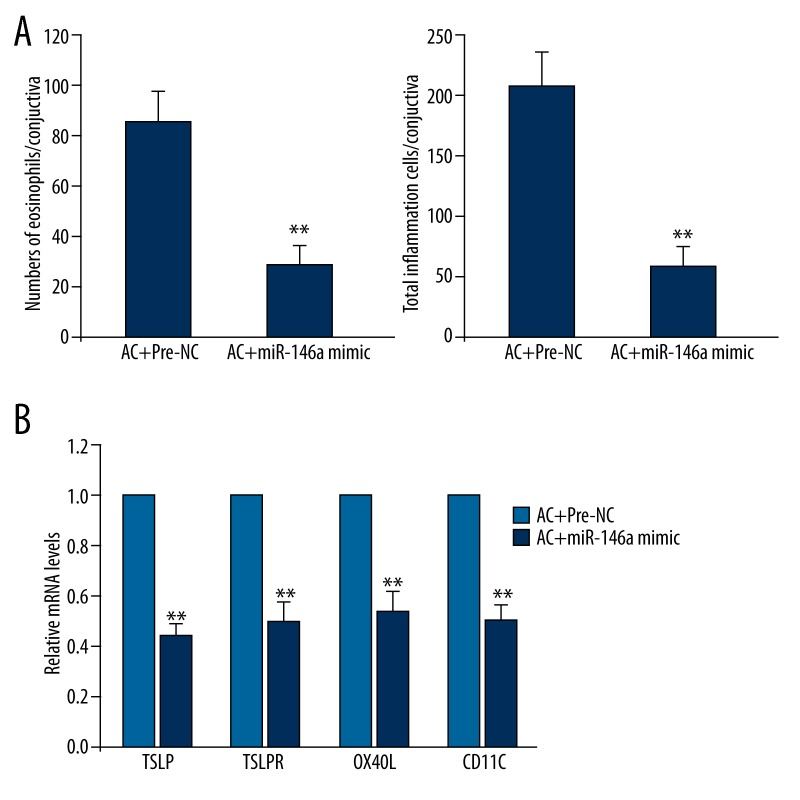
Overexpression of miR-146a reverses pollen effect in BALB/c mice
To explore whether miR-146a is the key regulator for pollen-triggered conjunctivitis, we observed inflammatory response and signaling pathway in miR-146a mimic-injected SRW-stimulated BALB/c mice. All these indexes were examined after 48 hours of miR-146a mimic vein intravenous injection. The numbers of eosinophils and total inflammatory cells were both decreased in conjunctiva simultaneous treated with pollen and miR-146a compared to pollen alone (Figure 5A). Pollen-induced increase of expression of TSLP and its downstream molecules, including TSLPR, OX40L, and CD11C, was also attenuated (Figure 5B).
Figure 5.
Effect of miR-146a overexpression on SRW-stimulated allergic conjunctivitis in mice. Before being challenged through ocular instillation with SRW, BALB/c mice were pretreated with miR-146a by injection. Animals were sacrificed 24 hours after the challenge and then (A) the numbers of eosinophils and total inflammatory cells and (B) mRNA levels of TSLP, TSLPR, OX40L, and CD11C were determined. Data are presented with mean ±s.d. ** P<0.01 compared with pre-NC+AC.
Discussion
Accumulating evidence suggests that disease occurrence is a process of genetic manipulation, in which miRNA, a ubiquitous non-coding RNA, has been shown to be the key regulator in disease pathogenesis. As such, recent studies proposed that miRNAs can serve as important therapeutic targets in a wide range of human diseases [15–17] in which they can regulate the expression of key genes [18]. In our present study, we identified miR-146a as a new regulator for pollen-induced AC, and that, combined with its downstream pathway of TSLP/TSLPR/OX40L/,
CD11C might be a potential therapy target in a well-characterized mouse AC model by SRW pollen. Furthermore, the data indicated that the miR-146a-mediated process of conjunctival inflammation was in a NF-κB-dependent pathway.
Although there have been numerous studies on the development of allergen-induced inflammation, the mechanisms leading to resolution of allergic inflammation remain poorly understood. This is an important challenge because failure to resolve allergen-driven inflammation potentially leads to recurrent or chronic allergic diseases. SRW pollen is a ubiquitous allergen that affects a large population worldwide and commonly induces AC [19]. It has been reported that AC is characteristic of inflammation occurrence in a Th2-dominant hypersensitivity that is mediated by the TSLP-induced inflammation reaction [5]. In the present study, we found that TSLP production and its downstream TSLPR/OX40L/CD11C molecules expression were elevated in repeatedly SRW pollen-triggered mice conjunctiva. Interestingly, we found that miR-146a, an inflammation-related miRNA, was downregulated in inflamed conjunctiva.
MiR-146a, extensively expressed in tissues, has been reported to be involved in various inflammation responses [20]. In this study, to identify whether miR-146a serves as a functional agonist capable of inducing TSLP triggered allergic inflammation, we demonstrated the stimulated TSLP/OX40L/OX40/CD11C signaling pathway in human corneal cells model by LPS stimulated inflammation. We selected the LPS-induced model of inflammation because there is a potential link between bacterial pathogens and allergic conjunctivitis [21]. From the result, we observed the evidence that miR-146a functioned as a key negative regulator for TSLP/TSLPR/OX40L/CD11C signaling in primary human corneal cells. This result suggested that miR-146a might serve as a potential therapeutic target for inflammation. We next demonstrated TSLP signaling in allergic inflammation via a miR-146a-dependent innate immune response. This novel phenomenon was revealed by evidence that LPS-induced elevated TSLP expression was abrogated by miR-146a mimic treatment in primary cultured human corneal cells, strongly confirmed by evidence that all clinical signs of allergic conjunctivitis (data not shown), the numbers of eosinophils and total inflammatory cells, as well as TSLP production and its downstream signals, were significantly reduced or eliminated in miR-146a mimic combined with SRW pollen-treated BALB/c mice.
In the human culture model, we observed that LPS stimulated TSLP expression and miR-146a expression were abrogated by the NF-κ B activation inhibitor, Bay [22]. This result further supports our hypothesis that miR-146a serves as a functional inflammation mediator because NF-κB is a well-known signaling pathway of inflammation response as well as allergic inflammation. Although this data suggested that allergen-induced miR-146a was NF-κB-dependent, further studies are necessary to explore the signaling pathways involved in the NF-κ B-mediated miR-146a innate immune response induced by SRW.
Conclusions
In conclusion, we have for the first time uncovered a novel miRNA – miR-146a – serving as a functional inflammation mediator, that induces TSLP/OX40L/OX40/CD11C signaling to trigger allergic inflammation via NF-κ B-dependent innate immunity pathways in a mouse model of short ragweed pollen-induced AC. These novel findings shed light on the understanding of innate immune reaction and pathogenesis of allergic inflammation, and may create new therapeutic targets to cure allergic disease.
Footnotes
Source of support: Science and technology foundation of Zhejiang province, China (No. 2013C33124)
Competing interests
The authors have no actual or potential conflicts of interest to declare.
Reference
- 1.Derrick T, Roberts C, Rajasekhar M, et al. Conjunctival MicroRNA expression in inflammatory trachomatous scarring. PLoS Negl Trop Dis. 2013;7:e2117. doi: 10.1371/journal.pntd.0002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dadaci Z, Borazan M, Kiyici A, Oncel Acir N. Plasma vitamin D and serum total immunoglobulin E levels in patients with seasonal allergic conjunctivitis. Acta Ophthalmol. 2014;92:e443–46. doi: 10.1111/aos.12398. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda K, Ishida W, Harada Y, et al. Prevention of allergic conjunctivitis in mice by a rice-based edible vaccine containing modified Japanese cedar pollen allergens. Br J Ophthalmol. 2015;99(5):705–9. doi: 10.1136/bjophthalmol-2014-305842. [DOI] [PubMed] [Google Scholar]
- 4.Takai T. TSLP expression: cellular sources, triggers, and regulatory mechanisms. Allergol Int. 2012;61:3–17. doi: 10.2332/allergolint.11-RAI-0395. [DOI] [PubMed] [Google Scholar]
- 5.Li DQ, Zhang L, Pflugfelder SC, et al. Short ragweed pollen triggers allergic inflammation through Toll-like receptor 4-dependent thymic stromal lymphopoietin/OX40 ligand/OX40 signaling pathways. J Allergy Clin Immunol. 2011;128(6):1318–25.e2. doi: 10.1016/j.jaci.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng X, Ma P, de Paiva CS, et al. TSLP and downstream molecules in experimental mouse allergic conjunctivitis. Invest Ophthalmol Vis Sci. 2010;51(6):3076–82. doi: 10.1167/iovs.09-4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathieu J, Ruohola-Baker H. Regulation of stem cell populations by microRNAs. Adv Exp Med Biol. 2013;786:329–51. doi: 10.1007/978-94-007-6621-1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelsvold DH, Utheim TP, Olstad OK, et al. miRNA and mRNA expression profiling identifies members of the miR-200 family as potential regulators of epithelial-mesenchymal transition in pterygium. Exp Eye Res. 2013;115:189–98. doi: 10.1016/j.exer.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reis AH, Vargas FR, Lemos B. More epigenetic hits than meets the eye: microRNAs and genes associated with the tumorigenesis of retinoblastoma. Front Genet. 2012;3:284. doi: 10.3389/fgene.2012.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suojalehto H, Lindstrom I, Majuri ML, et al. Altered microRNA expression of nasal mucosa in long-term asthma and allergic rhinitis. Int Arch Allergy Immunol. 2014;163(3):168–78. doi: 10.1159/000358486. [DOI] [PubMed] [Google Scholar]
- 11.Sheedy FJ, Palsson-McDermott E, Hennessy EJ, et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141–47. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 12.Mattes J, Collison A, Plank M, et al. Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc Natl Acad Sci USA. 2009;106:18704–9. doi: 10.1073/pnas.0905063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi B, Kim HA, Suh CH, et al. The Relevance of miRNA-21 in HSV-Induced Inflammation in a Mouse Model. Int J Mol Sci. 2015;16:7413–27. doi: 10.3390/ijms16047413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rebane A, Runnel T, Aab A, et al. MicroRNA-146a alleviates chronic skin inflammation in atopic dermatitis through suppression of innate immune responses in keratinocytes. J Allergy Clin Immunol. 2014;134(4):836–847.e11. doi: 10.1016/j.jaci.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Pan ZW, Lu YJ, Yang BF. MicroRNAs: a novel class of potential therapeutic targets for cardiovascular diseases. Acta Pharmacol Sin. 2010;31:1–9. doi: 10.1038/aps.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi J. Regulatory networks between neurotrophins and miRNAs in brain diseases and cancers. Acta Pharmacol Sin. 2015;36:149–57. doi: 10.1038/aps.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Figueira MF, Monnerat-Cahli G, Medei E, et al. MicroRNAs: potential therapeutic targets in diabetic complications of the cardiovascular and renal systems. Acta Physiol. 2014;211:491–500. doi: 10.1111/apha.12316. [DOI] [PubMed] [Google Scholar]
- 18.Imaizumi T, Tanaka H, Tajima A, et al. IFN-gamma and TNF-alpha synergistically induce microRNA-155 which regulates TAB2/IP-10 expression in human mesangial cells. Am J Nephrol. 2010;32:462–68. doi: 10.1159/000321365. [DOI] [PubMed] [Google Scholar]
- 19.Cordova C, Gutierrez B, Martinez-Garcia C, et al. Oleanolic acid controls allergic and inflammatory responses in experimental allergic conjunctivitis. PloS One. 2014;9:e91282. doi: 10.1371/journal.pone.0091282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rippo MR, Olivieri F, Monsurro V, et al. MitomiRs in human inflamm-aging: a hypothesis involving miR-181a, miR-34a and miR-146a. Exp Gerontol. 2014;56:154–63. doi: 10.1016/j.exger.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Deng R, Su Z, Lu F, et al. A potential link between bacterial pathogens and allergic conjunctivitis by dendritic cells. Exp Eye Res. 2014;120:118–26. doi: 10.1016/j.exer.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghashghaeinia M, Toulany M, Saki M, et al. The NFκ B pathway inhibitors Bay 11-7082 and parthenolide induce programmed cell death in anucleated Erythrocytes. Cell Physiol Biochem. 2011;27:45–54. doi: 10.1159/000325204. [DOI] [PubMed] [Google Scholar]