Abstract
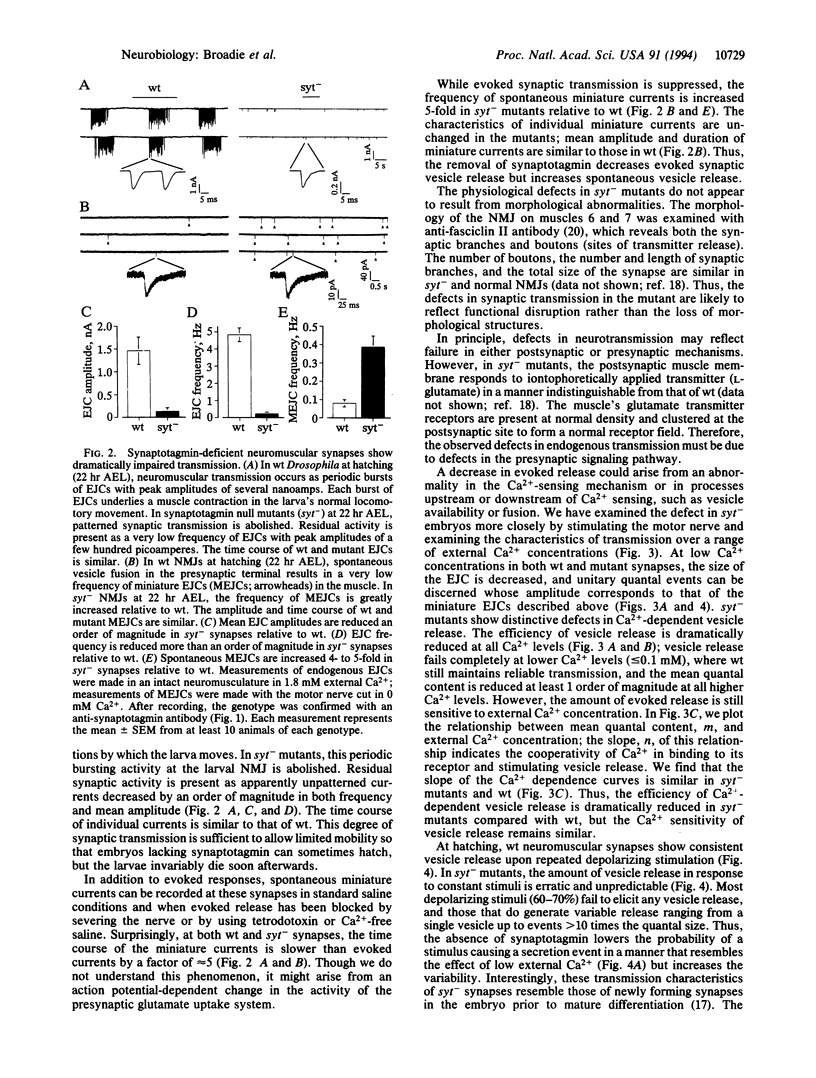
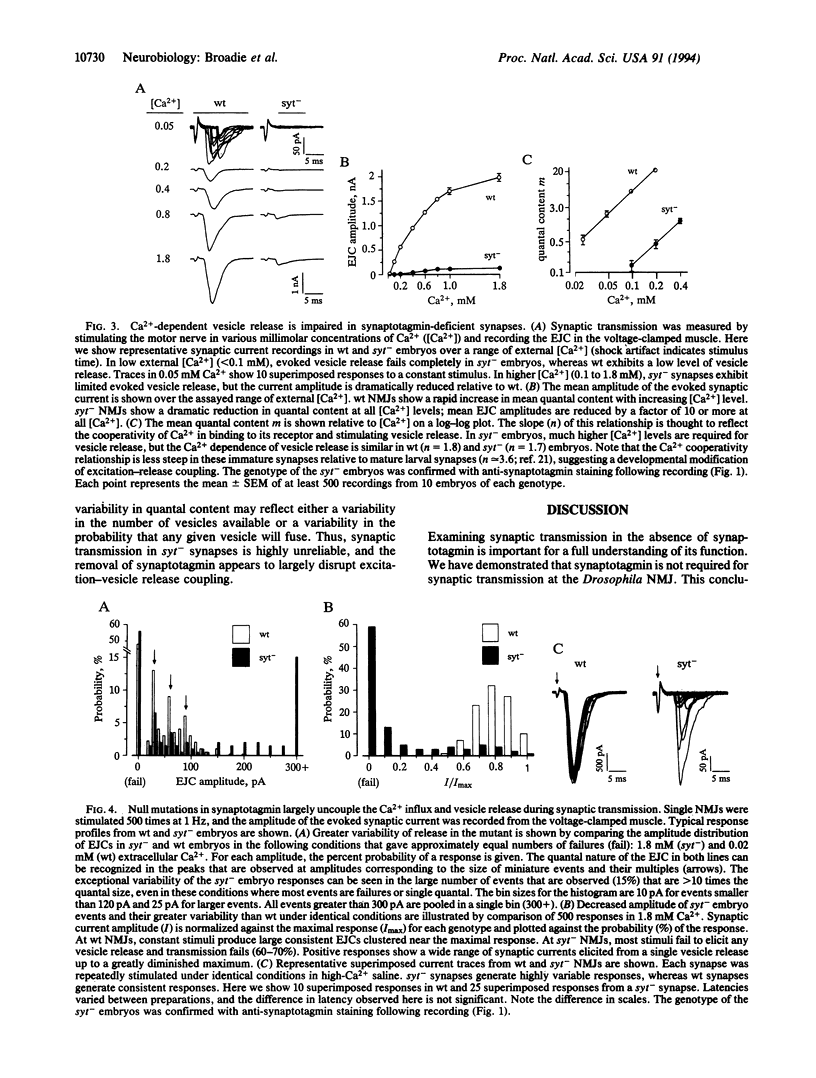
Synaptotagmin is an integral synaptic vesicle protein proposed to be involved in Ca(2+)-dependent exocytosis during synaptic transmission. Null mutations in synaptotagmin have been made in Drosophila, and the protein's in vivo function has been assayed at the neuromuscular synapse. In the absence of synaptotagmin, synaptic transmission is dramatically impaired but is not abolished. In null mutants, evoked vesicle release is decreased by a factor of 10. Moreover, the fidelity of excitation-secretion coupling is impaired so that a given stimulus generates a more variable amount of secretion. However, this residual evoked release shows Ca(2+)-dependence similar to normal release, suggesting either that synaptotagmin is not the Ca2+ sensor or that a second, independent Ca2+ sensor exists. While evoked transmission is suppressed, the rate of spontaneous vesicle fusion is increased by a factor of 5. We conclude that synaptotagmin is not an absolutely essential component of the Ca(2+)-dependent secretion pathway in synaptic transmission but is necessary for normal levels of transmission. Our data support a model in which synaptotagmin functions as a negative regulator of spontaneous vesicle fusion and acts to increase the efficiency of excitation-secretion coupling during synaptic transmission.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. K., Calakos N., Scheller R. H. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992 Jul 10;257(5067):255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Bommert K., Charlton M. P., DeBello W. M., Chin G. J., Betz H., Augustine G. J. Inhibition of neurotransmitter release by C2-domain peptides implicates synaptotagmin in exocytosis. Nature. 1993 May 13;363(6425):163–165. doi: 10.1038/363163a0. [DOI] [PubMed] [Google Scholar]
- Broadie K. S., Bate M. Development of the embryonic neuromuscular synapse of Drosophila melanogaster. J Neurosci. 1993 Jan;13(1):144–166. doi: 10.1523/JNEUROSCI.13-01-00144.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadie K., Bate M. Activity-dependent development of the neuromuscular synapse during Drosophila embryogenesis. Neuron. 1993 Oct;11(4):607–619. doi: 10.1016/0896-6273(93)90073-z. [DOI] [PubMed] [Google Scholar]
- Brose N., Petrenko A. G., Südhof T. C., Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992 May 15;256(5059):1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Dan Y., Poo M. M. Quantal transmitter secretion from myocytes loaded with acetylcholine. Nature. 1992 Oct 22;359(6397):733–736. doi: 10.1038/359733a0. [DOI] [PubMed] [Google Scholar]
- DiAntonio A., Parfitt K. D., Schwarz T. L. Synaptic transmission persists in synaptotagmin mutants of Drosophila. Cell. 1993 Jul 2;73(7):1281–1290. doi: 10.1016/0092-8674(93)90356-u. [DOI] [PubMed] [Google Scholar]
- DiAntonio A., Schwarz T. L. The effect on synaptic physiology of synaptotagmin mutations in Drosophila. Neuron. 1994 Apr;12(4):909–920. doi: 10.1016/0896-6273(94)90342-5. [DOI] [PubMed] [Google Scholar]
- Elferink L. A., Peterson M. R., Scheller R. H. A role for synaptotagmin (p65) in regulated exocytosis. Cell. 1993 Jan 15;72(1):153–159. doi: 10.1016/0092-8674(93)90059-y. [DOI] [PubMed] [Google Scholar]
- Elferink L. A., Scheller R. H. Synaptic vesicle proteins and regulated exocytosis. J Cell Sci Suppl. 1993;17:75–79. doi: 10.1242/jcs.1993.supplement_17.11. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Dennis M. J. Two mutations of synaptic transmission in Drosophila. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):87–108. doi: 10.1098/rspb.1977.0087. [DOI] [PubMed] [Google Scholar]
- Kelly R. B. Storage and release of neurotransmitters. Cell. 1993 Jan;72 (Suppl):43–53. doi: 10.1016/s0092-8674(05)80027-3. [DOI] [PubMed] [Google Scholar]
- Leveque C., Hoshino T., David P., Shoji-Kasai Y., Leys K., Omori A., Lang B., el Far O., Sato K., Martin-Moutot N. The synaptic vesicle protein synaptotagmin associates with calcium channels and is a putative Lambert-Eaton myasthenic syndrome antigen. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3625–3629. doi: 10.1073/pnas.89.8.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton J. T., Bellen H. J., Perin M. S. Expression of synaptotagmin in Drosophila reveals transport and localization of synaptic vesicles to the synapse. Development. 1993 Aug;118(4):1077–1088. doi: 10.1242/dev.118.4.1077. [DOI] [PubMed] [Google Scholar]
- Littleton J. T., Stern M., Schulze K., Perin M., Bellen H. J. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca(2+)-activated neurotransmitter release. Cell. 1993 Sep 24;74(6):1125–1134. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
- Matthew W. D., Tsavaler L., Reichardt L. F. Identification of a synaptic vesicle-specific membrane protein with a wide distribution in neuronal and neurosecretory tissue. J Cell Biol. 1981 Oct;91(1):257–269. doi: 10.1083/jcb.91.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M. L., Grundahl K., Meyer B. J., Rand J. B. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell. 1993 Jul 2;73(7):1291–1305. doi: 10.1016/0092-8674(93)90357-v. [DOI] [PubMed] [Google Scholar]
- Perin M. S., Brose N., Jahn R., Südhof T. C. Domain structure of synaptotagmin (p65) J Biol Chem. 1991 Jan 5;266(1):623–629. [PubMed] [Google Scholar]
- Perin M. S., Fried V. A., Mignery G. A., Jahn R., Südhof T. C. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990 May 17;345(6272):260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- Petrenko A. G., Perin M. S., Davletov B. A., Ushkaryov Y. A., Geppert M., Südhof T. C. Binding of synaptotagmin to the alpha-latrotoxin receptor implicates both in synaptic vesicle exocytosis. Nature. 1991 Sep 5;353(6339):65–68. doi: 10.1038/353065a0. [DOI] [PubMed] [Google Scholar]
- Popov S. V., Poo M. M. Synaptotagmin: a calcium-sensitive inhibitor of exocytosis? Cell. 1993 Jul 2;73(7):1247–1249. doi: 10.1016/0092-8674(93)90352-q. [DOI] [PubMed] [Google Scholar]
- Shirataki H., Kaibuchi K., Sakoda T., Kishida S., Yamaguchi T., Wada K., Miyazaki M., Takai Y. Rabphilin-3A, a putative target protein for smg p25A/rab3A p25 small GTP-binding protein related to synaptotagmin. Mol Cell Biol. 1993 Apr;13(4):2061–2068. doi: 10.1128/mcb.13.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T., Bennett M. K., Whiteheart S. W., Scheller R. H., Rothman J. E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993 Nov 5;75(3):409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Ushkaryov Y. A., Petrenko A. G., Geppert M., Südhof T. C. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992 Jul 3;257(5066):50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- Vactor D. V., Sink H., Fambrough D., Tsoo R., Goodman C. S. Genes that control neuromuscular specificity in Drosophila. Cell. 1993 Jun 18;73(6):1137–1153. doi: 10.1016/0092-8674(93)90643-5. [DOI] [PubMed] [Google Scholar]